Abstract
Chlorpyrifos (CPF) is one of the most widely used organophosphate insecticides in the United States. By December 2000, nearly all residential uses were voluntarily canceled, so that today, CPF is only used to control insect pests on a variety of crops. Periodic review of the potential effects of CPF on all developmental outcomes is necessary in the United States because the Food Quality Protection Act mandates special consideration of risk assessments for infants and children. This article reviews epidemiologic studies examining the association of potential CPF exposure with growth indices, including birth weight, birth length, and head circumference, and animal studies focusing on related somatic developmental endpoints. It differs from earlier reviews by including an additional cohort study and providing in-depth systematic evaluation of the patterns of association across different studies with respect to specificity of biomarkers for CPF, consistency, dose response, strength of association, temporality, and biological plausibility (Hill 1965), as well as consideration of the potential role of effect modification and bias. The review did not identify any strong associations exhibiting consistent exposure-response patterns that were observed in more than one of the four cohort studies evaluated. In addition, the animal data indicate that developmental effects occur at doses that produce substantial maternal toxicity and red blood cell (RBC) acetylcholinesterase (AChE) inhibition. Based on consideration of both the epidemiologic and animal data, maternal RBC AChE inhibition is a more sensitive endpoint for risk assessment than somatic developmental effects reviewed in this article.
Chlorpyrifos (CPF) is an organophosphorus insecticide, acaricide, and miticide currently used to control insect pests on a variety of food and feed crops, most commonly corn. Prior to June 2000, CPF was also widely used for indoor pest control and pet collars, but most household uses were phased out beginning in June 2000 and canceled by January 2001 (CitationU.S. EPA 2002; Citation2006). Therefore, the primary route of exposure to the general U.S. population of children and adults today is dietary, and such exposure has been estimated to be approximately 0.15–0.32 μg/kg-d for acute dietary food-only exposure and 8–34 ng/kg-d for chronic dietary food exposure with food handling establishment uses (U.S. EPA b). Children and adults, including pregnant females, who are farm workers or living on or near farms may be exposed through additional pathways, including dermal or inhalation routes.
CPF and other organophosphates (OP) are currently regulated based on red blood cell (RBC) or brain acetylcholinesterase (AChE) inhibition; the latter is considered to be a primary mode of action for toxicity, especially related to the acute clinical signs of neurotoxicity following short-term high-dose exposures. Other modes of action for acute toxicity have also been proposed, including alterations of presynaptic cholinergic functions or noncholinergic neurochemical processes that may contribute to differential expression of toxicity among different OP (CitationLiu and Pope 1998; CitationPope 1999; CitationUdarbe Zamora et al. 2008). Young rats are more sensitive to acute effects of CPF than adults, and these differences may be attributable, in part, to age differences in metabolic enzymatic activity, especially at higher doses (CitationEaton et al. 2008; CitationTimchalk et al. 2006). Plasma butyrylcholinesterase (BuChE) inhibition may occur at exposure levels below those that provide brain or RBC AChE inhibition and is used as a marker of exposure in occupational settings.
Human and mechanistic animal studies have led to the hypothesis that developmental effects occur at subclinical exposure levels by mechanisms other than AChE inhibition (CitationRauh et al. 2006). CitationSlotkin and Seidler (2007) stated that the fact that prenatal effects are elicited at “exposures below the threshold for inhibition of fetal brain cholinesterase reinforces the importance of other mechanisms underlying the developmental neurotoxicity of CPF, and potentially of other OP, and points to the inadequacy of cholinesterase activity as the sole factor for assessing exposure or safety” (426).
Therefore, a systematic evaluation of human and animal developmental studies is needed, including comparisons with AChE inhibition. Careful consideration of all developmental outcomes is important in providing the scientific basis for risk assessment, including science policy decisions required by the U.S. Environmental Protection Agency (EPA) Food Quality Protection Act (FQPA), which requires special protections for infants and children. A companion paper focuses on the developmental neurobehavioral data on CPF (CitationLi et al. 2012). This review focuses on epidemiologic studies that evaluated associations between CPF exposure and growth indices, including birth weight, birth length, and head circumference. This review assesses the evidence for and against a causal relationship between CPF exposure, as measured in umbilical cord blood, urinary metabolites, or air monitoring samples, and these outcomes.
