Abstract
The pyroligneous acid (PA), or wood vinegar, is a byproduct of wood carbonization during the slow pyrolysis process. PA is recognized globally as a safe compound for agriculture due to its various beneficial properties, such as antioxidant, antibacterial, antifungal, and termiticidal properties. However, the impact of different PA concentrations on beneficial soil organisms, such as earthworms has not been investigated. The present study aims to understand the effects of different PA concentrations on earthworm Eisenia fetida. The earthworms were exposed to nine different concentrations of PA in soils, including their control. The acute toxicity assay was performed after 14 days of exposure, and the chronic toxicity assay was performed up to 8 weeks after exposure. The results from the acute toxicity assay demonstrated no significant effect on earthworm mortality. The chronic toxicity assay showed that lower PA concentrations (0.01–0.2% of weight/weight PA in soil) promoted cocoon and juvenile production in soils, whereas higher PA concentrations (0.5 and 1%) had a negative effect. These findings highlight the potential of PA to enhance soil fertility at lower concentrations, up to 0.2%, by stimulating worm activity and subsequent manure production. The outcomes of this study have significant implications for the careful management of PA concentrations within agricultural operations.
Introduction
In recent years, organic agriculture has become an alternative agricultural system for sustaining soil health and ecosystems. Even though organic farming is gaining importance, synthetic fertilizers and pesticides are still heavily used to promote crop yield, with a strongly negative impact on the environment. The natural degradation of these chemical pesticides and fertilizers is practically implausible and their prevalence in the environmental matrices over long periods is of great concern. Further, several studies have reported the lethal and sublethal effects of chemical pesticides on soil organisms, particularly the earthworms.[Citation1]
One approach to overcome the adverse impact caused by synthetic fertilizer and pesticides is to use organic byproducts from agriculture like the pyroligneous acid (PA), also known as wood vinegar. PA is a liquid condensate obtained from the pyrolysis process and is increasingly being used to promote agricultural production by enhancing soil health.[Citation2] PA is a good resource for organic agriculture with proven promise, particularly in antifungal, termicidal, and antimicrobial activities. Also, a study on PA's effect on two pests, red spider mite and aphid, highlighted its possible use in crop protection.[Citation3] Such observations have led to a growing number of studies to explore the commercial value of PA, which was once considered as waste. However, the impact of PA on the soil organisms, such as earthworms is an area that mainly remains unexplored.
Earthworms are essential for improving soil fertility by breaking down soil organic matter, developing soil structure, and enhancing soil nutrient turnover. Agricultural management systems affect earthworm activity and population.[Citation4] Also, earthworms exhibit varying degrees of sensitivity to pesticides, heavy metals, and other soil pollutants. Thus, earthworm species Eisenia fetida and Eisenia andrei have been used extensively as model organisms and bioindicators to assess the ecological risks associated with contaminants in the soil systems.[Citation5] In past studies, the most commonly designed endpoint to assess the toxic effect of chemicals was by recording the earthworm survival/mortality rate in the presence of chemicals. Also, test endpoints, such as cocoon and juvenile production, reflect on the chronic reproductive endpoints of the tested pollutants.[Citation6]
The comet assay or the single cell gel electrophoresis (SCGE) has become a standard method for assessing the DNA damage caused by chemicals in sentinel organisms due to its high sensitivity.[Citation7] For earthworms, several studies have demonstrated comet assay’s effectiveness in determining the genotoxicity of chemicals by monitoring the extent of DNA damage.[Citation8–10] Two parameters used in the earlier comet assay studies were—% tail DNA, which reflects the exact level of DNA damage caused by pollutants at different concentrations, and Olive tail moment (OTM), which indicates the electrophoretic mobility of the damaged DNA induced by pollutants.[Citation11,Citation12] However, there are no reported studies on the effects of PA on acute, chronic, and cyto-genotoxicity to earthworms.
Therefore, the focus of the present study is to investigate the effects of PA on Eisenia fetida in two natural soils spiked with different concentrations of PA. The comet assay technique was used to monitor the resulting DNA damage. The study led to sensitive and reliable conclusions regarding the effects of PA on acute, chronic, and cyto-genotoxicity to earthworms.
