Abstract
Mercury (Hg) is a persistent element in the environment that has the ability to bioaccumulate and biomagnify up the food chain with potentially harmful effects on ecosystems and human health. Twenty-four streams remotely located in forested watersheds in northwestern PA containing naturally reproducing Salvelinus fontinalis (brook trout), were targeted to gain a better understanding of how Marcellus shale natural gas exploration may be impacting water quality, aquatic biodiversity, and Hg bioaccumulation in aquatic ecosystems. During the summer of 2012, stream water, stream bed sediments, aquatic mosses, macroinvertebrates, crayfish, brook trout, and microbial samples were collected. All streams either had experienced hydraulic fracturing (fracked, n = 14) or not yet experienced hydraulic fracturing (non-fracked, n = 10) within their watersheds at the time of sampling. Analysis of watershed characteristics (GIS) for fracked vs non-fracked sites showed no significant differences (P > 0.05), justifying comparisons between groups. Results showed significantly higher dissolved total mercury (FTHg) in stream water (P = 0.007), lower pH (P = 0.033), and higher dissolved organic matter (P = 0.001) at fracked sites. Total mercury (THg) concentrations in crayfish (P = 0.01), macroinvertebrates (P = 0.089), and predatory macroinvertebrates (P = 0.039) were observed to be higher for fracked sites. A number of positive correlations between amount of well pads within a watershed and THg in crayfish (r = 0.76, P < 0.001), THg in predatory macroinvertebrates (r = 0.71, P < 0.001), and THg in brook trout (r = 0.52, P < 0.01) were observed. Stream-water microbial communities within the Deltaproteobacteria also shared a positive correlation with FTHg and to the number of well pads, while stream pH (r = −0.71, P < 0.001), fish biodiversity (r = −0.60, P = 0.02), and macroinvertebrate taxa richness (r = −0.60, P = 0.01) were negatively correlated with the number of well pads within a watershed. Further investigation is needed to better elucidate relationships and pathways of observed differences in stream water chemistry, biodiversity, and Hg bioaccumulation, however, initial findings suggest Marcellus shale natural gas exploration is having an effect on aquatic ecosystems.
Introduction
Mercury (Hg) accumulation in ecosystems is of great concern because of the element's ability to persist, transform (to methylmercury, MeHg), biomagnify, and have neurotoxic effects on organisms.[Citation1–3] Mercury occurs in ecosystems as a result of both natural and anthropogenic processes[Citation4–9] and is considered a global contaminant due to its capacity for long-range transport in the atmosphere and ubiquitous deposition across landscapes.[Citation10-11] Mercury has the ability to contaminate land, air, water, and biota in aquatic and terrestrial environments, as well as threaten the long term health of organisms and ecosystems. Aquatic ecosystems are believed to be at increased susceptibility for accumulation of Hg, and human exposure to Hg occurs mostly through fish consumption. Currently all 50 states in the US have issued at least one fish eating advisory due to mercury, while 26 states have issued statewide mercury advisories, and coastal advisories have been posted for 15 states.[Citation12-13] In Pennsylvania, fish eating advisories due to mercury have been posted for over 1411 stream kilometers and 28 lakes (11,533 hectares).[Citation13–14]
Any perturbation to aquatic ecosystems (direct or indirect) not only has the ability to affect stream water quality and macro and micro biodiversity, but also the bioaccumulation of mercury. One such perturbation is the recent increase in extraction of Marcellus shale natural gas, which is changing land cover across the northeast, but specifically in Pennsylvania.[Citation15] Although there is a long history of natural gas and energy extraction in Pennsylvania, the emergence of horizontal drilling and hydraulic fracturing has only occurred within the past eight years.[Citation16] The most prominent formation, the Marcellus, underlies approximately 70% of Pennsylvania and much of West Virginia, as well as parts of Ohio, Maryland, and New York.[Citation16] Because hydrologic fracturing is in its relatively early stages in Pennsylvania, many of the cumulative and potentially long term impacts caused by the process are still unknown.[Citation17]
Stream water quality, aquatic biodiversity, and mercury levels all have the potential to be directly impacted by frackwater and flowback fluid produced during Marcellus shale extraction. Fracking and flowback fluids have been shown to contain toxic organic and inorganic chemicals such as hydrochloric acid, phenols, polycyclic aromatic hydrocarbons, BTEX, and even Hg.[Citation18–20] Recent research is beginning to define the microbial community structure of injected and flowback waters associated with hydraulic fracturing.[Citation21–22] In Pennsylvania alone, it has been estimated that over six billion liters of wastewater have already been generated,[Citation23] and the mismanagement of produced flowback waters is the significant threat to our water resources.[Citation24]
Frackwater has the potential to reach the stream through leaking equipment (waste-water hoses) and impoundments (evaporation pits and holding ponds/tanks), lateral blowouts and seepage, as well as backflow into the wellhead.[Citation25–33] Any flowback water reaching the stream would not only directly impact water quality and lead to changes in biodiversity,[Citation23,30] but may also cause changes in bioaccumulation of mercury. Furthermore, one study by the NY City department of Environmental Protection showed that flowback samples at two different sites contained Hg concentrations approaching 600 PPB.[Citation34] In addition to the potential for direct input of Hg, changes in water quality, such as lowering stream water pH, makes Hg more soluble thus increasing bioaccumulation in macroinvertebrates and fish.[Citation35,36]
Although potential contamination from frackwater and flowback fluid poses a significant threat to streams, infrastructure associated with Marcellus shale natural gas exploration may be currently having a larger impact on aquatic ecosystems. Large well pads are needed to execute horizontal drilling and horizontal fracturing. The average Marcellus well pad in Pennsylvania is from 1.2–2.0 hectares, but overall forest disturbance is much larger due to associated infrastructure including water and waste impoundments.[Citation37] If the collective impact of roads and pipelines are included, this area increases to about 3.5 ha of area cleared per well pad. The number of wells per pad range from one to around twelve or more, with an average of 2.15 wells per pad in 2010,[Citation38] making number of well pads a better indicator of landscape disturbance than number of wells.
If these disturbances occur in predominately small forested watersheds, impacts resulting from well pad construction and supporting infrastructure (i.e. roads, pipelines, waste-water pits) have the potential to increase transport of material from the terrestrial environments to stream ecosystems. Research has shown that forest disturbances (i.e. land clearing) can result in increased overland flow and transport of organic carbon, pollutants (i.e., Hg), and sediment to streams.[Citation39-41] Resulting changes in water quality also have the ability to alter species composition and biodiversity of macroinvertebrates and fish, change trophic food web structure, and impact Hg bioaccumulation and biomagnification properties within aquatic ecosystems.[Citation42-44]
Current estimates as to the number of Marcellus wells in Pennsylvania are just over 6000 active wells [Citation45] with over 10,000 permitted.[Citation46] However, this number is expected to increase, up to a total of 60,000 wells by 2030.[Citation37] Researchers have suggested that the recent rapid expansion of Marcellus shale development poses a significant threat to aquatic ecosystems in Pennsylvania.[Citation47,48] Here, we examined whether Marcellus shale natural gas exploration in northwestern PA is affecting stream water quality, aquatic biodiversity, and Hg concentration across media. We hypothesized that streams where fracking had occurred (within their watershed) would show differences in stream water chemistry (e.g., pH) and biodiversity, and increased Hg accumulation across compartments (stream water, macros, fish) when compared to similar non-fracked watersheds and streams.
More specifically, our objectives were (1) to determine whether differences in these three categories existed between fracked and non-fracked streams, (2) to assess whether the degree of forest disturbance (i.e. number of well pads) was associated with any variables from these three categories, and (3) to assess other ecosystem factors that may be controlling Hg accumulation in aquatic ecosystems.
Materials and methods
Study site selection
Stream selection targeted remotely located, forested watersheds within the Marcellus shale basin in northwestern Pennsylvania that contained naturally reproducing wild brook trout populations. Streams were first identified by utilizing GIS to overlay a PA Fish and Boat Commission designated wild trout stream layer with a constructed unconventional shale gas permit layer (PADEP database). Data regarding drilling and hydraulic fracturing dates were compiled from the PADEP SPUD wells report and from fracfocus.org for each unique well permit within the watershed of every potential study site.[Citation49–51] To determine the number of unique well pads constructed in each watershed, we plotted well permit GPS coordinates. Clusters (or single) of permits separated by more than 100 meters were categorized as a unique well pad. Well pad development status was initially assessed using SPUD dates to confirm construction of well pad. To further validate correct status and number of wells and well pads developed, we hiked to all permitted well and well pad coordinates to corroborated previous assessment.
A total of 24 streams were identified for use in this study, with 14 having experienced fracking at all well pads within their watershed prior to our sampling efforts (fracked group), and 10 streams that had not yet experienced fracking (or SPUD) within their watershed (non-fracked group) (). None of the streams in the non-fracked group had begun drilling, however, two of the ten streams (Dixon Run and Deer Creek) in the non-fracked group had roads constructed, land cleared, and well pad construction begun prior to our sampling.
Fig. 1. Map showing general sampling locations across Pennsylvania with county names and status, with their delineated upstream watersheds shown in inset maps A, B, and C. Numbers correspond to watershed characteristics found in Table 1.
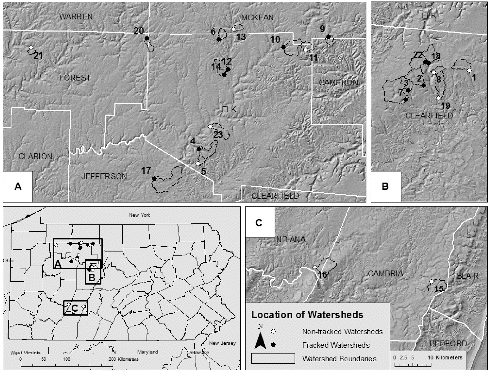
Specific 100-meter study sites were established on each stream study site downstream, and as close as possible to the well pad/permitted well pad site(s). Although some streams were in similar larger drainages, the study sites were located in separate sub-basins (not downstream/upstream of each other), separated by at least 1200 meters of stream-line distance, and not confounded. All watersheds were otherwise minimally disturbed with no evidence of mining legacy, and with few conventional oil wells and dirt roads being the only other observed within watershed anthropogenic disturbances. All sites were sampled under base flow conditions in June and July 2012.
Field methods
Water quality measurements including pH, conductivity, total dissolved solids (TDS), salinity and temperature were performed onsite using a PCSTestr 35 Multi-parameter test probe that was calibrated on a weekly basis. Stream water samples for Hg analysis were collected at each site using 2-L Polyethylene terephthalate copolyester, glycol-modified (PETG) containers following clean hands-dirty hands techniques[Citation52–53] and trace metal clean techniques.[Citation54] PETG containers were double bagged and stored on ice in darkened coolers until returning to the lab for filtration. Water samples collected for microbial analysis were collected according to Hazen et al.[Citation55] Additional water samples were collected in pre-cleaned amber glass bottles and pre-cleaned 500 mL polyethylene (HDPE) bottles which were stored on ice for later analysis of organic matter content (DOC) and basic water chemistry. All water samples were collected at the centroid of flow in riffles under base flow conditions.
