ABSTRACT
Flowback and produced wastewaters from unconventional hydraulic fracturing during oil and gas explorations typically brings to the surface Naturally Occurring Radioactive Materials (NORM), predominantly radioisotopes from the U238 and Th232 decay chains. Traditionally, radiological sampling are performed by sending collected small samples for laboratory tests either by radiochemical analysis or measurements by a high-resolution High-Purity Germanium (HPGe) gamma spectrometer. One of the main isotopes of concern is Ra226 which requires an extended 21-days quantification period to allow for full secular equilibrium to be established for the alpha counting of its progeny daughter Rn222. Field trials of a sodium iodide (NaI) scintillation detector offers a more economic solution for rapid screenings of radiological samples. To achieve the quantification accuracy, this gamma spectrometer must be efficiency calibrated with known standard sources prior to field deployments to analyze the radioactivity concentrations in hydraulic fracturing waste products.
Introduction
The field applications of a mobile shielded NaI gamma spectrometer used to perform radiological survey of Utica and Marcellus shale was described by Ying et al.Citation[1] The technologically-enhanced NORM (TENORM) radionuclides identified were the radium isotopes Ra226 and Ra228. Quantifications of activity concentrations were calculated by using a simple geometric modeling approximation to estimate the counting efficiency. The effective total counting efficiency of a spectrometer is a function of the intrinsic detector efficiency combined with the geometric form of the specific experimental sampling configuration. To accurately determine the total absolute efficiency, the complete system must be calibrated against known standard sources.
Radium isotopes are public health concerns because of their solubility in water, which increase their potential for leaching contaminations in flowback and produced wastewaters during the hydraulic fracturing processes used in unconventional gas and oil explorations. Current industrial recycling procedures applied to the wastewaters can further accumulate these radionuclides in the subsequent solid wastes. Disposal in landfill sites of these concentrated radium isotopes can potentially have an adverse long-term environmental impact due to emanation of gaseous highly-ionizing radon isotopes as part of the natural decay chains. Hence it is critical for proper environmental monitoring of the radiological impact of these industrial processes to develop an instrument and methodology that can provide accurate and rapid screening of hydraulic fracturing waste products throughout its complete life cycle from drill sites to midstream waste treatment plants to downstream landfill and water resource recovery facilities.
US Environmental Protection Agency (EPA) sets guideline levels of activity concentrations for Ra226. For drinking water the recommended maximum level is 5 pCi/L and for solid wastes is 5pCi/g that are regulated respectively under the Clean Water Act (CWA) and Title 40 Code of Federal Regulations (CFR) Part 192. EPA laboratory methods for the quantification of Ra226 typically involves a radiochemical procedure, Citation[2] whereby the radium atoms are chemically precipitated out of the samples and remnant gas are purged from the dried precipitates and allowed to stabilize in a sealed container. The radon daughter decay isotope of Ra226 is Rn222 which has a half-life of 3.8 days, and therefore a typical wait period of 21-days for secular equilibrium to be establish before alpha counting to determine the activity concentration of Ra226 from its progeny Rn222. Comparison of analytical methods used to quantify Ra226 by radiochemical, radon emanation and gamma spectroscopy for very high-salinity shale flowback wastewaters have indicated large error results using traditional radiochemical methods and 100% accurate determination through gamma spectroscopy.Citation[3]
Detection efficiencies
The absolute counting efficiency (ϵa) of a detector is defined as the total number of photons counted (Nc) out of the total number of photons (Nt) emitted by the sample. It is a function of the absorption cross-section and active thickness of the detector crystal (intrinsic efficiency ϵi) and the source to detector geometry (geometric efficiency ϵg), which can also be referred to as the geometric solid angle factor.Citation[4](1)
(2)
The total photons emitted by a source sample are a direct function of the activity (A) of the source and the measurement time (Tm):(3)
Detection efficiencies are determined by calibrating the detector systems with known standard sources. Because radioactive sources have decay half-lives (τ1/2), the source activity at any given time (AT) must be corrected for the age (Ta) of the source since its defining standard's calibration (T = 0).(4)
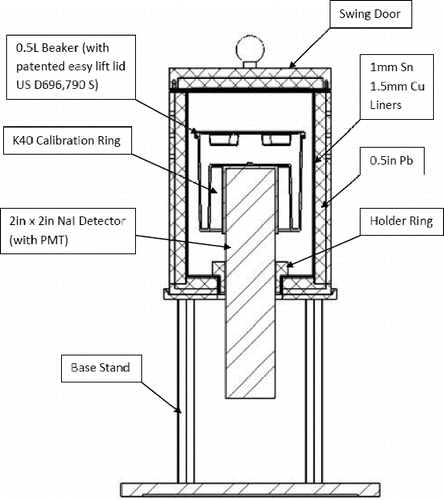
The analyzer system configuration consists of a 2-in diameter by 2-in length NaI detector (Thermo model RIIDEye-M) housed inside a 0.5 in thick shield (Kolga model A310-TT) constructed from ultra-low background lead (25 Bq/kg) lined with ultra-pure tin and copper. Samples are contained in 0.5 L Marinelli beakers and loaded through the top sliding door of the shield onto the detector head. illustrates the experimental setup.