Materials and methods
Experimental setup and test species
Northside Industries provided the refined PA (PyroAg®), a water-based condensate, for this project. All the solvents (analytical grade) were purchased from Sigma-Aldrich, Australia. Two soils with no history of pesticide application were collected from Lovedale, Hunter Valley (Soil A) and Boanbong Road, and Palm Beach (Soil B) were used in this study. The selected physicochemical properties of the soils (loamy sands) are Soil A: pH 5.9, electrical conductivity (EC) 32.07 μS cm−1, carbon 0.51%, sand 82.37%, silt 17.41%, clay 0.21%, and Soil B: pH 6.6, EC 119.95 μS cm−1, carbon 1.48%, sand 85.39%, silt 14.44%, and clay 0.17%. The test concentrations of PA were in the range of 0.01–2%, which were arrived at by spiking PA solution in 500 g of soil on a weight/weight basis. The PA concentrations (0.1–1%) used in this study represent the recommended application rates by the manufacturer in agricultural fields.[Citation2,Citation13] The PA concentration in soil was determined using Attenuated Total Reflection-Fourier-Transform Infrared Spectroscopy (ATR-FTIR) as described in our earlier study by Sivaram et al.[Citation13] Tests were conducted in triplicate. Each replicate consisted of 500 g of soil maintained at 35% moisture. The soil without any PA addition served as the control.
Eisenia fetida was selected for the study due to its common occurrence, the ease with which it can be cultured, and its widespread use in toxicity assays as a recommended organism by international agencies. The starter culture of E. fetida was purchased from Bunning’s Warehouse, Wallsend, NSW, and was maintained in earthworm bedding. The earthworms were fed with cow dung and maintained at 20 ± 2 °C, 72% humidity, and a 16:8 h light/dark cycle.[Citation9] Adult worms ranging from 500 to 600 mg weight and well-developed clitella were used for the experiments.
Acute toxicity assay
The effect of PA on E. fetida was studied according to the OECD guidelines.[Citation14] The tests were conducted under controlled conditions at room temperature 20 ± 2 °C, following 16 h light and 8 h dark cycles. Worms were cleaned and placed on moist filter paper in a ventilated container for depuration (24 h). Ten adult worms of uniform size with fully developed clitellum were released into each test container. The lids of all test containers were perforated to regulate aeration. The depurated earthworms were weighed before exposure to soil and at the end of 14 days of incubation. No feeding was given during the incubation period. Mortality after 14 days was recorded for each treatment and control. The data were analyzed statistically using Minitab 18 software to determine the concentration of the test chemicals that resulted in 50% mortality (LC50 value) of the earthworms for each treatment.
Chronic toxicity assay
The chronic toxicity assay was conducted in earthworms exposed to soils spiked with 0.01–1% PA concentrations. The experiment was conducted according to OECD guidelines[Citation15] and the setup was similar to the acute toxicity assay, except the worms were fed with 5 g of cow dung weekly. The moisture content of the soil was checked throughout the experiment and moisture was added when necessary. After four weeks (28 days) of exposure, the reproduction test was performed by removing adult worms from each test container. The cocoons produced in each treatment and control were counted, restored back to their respective containers, and maintained under the same conditions described above. The number of juveniles produced after eight weeks (56 days) of the experimental period was recorded, and the data were analyzed to determine the significance between treatment and control.
Comet assay
Comet assay was used to determine the cyto-genotoxic effects of PA on earthworms. After their exposure to a range of PA concentrations (0.01–1%), the earthworms in two different soils were analyzed for cyto-genotoxicity. The earthworms were washed with deionized water and allowed to depurate for 24 h. Following depuration, the worms were used for the cyto-genotoxicity assays. Coelomocytes from depurated worms were extracted following the protocol described by Dhawan et al.[Citation16] with a slight modification. Coelomocytes from the earthworms, extruded using an extrusion buffer, were washed thrice and centrifuged for 3 min at 8000 rpm and resuspended in 1× phosphate-buffered saline (PBS).[Citation9,Citation13] About 50 µL cell suspensions were added in 150 µL of 0.5% low-melting (37 °C) agarose and layered onto a slide and then allowed to solidify at 4 °C. Cells were lysed using a freshly prepared cold (4 °C) lysing solution whereby cellular proteins were removed, and the damaged DNA was liberated. Subsequently, DNA unwinding was done with an alkaline solution for 60 min. Electrophoresis was carried out after DNA unwinding for 30 min using 1× TBE. The slides were neutralized and then rinsed with ultrapure water. Finally, the slides were stained using SYBER green, fluorescent dye. The images were analyzed using a fluorescence microscope equipped with an excitation filter of 515–650 nm and a 580 nm barrier filter, and the images of selected cells from each treatment were examined using the Comet Score™ software.[Citation13]
Results and discussion
Studies on the effect of PA on earthworms were not done before, and information on the terrestrial effects of PA is not readily available. For assessing terrestrial ecotoxicity, earthworms are highly preferred as they are critical, non-target beneficial organisms in soils.[Citation17] Eisenia fetida was used in the present study since it is an internationally accepted model species for toxicity assessment with a cosmopolitan distribution.[Citation18] Earthworms’ interaction with soil contaminants is predominantly through dermal contact and ingestion, making it an ideal test candidate for assessing terrestrial ecotoxicity.[Citation19,Citation20] Several acute toxicity assays have recently been standardized to observe environmentally induced responses.[Citation21] Earthworms’ acute exposure causes severe weight reduction, whereas prolonged exposure tends to affect earthworms’ later generations due to a malfunction in reproduction.