Fish assemblages were assessed and Hg samples were collected on 100 meter unblocked segments using the wadeable electrofishing protocol[Citation56] and a Smith and Root LR 24 backpack electrofisher with pulsed direct currents ranging from 250–700 volts, depending on stream conductivity.[Citation57] Equal effort (i.e., electrofishing time, number participating) was employed for sampling fish at each stream site to allow for accurate cross-site comparison. At the completion of a pass all fishes were identified and relative abundances were recorded. Field measurements of brook trout included weight (in grams) using an OHAUS scale, and total length (in millimeters) using a fish board. Three to five brook trout were kept per stream, from three size ranges at approximately 140 mm, 170 mm, and 200 mm size for later Hg analysis of tissues and internal organs. Kept fish were stored on ice in darkened coolers until returning to the lab.
Upon completion of electrofishing, macroinvertebrate sampling was conducted using a 500-μm mesh D-frame dip net at three representative riffles within the 100-meter stretch to collect a composite macroinvertebrate sample for biodiversity estimates.[Citation58] On-site sorting was conducted to separate macroinvertebrates from detritus. All collected macroinvertebrates were placed into Nalgene bottles with a small volume of water (approximately 50 mL) and stored on ice until returning to the lab. Crayfish were targeted separately and collected through a combination of electrofishing and hand capture. The five largest crayfish caught per stream were kept on ice until later in lab processing.
One composite aquatic moss sample, consisting of two common water mosses, Fontinalis sphagnifolia and Fontinalis antipyretica, was collected from each stream following trace-metal clean sampling techniques.[Citation59] Each composite moss sample was collected from moss growing in five different locations within the 100 meter reach of the channel. Submerged aquatic mosses were targeted during sampling, and all mosses collected were within the active stream channel (i.e., frequently submerged by stream water). Mosses were typically collected from large rocks in the streambed where mosses were growing in large mats that in some cases were observed to contain a significant amount of sediment and biofilms. If mosses were mature, apical segments (3–4 cm) were separated from the shoots and rinsed in stream water for 30 secs to remove sediment. The samples were placed in pre-cleaned amber glass bottles and then stored on ice in darkened coolers.[Citation60–62]
Composite bed sediment samples were collected from five different areas within a 100m reach at each stream. The top 2 cm of deposited fines in streambeds were collected using Teflon scoops following USGS procedures[Citation63–64] and trace-metal clean sampling techniques.[Citation59] For each composite sample, approximately five scoops were used within the 100-m stream reach. Sediment samples were stored in pre-cleaned I-Chem brown borosilicate 40mL glass vials and put on ice in darkened coolers until returning to the lab. Sediment samples were not sieved to prevent volatilization of sulfides.[Citation65]
Lab methods
Macroinvertebrate identification
Macroinvertebrates were identified using morphological traits, geographic location, and habitat composition according to Merritt, Cummins, and Berg.[Citation66] Macroinvertebrates were identified to the lowest possible taxon (family or genus), relative abundances were recorded, and individuals were sorted according to feeding strategy (group). After all macroinvertebrates of a stream were identified and separated by feeding group, the samples were homogenized and frozen for later Hg analysis.
Crayfish were identified to species level following the dichotomous key constructed by Nutall, “Key to the Crayfishes of Pennsylvania,”[Citation67] weighed, and sexed. Using a digital caliper, measurements of body length (tip of rostrum to tip of tail), tail length, carapace length, carapace width, as well as left and right chela length and width were all taken to the nearest millimeter. The tail muscle was extracted by cutting through the underside of the exoskeleton directly below the gonopods towards the tip of the tail. Once the muscle tissue was extracted it was homogenized and refrozen until later mercury analysis.
Microbial analyses
Water samples from three fracked streams (Little Wolf Run, Iron Run, and Trout Run) and three non-fracked streams (Deer Creek, Ben's Creek, and Dead Man's Lick) were collected for microbial community analysis. Water collection, filtration, and DNA extractions for microbial analysis followed standard protocol.[Citation55] Illumina barcoded 16S rRNA amplicons were prepared,[Citation68] and 1 sequenced on the Illumina Miseq platform (250 bp paired end chemistry) at The Cincinnati Children's Hospital Medical Center. All sequenced reads were quality filtered with Usearch7[Citation69] and analyzed with the Qiime 1.7.0 platform.[Citation68, Citation70] Relative abundances of Operational Taxonomic Units (OTU's) were computed at the genus-level.
THg analysis
For THg analysis, subsamples of homogenized crayfish tail muscle, brook trout filets, and homogenized macroinvertebrates (by feeding group) were used. Also brook trout organs, including, kidney, liver and spleen were removed and homogenized, with subsamples being analyzed for THg. Stream bed sediment and aquatic mosses were oven dried at 45°C for 24-48 h[Citation61-62, Citation71] and then homogenized using Teflon stir rods. All samples were analyzed for Hg content by combustion atomic absorption spectrophotometry (CAAS) (Milestone DMA 80 direct mercury analyzer, Milestone, Monroe, CT) according to US EPA method 7473,[Citation72] which is known to produce statistically equivalent results to cold vapor atomic absorption.[Citation73] All fish tissue, crayfish tissue, and macroinvertebrate results were reported as wet weight values, while dry weight results were reported for stream sediments and aquatic mosses. All fish and crayfish samples were analyzed within 30 days of date of capture ensuring biopsy stability.[Citation74] THg analyses were conducted at Penn State University Institute for Energy and the Environment Lab (PSIEE).
Stream water samples
Water samples were prepped at Juniata College and analyzed for particulate (P) and dissolved (F) THg and MeHg by the USGS WRML lab, Middleton, Wisconsin. Samples were filtered at Juniata (on the same day as their collection) using quartz fiber filters (47-mm diameter, 0.7-μm pore size) with clean hands-dirty hands techniques[Citation52-53] and trace metal clean techniques[Citation54] to separate out particulate and dissolved constituents. Water samples and filters were shipped overnight to the USGS lab where cold vapor atomic fluorescence spectroscopy (CVAFS) was used according to US EPA Method 1631 (Revision E) and the WRML modifications in order to determine particulate and dissolved THg and MeHg concentrations in the stream water samples.[Citation75,76] Basic water chemistry was conducted at Juniata College, with dissolved organic carbon (DOC) analyzed at Penn State University by using a persulfate wet oxidation method.[Citation77]
Mercury detection limits and quality assurance
An instrument detection limit (IDL) of 0.002 μg /g mercury wet weight and 0.02 μg/g dry weight was determined for the DMA-80 by replicated analysis (and correction for water content) of dry weight values of standard reference materials (SRM 2976) to assess precision.[Citation77] For each run on the DMA-80 (30 samples), three randomly selected replicates were run and all showed <5% variability. Additionally, an intermediate liquid standard was prepared and three liquid standards (2 ppm, 4 ppm, and 8 ppm) were utilized along with blanks for every run to further ensure precision.
During filtration of stream water samples, distilled de-ionized water (DI) and samples of known concentrations (quality control samples, QC) were also filtered using the same techniques and reported results well below detection limits. Method detection limits of <0.004 for MeHg and <0.05 for THg were achieved by the USGS Wisconsin Water Lab.
Watershed analysis
Watersheds were delineated to show the area upstream of each sampling point. We used the watershed tool in ArcGIS 10.0 to calculate watersheds, using a 1/3 arcsecond (approximately 10 m) resolution National Elevation Dataset (NED),[Citation78] digital elevation model (DEM), and GPS coordinates for each sampling point as pour points. All elevation and slope metrics were calculated using the 1/3 arcsecond NED DEM, which is based on LiDAR-derived elevation data for the area studied in Pennsylvania. Once watersheds were delineated for each sampling point, land cover metrics (i.e., percent forest) were determined using the PAMAP Land Cover for Pennsylvania.[Citation79]
The geology of each watershed was determined using the Bedrock Geology of Pennsylvania, with each geologic unit being classified to its dominant lithology. Hydric soils data per watershed was calculated using the SSURGO (NRCS),[Citation80] with soil types either categorized as all hydric or partially hydric. Drainage density was calculated using the area of each watershed (km2) and the stream length (km) within each delineated watershed as measured using the networked streams of Pennsylvania shapefile.[Citation81]
Data analysis
Fish diversity measures were calculated utilizing the Simpson's Diversity Index,[Citation82] and macroinvertebrate diversity measures were calculated using Shannon's diversity index in which the proportion for each taxa is determined by dividing the number of individuals in each taxa by the total number of individuals per stream.[Citation82]
We used analysis of variance (ANOVA) to help us meet our first objective to assess differences in watershed characteristics, measures of water chemistry, biodiversity, and mercury concentration between fracked (n=14) and non-fracked (n = 10) streams. ANOVA was also used to compare Hg concentration among compartments (e.g., brook trout, crayfish, stream sediments) and among macroinvertebrate feeding groups (e.g., predators, collectors, shredders). Transformations of some data (log10 and square root) were used to better meet the assumptions of ANOVA. Where data could not be normalized, a Kruskal–Wallis non-parametric test was performed, and a Chi-squared test was used to assess differences in wetlands between fracked and non-fracked streams as most sites had no measurable wetlands. Mercury concentrations in brook trout were length normalized to account for any variation in size of captured fish between sites.
Towards meeting our second objective to further assess the degree of impacts of Marcellus development, Pearson's correlations were used to compare measures of water chemistry, macro and micro biodiversity, and mercury concentration to number of well pads within a watershed. These correlations were run including all streams (n = 24) and also for fracked streams sites only (n = 14). This was conducted to assure correlations were significant both ways, and the more conservative (lower correlation values) were displayed with scatterplots for discussion. Pearson's correlations were used to compare Hg concentration among brook trout tissues (e.g. muscle, liver, spleen, kidney), and other variables between and among water chemistry, macro and micro biodiversity, and mercury concentration to help us assess ecosystem factors controlling Hg accumulation.
Pearson correlations were also calculated to describe the relationship between the relative abundance of bacterial taxa (i.e., number of sequences belonging to an OTU) and the number of well pads and dissolved total mercury concentrations. The significance of these correlations was reported using FDR-corrected p-values. At a Fisher's alpha of 0.05 no significant correlations were observed, thus the Fisher's alpha was loosened to 0.1 to include more potentially significant correlations. All other statistical analyses were conducted utilizing Minitab (v.16) software, and were considered significant at P ≤ 0.05.
Results and discussion
Watershed characteristics
A number of environmental characteristics of watersheds were compared between stream sites where fracking had occurred in the watershed (n = 14) and sites that had not yet experienced any fracking within their watersheds (n = 10) at the time of sampling (). No significant differences existed between groups (at α = 0.05) for any variable, except number of well pads. Fracked and non-fracked groups had land use similarities, with the majority of their watersheds being forested (overall mean>90%), with approximately 76% in deciduous forest type, 17% mixed forest type, and 7% coniferous with no significant differences between groups.
