Abstract
Metastasis is the major cause of morbidity and mortality in cancer. Recent studies reveal a role of chemotaxis in cancer cell metastasis. Epidermal growth factor receptors (EGFR) have potent chemotactic effects on human breast cancer cells. Lipid rafts, organized microdomain on plasma membranes, regulate the activation of many membrane receptors. In the current study, we investigated the role of lipid rafts in EGFR-mediated cancer cell chemotaxis. Our confocal microscopy results suggested that EGFR co-localized with GM1-positive rafts. Disrupting rafts with methyl-β-cyclodextrin (mβCD) inhibited EGF-induced chemotaxis of human breast cancer cells. Supplementation with cholesterol reversed the inhibitory effects. Pretreatment with mβCD also impaired directional migration of cells in an in vitro “wound healing” assay, EGF-induced cell adhesion, actin polymerization, Akt phosphorylation and protein kinase Cζ (PKCζ) translocation. Taken together, our study indicated that integrity of lipid rafts was critical in EGF-induced chemotaxis of human breast cancer cells.
Introduction
Chemotaxis is based on the cellular capacity to detect an extracellular gradient of chemical stimuli and move to the higher concentration site (Iijima et al. [Citation2002], Devreotes & Janetopoulos [Citation2003]). Recent studies indicate that chemotaxis plays an important role in the metastatic spread of cancer cells (Muller et al. [Citation2001], Salcedo et al. [Citation2002]). Therefore, investigation of the molecular mechanism of cancer cell chemotaxis may provide important means of inhibiting metastasis and improving cancer survival. Activity of EGFR is closely associated with progression of many cancers (Bogdan & Klämbt [Citation2000], Olayioye et al. [Citation2000]). EGFR, a transmembrane receptor, belongs to the receptor tyrosine kinase family (Bogdan & Klämbt [Citation2000]). Upon dimerization of EGFR, typically induced by its ligands, phosphorylation of several tyrosine residues in the cytoplasmic tail regions results in activation of a spectrum of downstream signaling pathways (Bogdan & Klämbt [Citation2000], Schlessinger [Citation2000]). For example, mitogen-activated protein (MAP) kinase pathway regulates cell proliferation and survival (Davis [Citation2000]), and PKC pathway imposes a negative feedback on EGFR activity (Schlessinger [Citation2000]). Our recent results indicate that the PI3 kinase-Akt-PKCζ pathway is required for EGF-induced chemotaxis (Sun et al. [Citation2005]).
Recently, various cholesterol and sphingolipids-rich microdomains have been identified on plasma membranes as lipid rafts, which play a critical role in regulating a large collection of membrane receptors, and consequently orchestrating numerous biological processes, including virus infection, activation of lymphocyte T cell receptors, apoptosis, cell adhesion and migration, synaptic transmission, organization of the cytoskeleton, and protein sorting during both exocytosis and endocytosis (Brown & London [Citation1998], Langlet et.al. 2000, Simons & Toomre [Citation2000], Harris & Siu [Citation2002], Tsui-Pierchala et al. [Citation2002], Saknmoto et al. [Citation2005]). Several studies have shown that EGFR is a raft-associated receptor (Furuchi & Anderson [Citation1998], Mineo et al. [Citation1999], Yamabhai & Anderson [Citation2002]). Cholesterol depletion from rafts causes an increase in the amount of active MAP kinase upon EGF stimulation (Furuchi & Anderson [Citation1998]). However, it is still unclear whether EGFR is located in caveolae or GM1-positive rafts (Mineo et al. [Citation1999], Roepstorff et al. [Citation2002]). We hypothesized that lipid rafts played an important role in EGFR-mediated chemotactic signal transduction. To test this, we investigated the localization of EGFR relative to the rafts and the cellular chemotactic responses to EGF after raft disruption.
Materials and methods
Materials
Human breast cancer cell line MDA-MB-231 was obtained from American Type Culture Collection (Manassas, VA). Stable transfected MDA-MB-231 cells expressing PKCζ-GFP were generated in our laboratory. Cell lines, T47D and MCF-7, as well as RPMI 1640 medium, were from Invitrogen (Carlsbad, CA). Fetal calf serum was purchased from Hyclone (South Logan, UT). Methyl-β-cyclodextrin (m?CD), cholesterol and 0.1% fibronectin were from Sigma (St Louis, MO). Chemotaxis chambers and membranes were purchased from Neuroprobe (Gaithersburg, MD). Human EGF and recombinant human SDF-1α were from Peprotech (Rocky Hill, NJ). LY294002 and PD98059 were from Calbiochem (La Jolla, CA). Antibodies toward EGFR, Akt and integrin β3 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibody to caveolin was from BD Transduction (San Jose, CA). Antibodies to MAP kinase and phosph-MAP kinase were purchased from Cell Signaling Technology Inc. (Beverly, MA). Goat anti-rabbit IgG-FITC and goat anti-mouse IgG-FITC were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Oregon Green 514-phalloidin, CTB-Alexa 555 and goat anti-mouse IgG-Alexa 546 were from Molecular Probes, Inc. (Eugene, OR). Protease inhibitors cocktail was from Roche. (Penzberg, Germany). BCA protein analysis kit, PVDF membranes and enhanced chemiluminescence reagent were from Pierce (Rockford, IL).