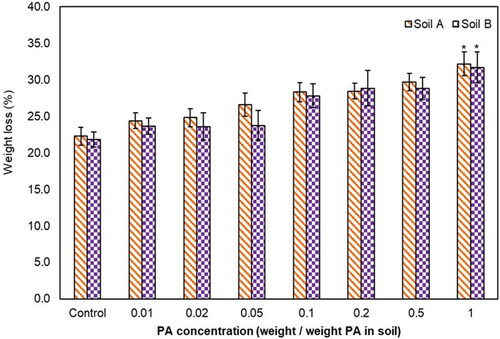
In this study, PA concentrations varying from 0.01 to 2% (weight/weight PA in soil) were used for the earthworm acute toxicity assays. The earthworms exhibited avoidance at 0.5 and 1% PA spiked soils a few hours after release, and after one day, the earthworms started burrowing into the soils. There was no change in their survival rate up to 0.5% PA, and at concentrations above 1%, the survival percentage was finally affected (). The earthworm weight-loss percentage consistently increased with increasing PA concentration, and the outcome was statistically significant at 1% of PA spiked soils ().
Figure 1. Effect of PA on E. fetida survival in Soil A and Soil B. *Statistically significant based on Student’s t-test at P = 0.05, **P = 0.01.
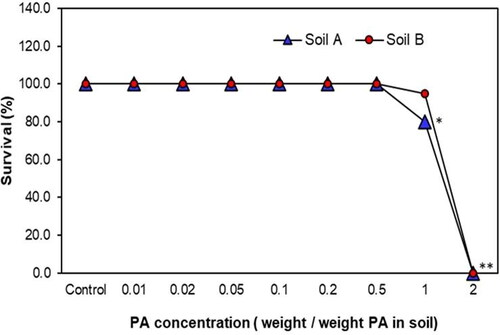
Figure 2. Effect of PA on E. fetida weight loss in Soil A and Soil B. *Statistically significant based on Student’s t-test at P = 0.05.
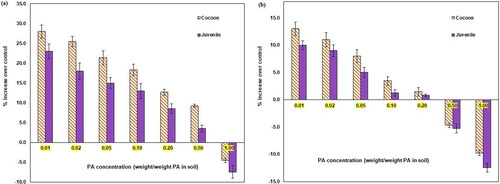
As a sensitive indicator, reproductive parameters, such as cocoon and juvenile production have been used in this study to reflect any chronic toxicity of PA to the organisms. The results demonstrate that chronic toxicity of PA in earthworms was not observed at lower concentrations (0.01–0.2% PA). Moreover, lower PA concentrations promoted cocoon and juvenile hatching compared to the control soils. This could be due to the enhancement of soil organic matter and nutrient availability afforded by PA.[Citation22] This soil improvement by PA indirectly enhanced the growth and reproduction of soil earthworms. In contrast, the higher PA concentrations of 0.5 and 1% reduced cocoon and juvenile hatching compared to the control soils (). These results indicate that PA at high concentrations adversely affects earthworm reproduction, indirectly by reducing the energy available for the reproduction.[Citation23,Citation24]
In all organisms, molecular, biochemical, and physiological compensatory mechanisms change or get affected upon exposure to environmental contaminants. These parameters serve as biomarkers/indicators in assessing toxicity.[Citation25] Among the molecular components, the DNA of aquatic and terrestrial organisms is the crucial target of environmental stress.[Citation26] The loss of DNA integrity indicated by the level of strand breakage was proposed as a sensitive indicator of genotoxicity.[Citation27,Citation28] For detecting DNA strand breakage, alkaline comet assay was reported to be a susceptible method.[Citation29] The genotoxicity of different concentrations of PA to earthworm (E. fetida) was measured using the alkaline comet assay. DNA strand breakage/damage was calculated in terms of the percent DNA in the comet tail and the Olive tail moment (OTM). The different concentrations of PA were evaluated for their potential to cause DNA damage in earthworms through alkaline comet assay. The results exhibited no DNA damage in all the tested PA concentrations. Therefore, the comet assay results indicate that PA is unlikely to induce genotoxicity in earthworms. Overall, the application of PA in organic agriculture at lower concentrations up to 0.2% provides favorable conditions and enhances the production of cocoons and juveniles, which in turn improves soil fertility.