Table 1. Watershed (WS) characteristics of streams sampled where N stands for sites with no fracking within their watershed (n = 10) and F stands for sites where fracking has occurred within their watershed (n = 14) (df = 21). Conventional wells are oil wells pre-dating Marcellus shale natural gas exploration.
Sandstone was the predominant geology at all sites, accounting for greater than 99% of the geology in most watersheds. Percent of watershed area with wetlands was low (overall mean = 0.06%, fracked median 0.39% and non-fracked median = 0.00%), with 19 of 24 watersheds experiencing less than 1% wetlands and all remaining watersheds at or below 3% wetlands. Further, no significant differences in wetland area were observed between fracked and non-fracked sites (P > 0.05). The number of conventional oil wells was also compared between fracked and non-fracked sites and no significant differences were observed (P > 0.05).
Further, the number of conventional oil wells in a watershed were weakly negatively correlated with the number of Marcellus well pads (r = −0.48, P = 0.082), suggesting greater Marcellus development in areas with fewer conventional natural gas wells. Non-fracked sites were observed to have somewhat steeper slopes, while fracked sites had slightly larger watershed sizes (although neither significant at α = 0.05). Research has not generally shown these two variables to be important predictors of mercury bioaccumulation. Overall, similarities in watersheds between groups allowed for robust comparative analysis.
Well pads
Significant similarities in watershed characteristics existed between streams in both the fracked and non-fracked groups. However, across fracked sites (n = 14) it was observed that the number of well pads varied greatly among watersheds (1-11). Well pad density within a watershed was not correlated with watershed size (r = 0.19, P = 0.93), and the highest number of well pads were often found in smaller watersheds. For these reasons, we hypothesized that the number of well pads within a watershed could be used as a proxy for the degree of impact/disturbance to a watershed and the receiving water body. Even distribution of streams across all number of well pads did not exist. The majority of streams (12) contained between one and five well pads within their watershed; with the remaining two streams, Alex Branch and Little Laurel Run, having 9 and 11 well pads respectively. Ideally, we would have had more stream sites with a greater number of well pads within their watershed. However, no additional sites fitting these criteria existed, and we are representing the population (not a sample) of streams in NW PA with high numbers of well pads that met our rigorous selection criteria (i.e. forested, minimally anthropogenic impacts except for Marcellus, brook trout stream).
Alex Branch and Little Laurel Run are also streams that have had documented frackwater contamination within their watersheds. The PA Department of Environmental Protection (PADEP) and the PA Fish and Boat Commission (PAFBC) determined that 7,980 gallons of fracking fluids leaked from a tank into Alex Branch and flowback water from a faulty wastewater hose and a leaking pit was reaching Little Laurel Run in 2009.[Citation47] Ideally, we would have additionally used sites with high numbers of well pads with no contamination, but they did not exist.
Water quality indicators
Observed differences in water quality and chemistry between fracked and non-fracked streams are likely the result of within watershed disturbances (). Increased concentrations of DOC in stream water at fracked sites is indicative of land clearing practices for road, pipeline, and well pad construction (P = 0.001, ). A number of studies have shown that forest disturbances, even on a relatively small scale, can significantly increase DOC in streams.[Citation39,Citation83–84] Increases in stream water DOC have also been linked to increases in wetlands;[Citation85–86] however, no significant correlations existed between percent wetland area and DOC or pH (P > 010). Measured increases in dissolved THg (FTHg) in stream water in the fracked group (P = 0.007, ) are likely reflective of complex formation with DOC (r = 0.88, P < 0.001), allowing for increased transport of Hg to streams, which is a conclusion supported by a large body of literature.[Citation85–89]
Table 2. Comparison of water quality and chemistry, mercury concentrations, and biodiversity measures between fracked and non-fracked study sites. TDS represents total dissolved solids, DOC represents dissolved organic carbon, PTHg represents particulate total mercury, PMeHg represents particulate methylmercury, FTHg represents dissolved total mercury and FMeHg represents dissolved methylmercury.
Others have also observed variation in Hg concentrations in streams without the presence of wetlands in their watersheds.[Citation1,90] Further, if increased DOC and Hg was the result of differences in wetlands, we would have expected to see increases in stream water MeHg as well, as significant methylation is believed to occur in wetlands.[Citation88,Citation91,92] While we anticipated DOC would be positively correlated with number of well pads within a watershed (further supporting the idea of disturbance regime), it was not.
However, differences in hydrologic conditions (e.g. flow paths) and catchment characteristics including the number of roads, number of roads crossing streams, number of pipelines, or distance of any of these disturbances to the stream, were not accounted for and likely vary significantly among watersheds. Several studies have suggested that pipelines may even be a more significant watershed disturbance than well pad construction.[Citation15,Citation37] Although the ultimate source of the FTHg is uncertain, other research suggests it is likely either from atmospherically deposited Hg in the watershed or from flowback fluid.[Citation5-7,Citation10,34] Regardless of the source, the coupled increase in DOC at fracked streams suggests that increased overland flow and transport to streams (from well pad, pipeline, and road construction) contributed to the increased stream water FTHg.
Differences in stream water pH between fracked and non-fracked groups are believed to be either the result of unmeasured and unobserved differences in watershed characteristics, differences in acid rain, or the result of fracking activities (P = 0.03, ). Watershed characteristics such as wetlands and conifer stands have the ability to significantly decrease stream water pH;[Citation93] however, no differences were observed for wetlands, hydric soils, or percent conifer between groups. Small wetlands (not captured by GIS analysis) or acid-rich peat soils adjacent to the streams could account for a decrease in stream water pH in the fracked group, however, no small wetlands were observed at sites during the field work, and no significant differences in hydric soils were observed between fracked and non-fracked streams ().
Another potential etiology for pH variation may be due to differences in atmospheric deposition of acid rain. Research has shown that both chronic and episodic acidification from atmospheric deposition have the potential to significantly alter stream water pH,[Citation94–96] even in geographically close sites. However, if the cause of lower pH stream water at fracked sites was due to increased acid rain, we would have anticipated seeing similar trends in stream water nitrogen levels, as others have shown.[Citation97] We saw no significant differences in stream water total nitrogen levels between fracked and non-fracked streams (P = 0.60).
Fracking activities may be having a direct impact on stream water pH, as research has shown that fracking uses a number of acidic agents and solutions during the extraction of natural gasses.[Citation18,20] Well pad construction, drilling, and fracturing processes unearth and cross a number of geologic formations, potentially exposing pyritic sandstone. Other research in Pennsylvania has shown that exposing pyritic sandstone can significantly affect stream and groundwater pH.[Citation98] Stream water pH was found to be negatively correlated with the number of well pads, potentially indicating a link between stream pH and fracking activities occurring at the well site (). If acidic agents are being introduced to the watershed via fracking activities, these agents are likely being flushed to the fracked streams as a result of increased overland flow and transport (supported by increased DOC and THg levels at fracked streams).
Fig. 2. Significant negative correlation between number of well pads within a watershed and stream water pH (Pearsons r = -0.74, P = 0.0001). Blackened circles represent fracked sites and open circles represent non-fracked sites.
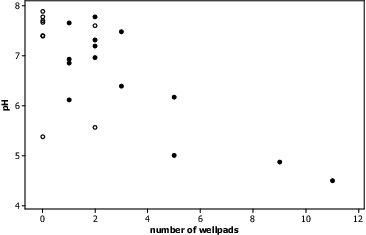
Differences in stream water conductivity and salinity were not observed between fracked and non-fracked groups under baseflow conditions (P > 0.05, ). Others have also shown no significant increases in ion concentrations in stream water where fracking had occurred within the watershed.[Citation47] If frackwater or flowback water reached the stream, it is anticipated that observed differences would have been noticed as research has suggested flowback waters have high salinities (>200,000 mg/L TDS) and conductivities due to increased ion concentrations.[Citation18,99] However, unless measured directly following acute contamination or under stormflow conditions, differences in salinity and conductivity may not be observable due to the first flush effect and residence time differences when compared to other stream water constituents (DOC, FTHg).
Biodiversity in aquatic ecosystems
Fish biodiversity differences observed between groups were believed to be largely driven by stream water pH. Although no significant differences were observed for fish richness or biodiversity between fracked and non-fracked groups (), a one-way ANOVA showed significantly higher biodiversity in watersheds with zero to two well pads compared to watersheds with more than three well pads (P < 0.01). Further, fish biodiversity was significantly correlated with number of well pads, but more strongly with stream water pH (), implicating pH as the driving factor.Although brook trout were found at all stream sites (excluding Little Laurel Run-recently extirpated), they are known to have a higher tolerance to acidic waters than other fish species.[Citation100–102]
Fig. 3. Above scatterplot A shows decreasing fish diversity (Simpson's Diversity Index) with increasing number of well pads for fracked sites (n = 14, r = -0.60, P = 0.02). Scatterplot B shows significant positive correlation between pH and fish species diversity (r = 0.70, P = 0.0001) with blackened circles representing fracked sites and open circles representing non-fracked sites. Similar strength correlation observed for scatterplot A when non-fracked sites included.
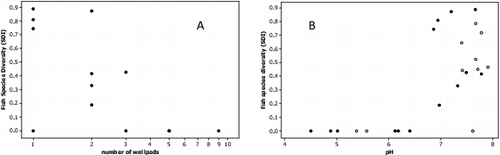
Blacknosed dace and long-nosed dace (Rhinichthys atratulus and Rhinichthys cataractae) were found in abundance at streams with zero to two well pads within their watersheds, but were not found at any stream sites with more than three well pads in the watershed where pH levels were below six. Others have suggested that dace, unlike brook trout, are not tolerant of acidic waters; likely due to their inability to regulate ion concentrations at low pH.[Citation102–104] Overall, we observed decreased fish biodiversity and pH-sensitive fish at more acidic streams that were experiencing increased Marcellus activities.
Unlike fish biodiversity, macroinvertebrate biodiversity was not observed to vary with stream water pH. While no measures of macroinvertebrate biodiversity were observed to be different between fracked and non-fracked groups (), macroinvertebrate taxa richness was found to be negatively correlated with number of well pads (r = −0.60, P = 0.02). Based on the literature, it was expected that pH would be the factor driving macroinvertebrate biodiversity,[Citation105] however stream water pH was not significantly correlated with macroinvertebrate richness or diversity (P > 0.10). Landscape disturbances that increase instream sedimentation have also been reported to negatively impact macroinvertebrate biodiversity.[Citation41,106] Although we did not measure substrate embededness, we hypothesize that increased development in the watershed (e.g., well pad, road, and pipeline construction) may have been increasing instream sedimentation and impact macroinvertebrate richness.