The human data for growth indices are of interest because analyses were reported for birth outcomes before and after cancelation of residential uses and included data based on personal maternal monitoring of CPF exposures. In addition, this review evaluates whether the PON1 genotype, associated with detoxification of the toxic metabolite chlorpyrifos oxon (CPO), can modify associations between CPF exposure and fetal growth. Finally, the human data were compared with animal data for similar outcomes from developmental or reproductive animal studies published in the literature that include at least 3 CPF dose levels and 20 litters/dose group, which are the standard requirements for developmental toxicity studies based on the U.S. EPA Guidelines for Developmental Toxicity Risk Assessment (CitationU.S. EPA 1991).
The present review of the animal and epidemiologic studies on birth and growth outcomes differs from previous reviews (CitationClegg and van Gemert 1999a; CitationEaton et al. 2008; Weselak et al. 1999b; Citation2007; CitationZhao et al. 2005; Citation2006;) by contributing in-depth analyses of outcomes and including an additional study published in 2010 (CitationBarr et al. 2010). The two 1999 expert panel reports (CitationClegg and van Gemert 1999a; Citation1999b) were written prior to publication of the epidemiologic studies included in this review. CitationEaton et al. (2008) summarized each of the major epidemiologic studies of CPF exposure and birth outcomes in children, based on authors' reported findings, and provided comprehensive analyses of exposures. This review differs from the CitationZhao et al. (2005) review of CPF and birth weight, the CitationWeselak et al. (2007) review of pesticides and birth outcomes, and the CitationEaton et al. (2008) reviews by (1) providing comparisons of methodologies (including exposure measurement) and results; (2) reporting in tables the magnitude and direction of associations and 95% confidence intervals so that the reader can evaluate the precision of estimates and whether the direction of associations are consistent for similar exposure-outcome associations across studies; (3) considering the potential role of systematic error (bias); (4) discussing findings from the human studies in a causal framework (e.g., data addressing strength of the association, consistency across studies, exposure-response patterns of association); and (5) directly comparing the human data with the relevant animal data for biological plausibility.
In summary, this article evaluates whether the epidemiologic data indicate a strong or otherwise meaningful pattern of association of CPF exposure with birth weight, birth length, and head circumference at birth across different cohort studies. This is one of the scientific issues considered to be of primary importance in evaluating children's health assessment for CPF, including determination of uncertainty factors required under FQPA.
METHODS
Scope of the Review
The scope of the literature search included epidemiologic studies that investigated postulated associations between in utero exposure to CPF and growth endpoints in neonates, including head circumference, birth weight and length, and longitudinal growth indices in children; however, no studies met our inclusion criteria that included longitudinal growth indices in children. Abdominal circumference and ponderal index (PI; analogous to the body mass index in adults) were evaluated in some studies and results are included. Studies that reported results for adolescents or adults were excluded. The literature search was not limited by the geographic location of the study; however, our review includes only peer-reviewed studies that were published in English. Case reports and case series were excluded because they do not test hypotheses, estimate effects, or otherwise provide information on associations between an exposure and an outcome. Studies based on accidental or intentional poisonings were also excluded because this review is focused on evaluating effects of exposures resulting from standard uses (e.g., agricultural and residential).
All studies that inferred CPF exposure but did not directly measure and quantify exposure levels were excluded. This included any study that evaluated CPF exposure based on the residential location of the study participant with respect to agricultural activities (e.g., distance to nearest farm). Studies that measured air CPF concentrations at the individual level were included. Studies that described CPF biomonitoring but did not evaluate an association between exposure and the growth indices of interest were evaluated, but were not included in our final review. Because AChE inhibition is considered to be a mode of action for CPF, results on AChE levels and these outcomes from one of the studies are also reported.
Outcomes of interest were based strictly on the epidemiologic studies. To address the question of biological plausibility, animal data from robust study designs for endpoints related to those measured in the epidemiologic studies were evaluated. The inclusion criteria for in vivo animal studies were at least 3 CPF dose levels and 20 litters/dose level, the minimum regulatory requirements for reproduction and developmental studies to enhance confidence in the data. In addition, the route of exposure had to be relevant to human exposures, and the period of exposure had to include gestational exposures. Because there were no longitudinal epidemiologic studies measuring height and weight at different ages in children, the literature search for animal studies was restricted to outcomes of fetal weight or birth weight. Other relevant findings reported in these selected animal studies (e.g., AChE inhibition, maternal toxicity, and general developmental toxicity data) that aid in evaluation of birth or fetal weight were also evaluated.