Cell culture
Human breast cancer cell lines MDA-MB-231, T47D and MCF-7 were cultured in RPMI 1640 containing 10% fetal calf serum.
Cholesterol depletion and cholesterol repletion
The same concentration of mβCD and mβCD/cholesterol complex were used in all experiments. The mβCD/cholesterol complex was prepared as described previously (Furuchi & Anderson [Citation1998]). Cholesterol depletion was carried out as reported previously (Roepstorff et al. [Citation2002]). For cholesterol depletion, cells were serum-starved for 3 h before being incubated with a medium containing 100 µmol l−1 mβCD for 1 h at 37°C. Cholesterol repletion was carried out by incubating cells in the presence of mβCD/cholesterol complex (100 µmol l−1 mβCD-10 µmol l−1 cholesterol) for 1 h at 37°C. Cells were then washed once with PBS before further experiments.
Isolation of raft
Rafts were isolated from the MDA-MB-231 cells as described previously (Pike & Casey [Citation1996]). After a 2-d culture, cells were starved for 3 h. The pellet of treated or untreated cells with 100 µmol l−1 mβCD was then lysed with 0.42 ml lysis buffer (25 mmol l−1 MES, pH 6.5, 150 mmol l−1 NaCl, 1% Triton X-100, 1 mmol l−1 EGTA, 1 mmol l−1 PMSF, 1 mmol l−1 NaF, 1 mmol l−1 Na3VO4, 2.5 mmol l−1 Na4P2O7, and 1×protease inhibitors cocktail) on ice for 10 min with frequent agitation. The lysate was mixed with equal volumes of buffer A (25 mmol l−1 MES, pH 6.5, 150 mmol l−1 NaCl, 1 mmol l−1 EGTA, and 80% (w/v) sucrose). 2.5 ml of buffer B (25 mmol l−1 MES, pH 6.5, 150 mmol l−1 NaCl, 1 mmol l−1 EGTA, and 30% (w/v) sucrose) and 1.66 ml of buffer C (25mmol l−1 MES, pH 6.5, 150 mmol l−1 NaCl, 1 mmol l−1 EGTA, and 5% (w/v) sucrose) were layered on top of the cell lysate. The gradient was then ultra-centrifuged for 16 h at 4°C at 175,000 g in a RPS50-2 rotor; 0.5 ml of each fraction was drawn from the top of the gradient. Pellets were resuspended in lysis buffer and sonicated twice, each for 2 sec; 20 µl of each fraction was loaded onto the SDS-PAGE. The western blotting analysis was carried out using anti-EGFR and anti-caveolin antibodies.
Chemotaxis assay
Chemotaxis assay was performed as described previously (Sun et al. [Citation2005]). Control and pretreated cells were suspended in binding medium (RPMI 1640, 0.1% BSA, and 25 mmol l−1 HEPES) at the density of 0.5×106 cells/ml. Chemoattractant, EGF or SDF-1α, was loaded into the lower chemotaxis chamber. A 10 µm filter membrane pre-coated overnight at 4°C with 10 µg ml−1 fibronectin in serum-free medium was put between the chambers. Cell suspension was then added into the upper chamber and incubated at 37°C in 5% CO2 for 3 h. Filter membranes were then rinsed and stained afterwards. The number of cells that migrated into the membrane was counted with a light microscope at 400×. Chemotaxis index was calculated as the ratio of the migrated cell number in a chemoattractant gradient versus that in a medium control. For chemokinesis assay (checkerboard assay), control or pretreated MDA-MB-231 cells were suspended in medium containing different concentrations of EGF before addition to the upper chamber.
Adhesion assay
Adhesion assay was carried out as described earlier in our lab (Sun et al. [Citation2005]). 1.5 ml of control or pretreated MDA-MB-231 cell suspension with or without 10 ng ml−1 EGF was added to a 35-mm dish containing a dried glass coverslip that had been pretreated with 10 µg ml−1 fibronectin in serum-free medium at 37°C for 2 h. After 5 min, cells were gently washed and fixed. Cells attached to the coverslip were counted under a light microscope at 200×.
Scratch assay/wound healing assay
MDA-MB-231 cells were cultured for two days in 35-mm dishes followed by serum-starvation for 3 h. Cells were then incubated with medium containing 100 µmol l−1 mβCD or mβCD/cholesterol complex at 37°C for 1 h. After a gentle wash, binding medium, with or without EGF (10 ng ml−1), was added to appropriate dishes. A “wound” was scratched in a confluent monolayer of cells on the coverslips. The width of wound was measured at different time points. The data were collected using time-lapse photography. Cell migration distances were calculated accordingly.