In addition to macro-scale biodiversity (fishes and macroinvertebrates), recent research has also noted that aquatic microbial communities differ in diversity and composition between fracked and non-fracked streams.[Citation70] Analyzing a subset of our streams (6/24) for microbial communities in stream water revealed that specific microbial taxa within the Deltaproteobacteria shared a positive correlation to dissolved total mercury (FTHg) and to the number of well pads within a watershed (). Geobacter, which are known to methylate mercury in diverse environments, were positively correlated to dissolved THg.[Citation107–111]
Table 3. Pearsons correlations of bacterial taxonomic relative abundances to the number of Well pads and FTHg (dissolved total mercury)
Furthermore, taxa of the Verrucomicrobia, Delta-Proteobacteria, and Elusimicrobia were positively correlated to the number of well pads in a given watershed (). Verrucomicrobia have also been observed in hydrocarbon-contaminated soil[Citation112] and found to be positively associated with THg and MeHg in mercury contaminated streams in Tennessee.[Citation112] Similarly we observed Elusimicrobia,[Citation113] which are found in a number of environments, but have been shown to inhabit contaminated systems.[Citation114] Increased abundance of bacterial taxa with potential biodegrading capabilities in watersheds with more well pads could suggest that these autochthonous populations are responding to the increased land disturbances or potential releases of fracking fluids into the environment. However, future studies will need to incorporate a greater number of samples for microbial community analysis and more robust genomics studies (i.e., metagenomics, metatrancriptomics) that assay the functional potential of these microbial communities.
Mercury as an indicator of change
Brook trout
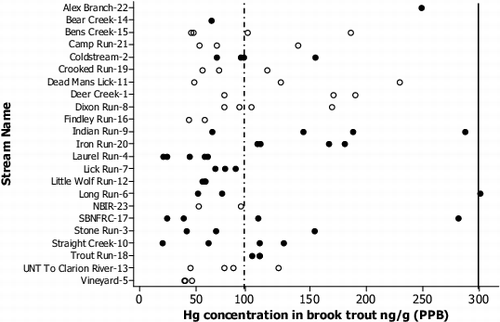
Mercury concentration in aquatic compartments increased between trophic levels, with brook trout experiencing the highest THg concentration (). This was expected as biomagnification of MeHg through the food web is the primary pathway of Hg to fish tissue, and existing data suggests that the majority (>95%) of mercury found in fish is in the methylated form.[Citation115,116] Overall, mercury concentrations in brook trout were lower than the majority of geographically larger studies that included streams, lakes, and reservoirs,[Citation65,Citation117–120] but more similar to studies focused on smaller streams in the northeastern United States.[Citation121,122] Across all brook trout (n = 73), 35% (26 fish) were above the U.S. Fish and Wildlife Service piscivorous bird and wildlife advisory (0.1 PPM;[Citation63]), with 40% (17) of fish from fracked sites and 30% (9) from non-fracked sites above the advisory (). One fish from Long Run (fracked site) was above EPA Hg Fish eating advisory for human consumption (0.3 PPM;[Citation12]). It should be noted that filets were used for Hg analysis, while consumption criteria are set for whole fish Hg levels.
Fig. 4. Above boxplot represents stream mean (trout, crayfish, macroinvertebrates) or composite (sediment and moss) THg values for each compartment. Grey boxplots indicate fracked sites (n = 14), while white boxplots indicate non-fracked sites (n = 10). P values on figure indicate significant differences (P < 0.05) among compartments were observed with trout>moss>crayfish>macroinvertebrates/sediment. No significant differences were observed across all sites between mean macroinvertebrate Hg concentration (across feeding groups) and Hg concentration in stream bed sediments (P > 0.10). One fish Hg concentration value does not appear on the above boxplot (249.10 ng/g). Boxplots show first quartile (25%), median line, and third (75%) quartiles represented by boxes. Upper and lower whiskers extend to data within 1.5 box height, and outliers are represented by asterisks.
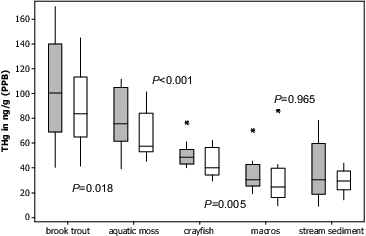
Fig. 5. Individual value plot showing Hg concentration in fish filets for all fish (n = 73) across all sites (n = 24) with Map ID numbers. Blackened circles represent fish from fracked sites and opened circles represent non-fracked sites. The dotted line represents the USFWS fish eating bird and wildlife Hg regulatory criteria (100 PPB) and the solid line represents the USEPA's regulatory criteria for Hg for human consumption of fish (300 PPB).
Increased dissolved THg in stream water and low pH conditions are believed to be affecting mercury uptake in brook trout at fracked stream sites. Although we saw no significant differences in Hg concentration in brook trout between fracked and non-fracked groups (), a weak positive trend existed between Hg concentration in brook trout and number of well pads within the watershed (r = 0.52, P = 0.01). Increased land-clearing disturbances are likely the cause of increased THg-DOC complex transport to streams where fracking has occurred. Low pH stream water and higher DOC, like those observed at fracked sites, increases solubility and uptake of Hg not only in lower trophic level biota,[Citation35–36,Citation123] but also in higher order fish as a result of reductions in growth efficiency.[Citation42,Citation124–126] Comparing mercury concentration across muscle and the internal organs of brook trout (spleen, kidney, liver) suggest that fish are being impacted by increased FTHg. Our analysis showed strong positive correlations between Hg in spleen, liver, kidney and muscle mercury concentration for the fracked group, but not for the non-fracked group (). The consistent accumulation of Hg in organs and muscle tissue at fracked sites is suggestive of inorganic mercury uptake (THg) from the aqueous environment, across the gills, gut, or skin.[Citation127-128] Contrastingly, the dissimilarity in mercury accumulation between organs and muscle tissue at non-fracked sites is suggestive of dietary uptake of MeHg and subsequent thiol binding for accumulation in muscle tissue.[Citation127,129] These Hg results are suggestive of a relatively recent increase in THg in stream water that has not had time to methylate in stream sediments and pass through the food chain (which is supported by increased FTHg in stream water at fracked sites).
Table 4. Correlation analysis matrix showing relationships aHg concentration in brook trout muscle tissue and organs when divided into fracked (F) and non-fracked (N) streams. Data was normalized using log10 transformation.
Crayfish
Crayfish mercury concentrations were found to be lower than brook trout mercury levels, but significantly higher than stream mean macroinvertebrate and stream sediment Hg concentrations (). Others have also found crayfish to group between fish and other macroinvertebrates with regard to THg concentration.[Citation130] This is presumably a result of trophic position, food chain length, and other biomagnification properties. We also observed that stream means for Hg concentration in crayfish and brook trout were positively correlated (r = 0.62, P = 0.02), suggesting crayfish are significant food source for brook trout, and another vector of Hg accumulation. Overall, observed THg concentrations in crayfish for our study were low when compared to other research.[Citation130–132] However, making accurate comparisons proved difficult as we used wet weight values when calculating our Hg concentrations, while other reported values were dry weights or unspecified.
Relationships between Hg concentration, status (fracked vs. non-fracked), and degree of impact within a watershed (number of well pads) were noticeably stronger for crayfish than brook trout ( and ). Crayfish in the fracked group had significantly higher THg than crayfish captured from the non-fracked group (P = 0.001, ) and a strong positive correlation between number of well pads and stream mean crayfish THg concentration was observed (r = 0.79, P < 0.001, ). This relationship further strengthened when only including fracked sites (r = 0.83, P < 0.001). Observed low pH stream water and increased THg in stream water conditions at fracked sites are likely the driving forces controlling bioaccumulation of Hg in crayfish (). Others have suggested that streams with low pH (even in the absence of wetlands) significantly increase accumulation of Hg in aquatic invertebrates.[Citation133] Researchers have also shown that species of lower trophic levels typically exhibit higher THg concentrations than species of higher trophic status.[Citation134] Presumably this is the result of a strong and irreversible interaction between Hg and chitin,[Citation135,136] allowing for absorption of THg across the exoskeleton.[Citation137] Further, crayfish and other macroinvertebrates have been shown to uptake significant amounts of Hg across their gills.[Citation138,139] These results further support our findings from the brook trout organ Hg data, and our theory of a relatively recent increase in stream water FTHg, which would show differences in Hg concentrations in crayfish first.
Fig. 6. Scatterplot A shows a significant positive correlation between number of well pads within a watershed and mean crayfish Hg concentration for given stream (r = 0.79, P < 0.001, n = 24). Scatterplot B shows a positive correlation between number of well pads and THg concentration in predatory macroinvertebrates (r = 0.71, P = 0.001, n = 22). Blackened circles represent fracked sites and open circles represent non-fracked sites. Relationships strengthened when stream sites with no Marcellus well pads were removed (A-r = 0.83, B-r = 0.76).
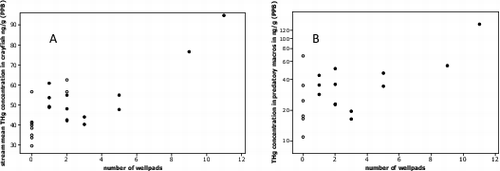
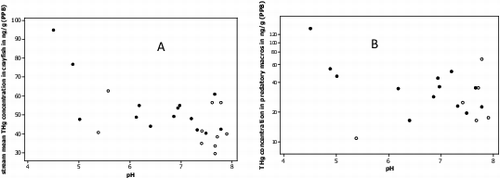
Fig. 7. Scatterplot A shows a significant negative correlation between stream pH and mean crayfish Hg concentration for given stream (r = 0.63, P = 0.001, n = 24). Scatterplot B shows a weak negative correlation between stream pH and THg concentration in predatory macroinvertebrates (r = 0.50, P = 0.03, n = 22). Blackened circles represent fracked sites and open circles represent non-fracked sites.
Crayfish proved to be a useful bioindicator of mercury accumulation in aquatic environments. THg concentrations in crayfish were well above detection limits allowing for easy sample preparation and analysis. Also, we observed no differences in sex or size between fracked and non-fracked sites, and no correlations existed between THg concentration and crayfish size across all sites (P > 0.05, ). Further, similar species were found across all sites, with mature C. carinirostris found in abundance at 23 of 24 streams (). Crayfish are also not able to migrate as far or as quickly as fish, making them potentially more reflective of local conditions than fish. Other research has suggested similar ideas about the utility of crayfish as ideal bioindicators due to their Hg accumulation in muscle tissue, association with sediment, lack of migration, resistance to toxicity, and relative ease of capture.[Citation131,140,141]
Table 5. Crayfish data including species, sizes, and Hg concentration by stream and status of watershed. N stands for sites with no fracking within their watershed and F stands for sites where fracking has occurred within their watershed.
Macroinvertebrates
Differences in Hg concentration between feeding groups and among streams were observed for aquatic macroinvertebrates ( and ). Mercury concentrations in stream mean THg for macroinvertebrates were significantly lower than concentrations in crayfish and similar to THg concentrations in stream sediments (). Comparisons across all feeding groups and streams showed significantly higher THg in predators and collectors than shredders (), which is similar to what others have shown.[Citation142] Stream mean THg concentrations in macroinvertebrates (across feeding groups) showed somewhat higher THg concentrations at fracked sites (at α = 0.10, ). The predatory feeding group was observed to have increased THg concentrations at fracked sites (P < 0.05, ), and to be strongly correlated with number of well pads within a watershed (r = 0.71, P = 0.001, ).