Literature Search
The literature search included articles published in English through May 31, 2011. For the epidemiologic studies, a comprehensive search of the published literature was conducted in MEDLINE using the following search terms: “organophosphate,” OR “organophosphorus,” OR “chlorpyrifos,” “head circumference,” “head size,” “birth weight,” “birth length,” “weight,” “length,” “fetal growth,” OR “infant growth,” “Ponderal Index,” “small for gestational age,” “small-for-gestational-age,” “small size,” “birth outcome.” In addition, reference lists in recent reviews on CPF were cross-checked to identify any relevant papers that may have been missed by our search terms.
For the animal studies, MEDLINE was used to search for published journal animal articles written in English using the following search terms: chlorpyrifos and (development or reproduction or developmental) and (“birth weight” or “body weight” or “fetal weight” or “weight”) and (rat or rats or mice or mouse or rodent or mammal or monkey or primate or animal) NOT (“bugs” or “cockroach” or “in vitro” or “zebrafish” or “cows” or “cow”). The following inclusion criteria were intended to ensure robustness and reliability of the animal studies and relevance to the human studies on birth outcomes: gestational exposures, 3 CPF dose levels, 20 or more litters per dose group, the litter was the experimental unit of analyses for fetal or pup birth weight, route of exposure was relevant to humans (oral, dermal, or inhalation), and data were presented. Of the 43 papers found, only 11 were original papers involving gestational exposures. Of these 11 papers, 5 were eliminated because the sample size was 5–10, the litter was not the experimental unit, and/or the route of exposure was subcutaneous (sc) injections. Although the sc injection route might have advantages for investigative mechanistic studies, it can affect the pharmacokinetics of CPF, such as bypassing first-pass metabolism (Marty et al. 2007; Slotkin et al. 2006), and degree of cholinesterase (ChE) inhibition and toxicity (Carr and Nail 2008). An exception was made for one oral study with a sample size of 10 that was included because the primary focus of this study was to evaluate the effect of CPF on body weight (CitationLassiter et al. 2008).
CPF and OP Biomarkers Included in This Review
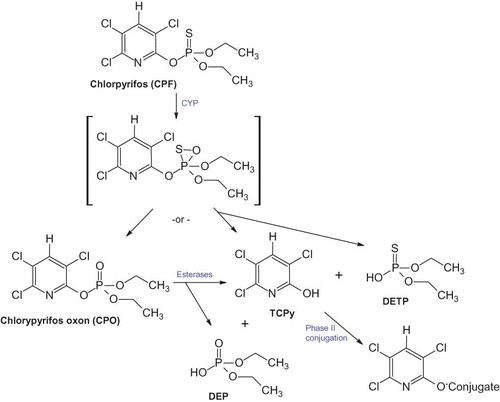
This review focused on studies that analyzed the associations between biomarkers of CPF and birth outcomes. The CPF and other OP biomarkers () included in this review and their specificity to CPF exposure are as follows (CitationBarr and Angerer 2006; CitationBravo et al. 2004; CitationNeedham 2005):
| • | CPF and chlorpyrifos-oxon (CPO) are biomarkers of highest specificity for CPF that are measured in blood plasma or serum. CPF is bioactivated to CPO, the primary toxic active metabolite of concern. Environmental exposure to CPO is also possible (CitationBarr and Angerer 2006). | ||||
| • | 3,5,6-Trichloro-2-pyridinol (TCPy) is the most common urinary biomarker of CPF exposure, but with important limitations including that it may reflect exposures other than the parent compound CPF. Briefly, CPO is rapidly hydrolyzed to TCPy and diethylphosphate (DEP) (). CPF can also be dearylated to form TCPy and diethylthiophosphate (DETP) (). However, TCPy is also a metabolite of chlorpyrifos-methyl and triclopyr (CitationBarr and Angerer 2006; CitationWhyatt et al. 2009). It is an environmental degradate present in food, the environment, or homes, as a breakdown product from exposure to CPF, CPO, or chlorpyrifos-methyl (CitationBarr and Angerer 2006; CitationEaton et al. 2008; CitationWhyatt et al. 2009). Significant intra-individual variability in repeat urine samples from the same individual has been observed (CitationWhyatt et al. 2009). | ||||
| • | Diethylphosphates (DEPs) represent a broad class of OP metabolites measured in urine that include DEP, DETP, and diethyldithiophosphate (DEDTP). Only DEP and DETP are metabolites of CPF (, ). DEPs are relatively nonspecific as a biomarker of CPF because other OP are metabolized to DEPs and may be present in the environment (). | ||||