F-actin content assay
F-actin content was detected as described previously (Sun et al. [Citation2005]). In brief, MDA-MB-231 cells were treated with mβCD or mβCD/complex followed by stimulation of 50 ng ml−1 EGF at 37°C at different time points. Cells were then fixed, permeablized, and incubated with Oregon Green 514/phalloidin in F-actin buffer (10 mmol l−1 HEPES, 20 mmol l−1 KH2PO4, 5 mmol l−1 EGTA, 2 mmol l−1 MgCl2, PBS, pH 7.4) at room temperature for 2 h. Cells were washed five times. Labeled phalloidin that were bound to F-actin was extracted by methanol at 4°C for 90 min. Fluorescence was captured at Ex/Em 511/529 nm in each sample and normalized against total protein content as analysed by a BCA kit. The relative F-actin content over different time period was calculated by the following equation:
Western blotting assay
Western blotting assay was performed as described previously (Sun et al. [Citation2005]). In MAP kinase and Akt/PKB activation assay, treated or untreated MDA-MB-231 cells were stimulated with 10 ng ml-1 EGF for 1 min or 5 min. Cells were then collected and lysed. Cell lysate was loaded onto a 10% SDS-PAGE at 40 µg total protein per lane. Following gel separation, the proteins were transferred onto a PVDF membrane which was then blocked with 5% nonfat milk followed by incubation with primary antibody, anti-phosphorylated-Akt, anti-Akt, anti-phosphorylated-MAP kinase or anti-MAP kinase, respectively, and HRP-conjugated secondary antibody. Enhanced chemiluminescence reagent was used in the antibody detection process. In EGFR internalization assay, treated or untreated MDA-MB-231 cells were stimulated with 50 ng ml−1 EGF for 5 min, 15 min, 30 min and 60 min, respectively. And the stimulation was terminated by cold PBS. Cells were then separated into two parts. One part was lysed directly to detect total EGFR. The other was centrifugated at 12,000 rpm at 4°C for 15 min to isolate membrane portion from the whole cell. Then membrane partition was lysed and loaded on 8% SDS-PAGE at 50 µg total protein per lane. The following steps were similar to the process of Akt activation western blotting. The primary antibody was anti-EGFR (1:1000).
Flow cytometry assay
Cell surface expression level of EGFR was monitored by FACS analysis as described previously (Sun et al. [Citation2005]). MβCD-treated or normal MDA-MB-231 cells (5×105 cells/sample) were stimulated with 50 ng ml−1 EGF for various time, washed twice with ice-cold FACS buffer (Dulbecco's PBS (DPBS), 1% FCS, 5 mmol l−1 EDTA, and 0.1% sodium azide, pH 7.4), stained with anti-EGFR monoclonal antibody for 1 h on ice. After three complete washes with ice-cold FACS buffer, Alexa 546-labeled secondary antibody was applied for another 30 min on ice. The cells were washed twice, resuspended in FACS buffer and analysed immediately by a flow cytometry (BD Bioscience, San Jose, CA).
Immunofluorescence microscopy
The MDA-MB-231 cells were cultured in chamber slides one day before staining. For double staining of GM1 and EGFR/caveolin/integrin β3, cells were incubated with CTB-Alexa 555 conjugate at concentration of 1 µg ml−1 for 10 min at 4°C to label GM1. Cells were gently washed with chilled PBS, fixed with 4% paraformaldehyde for 10 min at room temperature, then permeablized with 0.1% Triton X-100 in PBS. After blocking with serum, cells were incubated overnight with primary antibody (anti-EGFR, anti-caveolin, or anti-integrin β3) at 4°C, washed, and incubated with secondary antibody. For double staining of EGFR and caveolin or, EGFR and integrin β3, cells were directly fixed before antibodies were added. Cells were visualized with an Olympus FV1000 Spectrum inverted fluorescent confocal microscope (Olympus, Inc., Japan).
Translocation of Akt in MDA-MB-231 cells was analysed as described previously (Sun et al. [Citation2005]). In brief, treated or untreated cells were stimulated with 10 ng ml−1 EGF at 37°C for 5 min before processed for immunofluorescence staining. Over 100 cells were examined for their fluorescent patterns. Cells with membrane-distribution of Akt were counted as positive cells. Percentage of positive cells of all examined cells was recorded.
MDA-MB-231 cells transfected with PKCζ-GFP were used in PKCζ translocation assay. Treated or untreated cells were stimulated with 10 ng ml−1 EGF at 37°C for 10 min. Cells were fixed, kept in PBS and directly visualized. Over 100 cells were examined for their fluorescent patterns. Cells with membrane-distribution of PKCζ-GFP were counted as positive cells. Percentage of positive cells of all examined cells was recorded.
Statistical analysis
Prism 3.0 software was used for data analysis. One-way ANOVA was applied for three groups of data. Two-way ANOVA was used for two groups of data.