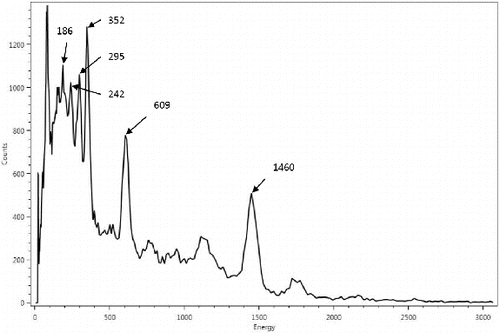
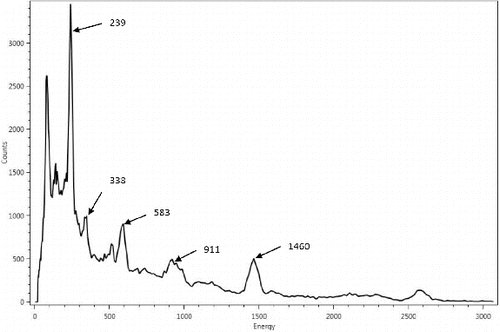
Energy calibrations of the detector are performed in the factory with Co57 (122 keV), Cs137 (662 keV) and Co60 (1173 keV and 1332 keV) radioactive sources. For efficiency calibrations of the integrated system as an analyzer for sampling hydraulic fracturing waste products, Ra226 and Ra228 standard sources housed in homogenous matrix suspension inside sealed 0.5 L Marinelli beakers are used. The time of original defining calibrations (T = 0) of these standard sources are certified for traceability to US National Institute of Standards and Technology (NIST). lists the parameters for a set of these calibrated sources. and are spectra taken using the analyzer instrument over measurement time periods of 30 minutes with Ra226 and Ra228 standard sources respectively. The energy peaks observed in the two spectra at 1460 keV belongs to K40 NORM incorporated into the RIIDEye isotope identifier as part of its auto-calibration and thermal stabilization operating functions.
Table 1. NIST-traceable radium standard sources for instrument efficiency calibration.
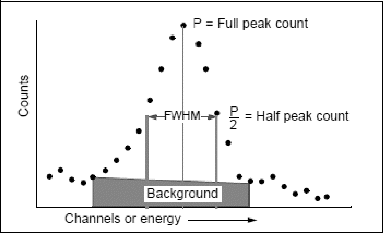
Quantitative analysis integrates the total counts in an energy peak attributable to the radionuclide of interest. illustrates the determination of the net counts under a peak area of interest.