Conclusion
The results from this study show that the effects of PA on earthworms are strongly dependent on its concentrations. PA concentrations of 0.01–0.2% (weight/weight PA in soil) had a positive effect on earthworms by increasing the cocoon and juvenile production, whereas the effect was the opposite at PA concentrations of 0.5 and 1%. The implication is the beneficial effects of PA in organic agriculture to produce worm and worm manure at concentrations of up to 0.2% to enhance soil fertility.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Rico, A.; Sabater, C.; Castillo, M. Á. Lethal and Sub-Lethal Effects of Five Pesticides Used in Rice Farming on the Earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2016, 127, 222–229. DOI: 10.1016/j.ecoenv.2016.02.004.
- Sivaram, A. K.; Panneerselvan, L.; Mukunthan, K.; Megharaj, M. Effect of Pyroligneous Acid on the Microbial Community Composition and Plant Growth-Promoting Bacteria (PGPB) in Soils. Soil Syst. 2022, 6, 10. DOI: 10.3390/soilsystems6010010.
- Mmojieje, J.; Hornung, A. The Potential Application of Pyroligneous Acid in the UK Agricultural Industry. J. Crop. Improv. 2015, 29, 228–246. DOI: 10.1080/15427528.2014.995328.
- Kladivko, E.; Timmenga, H. Earthworms and Agricultural Management, Rhizosphere Dynamics; CRC Press, New York, 2019; pp 192–216.
- Fründ, H. C.; Graefe, U.; Tischer, S. Earthworms as Bioindicators of Soil Quality, Biology of Earthworms; Springer, Berlin, Heidelberg, 2011; pp 261–278.
- Li, L.; Yang, D.; Song, Y.; Shi, Y.; Huang, B.; Bitsch, A.; Yan, J. The Potential Acute and Chronic Toxicity of Cyfluthrin on the Soil Model Organism, Eisenia fetida. Ecotoxicol. Environ. Saf. 2017, 144, 456–463. DOI: 10.1016/j.ecoenv.2017.06.064.
- Collins, A. R. The Comet Assay for DNA Damage and Repair. Mol. Biotechnol. 2004, 26, 249–261. DOI: 10.1385/MB:26:3:249.
- Button, M.; Jenkin, G. R. T.; Bowman, K. J.; Harrington, C. F.; Brewer, T. S.; Jones, G. D. D.; Watts, M. J. DNA Damage in Earthworms from Highly Contaminated Soils: Assessing Resistance to Arsenic Toxicity by Use of the Comet Assay. Mutat. Res. 2010, 696, 95–100. DOI: 10.1016/j.mrgentox.2009.12.009.
- Sivaram, A. K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Phytoremediation Efficacy Assessment of Polycyclic Aromatic Hydrocarbons Contaminated Soils Using Garden Pea (Pisum sativum) and Earthworms (Eisenia fetida). Chemosphere. 2019, 229, 227–235. DOI: 10.1016/j.chemosphere.2019.05.005.
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological Effects of Polystyrene Microplastics on Earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. DOI: 10.1016/j.envpol.2019.113896.
- Kumaravel, T.; Jha, A. N. Reliable Comet Assay Measurements for Detecting DNA Damage Induced by Ionising Radiation and Chemicals. Mutat. Res. 2006, 605, 7–16. DOI: 10.1016/j.mrgentox.2006.03.002.
- Ma, T.; Chen, L. K.; Wu, L.; Zhang, H.; Luo, Y. Oxidative Stress, Cytotoxicity and Genotoxicity in Earthworm Eisenia fetida at Different Di-n-Butyl Phthalate Exposure Levels. PLOS One 2016, 11, e0151128. DOI: 10.1371/journal.pone.0151128.
- Sivaram, A. K.; Logeshwaran, P.; Abinandan, S.; Mukunthan, K.; Megharaj, M. Cyto-Genotoxicity Evaluation of Pyroligneous Acid Using Allium cepa Assay. J. Environ. Sci. Health A 2022, 57, 852–857.
- Organization for Economic Cooperation and Development (OECD) Test No. 207: Earthworm, Acute Toxicity Tests; OECD Publishing, Paris, 1984.
- Organization for Economic Cooperation and Development (OECD). Earthworm Reproduction Test. Guideline for Testing Chemicals; OECD Publishing, Paris, 2004; Vol. 222.