Results
Localization of EGFR on breast cancer cell membrane
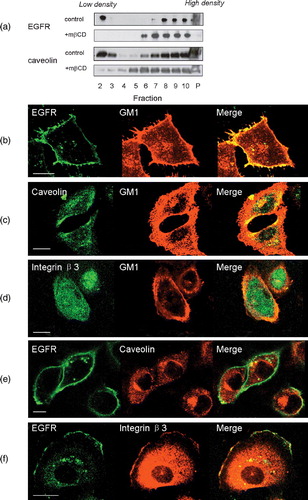
We first assessed the localization of EGFR on the plasma membrane of human breast cancer cells, MDA-MB-231. Both caveolae and GM1-positive rafts were lower in density than the remainder of the plasma membrane and could be separated from the rest of membrane by 1% Triton X-100 extraction followed by ultra-centrifugation in a sucrose-density gradient (Pike & Casey [Citation1996], Yamabhai & Anderson [Citation2002]). This was confirmed by our control experiment where caveolin, a marker of caveolae, was detected in these low density fractions (a). A considerable portion of EGFR was also detected in the low-density membrane fractions (a), consistent with previous reports (Pike & Casey [Citation1996], Furuchi & Anderson [Citation1998], Mineo et al. [Citation1999], Yamabhai & Anderson [Citation2002]). After treatment with mβCD, distribution of EGFR and Caveolin shifted to higher density membrane fractions, consistent with previous report (a, Pike & Miller [Citation1998], Pike & Casey [Citation2002], Peres et al. [Citation2003]). Next, we used a specific fluorescent marker to detect EGFR on cancer cell membrane. Fluorescent signals from EGFR-recognizing antibodies colocalized with those from Alexa 555 labeled cholera toxin B-chain (CTB-Alexa 555), a proven marker specific for GM1 (b). However, the signals from EGFR did not overlap with those from caveolin (e). Neither did fluorescence from EGFR-labeling overlap with those from integrin β3, a well-characterized non-raft membrane protein (f; Mineo et al. [Citation1996]). In control experiments, we showed that neither integrin β3 nor caveolin colocalized with GM1 (c and d; Mineo et al. [Citation1996], Roepstorff et al. [Citation2002]). These results suggested that a large portion of EGFR was associated with non-caveolae, GM1-containing rafts.
Figure 1. Localization of EGFR on cell membrane. (a) Distribution of EGFR and caveolin on membrane fractions analysed by western blotting with or without mβCD treatment. Fractions 2 to 10 were membrane fractions from low density to high density. P indicated the resuspended pellet fraction. Figure showed the representative data from two repeated experiments. (b–d) Colocalization analysis of GM1 with EGFR, caveolin or integrin β3 by immunofluorescence assay. (e and f) Colocalization analysis of EGFR with caveolin or integrin β3. Figures showed representative images from three repeated experiments. Scale bar, 10 µm. This figure is reproduced in color in Molecular Membrane Biology online.
EGF-induced chemotaxis in breast cancer cells with cholesterol depletion
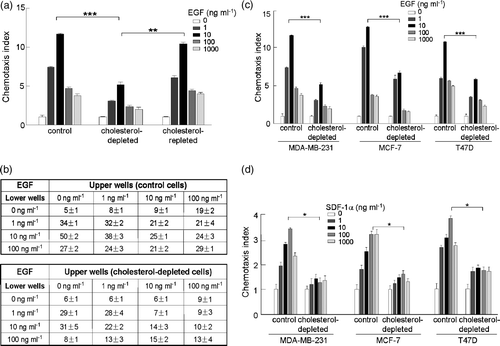
We next investigated the capacity of EGF to induce chemotaxis of human breast cancer cells after raft disruption. Cholesterol is a key component of lipid rafts. It has been well established that mβCD can form barrel-shape structures, specifically sequester cholesterol from cell membranes, and disrupt lipid rafts without affecting cell viability or other cytosolic activities (Ilangumaran & Hoessli [Citation1998], Rodal et al. [Citation1999]). EGF induced chemotaxis of MDA-MB-231 cells in a dose-dependent manner (a; Sun et al. [Citation2005]). After cholesterol depletion by pretreatment with 100 ?M mβCD, the chemotactic responses of MDA-MB-231 cells to EGF were impaired (a). Supplementation with of cholesterol (100 µmol l−1 mβCD-10 µM cholesterol) reversed the mβCD inhibitory effects, indicating that the inhibitory effects of mβCD resulted specifically from cholesterol depletion. Chemokinesis, a non-directional enhancement of cell migration, was examined by checkerboard analysis (b). Results showed that cholesterol depletion had only modest inhibitory effects on the EGF-induced chemokinesis and could not totally account for the loss in chemotaxis. Thus, the impairment in chemotaxis after mβCD pretreatment may also result from a loss in directional sensing. MβCD pretreatment also impaired EGF-induced chemotaxis of two additional human breast cancer cell lines, T47D and MCF-7 (c). Chemokine receptors, such as CXCR4, are known to play an important role in chemotaxis and metastasis (Muller et al. [Citation2001]). Our results showed that lipid rafts were also required for CXCR4-mediated chemotaxis of the three human breast cancer cell lines (d). Taken together, our results indicated that lipid rafts played an important role in EGFR-mediated chemotaxis of human breast cancer cells, as well as in CXCR4 chemokine receptor-mediated chemotaxis.