The straight line trapezoidal area under the main peak channels represents the Compton-scattered background, which is subtracted from the total integrated peak counts to determine the net counts (Nc) under the peak area attributed to the detected gamma-rays of interest. These net counts are then cross-reference to the known activity of the age-compensated standard source (as defined in Equationequation 4The gamma energy emitted by the decay of a radionuclide has an associated branching intensity ratio (I), which must also be factored into the efficiency formula:(5)
Since the standard calibration source Ra226 has been sealed for longer than 21-days, secular equilibrium is assumed to be established between this parent isotope and its gaseous progeny Rn222 and succeeding short-lived daughter isotopes. lists the absolute efficiency of the calibrated system determined from the energy peaks highlighted in and (excluding 1460 keV which belongs to K40). The age of these standard Ra226 and Ra228 (Ta) at the time of the instrument efficiency calibration is 216 days, and the measurement period (Tm) is 30 minutes.
Table 2. Determination of detection efficiency using standard radium calibrated sources.
Field analysis of shale produced samples
With the instrument fully characterized by calibrating with radium standard sources, field samples were collected and tested with the mobile shielded analyzer that integrates the battery-operated NaI spectrometer and lightweight lead shield. The following site locations were selected and samples placed inside sealed 0.5 L beakers; Seneca stone quarry (Lodi, New York): rock samples taken from the marcellus shale outcrop. Blacklick Creek (Blacklick, Pennsylvania): sedimentary soil samples and brine water were collected from a wastewater treatment plant (WWTP) pipe discharging treated water directly into the creek. Samples were retrieved at the exhaust point and approximately 0.5 km and 1.7 km further downstream from the discharge pipe. lists the analyzed results on these varieties of field samples based on the standard 30 minutes measurement time.
Table 3. Analyzed results of shale produced field samples.
Tests on the field collected samples indicate that a 30 minutes measurement time can yield minimum detectable activity (MDA) on the radium decay isotopes of interest for solids above nominally 1 pCi/g and for liquids above 1000 pCi/L levels. For increased sensitivities the use of a thicker lead shield to reduce the background levels or longer measurement times would be required. The continuous discharges from the WWTP into the Blacklick creek over many years have impacted on the accumulated radioactivity in the soil sediment directly at the exhaust point. Although dilution occurs downstream of the discharge pipe, there is still measureable levels of radiological contaminations in the stream sediments approximately 0.5 km from source. Previous analysis in the same Western Pennsylvania locations reported Marcellus shale produced wastewater average concentrated radioactivity levels for Ra226 and Ra228 of 3231 pCi/L and 452 pCi/L, and for treated effluent water of 4 pCi/L and 2 pCi/L respectively. Chemical analysis also indicates associated high contaminated levels of salinity and toxic metals.Citation[5]
Conclusion
The application of a mobile analyzer consisting of a shielded NaI spectrometer for rapid screening of hydraulic fracturing waste products has been successfully implemented. Accurate quantifications of contaminated radium isotopes and its associated decay daughters can be achieved by proper efficiency calibrations of the instrument with known standard sources.
Acknowledgments
We would like to thank Kolga for providing hardware and technical support for these field experiments and offer our gratitude to Nathaniel Warner (Dartmouth University) for the invitation to participate in the on-site sampling at Blacklick Creek reported in this article.
References
- Ying, L.; O’Connor, F. TENORM radiological survey of Utica and Marcellus Shale. Appl. Radiat. Isotopes 2013, 80, 95–98.
- Petrow, H.G.; Allen, R.J. Estimation of the isotopic composition of separated radium samples. Anal. Chem. 1961, 33, 1303–1305.
- Nelson, A.W., May, D.; Knight, A.W.; Eitrheim, E.S.; Mehrhoff, M.; Shannon, R.; Litman, R.; Schultz, M.K. Matrix complications in the determination of radium levels in hydraulic fracturing flowback water from Marcellus shale. Environ. Sci. Tech. Lett. 2014, 1, 204–208.
- Kaplanis, S.N. Geometric, effective solid angles and intrinsic efficiencies of a 3×3 NaI(Tl) for isotopic and non-isotopic photon emission. Int. J. Appl. Radiat. Is. 1982, 33, 127–135.
- Warner, N.R.; Christie, C.A.; Jackson, R.B.; Vengosh, A. Impacts of shale gas wastewater disposal on water quality in western Pennsylvania. Environ. Sci. Tech. 2013, 47, 11849–11857.