Fig. 8. Above boxplot represents distribution of Log10 of THg concentration in feeding groups of macroinvertebrates. Grey boxplots indicate samples from fracked sites, while white boxplots indicate non-fracked sites. Predator and collector concentrations were found to be significantly higher than shredders (P < 0.05), with predators also showing significant differences between fracked and non-fracked sites (P < 0.05). Hg concentration in shredders and scrapers were not found to be significantly different between fracked and non-fracked streams (P > 0.10). Boxplots show first quartile (25%), median line, and third (75%) quartiles represented by boxes. Upper and lower whiskers extend to data within 1.5 box height, and outliers are represented by asterisks.
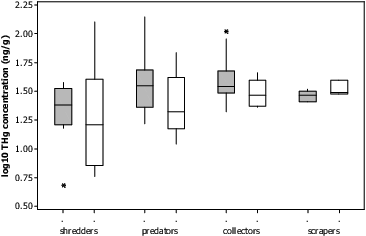
This correlation further strengthened when only including fracked sites (r = 0.77). No significant differences in Hg concentration existed between fracked and non-fracked stream sites for shredders, collectors, and scrapers. However, we believe this is due small and unbalanced sample sizes resulting from insufficient mass of feeding groups being acquired (for Hg analysis) at many streams (e.g., sufficient collector mass found at 16/24 streams, scrapers mass at 8/24 sites).
Low stream water pH and increased THg-DOC in stream water are believed to be affecting Hg accumulation in macroinvertebrates, particularly predatory macroinvertebrates. Research has shown evidence of macroinvertebrates directly consuming DOC suspended in the water column[Citation143] suggesting the possibility for uptake of DOC bound THg. Moreover, it is believed that macroinvertebrates retain the majority of mercury in their gut and exoskeletons with the larger, predatory species showing increased mercury level.[Citation140] Our observed negative trend between stream water pH and THg in predatory macroinvertebrates () aligns with what others have found, that macroinvertebrate THg levels can be predicted by stream water pH. [Citation40] We also observed a weak trend between predatory THg levels and dissolved THg in stream water (r = 0.44, P = 0.068), further supporting the direct uptake of DOC bound elemental Hg from stream water.[Citation143] The correlation between number of well pads and increasing THg accumulation in predatory macroinvertebrates is suggestive of a link between well pad construction, decreased pH, and increased THg in stream water.
Aquatic mosses and stream bed sediments
Aquatic mosses were believed to be a good potential indicator of Hg accumulation because of their relatively high THg concentrations (), their predisposition to cell surface adsorption of inorganic heavy metals such as mercury,[Citation62,144,145] and their proven use as biomonitors of atmospheric deposition of Hg.[Citation144,146,147] Knowing this, we expected to see differences that aligned with our Hg results for brook trout, crayfish, and macroinvertebrates. However, no significant differences in THg in aquatic mosses were observed between fracked and non-fracked sites (), and no relationships existed between any other aquatic (i.e., pH, FTHg) or terrestrial variables (i.e., number of well pads). We believe unobserved relationships between THg in mosses and other environmental correlates are likely the result of differences in species composition, age and size, and observed biofilms growing within the mosses that were noted during sample collection. We believe accounting for aforementioned confounding variables in future sampling may increase their utility for monitoring Hg accumulation in aquatic ecosystems as others have demonstrated.[Citation71,148]
Many researchers have shown the role of stream sediments as a sink and a source for Hg.[Citation62,Citation149-152] No significant differences in THg in stream bed sediment were observed between fracked and non-fracked sites (), and no significant correlations existed with any other environmental variables. Others have suggested that stream bed sediments are an excellent long-term indicator of watershed conditions;[Citation65] however, our previous Hg results suggest that more recent changes in stream water THg have occurred and may not yet be well reflected in stream bed sediments Hg. Percent organic matter in stream sediments has also been shown to be important in determining amount of THg in stream sediments.[Citation116,153] During sediment sample collection, significant variation in the amount of organic material across sites was observed, and we did not analyze samples for percent organic matter, which may explain more of the variation of THg in stream sediments than stream status.
Conclusion
Differences in stream water quality, aquatic biodiversity, and Hg concentration were observed between fracked and non-fracked streams in northwestern Pennsylvania. All streams were located in forested basins, with the only major within watershed disturbance and differences being related to Marcellus shale development for natural gas extraction. Streams where fracking had occurred were exhibiting lower stream water pH, higher FTHg, and higher DOC. The increased stream water acidity and land disturbance at fracked stream sites were observed to decrease biodiversity (fish, macros) and increase THg concentrations across several trophic levels (crayfish, predatory macros), but not at the top of the food chain-brook trout. The number of well pads within a watershed proved to be a good proxy for the degree of impact, as it was highly correlated with stream water pH and measures of biota Hg (crayfish, predatory macroinvertebrates, and fish).
Our findings from this novel research suggest the observed differences between streams to be related to Marcellus shale development; however, uncertainties remain about the absolute source of acidic agents and Hg to the fracked streams. Land disturbance (well pads, pipelines, roads) and fracking activities (fracking, fluid transport and storage, spills) are both believed to play a role, but more work is needed to further detail pathways and sources. It should be noted that our study did not directly account for the potential effects of variation in atmospheric deposition of Hg, episodic acidification, the capacity of microbial transformation of mercury, and food chain/web structure.
Future work including a temporal component is needed to allow for a better understanding of the source of acidic agents and mercury driving the changes we observed between fracked and non-fracked stream sites. Sampling the same streams pre/post fracking would greatly build upon our initial data set, while measuring MeHg (in addition to THg in biota), would allow for more definitive determination of recent changes in THg in streams. Constructing food chains and webs for each stream (using isotopic fractionation) and using a more comprehensive microbial community structure approach (including analyzing all streams and a functional gene analyses) would help to further elucidate potential pathways of Hg biomagnification and contamination of aquatic ecosystems.
Acknowledgments
Collaboration with Dave Krabbenhoft, John Dewild, and their colleagues at the US Geological Survey Wisconsin Water Science Center was instrumental in allowing for measure of stream water mercury levels. We thank Krista Leibensperger for her significant help in the field collection and laboratory analysis. We would also like to thank Beth Boyer, Mike Brown, Scott Atkinson, Carol Confer, and the staff at the Penn State Institute for Energy and the Environment for their cooperation in processing samples. We are grateful that John Stolz and Samantha Malone spearheaded efforts to secure this special issue of the Journal of Environmental Science and Health. We would like to thank Mike Keating, Genna Kasun, Karla Wiser, Joan Engle, and the Juniata College Grants office for their support throughout this process. We would like to thank the anonymous reviewers of this manuscript who significantly improved the focus of the paper. This work would not have been possible without the approval for sample collection by the PA Fish and Boat Commission and the Institute for Animal Care and Use Committee (IACUC) at Juniata College. Finally, we would like to thank the many people we encountered at hunting camps, diners, on the roads, and in the woods, for their directions to sampling sites and their interest in and support of our research.
Funding
We would like to thank the Colcom Foundation for providing primary financial support for this research. The microbial component of this research was supported by a grant to Juniata College from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program.
References
- Chen, C.Y.; Stemberger, R.S.; Kamman N.C.; Mayes, B.M; Folt, C.L. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicology 2005, 14, 135–147.
- Fitzgerald, W.F.; Lamborg, C.H.; Hammerschmidt, C.R. Marine biogeochemical cycling of mercury. Chem. Rev. 2007, 107, 641–662.
- Paller, M.H.; Jagoe, C.H.; Bennett, H.; Brant, H.A.; Bowers, J.A. Influence of methylmercury from tributary streams on mercury levels in Savannah River Asiatic clams. Sci. Total Environ. 2004, 325, 209–219.
- Gray, J.E.; Hines, M.E.; Biester, H. Mercury methylation influenced by areas of past mercury mining in the Terlingua district, Southwest Texas, USA. Appl. Geochem. 2006, 21(11), 1940–1954.
- Gustin, M.S.; Lindberg S.E.; Weisberg, P.J. An update on the natural sources and sinks of atmospheric mercury. Appl. Geochem. 2008, 23, 482–493.
- Pacyna, E.G.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindborn, K.; Wilson, S.; Steenhuisen, F.; Maxson, P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos. Environ. 2010, 44(20), 2487–2499.
- Seigneur, C.; Jayaraghavan, K.; Lohman, K.; Karamchandani, P.; Scott, C. Globalsource attribution for mercury deposition in the United States. Environ. Sci. Technol. 2004, 38, 555–569.
- Shetty, S.K.; Lin, C.J.; Streets, D.G.; Jang, C. Model estimate of mercury emission from natural sources in East Asia. Atmos. Environ. 2008, 42, 8674–8685.
- Electronic report on the environment for 2008; mercury indicator. NTIS PB2008-112484 U.S. Environmental Protection Agency: Washington, DC, 2009/600/R-07/045F, 2009. Available at http://cfpub.epa.gov/eroe/index.cfm?fuseaction=detail.viewInd&lv=list.listByAlpha&r=188199&subtop=341. (accessed Dec 2013).
- Lindberg, S.; Bullock, O.R.; Ebinghaus, R.; Engstrom, D.; Feng, X.; Fitzgerald, W.; Pirrone, N.; Prestbo, E.; Seigneur, C. A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 2007, 36, 19–32.
- Mason R.P.; Abbott, M.L.; Bodaly, R.A.; Bullock, O.R.; Evers, D.; Lindberg, S.E.; Murray, M.; Swain, E.B. Monitoring the response to changing mercury deposition. Environ. Sci. Technol. 2009, 39(1), 14–22.
- U.S. Environmental Proection Agency (USEPA). National Listing of Fish and Wildlife Advisories (NLFWA). Office of Water: Washington, DC, 2001. Available at http://www.epa.gov/ost/fish/listing.html (accessed Dec 2013).
- U.S. Environmental Proection Agency (USEPA). National listing of fish advisories: technical fact sheet; 2008 biennial national listing. U.S. Environmental Protection Agency: Washington, DC, EPA-823-F-09-007, 2009. Available at http://water.epa.gov/scitech/swguidance/fishshellfish/fishadvisories/tech2008.cfm (accessed Dec 2013).
- U.S. Environmental Protection Agency (USEPA) Great Lakes water quality initiative technical support document for wildlife criteria: EPA 820-B95-009 U.S. Environmental Protection Agency (USEPA). Office of Water: Washington, DC, 1995.
- Drohan, P.J.; Brittingham, M.; Bishop, J.; Yoder, K. Early trends in landcover change and forest fragmentation due to shale-gas development in Pennsylvania: A potential outcome for the northcentral Appalachians. Environ. Manage. 2012, 49(5), 1061–1075.
- US DOE. Modern shale gas development in the United States: A primer. Office of Fossil Energy and National Energy Technology Laboratory, United States Department of Energy; Oklahoma City, OK, 2009; 96 pp.
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J D. Impact of Shale Gas Development on Regional Water Quality. Science 2013, 340(6134), 1235009.
- Kharaka, Y.K.; Thordsen, J.J.; Conaway, C.H.; Thomas R.B. The energy-water nexus: potential groundwater-quality degredation associated with production of shale gas. Procedia Earth Planet. Sci. 2013, 7, 417–422.