Figure 2. The effect of cholesterol-depletion on EGF-induced chemotaxis in human breast cancer cell lines. (a) Chemotactic dose-responses of normal, cholesterol-depleted and cholesterol-repleted MDA-MB-231 cells toward EGF stimulation (p*** <0.0001, p** =0.0154, two-way ANOVA). Chemotaxis index was calculated as that stated in the Materials and methods section. (b) Chemokinesis of normal and cholesterol-depleted MDA-MB-231 cells induced by EGF. (c) Chemotactic responses of normal and cholesterol-depleted cells toward EGF stimulation in three different human breast cancer cell lines, MDA-MB-231, MCF-7 and T47D cells (p*** ≤0.0001, two-way ANOVA). (d) Chemotactic responses of normal and cholesterol-depleted cells toward SDF-1α stimulation in three different human breast cancer cell lines, MDA-MB-231, MCF-7 and T47D cells (p* <0.05, two-way ANOVA). Data collected in this set of figures were from a representative of at least three repeated experiments. In each experiment, each data point was an average calculated from three chemotactic microchambers.
EGF-induced cell adhesion and migration
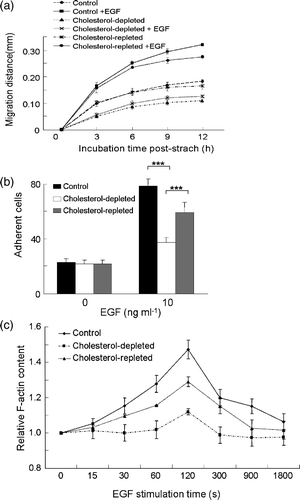
To confirm the involvement of rafts in EGF-induced chemotaxis, we further performed scratch assay, EGF-induced adhesion and actin polymerization assays. Scratch assay, an in vitro “wound healing” assay, is another method to assess directional migration capacity of cells (Manneville & Hall [Citation2002]). Cells sense the “wound” made by the scratch, reorganize their microtubule organization center, and move perpendicularly to the scratch to fill the “wound” in the absence of a chemical gradient (Wittmann & Waterman-Storer [Citation2001], Manneville & Hall [Citation2002]). As shown in a, cells pretreated with mβCD filled the “scratch wound” at a slower rate. Moreover, EGF-induced “wound healing” effects were also inhibited by mβCD pretreatment, consistent with the inhibitory effects of mβCD on chemotaxis. Furthermore, cholesterol supplement partially rescued the ability of wound healing. Ligand-induced cell adhesion is closely associated with chemotaxis. Following 5 min treatment, 10 ng ml−1 EGF induced a marked increase of cells adhesion to fibronectin-coated glass coverslips (b). Cholesterol depletion by mβCD inhibited EGF-stimulated rapid adhesion, which could be rescued by supplementation with cholesterol (b). Actin polymerization at the leading edges of a cell is the driving force for chemotaxis (Devreotes & Zigmond [Citation1988], Vasioukhin et al. [Citation2000], Manneville & Hall [Citation2002]). Ligand-induced transient actin polymerization correlates with chemotaxis activity of a cell (Devreotes & Zigmond [Citation1988]). As shown in c, EGF induced a transient polymerization of globular actin in MDA-MB-231 cells (Chan et al. [Citation1998], Sun et al. [Citation2005]). In the presence of mβCD, actin polymerization induced by 50 ng ml−1 EGF was significantly reduced (c). Supplementation with cholesterol reversed the inhibitory effects of mβCD. Taken together, these data showed that lipid rafts played a role in EGF-induced in vitro “wound healing”, cell adhesion and actin polymerization. These results provided further support to our observation that rafts were involved in EGFR or CXCR4-mediated chemotaxis of human breast cancer cells.
Figure 3. The effect of cholesterol depletion on EGF-induced cell migration and cell adhesion in MDA-MB-231 cells. (a) In vitro wound-healing assay on normal, cholesterol-depleted and cholesterol-repleted cells with or without EGF stimulation. Cell migration distance upon incubation time was measured after wound was created. EGF, 10 ng ml−1. (b) Cell adhesion of normal, cholesterol-depleted and cholesterol-repleted cells upon EGF stimulation (EGF, 10 ng ml−1, p*** ≤0.0001, two-way ANOVA). (c) Time course of EGF-induced F-actin polymerization on normal, cholesterol-depleted, and cholesterol-repleted cells (EGF, 50 ng ml−1, p=0.0004, one-way ANOVA). The data were collected from one representative of three repeated experiments. Each data point was an average of triplicate assays.