| • | Dimethyl phosphates (DMPs) are urinary metabolites that cannot be formed from CPF (). They are metabolites of several methyl OP, including malathion and chlorpyrifos methyl (a pesticide registered separately from CPF). These metabolites are important to consider in evaluation of whether associations between DAPs and birth outcomes are more relevant to DMPs compared to DEPs. | ||||
| • | Dialkyl phosphates (DAPs) are a class of OP metabolites measured in urine, which include both DEPs and DMPs (). Therefore, DAPs are highly questionable biomarkers of CPF compared to DEPs because they include metabolites that cannot be formed from CPF. As with DEPs, DAPs are also a nonspecific environmental degradate of OP. | ||||
TABLE 1. Biomarkers of Organophosphate Exposures and Their Relevance to Chlorpyrifos
EPIDEMIOLOGIC STUDY RESULTS
Epidemiologic Studies Included in This Review
summarizes the study characteristics (including consideration of smoking, alcohol, and drug exposure) of the eight epidemiologic reports evaluating the association between CPF exposure and growth outcomes in neonates. These were reports from the following four cohorts: the Columbia Center for Children's Environmental Health (CCCEH) (CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005), the Mount Sinai Center for Children's Environmental Health and Disease Prevention Research (Mt. Sinai) (CitationBerkowitz et al. 2004; CitationWolff et al. 2007), the Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) (CitationEskenazi et al. 2004), and the New Jersey Cohort of Pregnant Women and their Children (New Jersey) (CitationBarr et al. 2010).
TABLE 2. Characteristics of the Epidemiologic Studies Reporting Associations Between Chlorpyrifos or Relevant Metabolites and Fetal Growth Outcomes
All four cohort studies presented results for head circumference, birth length, and birth weight. Two studies, the Mt. Sinai study (CitationWolff et al. 2007) and CHAMACOS study (CitationEskenazi et al. 2004), reported results for the ponderal index, calculated as (birth weight in g × 100)/(length in cm)3 (CitationEskenazi et al. 2004). Results for abdominal circumference were reported only by the New Jersey cohort study (CitationBarr et al. 2010). Although all four publications cited from the CCCEH cohort reported results that met our inclusion criteria, the purpose of the publication by CitationRauh et al. (2006) was to evaluate associations between prenatal CPF exposures and neurobehavioral outcomes. We review those results in a separate article (CitationLi et al. 2012).
The CCCEH cohort included nonsmoking women, ages 18–35 yr, self-identified as African American or Dominican residing in northern Manhattan or the South Bronx for at least 1 yr before pregnancy (CitationPerera et al. 2003; CitationWhyatt et al. 2004; Citation2005). The women were included if they were free of diabetes, hypertension, or known HIV (human immunodeficiency virus). Covariates included in the final models were race/ethnicity, gestational age, parity, maternal prepregnancy weight and new weight gain during pregnancy, maternal self-reported environmental tobacco smoke in the home, sex of the newborn, and season of delivery. Annual household income, maternal education, maternal marital status, material hardship during pregnancy, and degree of housing disrepair were not included in the final model because they did not affect the results (CitationWhyatt et al. 2004)
The Mt. Sinai cohort included women pregnant for the first time with one child without serious chronic diseases such as diabetes, hypertension, thyroid disease, or serious pregnancy complication that could affect fetal growth and development. The mother and infant were excluded if there was severe prematurity or congenital malformation. The largest ethnic group was Hispanics, followed by African-Americans and whites. Models included race/ethnicity, infant sex, and gestational age. Prepregnancy body mass index, maternal weight gain, blood lead levels, and cesarean section delivery were not included in the final models because they did not affect the results. Marital status and educational levels were not included in the analysis because they were “too closely correlated with race/ethnicity” (CitationBerkowitz et al. 2004).