- Boswell, Z. A study of natural gas extraction in Marcellus Shale. Master's Thesis, submitted to the Department of Civil and Environmental Engineering at the Massachusetts Institute of Technology, 2011, 73 pp.
- Well Permit Issuance for Horizontal Drilling and High-volume Hydraulic Fracturing to Develop the Marcellus Shale and Other Low-permeability Gas Reservoirs. Revised Draft Supplemental Generic Environmental Impact Statement on the Oil, Gas and Solution Mining Regulatory Program, New York State Department of Environmental Conservation. Division of Mineral Resources: Albany, NY, 2011.
- Struchtemeyer, C.G.; Elshahed, M.S. Bacterial communities associated with hydraulic fracturing fluids in thermogenic natural gas wells in North Central Texas, USA. FEMS Microbiol. Ecol. 2012, 81, 13–25.
- Murali Mohan, A.; Hartsock, A.; Hammack, R.W.; Vidic, R.D.; Gregory, K.B. Microbial communities in flowback water impoundments from hydraulic fracturing for recovery of shale gas. FEMS Microbiol. Ecol. 2013, 86, 576–580.
- Rahm, B.; Bates, J.T.; Bertoia, L.R.; Galford, A.E.; Yoxtheimer, D.A.; Riha, S.J. Wastewater management and Marcellus shale gas development: trends, drivers, and planning implications. J. Environ. Manage. 2013, 120, 105–113.
- Vengosh, A.; Warner, N.; Jackson, R.; Darrah, T. The effects of shale gas exploration and hydraulic fracturing on the quality of water resources in the United States. Procedia Earth Planet. Sci. 2013, 7, 863–866.
- Kargbo, D.; Wilhelm, R.; Campbell, D. Natural gas plays in the Marcellus shale: challenges and potential opportunities. Environ. Sci. Technol. 2010, 44, 5679–5684.
- Lee, S. Challenges and strategies of shale gas development. Master's Thesis, University of Texas at Austin: Austin, TX, 2012.
- Rahm, D. Regulating hydraulic fracturing in shale gas plays: the case of Texas. Energy Pol. 2011 39, 2974–2981.
- Considine, T.; Watson, R.; Considine, N.; Martin, J. Environmental impacts during Marcellus Shale gas drilling: causes, impacts, and remedies, Shale Resources and Society Institute Report 2012-1, State University of New York at Buffalo, 2012.
- Warner, N.; Jackson, R.; Darrah, T.; Osborn, S.; Down, A.; Zhao, K.; White, A.; Vengosh, A. Geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc. Natl. Acad. Sci. 2012, 109(30), 11961–11966.
- Peduzzi, P.; Ruth, H.R.R. Gas fracking: can we safely squeeze the rocks? Environ. Develop. 2013, 6, 86–99.
- New York City Department of Environmental Protection. Impact assessment of natural gas production in the New York City water supply watershed. City of New York: New York, NY, 2009.
- Rahm, B.; Riha, S. Toward strategic management of shale gas development: Regional, collective impacts on water resources. Environ. Sci. Poll. 2012, 17, 12–23.
- Broomfield, M. Support to the identification of potential risks for the environment and human health arising from hydrocarbons operations involving hydraulic fracturing in Europe. AEA 2012, 17c, 1–210.
- Leff, E. Supplemental Generic Environmental Impact Statement on the Oil, Gas and Solution Mining Regulatory Program, Well Permit Issuance for Horizontal Drilling and High-Volume, Hydraulic Fracturing to Develop the Marcellus Shale and Other Low-Permeability Gas Reservoirs. New York State Department of Environmental Conservation: New York, NY, 2011.
- Ward, D.M.; Nislow, K.H.; Folt, C.L. Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann. NY Acad. Sci. 2010, 1195, 62–83.
- Pickhardt, P.C.; Fisher, N.S. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ. Sci. Technol. 2007, 41(1), 125–131.
- Johnson, N. 2010. Pennsylvania energy impacts assessment. The Nature Conservancy, Harrisburg, PA. Available at http://www.marcellus.psu.edu/resources/PDFs/tncenergy.pdf (accessed Jan 2014).
- Ladlee, J.; Jacquet, J. The implications of multi-well pads in the Marcellus Shale. Community and Regional Development Institute at Cornell (CaRDI) Research and Policy Brief Series, Cornell University, Ithaca NY, 2011, 43.
- Schelker, J.; Eklöf, K.; Bishop, K.; Laudon, H. Effects of forestry operations on dissolved organic carbon concentrations and export in boreal first-order streams. J. Geophys. Res. 2012, 117 (G1).
- Jardine, T.; Kidd, K.; Rasmussen, J. Aquatic and terrestrial organic matter in the diet of stream consumers: implications for mercury bioaccumulation. Ecol. Appl. 2012, 22(3), 843–855.
- Bryce, S.A.; Lomnicky, G.A.; Kaufmann, P.R. Protecting sediment-sensitive aquatic species in mountain streams through the application of biologically based streambed sediment criteria. J. North Am. Benthol. Soc. 2010, 29(2), 657–672.
- Trudel, M.; Rasmussen, J.B. Bioenergetics and mercury dynamics in fish: a modelling perspective. Can. J. Fish. Aquat. Sci. 2006, 63, 1890–1902.
- Chasar, L.C.; Scudder, B.C.; Stewart, A.R.; Bell, A.H.; Aiken, G.R. Mercury cycling in stream ecosystems. 3. Trophic dynamics and methylmercury bioaccumulation. Environ. Sci. Technol. 2009, 43, 2733–2739.
- Cabana, G.; Rasmussen, J.B. Modeling food-chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 1994, 372, 255–257.
- Pennsylvania Department of Environmental Protection (PADEP). Oil and Gas Reports. Commonwealth of Pennsylvania: Harrisburg, PA, 2013. Available at http://www.portal.state.pa.us/portal/server.pt/community/marcellusshale/20296 (accessed Dec 2013).
- Penn State Marcellus Center for Outreach and Development. Map of Issued Permits for Unconventional Wells. Penn State University: University Park, PA, 2012. Available at http://www.marcellus.psu.edu/resources/maps.php (accessed Dec 2013).
- Olmstead, S.; Muehlenbachs, L.; Shih, J.; Chu, Z.; Krupnick, A. Shale gas development impacts on surface water quality in Pennsylvania. Proc. Natl. Acad. Sci. 2013, 110(13), 4962–4967.
- Entrekin, S.; Evans-White, M.; Johnson, B.; Hagenbuch, E. Rapid expansion of natural gas development poses a threat to surface waters. Front. Ecol. Environ. 2011, 9(9), 503–511.
- Pennsylvania Department of Environmental Protection (PA DEP). Permits Issued Detail Report. Harrisburg, PA, 2012. http://www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/Permits_Issued_Detail (accessed July 2013).
- Pennsylvania Department of Environmental Protection (PA DEP). SPUD Data Report. PA DEP: Harrisburg, PA, 2012. http://www.depreportingservices.state.pa.us/ReportServer/Pages/ReportViewer.aspx?/Oil_Gas/Spud_External_Data (accessed July 2013).
- Ground Water Protection Council (GWPC), & Interstate Oil and Gas Compact Commission (IOGCC). FracFocus: Chemical Disclosure Registry. FracFocus Chemical Disclosure Registry, 2010. Available at http://www.fracfocusdata.org/DisclosureSearch/ (accessed Jul 2013).
- Olson, M.L.; DeWild, J.F. Techniques for the collection and species-specific analysis of low levels of mercury in water, sediment, and biota: U.S. Geological Survey, Water Resources Investigations Report 99–4018-B, 11 pp, 1999.
- John, F.B. Collection water-quality samples for dissolved metals-in-water. USA Environmental Protection Agency: Washington, DC, 2000. http://www.epa.gov/region6/qa/qadevtools/mod5_sops/surface_water_sampling/low_level_metals/r6wtr-sampling-metals.pdf (accessed Dec 2013).
- Lewis, M.E.; Brigham, M.E. Low-level mercury. In Processing of Water Samples, F. D. Wilde, D.B. Radtke, J. Gibs, R. T. Iwatsubo, Eds. US Geological Survey Techniques of Water-Resources Investigations, Book 9, Chapter A5, 2004.
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jansson, J.K.; Probst, A.; Borglin, S.E.; Fortney, J.L.; Stringfellow, W.T.; Bill, M.; Conrad, M.E.; Tom, L.M.; Chavarria, K.L.; Alusi, T.R.; Lamendella, R.; Joyner, D.C.; Spier, C.; Baelum, J.; Auer, M.; Zemla, M.L.; Chakraborty, R.; Sonnenthal, E.L.; D’haeseleer, P.; Holman, H.N.; Osman, S., Lu’ Z., Van Nostrand, J.D.; Deng, Y.; Zhou, J.; Mason, O.U. Deep-Sea oil plume enriches indigenous oil-degrading bacteria. Science 2010, 330, 204–208.
- Peck, D.V.; Lazorchak, J.M.; Klemm, D.J. Field operations and methods for wadeable streams; U.S. Environmental Protection Agency: Corvallis, OR, 2003; 203–232.
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Chapter 8: Fish Protocol. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, Second Edition. EPA 841-B-99-002. U.S. Environmental Protection Agency: Office of Water: Washington, DC, 1999; 1–20.
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Chapter 7 (part A): Benthic Macroinvertebrate Protocols. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, Second Edition. EPA 841-B-99-002. U.S. Environmental Protection Agency: Office of Water: Washington, DC, 1999; 1–35.
- Lutz, M.A.; Brigham, M.E.; Marvin-DiPasquale, M. Procedures for collecting and processing streambed sediment and pore water for analysis of mercury as part of the National Water-Quality Assessment Program; Open-File Report 2008-1279, U.S. Geological Survey: Reston, VA, 2008.
- Fernandez, J.A.; Aboal, J.R.; Carballeira, A. Identification of pollution sources by means of moss bags. Ecotoxicol. Environ. Safety 2004, 59, 76–83.
- US EPA. Chapter Three: Inorganic Analytes. SW-846, Revision 4; US Environmental Protection Agency: Washington, DC, 2007; 1–28. Available at (http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/chap3.pdf" (accessed Dec 2013).
- Cesa, M.; Campisi, B.; Bizzotto, A.; Ferraro, C.; Fumagalli, F.; Nimis, P.L. A Factor Influence study of trace element bioaccumulation in moss bags. Arch. Environ. Contam. Toxicol. 2008, 55, 386–396.
- Brightbill, R.A.; Murray, K.R.; Bilger, M.D.; Byrnes, J.D. Total mercury and methylmercury in fish fillets, water, and bed sediments from selected streams in the Delaware River Basin, New Jersey, New York, and Pennsylvania, 1998-2001. Water Resources Investigation Report 03-4183, 2004.
- US EPA. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual. US EPA 823-B-01-002. U.S. Environmental Protection Agency, Office of Water: Washington, DC, 2001; 1–208.