EGFR expression and internalization
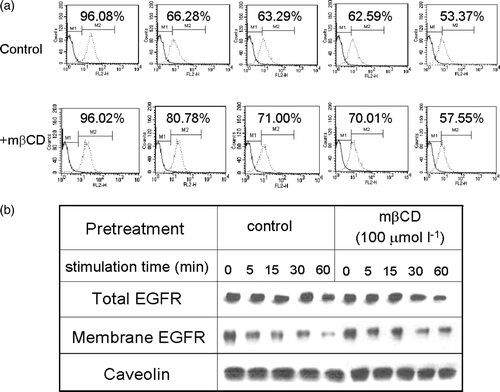
In a previous study, we have shown that disruption of lipid rafts reduced the level of chemokine receptors on the surface of HEK293 cells (Jiao et al. [Citation2005]). We suspected that disruption of lipid rafts may also reduce the level of EGFR on the surface of human breast cancer cells. Fluorescence activated cell sorter (FACS) analysis revealed the expression of EGFR on the cell surface (a). Treatment with EGF at 50 ng ml−1 elicited a decrease in EGFR in a time-dependent manner, probably due to receptor internalization and subsequent degradation (a). Depletion of cholesterol with 100 µmol l−1 m?CD slowed down the decrease in surface EGFR, but didn't alter the level of EGFR at 0 min. The results from western blotting analysis also showed a similar level of total EGFR after treatment with m?CD (b). Loss of EGFR after EGF treatment was consistent with the FACS analysis results. A slight higher level of EGFR was detected on the membrane fraction of mβCD-treated cells (b). Taken together, both FACS and western blotting analysis showed that disruption of lipid rafts didn't result in a decrease of cell surface EGFR.
Figure 4. The effect of cholesterol depletion on EGF expression and internalization. (a) MDA-MB-231 cells were treated with (the bottom panel) or without (the top panel) mβCD, stimulated with 50 ng ml−1 EGF for 0, 5, 15, 30, and 60 min, then followed by FACS analysis of surface EGFR. (b) MDA-MB-231 cells were treated with or without m?CD, followed by stimulation with 50 ng ml−1 EGF for 0, 5, 15, 30, 60 min. The levels of EGFR in total cell lysate (the top panel) or in the membrane fraction (the bottom panel) were analysed by western blotting analysis. The amount of caveolin in membrane fraction in each sample was analysed by western blotting and shown at the bottom.
EGF-induced activation of some important signaling molecules with cholesterol depletion
How did mβCD pretreatment inhibit EGF-induced chemotaxis since the level of surface EGFR did not alter? We hypothesized that raft disruption by mβCD pretreatment might block EGFR-mediated chemotactic signaling transduction. To test this hypothesis, we examined the activation of downstream chemotactic signaling components upon cholesterol depletion. In a control experiment, we first analysed EGF-induced MAP kniase activation, which was not involved in the chemotaxis pathway (Sun et al. [Citation2005]). Treatment with mβCD did not impair MAP kinase phosphorylation, consistent with a previous report (Furuchi & Anderson [Citation1998]). Thus, disruption of lipid rafts did not impose a pleiotropic inhibitory effect specifically on EGFR-mediated signal transdution.
Akt/PKB is a critical component of EGFR-mediated chemotactic signaling pathway (Sun et al. [Citation2005]). In MDA-MB-231 cells, EGF at 10 ng ml−1 induced a robust phosphorylation of Akt in 5 min, indicative of activation of the chemotactic signaling pathway (b; Sun et al. [Citation2005]). Pretreatment with mβCD caused a marked reduction of EGF-induced Akt phosphorylation (b). In a control experiment, treatment with LY294002, a PI3 kinase inhibitor, also impaired EGF-induced Akt phosphorylation (Okano et al. [Citation2000]). When cholesterol was supplied, EGF-induced Akt phosphorylation was partially restored (c). Since Akt translocation from cytosol to plasma membrane is the hallmark of Akt activation (Vivanco & Sawyers [Citation2002]), we further investigated whether mβCD pretreatment would inhibit EGF-induced Akt translocation. As shown in d, immunofluorescence staining using anti-Akt antibody revealed that Akt was mainly distributed in the cytosol of resting cells. We counted membrane-distribution of Akt as criteria for positive cells. In the absence of EGF, the proportion of positive cells in control, cholesterol-depleted, and cholesterol-repleted cells were 8.7±1.0%, 7.8±2.1%, and 6.8±0.7%, respectively (d). Stimulation with EGF increased the population of positive cells to 41.0±0.6% in control cells. Disruption of rafts with mβCD reduced this percentage to 20.5±1.7%. Supplementation with cholesterol partially redistributed Akt to the membrane (d). In summary, our results clearly indicated that lipid rafts were involved in EGF-induced activation of Akt/PKB, a critical chemotactic signaling molecule.
Figure 5. The effect of cholesterol depletion on EGF-induced MAP kinase phosphorylation, Akt activation and PKC?-GFP translocation in MDA-MB-231cells. (a) EGF-induced MAP kinase phosphorylation in the presence or absence of mβCD treatment. Total MAP kinase amount in each sample was analysed by western blotting and shown at the bottom. (b) Akt activation was analysed by western blotting for phosphorylated Akt (p-Akt/PKB) in control, LY294002 treated, and cholesterol-depleted (mβCD) cells upon EGF stimulation. Total Akt/PKB amount in each sample was analysed by western blotting and shown at the bottom. (c) Phosphorylated Akt (p-Akt/PKB) amount in control, cholesterol-depleted, and cholesterol-repleted cells upon EGF stimulation analysed by western blotting. Total input of Akt/PKB in each sample was shown at the bottom of the panel. (d) Akt/PKB cellular translocation of control, cholesterol-depleted, and cholesterol-repleted cells upon EGF stimulation (EGF, 10 ng ml−1). Bar graph, percentage of cells that exhibited Akt/PKB translocation of total examined cells under indicated assay conditions. Data were the mean of triplicate experiments (p*** <0.0001, p**? = 0.0030, two-way ANOVA????Images, representative cells that exhibited Akt/PKB translocation upon EGF stimulation. Arrows showed activated Akt/PKB on the membrane. Scale bar, 20 µm. (ecddd ) PKCζ?GFP translocation of control, cholesterol-depleted, and cholesterol-repleted cells upon EGF stimulation (EGF, 10 ng ml−1). In the bar graph, percentage of cells exhibited PKCζ?GFP translocation of total examined cells under indicated assay conditions. Data were the mean of triplicate experiments (p*** <0.0001, p**=0.0054, two-way ANOVA???In?images, representative cells displayed PKCζ-GFP cellular translocation upon EGF stimulation. Arrows showed activated PKCζ-GFP on the membrane. Scale bar, 30 µm. This figure is reproduced in color in Molecular Membrane Biology online.