The CHAMACOS cohort consisted primarily of low-income Latina women living in an agricultural community in the Salinas Valley, California, without gestational or preexisting diabetes, hypertension, twin births, or stillbirths (CitationEskenazi et al. 2004). Women with infants diagnosed with congenital anomalies at birth were included. Approximately 42% of the women worked in the field during pregnancy or worked at other agricultural jobs (e.g., packing shed, nursery and greenhouse work), and 85% had agricultural workers living in their homes during pregnancy. All models were adjusted for gestational age, and included variables for maternal age, pregnancy weight gain, week of initiating prenatal care, parity, infant sex, mother's country of birth, body mass index, and family income.
The New Jersey cohort included women pregnant with one nonanomalous fetus scheduled for an elective cesarean birth at term at Saint Peter's University Hospital in New Bruswick, NJ. Women were excluded if the hemoglobin level was less than 8 mg/dl, if there was evidence for labor or rupture of membranes at the time of operative delivery, and if they were taking medications that might interfere with metabolism of environmental chemicals. The statistical models were adjusted for maternal age, primagravida, maternal prepregnancy body mass index, infant sex, and gestational age.
Biomarkers of CPF Exposures
The main CPF exposures reported in the reviewed studies were measured and quantified from biological specimens obtained directly from women during pregnancy or from the umbilical cord at delivery (). This included measuring the parent compound CPF in maternal or umbilical cord blood, as well as measuring metabolites of CPF in maternal urine during pregnancy, including TCPy, DEPs, and DAPs. As discussed in greater detail in the methods section, TCPy is a more specific biomarker of CPF than the nonspecific OP urinary metabolites DEPs and DAPs. All of these urinary metabolites have limitations as biomarkers for CPF because they may also reflect exposure to environmental degradates of OP (including CPF) or to other OP (CitationNeedham 2005).
The New Jersey cohort (CitationBarr et al. 2010) analyzed birth outcomes based on maternal (immediately prior to birth by Cesarean section) and umbilical cord serum levels of CPF. Maternal and cord serum samples were not correlated in the New Jersey cohort (r = 0.12), and results for these two measures were reported separately (CitationBarr et al. 2010). In contrast, the CCCEH cohort study (CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005) analyses were based on umbilical cord plasma levels of CPF at birth and air measurements collected during the third trimester of pregnancy. In the CCCEH cohort, maternal plasma levels were used only in cases where cord samples were not collected because maternal and cord plasma samples were correlated (r = .76), and estimates of cord plasma levels from maternal plasma were based on formulas derived from a regression analysis (CitationWhyatt et al. 2004). The CPF levels in maternal air samples were collected via a personal air monitor that was worn in a backpack during the daytime for two consecutive days and placed by the bed while sleeping during the third trimester of pregnancy (CitationWhyatt et al. 2005).
The urinary metabolites TCPy, DEPs, and DAPs were measured in the Mt. Sinai and CHAMACOS studies (CitationBerkowitz et al. 2004; CitationEskenazi et al. 2004; CitationWolff et al. 2007). The CHAMACOS and Mt Sinai studies measured the following six DAPs (CitationEskenazi et al. 2004; CitationWolff et al. 2007): DEP, DETP, DEDTP, DMP, DMDTP, and DMTP. The first three were summed to obtain the total concentration of DEPs, and the latter three were summed to obtain the total concentration of DMPs. DAPs were defined as the sum of all the DEPs and DMPs. DMPs are discussed in this review only when relevant to interpreting associations reported for DAPs.
Exposure to OP pesticides was also assessed in the CHAMACOS study by measuring AChE in whole blood and BuChE in plasma from mothers during pregnancy and from the umbilical cord at delivery (CitationEskenazi et al. 2004; CitationWolff et al. 2007). CitationWolff et al. (2007) also measured BuChE in maternal plasma during the third trimester of pregnancy in the Mt. Sinai study. AChE and BuChE are nonspecific measures of general exposure to OP and possibly carbamates. Neither cohort study measured CPF in maternal plasma.