- Scudder, B.C.; Chasar, L.C.; Wentz, D.A.; Bauch, N.J.; Brigham, M.E.; Moran, P.W.; Krabbenhoft, D.P. Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. U.S. Geological Survey Scientific Investigations Report 2009–5109, Reston, VA, 2009.
- Merritt, R.W., Cummins, K.W., Berg, M.B., Eds. An Introduction to Aquatic Insects of North America, 4th ed.; Kendall/Hunt Publishing Company: Dubuque, IA 2008; 1158 pp.
- Nuttall, T. Key to the crayfishes of Pennsylvania. The Carnegie Museum of Natural History: Pittsburgh, PA. Available at http://iz.carnegiemnh.org/crayfish/key_to_pa_crayfishes.html (accessed Dec 2013).
- Caporaso, G.J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; Huttley, G.A.; Kelley, S.T.; Knights, D.; Koenig, J.E.; Ley, R.E.; Lozupone, C.A.; McDonald, D.; Muegge, B.D.; Pirrung, M.; Reeder, J.; Sevinsky, J.R.; Turnbaugh, P.J.; Walters, W.A.; Widmann, J.; Yatsunenko, T.; Zaneveld, J.; Knight, R. QIIME allows analysis of high-throughput community sequencing data. Nat. Meth. 2010, 7, 335–336.
- Edgar, R. CUPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Meth. 2013, 10, 996–998.
- Trexler, R.; Solomon, C.; McClure, E.; Brislawn, C.; Peterson, M.; Keddache, M.; Mason, O.; Hazen, T.; Hazen, T.; Grant, C.; Lamendella, R. The response of freshwater aquatic microbial communities to Marcellus Shale natural gas extraction. Front. Microbiol. 2014, 5, doi: 10.3389/fmicb.2014.00522.
- Cenci, R.M. The use of aquatic moss (Fontinalis antipyretica) as monitor of contamination in standing and running waters: limits and advantages. Scientific and legal aspects of biological monitoring in freshwater J. Limnol. 2000, 60, 53–61.
- U.S. Environmental Protection Agency (USEPA). Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrometry, Draft method 7473; U.S. Environmental Protection Agency: Washington, DC, 1998.
- Cizdziel, J.V.; Hinners, T.A.; Heithmar, E.M. Determination of total mercury in fish tissues using combustion atomic absorption spectrometry with gold amalgamation. Water Air Soil Pollut. 2002, 135, 355–370.
- Peterson, S.A.; Van Sickle, J.; Hughes, R.M.; Schacher. J.A., Echols, S.F. A biopsy procedure for determining filet and predicting whole-fish mercury concentration. Arch. Environ. Contam. Toxicol. 2005, 48, 99–107.
- U.S. Environmental Protection Agency (USEPA). Method 1631 Revision E-Mercury in water by oxidation, purge, and trap, and cold vapor atomic fluorescence spectrometry: EPA-821-R-02-019: 38, US EPA: Washington, DC, 2002.
- Olund, S.D.; DeWild J.F.; Olson, M.L.; Tate, M.T. Methods for preparation and analysis of solids and suspended solids for total mercury: U.S. Geological Survey Techniques of Water-Resources Investigations, vol. 5, chap. A8; U.S. Geological Survey: Reston, VA, 2004.
- Aiken, G.R. Chloride interference in the analysis of dissolved organic carbon by the wet oxidation method. Environ. Sci. Technol. 1992, 26, 2435–2439.
- Taylor, J.K. Quality Sssurance of Chemical Measurements; Lewis Publishers: Chelsea, MI, 1987.
- Gesch, D.B. The National Elevation Dataset. In Maune, D. Digital Elevation Model Technologies and Applications: The DEM Users Manual, 1st Ed.; Maune, D. Ed.; American Society for Photogrammetry and Remote Sensing: Bethesda, Maryland, 2007; 99–118.
- PAMAP. PAMAP program land cover for Pennsylvania. Pennsylvania geospatial data clearinghouse; University Park, PA, 2005. Available at http://www.pasda.psu.edu/uci/FullMetadataDisplay.aspx?file=palanduse05utm18nad83.xml. (accessed Mar 2013).
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Soil Survey Geographic (SSURGO) Database for Pennsylvania. Available at http://soildatamart.nrcs.usda.gov (accessed Mar 2013).
- PASDA. Networked streams of Pennsylvania. Pennsylvania Geospatial Data Clearinghouse, 1998. Available at http://www.pasda.psu.edu/uci/FullMetadataDisplay.aspx?file=netstreams1998.xml (accessed Mar 2013).
- Eros, T. Partitioning the diversity of riverine fish: the roles of habitat types and non-native species. Freshwater Biol. 2007, 52(7), 1400–1415.
- Kreutzweiser, D.P.; Hazlett, P.W., Gunn, J.M. Logging impacts on the biogeochemistry of boreal forest soils and nutrient export to aquatic systems: A review. Environ. Rev. 2008, 16, 157–179.
- Johnston, C.A.; Shmagin, B.A.; Frost, P.C.; Cherrier, C.; Larson, J.H.; Lamberti, G.A.; Bridgham, S.D. Wetland types and wetland maps differ in ability to predict dissolved organic carbon concentrations in streams. Sci. Total Environ. 2008, 404(2–3), 326–334.
- Laudon, H.; Berggren, M.; Ågren, A.; Buffam, I.; Bishop, K.; Grabs, T.; Jansson, M.; Köhler, S. Patterns and dynamics of dissolved organic carbon (DOC) in boreal streams: The role of processes, connectivity, and scaling. Ecosystems 2011, 14, 880–893.
- Dittman, J.; Shanley, J.; Driscoll, C.; Aiken, G.; Chalmers, A.; Towse, J.; Selvendiran, P. Mercury dynamics in relation to dissolved organic carbon concentration and quality during high flow events in three northeastern U.S. streams. Water Resour. Res. 2010, 46(7), W07522, doi:10.1029/2009WR008351.
- Burns, D.; Riva-Murray, K.; Bradley, P.; Aiken, G.; Brigham, M. Landscape controls on total and methyl Hg in the upper Hudson River basin, New York, USA. J. Geophys. Res. 2007, 117, (G1).
- Selvendiran, P.; Driscoll, C.; Bushey, J.; Montesdeoca, M. Wetland influence on mercury fate and transport in a temperate forested watershed. Environ. Pollut. 2008, 154, 46–55.
- Liu, G., Cai, Y., O’Driscoll, N., Eds. Environmental Chemistry and Toxicology of Mercury; John Wiley & Sons, Inc: Somerset, NJ, 2012; 468–471.
- Munthe, J.; Hultberg, H. Mercury and methylmercury in runoff from a forested catchment – concentrations, fluxes, and their response to manipulation. Water Air Soil Poll..2004, 4, 607–618.
- Tsui, M.T.K.; Finlay, J.C.; Nater, E.A. Mercury bioaccumulation in a stream network. Environ. Sci. Technol. 2009, 43, 7016–7022.
- Grigal, D.F. Inputs and outputs of mercury from terrestrial watersheds: a review. Environ. Rev. 2002, 10, 1–39.
- St. Louis, V.L.; Rudd, J.W.; Kelly, C.A.; Beaty, K.G.; Bloom, N.S.; Flett, R.J. Importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Can. J. Fish. Aquat. Sci. 1994, 51(5), 1065–1076.
- Agren, A.; Löfgren, S. pH sensitivity of Swedish forest streams related to catchment characteristics and geographical location – Implications for forest bioenergy harvest and ash return. For. Ecol. Manage. 2012, 276, 10–23.
- Driscoll, C.T. Ecological Effects of Acidic Deposition, In Reference Module in Earth Systems and Environmental Sciences. Elsevier: Amsterdam, 2013.
- Driscoll, C.; Driscoll, K.; Mitchell, M.; Raynal, J. Effects of acidic deposition on forest and aquatic ecosystems in New York State. Environ. Pollut. 2003, 123, 327–336.
- Baker, J.; Van Sickle, J.; Gagen, C.J.; DeWalle, D.R.; Sharpe, W.E.; Corlin, R.F.; Baldigo, B.P.; Murdoch, P.S.; Bath, D.W.; Krester, W.A.; Simonin, H.A.; Wigington, P.J. Episodic acidification of small streams in the northeastern United States: Effects on fish populations. Ecol. Soc. Am. 1996, 6, 422–437.
- Hammarstrom, J.M.; Brady, K.; Cravotta, C.A. Acid-Rock Drainage at Skytop, Centre County, Pennsylvania. U.S. Geological Survey Open-File Report 2005-1148. USGS: Reston, VA. 2004.
- Fisher, C.; Jack, R.; Lopez, L. Determination of anions in fracking flowback water from the Marcellus shale using automated dilution and ion chromatography. Thermo Scientific Technical Note 139, 2013.
- Jardine, T.D.; Kidd, K.A.; O’ Driscoll, N. Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquat. Toxicol. 2013, 132–133, 46–52.
- Pedder, S.C.J.; Maly, E.J. The avoidance response of groups of juvenile brook trout, Salvelinus fontinalis to varying levels of acidity. Aquat. Toxicol. 1986, 8(2), 111–119.
- Simonin H.A.; Krester W.A.; Bath D.W.; Olson M.; Gallagher J. In situ bioassays of brook trout (Salvelinus fontinalis) and blacknose dace (Rhinichthys atratulus) in Adirondack streams affected by episodic acidification. Can. J. Fish. Aquat. Sci. 1993, 50(5), 902–912.
- Baldigo B.P.; Lawrence G.B. Effects of stream acidification and habitat on fish populations of a North American River. Aquat. Sci. 2001, 63, 196–222.
- Peterson R.H.; Coombs K.; Power J.; Paim U. Responses of several fish species to pH gradients. Can. J. Zool. 1989, 67(6), 1566–1572.
- Shami, S.; Heino, J.; Salmah, M.; Hassan, A.; Suhaila, A.; Madrus, M. Drivers of beta diversity of macroinvertebrate communities in tropical forest streams. Freshwater Biol. 2013, 58(6), 1126–1137.
- Kaller, M.; Hartman K. Evidence of a threshold level of fine sediment accumulation for altering benthic macroinvertebrate communities. Hydrobiologia 2004, 518, 95–104.
- Wilson, M.; Winsor, L.; Nislow, K. What predicts the use by brook trout (Salvelinus fontinalis) of terrestrial invertebrate subsidies in headwater streams? Freshwater Biol. 2013, 59(1), 187–199.
- Compeau, G.C.; Bartha, R. Sulfate-reducing bacteria: Principal methylators of Mercury in anoxic estuarine sediment. Appl. Environ. Microbiol. 1985, 50, 498–502.
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate stimulation of Mercury methylation in Freshwater Sediments. Environ. Sci. Technol. 1992, 26, 2281–2287.
- Fleming, E.J.; Mack, E.E.; Green, P.G.; Nelson, D.C. 2006. Mercury Methylation from unexpected sources: Molybdate-inhibited freshwater sediments and Iron-reducing bacterium. Appl. Environ. Microbiol. 2006, 72, 457–464.