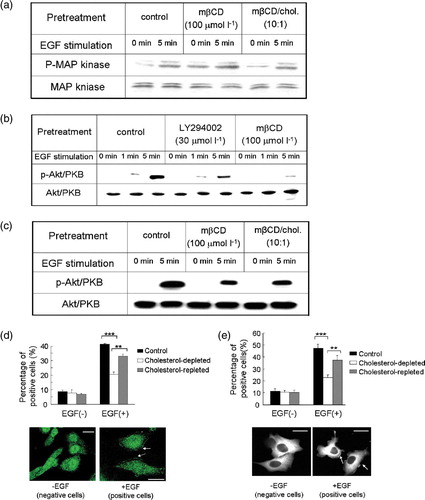
PKCζ? another signaling molecule, is required for EGFR-mediated chemotaxis (Sun et al. [Citation2005]). We examined PKCζ activation by monitoring PKCζ-GFP?translocation from cytosol to plasma membrane (Mochly-Rosen [Citation1995], Sun et al. [Citation2005]; our unpublished results). We designated cells containing fluorescent signals on their plasma membrane as positive cells. In the absence of EGF, control, cholesterol-depleted, and cholesterol-repleted cells exhibited minimal PKCζ activation, consisting of only 11.6±1.9%, 10.6±1.3%, and 10.4±1.8% positive cells, respectively (e). EGF induced a marked increase in positive cell population to 47.1±3.3% in control cells. Cholesterol depletion reduced this to 22.9±2.2%. Cholesterol addition restored the positive cell portion to 37.1±4.2% (e). The fact that cholesterol depletion caused a striking decrease in PKCζ activation further supported our hypothesis that lipid rafts were required for EGFR-mediated chemotactic signaling transduction.
Discussion
Our investigation clearly indicated that raft integrity was critical for EGFR-mediated chemotaxis of human breast cancer cells. Results of sucrose density gradient ultra-centrifugation and confocal fluorescent microscopy studies suggested that the EGFR was probably localized in GM1-positive rafts. Disruption of rafts by cholesterol depletion impaired EGF-induced chemotaxis, cell adhesion, and actin polymerization in MDA-MB-231 cells. The fact that m?CD pretreatment also inhibited chemotaxis of MCF-7 and T47D cells suggested that lipid rafts were generally involved in chemotaxis of human breast cancer cells. In addition, the integrity of rafts was also required for CXCR4-mediated chemotaxis. Studies on Akt/PKB and PKCζ activation revealed that pretreatment with mβCD interfered with EGF-induced chemotaxis signaling transduction. However, the detailed molecular mechanism of how lipid rafts are involved in EGFR-mediated signaling transduction is still unknown. We speculate that the integrity of rafts is essential for recruitment of downstream effectors to cytoplasmic tail of EGFR upon ligand binding. We are in the process of testing this hypothesis.
Lipid rafts played a different role in regulating EGFR-mediated signaling transduction in human breast cancer cells. Previous studies have shown that cholesterol depletion enhances EGFR-mediated activation of MAP kinase pathway, which plays a critical role in cell transformation and proliferation (Furuchi & Anderson [Citation1998], Davis, [Citation2000]). In our system, we also found disruption of rafts led to higher EGF-induced activation of MAP kinase in MDA-MB-231 cells by western blotting assay. The enhancement effects may result from hyperphosphorylation of MAP kniase by MAP kinase kinase caused by the decreased concentration of this enzyme in raft after cholesterol depletion (Burack & Sturgill [Citation1997], Furuchi & Anderson [Citation1998]), or an increase in ligand binding upon cholesterol depletion (Roepstorff et al. [Citation2002]). Our results showed an inhibitory effect in EGFR-mediated chemotaxis after disruption of lipid rafts. Clearly, our FACS and western blotting analysis indicated that the impairment of chemotaxis was not due to a loss of EGFR on cell surface. It seemed that lipid rafts were rather critical for the recruitment of downstream chemotactic signaling components, such as PKCζ, to the plasma membranes. In a previous report, we showed that lipid rafts played a critical role in G-protein coupled receptor-mediated chemotaxis (Jiao et al. [Citation2005]). Taken together, our results suggested that the integrity of lipid rafts was generally required in the chemotaxis of mammalian cells.