- Dhawan, A.; Bajpayee, M.; Parmar, D. Comet Assay: A Reliable Tool for the Assessment of DNA Damage in Different Models. Cell Biol. Toxicol. 2009, 25, 5–32. DOI: 10.1007/s10565-008-9072-z.
- Lionetto, M. G.; Calisi, A.; Schettino, T. Earthworm Biomarkers as Tools for Soil Pollution Assessment, Soil Health and Land Use Management; InTech-Open Access Publisher in Science, Technology, and Medicine: Rijeka, 2012; pp 305–332.
- Edwards, C. A. Development of a Standardized Laboratory Method for Assessing the Toxicity of Chemical Substances to Earthworms. In Earthworm Acute Toxicity Tests, EUR 8714 EN/W/80/574, No 207. Technical Report; Organisation for Economic Co-Operation and Development, Ed.; OECD: Brussels, 1984; pp 35–40.
- Vijver, M. G.; Vink, J. P.; Miermans, C. J.; van Gestel, C. A. Oral Sealing Using Glue: A New Method to Distinguish between Intestinal and Dermal Uptake of Metals in Earthworms. Soil Biol. Biochem. 2003, 35, 125–132. DOI: 10.1016/S0038-0717(02)00245-6.
- Morgan, A.; Stürzenbaum, S.; Winters, C.; Grime, G.; Aziz, N. A. A.; Kille, P. Differential Metallothionein Expression in Earthworm (Lumbricus rubellus) Tissues. Ecotoxicol. Environ. Saf. 2004, 57, 11–19. DOI: 10.1016/j.ecoenv.2003.08.022.
- Pauwels, M.; Frérot, H.; Souleman, D.; Vandenbulcke, F. Using Biomarkers in an Evolutionary Context: Lessons from the Analysis of Biological Responses of Oligochaete Annelids to Metal Exposure. Environ. Pollut. 2013, 179, 343–350. DOI: 10.1016/j.envpol.2013.05.005.
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative Study of Individual and Co-application of Biochar and Wood Vinegar on Blueberry Fruit Yield and Nutritional Quality. Chemosphere 2020, 246, 125699. DOI: 10.1016/j.chemosphere.2019.125699.
- Wallwork, J. A. Earthworm Biology; E. Arnold (Publishers) Ltd., London; Baltimore, Md., USA, 1983.
- Melo, T. M.; Schauerte, M.; Bluhm, A.; Slaný, M.; Paller, M.; Bolan, N.; Bosch, J.; Fritzsche, A.; Rinklebe, J. Ecotoxicological Effects of Per-and Polyfluoroalkyl Substances (PFAS) and of a New PFAS Adsorbing Organoclay to Immobilize PFAS in Soils on Earthworms and Plants. J. Hazard. Mater. 2022, 433, 128771. DOI: 10.1016/j.jhazmat.2022.128771.
- Reinecke, S. A.; Reinecke, A. J. The Comet Assay as a Biomarker of Heavy Metal Genotoxicity in Earthworms. Arch. Environ. Contam. Toxicol. 2004, 46, 208–215. DOI: 10.1007/s00244-003-2253-0.
- Frenzilli, G.; Nigro, M.; Scarcelli, V.; Gorbi, S.; Regoli, F. DNA Integrity and Total Oxy Radical Scavenging Capacity in the Mediterranean Mussel, Mytilus galloprovinciales: A Field Study in a Highly Eutrophicated Coastal Lagoon. Aquat. Toxicol. 2001, 53, 19–32. DOI: 10.1016/s0166-445x(00)00159-4.
- Belpaeme, K.; Delbeke, K.; Zhu, L.; Kirsch-Volders, M. Cytogenetic Studies of PCB77 on Brown Trout (Salmo trutto fario) Using the Micronucleus Test and the Alkaline Comet Assay. Mutagenesis 1996, 11, 485–492. DOI: 10.1093/mutage/11.5.485.
- Sivaram, A. K.; Logeshwaran, P.; Subashchandrabose, S. R.; Lockington, R.; Naidu, R.; Megharaj, M. Comparison of Plants with C3 and C4 Carbon Fixation Pathways for Remediation of Polycyclic Aromatic Hydrocarbon Contaminated Soils. Sci. Rep. 2018, 8, 2100. DOI: 10.1038/s41598-018-20317-0.
- Fairbairn, D. W.; Olive, P. L.; O'Neill, K. L. The Comet Assay: A Comprehensive Review. Mutat. Res. 1995, 339, 37–59. DOI: 10.1016/0165-1110(94)00013-3.