- Kerin, E.J.; Gilmour, C.C.; Roden, E.; Suzuki, M.T.; Coates, J.D.; Mason, R.P. Mercury methylation by dissimilatory iron-reducing bacteria. Appl. Environ. Microbiol. 2006, 72, 7919–7921.
- Cébron, A.; Beguiristain, T.; Faure, P.; Norini, M.P.; Masfaraud, J.F.; Leyval, C. Influence of vegetation on the in situ bacteria community and Polycyclic Aromatic Hydrocarbon (PAH) degraders in aged PAH-contaminated or Thermal-Desorption-treated soil. Appl. Environ. Microbiol. 2009, 75(19), 6322–6330.
- Vishnivetskaya, T.A.; Mosher, J.J.; Palumbo, A.V.; Yang, Z.K.; Podar, M., Brown; S.D., Brooks; S.C., Gu, B.; Southworth, G.R.; Drake, M.M.; Brandt, C.C.; Elias, D.A. Mercury and other heavy metals influence bacterial community structure in contaminated Tennessee streams. Appl. Environ. Microbiol. 2011, 77, 302–311.
- Herlemann, D.P.; Geissinger, O.; Brune, A. The Termite Group I Phylum is highly diverse and widespread in the environment. Appl. Environ. Microbiol. 2007, 73, 6682–6685.
- Dojka, M.A.; Hugenholtz, P.; Haack, S.K.; Pace, N.R. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 1998, 64, 3896–3977.
- Marvin-Dipasquale, M.; Lutz, M.A.; Brigham, M.E.; Krabbenhoft, D.P.; Aiken, G.R.; Orem, W.H.; Hall, B.D. Mercury cycling in stream ecosystems: Benthic methylmercury production and bed sediment-pore water partitioning. Environ. Sci. Tech. 2009, 43, 2726–2732.
- Paulson, A.J. Sources of mercury in sediments, Water, and fish of the lakes of Whatcom County, Washington. USGS, Scientific Investigations Report 2004–5084. 2004.
- Kamman, N.C.; Burgess, N.M.; Driscoll, C.T.; Simonin, H.A.; Goodale, W.; Linehan, J.; Estabrook, R.; Hutcheson, M.; Major, A.; Scheuhammer, A.M.; Scruton, D.A. Mercury in freshwater fish of Northeast North America: a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 2005, 14, 163–180.
- Peterson, S.A.; Sickel, J.V.; Herlihy, A.T.; Hughes, RM. Mercury concentrations in fish from streams and rivers throughout the western United States. Environ. Sci. Technol. 2007, 41(1), 58–65.
- Wiener, J.G.; Knights, B.C.; Sandheinrich, M.B.; Jeremiason, J.D.; Brigham, M.E.; Engstrom, D.R.; Woodruff, L.G.; Cannon, W.F.; Balogh, S.J. Mercury in soils, lakes, and fish in Voyageurs National Park: importance of atmospheric deposition and ecosystem factors. Environ. Science Technol. 2006, 40, 6261–6268.
- Driscoll, C.T.; Han, Y.; Chen, C.Y.; Evers, D.C.; Fallon, L.K.; Holsen, T.M.; Kamman, N.C.; Munson, R.K. Mercury Contamination in Forest and Freshwater Ecosystems in the Northeastern United States. Bioscience 2007, 57(1), 17–28.
- Castro, M.S.; Hilderbrand, R.H.; Thompson, J.; Heft, A.; Rivers, S.E. Relationship between wetlands and mercury in brook trout. Arch. Environ. Contam. Toxicol. 2007, 52, 97–103.
- Mierzykowski, S.E.; Ruksznis, P.; McCaw, D.; Czapiga, J. Environmental contaminants in brook trout (Salvelinus fontinalis) from Cove Brook and two tributaries of the Sheepscot River. USFWS. Spec. Proj. Rep. FY07-MEFO-5-EC. Maine Field Office. Old Town, ME, 2008. 41 pp.
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M.M. Uptake, Toxicity, and Trophic Transfer of Mercury in a Coastal Diatom. Environ. Sci. Technol. 1996, 30(6), 1835–1845.
- Watras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.M.; Morrisson, K.A.; Wente, S.P. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 1998, 219, 183–208.
- Greenfield, B.K.; Hrabik, T.R.; Harvey, C.J.; Carpenter, S.R. Predicting mercury levels in yellow perch: use of water chemistry, trophic ecology, and spatial traits. Can J. Fish. Aquat. Sci. 2001, 58, 1419–1429.
- Karimi, R.; Chen, C.Y.; Pickhardt, P.C.; Fisher, N.S.; Folt, C.L. Stoichiometric controls of mercury dilution by growth. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 7477–7482.
- Homeostasis and Toxicology of Non-Essential Metals, In Fish Physiology, Wood, Chris M., Farrell, Anthony P., Brauner, Colin J. Eds.; Academic Press, 2011, Vol. 31B, 1–531.
- Homeostasis and Toxicology of Non-Essential Metals, In Fish Physiology, Wood, Chris M., Farrell, Anthony P., Brauner, Colin J. Eds.; Academic Press, 2011, Vol. 31B, 1–531.
- Boudou; A., Ribeyre, F. Contamination of aquatic biocenoses by mercury compounds: an experimental ecotoxicological approach. In Aquatic Toxicology (Advances in Environmental Sceince and Technology), John Wiley and Sons: New York, 1983, Vol. 13, 73–116.
- Lemes, M.; Wang, F. Methylmercury speciation in fish muscle by HPLC-ICP_MS following enzymatic hydrolysis. J. Anal. At. Spectrom. 2009, 24, 663–668.
- Gentes, S.; Maury-Brachet, R.; Guyoneaud, R.; Monperrus, M.; Andre, J.; Davail, S.; Legeay, A. Mercury bioaccumulation along food webs in temperate aquatic ecosystems colonized by aquatic macrophytes in south western France. Ecotoxicol. Environ. Safety 2013, 91, 180–187.
- Kouba, A.; Buric, M.; Kozak, P. Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air and Soil Pollut. 2010, 211, 5–16.
- Rizzo, A.; Arcagni, M.; Arribére, M.A.; Bubach, D.; Ribeiro Guevara, S. Mercury in the biotic compartments of Northwest Patagonia lakes, Argentina. Sci. Total Environ. 2013, 454–455, 170–180.
- Mason, R.; Laporte, J.M.; Andres, S. Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch. Environ Contam. Toxicol. 2000, 38, 283–297.
- Suchanek, T.H.; Richerson, P.J.; Zierenberg, R.A.; Eagles-Smith, C.A.; Slotton, D.G.; Harner, E.J.; Osleger, D.A.; Anderson, D.W.; Cech, J.J.; Schladow, S.G.; Colwell, A.E.; Mount, J.F.; King, P.S.; Adam, D.P.; McElroy, K.J. The Legacy of Mercury Cycling from Mining Sources in an Aquatic Ecosystem: From Ore to Organism. Ecol. Appl. 2008, 18(8), A12–A28.
- Simon, O.; Boudou, A. Simultaneous experimental study of direct and direct plus trophic contamination of the crayfish Astacus astacus by inorganic mercury and methylmercury. Environ Toxicol. Chem. 2001, 20(6), 1206–1215.
- Barriada J.L.; Herrero R.; Prada-Rodriguez D.; Sastre de Vicente M.E. Interaction of mercury with chitin: A physicochemical study of metal binding by a natural biopolymer. React. Funct. Polym. 2008, 68(12), 1609–1618.
- Boening D.W. Ecological effects, transport, and fate of mercury: a general review. Chemosphere 2000, 40 (12), 1335–1351.
- Coelho J.P.; Reis A.T.; Ventura S.; Pereiera M.E.; Duarte A.C.; Pardal M.A. Pattern and pathways for mercury lifespan bioaccumulation in Carcinus maenas. Mar. Pollut. Bull. 2008, 56(6), 1104–1110.
- Zizek S.; Horvat M.; Gibicar D.; Fajon V.; Toman M.J. Bioaccumulation of mercury in benthic communities of a river ecosystem affected by mercury mining. Sci. Total Environ. 2007, 377(2-3), 407–415.
- Pennuto, C.M.; Lane, O.P.; Evers, D.C; Taylor, R.J.; Loukmas, J. Mercury in the Northern Crayfish, Orconectes virilis (Hagen), in New England, USA. Ecotoxicology 2005, 14(1-2), 149–162.
- Kuklina, I.; Kouba, A.; Kozák, P. Real-time monitoring of water quality using fish and crayfish as bio-indicators: a review. Environ. Monit. Assess. 2013, 185(6), 5043–5053.
- Riva-Murray, K.; Chasar, L.C.; Bradley, P.M.; Burns, D.A.; Brigham, M.E.; Smith, M.J.; Abrahamson, T.A. Spatial patterns of mercury in macroinvertebrates and fishes from streams of two contrasting forested landscapes in the eastern United States. Ecotoxicology 2011, 20, 1530–1542.
- Harding, K.M.; Gowland, J.A.; Dillon, P.J. Mercury concentration in black flies Simulium spp. (Diptera, Simuliidae) from soft-water streams in Ontario, Canada. Environ. Pollut. 2006, 143(3), 529–535.
- Moore, T.R.; Bubier, J.L.; Heyes, A.; Flett, R.J. Methyl and total mercury in boreal wetland plants, experimental lakes area, northwestern Ontario. J. Environ. Qual. 1995, 24(5), 845–850.
- Veglio, F.; Beolchini, F. Removal of metals by biosorption: a review. Hydrometallurgy 1997, 44, 301–316.
- Mouvet, C.; Morhain, E.; Sutter, C.; Couturieux, N. Aquatic mosses for the detection and follow-up of accidental discharges in surface waters. Water Air Soil Pollut. 1993, 66, 333–348.
- Poikolainen, J.; Kubin, E.; Piispanen, J.; Karhu, J. Atmospheric heavy metal deposition in Finland during 1985–2000 using mosses as bioindicators. Sci. Total Environ. 2004, 318, 171–185.
- Yu, R.; Adatto, I.; Monesdeoca, M.R.; Driscoll, C.T.; Hines, M.E.; Barkay, T. Mercury methylation in Sphagnum moss mats and its association with sulfate-reducing bacteria in an acidic Adirondack forest lake wetland. FEMS Microbiol. Ecol. 2010, 74(3), 655–668.
- 655–bert, K.F.; Holsen, T.M.; Chen, C.Y.; Clair, T.A.; Butler, T. Biological mercury hotspots in the northeastern United States and southeastern Canada. Biosci. Mag. 2007, 57, 29–43.
- Hultberg, H.; Iverfeldt, A.; Lee, Y.H. Methylmercury input and accumulation in forested catchments and critical loads for lakes in southewestern Sweden. In Mercury Pollution: Integration and Synthesis: Watras, J., Huckabee, J.W. Eds. Lewis Publishers: Boca Raton, FL; 1994; 313–322.
- Lee, Y.H.; Bishop, K.H.; Munthe, J.; Iverfeldt, A.; Verta, M.; Parkman, H.; Hulberg, H. An examination of current Hg deposition and export in Fenno-Scandian cathments. Biogeochemistry 1998, 40, 125–135.