Our results provide a novel approach involving disruption of lipid rafts to inhibit cancer cell metastasis. Increasing evidence indicates a pivotal role of rafts in the signaling transduction of many biological processes, such as the immune responses, and neurotransmission (Langlet et al. [Citation2000], Tsui-Pierchala et al. [Citation2002]). A recent report shows that NA255, a lipophilic long-chain base compound, can block hepatitis C virus (HCV) infection by inhibiting synthesis of sphingolipid, a major raft component (Saknmoto et al. [Citation2005]). Our findings suggest that lipid rafts on cancer cells may be used as a drug target to develop novel therapeutics to counter tumor spread.
This paper was first published online on iFirst on 6 February 2007.
We would like to thank Dr Xinsheng Zhao for inspiring discussion, Ms Ganghong He and Mr Dong Qi of Olympus Company for technique assistance with confocal microscopy. This research is supported by Chinese National Science Foundation Grant (30400401), 973 program (2006CB705600) and NCI intramural research funding.
References
- Bogdan S, Klämbt C. Epidermal growth factor receptor signaling. Curr Biol 2000; 11: R292–295
- Brown DA, London E. Functions of lipid rafts in biological membranes. Ann Rev Cell Dev Biol 1998; 14: 111–136
- Burack WR, Sturgill TW. The activating dual phosphorylation of MAPK by MEK is nonprocessive. Biochemistry 1997; 36: 5929–5933
- Chan AY, Raft S, Bailly M, Wyckoff JB, Segall JE, Condeelis JS. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J Cell Sci 1998; 111: 199–211
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–252
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Ann Rev Cell Biol 1988; 4: 649–686
- Devreotes PN, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem 2003; 278: 20445–20448
- Furuchi T, Anderson RGW. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J Biol Chem 1998; 273: 21099–21104
- Harris TJ, Siu CH. Reciprocal raft–receptor interactions and the assembly of adhesion complexes. Bioessays 2002; 24: 996–1003
- Iijima M, Huang YE., Devreotes PN. Temporal and spatial regulation of chemotaxis. Dev Cell 2002; 3: 469–478
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 1998; 335: 433–440
- Jiao XM, Zhang N, Xu XH., Oppenheim JJ, Jin T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol Biol Cell 2005; 25: 5752–5762
- Langlet C, Bernard AM, Drevot P, He HT. Membrane rafts and signaling by the multichain immune recognition receptors. Curr Opin Immunol 2000; 12: 250–255
- Manneville SE, Hall A. Rho GTPases in cell biology. Nature 2002; 420: 629–635
- Mineo C, James GL, Smart EJ, Anderson RGW. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem 1996; 271: 11930–11935
- Mineo C, Gill GN, Anderson RGW. Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem 1999; 274: 30636–30643
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science 1995; 268: 247–251
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrerak JL, Mohark A, Vera steguik E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56
- Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem 2000; 274: 30934–30942
- Olayioye MA, Neve RM, Lane HA., Hynes NE. The erbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000; 19: 3159–3167
- Peres C, Yart A, Perret B, Salles JP, Raynal P. Modulation of phosphoinositide 3-kinase activation by cholesterol level suggests a novel positive role for lipid rafts in lysophosphatidic acid signaling. FEBS Lett 2003; 234: 164–168
- Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5- bisphosphate in caveolin-enriched membrane domains. J Biol Chem 1996; 271: 26453–26456
- Pike LJ, Casey L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry 2002; 41: 10315–10322
- Pike LJ, Miller JM. 1998. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and Inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem 273:22298–22304.
- Rodal SK., Skretting G, Garred Ø, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 1999; 10: 961–974
- Roepstorff K, Thomsen P, Sandvig K, van Deurs B. Sequestration of epidermal growth factor receptors in non-caveolar lipid rafts inhibits ligand binding. J Biol Chem 2002; 277: 18954–18960
- Saknmoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A, Tsukuda T, Shimma N, Aoki Y, Arisawa M, Kohara M, Sudoh M. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol 2005; 1: 333–337
- Salcedo R, Martins-Green M, Gertz B, Oppenheim JJ, Murphy WJ. Combined administration of antibodies to human interleukin 8 and epidermal growth factor receptor results in increased anti-metastatic effects on human breast carcinoma xenografts. Clin Cancer Res 2002; 8: 2655–2665
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211–225
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31–41
- Sun RH, Gao P, Chen L, Ma DL, Wang JM, Oppenheim JJ, Zhang N. Protein kinase Cζ is required for epidermal growth factor–induced chemotaxis of human breast cancer cells. Cancer Res 2005; 65: 1433–1441
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM, Jr. Lipid rafts in neuronal signaling and function. Trends Neurosci 2002; 25: 412–417
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000; 100: 209–219
- Vivanco I, Sawyers CL. The PI3K/Akt pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501
- Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way?. J Cell Sci 2001; 114: 3795–3803
- Yamabhai M, Anderson RGW. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem 2002; 277: 24843–24846