Maternal Information and Self-Reported CPF Exposure
Each cohort study administered a questionnaire to obtain information on study participants; however, the specific contents of the survey varied across the cohorts. The New Jersey cohort (CitationBarr et al. 2010) also obtained information about maternal characteristics from hospital records. In general, the objectives of the surveys were to obtain information about demographic characteristics, personal habits, maternal medical history, and the personal use of pesticides among the study participants. As described earlier and in and , information obtained from the questionnaires may or may not have been used in the final multivariate models presented in each study. The CHAMACOS study (CitationEskenazi et al. 2004) administered the questionnaire two times during the pregnancy (first interview: mean = 13 wk, range = 4–29 wk; second interview: mean = 26 wk, range = 18–39 wk). The women who participated in the CCCEH study (CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005) and the Mt. Sinai study (CitationBerkowitz et al. 2004; CitationWolff et al. 2007) completed their surveys during the third trimester of pregnancy. The timing of questionnaire administration was not stated for the New Jersey cohort (CitationBarr et al. 2010). Women in that study were recruited from lists of patients scheduled for elective cesarean birth, so it seems plausible that questionnaires were administered during the third trimester of pregnancy. Although pesticide exposure data were collected via questionnaire, none of the studies reported associations between self-reported CPF exposure and any of the birth outcomes.
TABLE 3. Summary of growth indices by metabolite
Associations of CPF and Metabolites With Growth Outcomes
provides the results of analyses of growth indices by CPF or metabolite for each of the reports included in this review. summarizes results relevant to the potential interaction of PON1 enzyme activity (phenylacetate as a substrate) or PON192 genotype and CPF-related biomarkers of exposure for each of the growth indices.
TABLE 4. Summary of PON1 results presented by the Mt. Sinai Cohort that are related to potential CPF biomarker exposure
Head Circumference
No statistically significant associations were observed between head circumference and CPF in blood (maternal serum or umbilical cord plasma or serum) or with TCPy measured in the urine in the CCCEH (CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005), New Jersey (CitationBarr et al. 2010), Mt. Sinai (CitationBerkowitz et al. 2004), or CHAMACOS (CitationEskenazi et al. 2004) cohorts. There was no statistically significant association between maternal personal air samples of CPF during the third trimester of pregnancy and head circumference in the CCCEH cohort (CitationWhyatt et al. 2004).
Associations between head circumference and the less specific biomarkers DEP and DAP were in opposite directions in the two studies that evaluated these associations. Specifically, the CHAMACOS cohort reported a statistically significant 0.32-cm increase in head circumference associated with each 1-unit (nmol/L, log10 scale) increase of the DAP metabolite (no creatinine adjustment) (CitationEskenazi et al. 2004). In contrast, the Mt. Sinai cohort study reported a statistically significant 0.26 cm decrease in head circumference for every 1-unit (nmol/L, log10 scale) increase of the DAP metabolite (no creatinine adjustment) (CitationWolff et al. 2007). CitationWolff et al. (2007) also reported a numerical 0.25 cm decrease in head circumference associated with the DAP metabolite when the model was adjusted for creatinine. A similar discordant pattern was reported across the CHAMACOS and Mt. Sinai cohorts for the DEP metabolite; however, these associations were statistically nonsignificant ().
Birth Weight
The CCCEH cohort study reported no statistically significant associations between personal air levels of CPF collected over a period of 48 h during the third trimester of pregnancy and birth weight. However, there were statistically significant inverse associations between umbilical cord plasma CPF and birth weight in four separate publications of data from the CCCEH cohort. Specifically, CitationPerera et al. (2003) reported a statistically significant 0.04-g decrease in the log of birth weight among all participants in the cohort for every unit increase (log-transformed pg/g) in CPF. Statistically significant results of a similar magnitude were observed for African American participants but not Dominican participants. Subsequent analyses did not stratify results on ethnicity, because the tests for interaction were statistically nonsignificant. Information beyond the lack of significance of the statistical tests of interaction was not provided in the other CCCEH reports. Thus, in the absence of stratified data, it is unknown whether patterns of association were materially similar or different for the two ethnic groups, regardless of the results of the statistical tests for other CCCEH publications.
CitationWhyatt et al. (2004) reported a statistically significant 42.6-g decrease for each unit increase (log transformed pg/g) of CPF. Additional analyses indicated that this association was not strictly monotonic, and was driven by stronger associations at higher levels of exposure. Specifically, when stratified into 4 exposure groups (CPF levels < LOD [level of detection], CPF levels > LOD divided into tertiles), the birth weight in the highest exposure tertile averaged 150 g lower (95% CI: –28.7 to –12.5) than the undetectable group, whereas the birth weight in the lowest exposure tertile was 39.2 g higher on average (95% CI: –107.3 to 185.7g) (). CitationRauh et al. (2006) reported that participants with CPF levels > 6.17 pg/g had a statistically significant lower mean birth weight than participants with CPF < 6.17 pg/g (3239.58 g versus 3450.93 g, respectively). This cutoff point (6.17 pg/g) was based on combining the undetectable group with the two lower tertiles into a new “low-exposure” group and comparing to the upper tertile.
In 2000–2001, the U.S. EPA implemented regulatory actions to phase out the residential use of CPF. CitationWhyatt et al. (2005) reported that children in the CCCEH cohort born prior to January 1, 2001, had a statistically significant 67.3-g decrease in birth weight per unit increase in CPF (log transformed pg/g; n = 237). In contrast, the association was positive and statistically nonsignificant among children born after that date (n = 77). A similar analysis using third-trimester personal ambient air samples of CPF was statistically nonsignificant for children born both before and after January 1, 2001 (CitationWhyatt et al. 2004).
There were no statistically significant associations between CPF in maternal serum and umbilical cord serum and birth weight in the New Jersey cohort study, which enrolled participants beginning in July, 2003, after cancelation of residential uses (CitationBarr et al. 2010) (). None of the reported associations between TCPy (CitationBerkowitz et al. 2004; CitationEskenazi et al. 2004), DEP or DAP (CitationEskenazi et al. 2004; CitationWolff et al. 2007), and birth weight in the Mt. Sinai and CHAMACOS studies was statistically significant, and the associations varied in magnitude, with both negative (inverse) and positive findings. Residential exposures to OP pesticides, including CPF, in the Mt. Sinai study have been considered to be relatively similar to the CCCEH study in terms of pathway, amount, rate, and type (CitationNeedham 2005), and TCPy is a relatively specific biomarker of CPF compared to other urinary metabolites. As described previously, however, TCPy levels may reflect exposures other than, or in addition to, CPF (parent compound) (CitationBarr and Angerer 2006; CitationNeedham 2005). Nevertheless, the inverse associations between umbilical cord CPF levels and birth weight reported by the CCCEH cohort study were not corroborated by a similar cohort in New York with residential exposures and data on TCPy and DEP; nor did the CCCEH investigators observed similar findings based on air monitoring data.
Birth Length
The CCCEH (CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005) and New Jersey cohorts (CitationBarr et al. 2010) evaluated the association between CPF in serum and birth length. There were no statistically significant associations between CPF in maternal serum and umbilical cord serum and birth length in the New Jersey cohort (CitationBarr et al. 2010). The CCCEH cohort studies reported mixed results, depending on the analysis. Specifically, CitationPerera et al. (2003) reported a statistically significant 0.02-cm decrease in birth length among all participants for every unit increase (log transformed pg/g) in cord plasma CPF. Statistically significant results of similar magnitude were observed among the Dominican participants but not the African American participants. This was in contrast to the pattern observed by CitationPerera et al. (2003) for birth weight, a pattern that appeared to be limited to African American infants. As discussed previously for birth weight, subsequent analyses that reported data on birth length did not stratify on ethnicity, because the statistical tests for interaction were nonsignificant (CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005). CitationWhyatt et al. (2005) observed that children born prior to January 1, 2001, had a significant 0.43-cm decrease in birth length for every unit (log transformed pg/g) increase in cord plasma CPF, but there was essentially no association among newborns after this date.
When stratified into 4 exposure groups (CPF umbilical cord levels < LOD, CPF levels > LOD divided into tertiles), the birth length in the highest exposure group averaged 0.75 cm less than in the group with CPF levels below the LOD; however, this association was statistically nonsignificant and the overall pattern did not appear monotonic (CitationWhyatt et al. 2004) (). CitationRauh et al. (2006) reported similar mean birth lengths (50.02 cm versus 51.05 cm, respectively) among participants with CPF > 6.17 pg/g versus those with CPF < 6.17 pg/g. Despite the statistically significant inverse association reported in the linear regression analyses (CitationPerera et al. 2003; CitationWhyatt et al. 2004; Citation2005), analyses that compared each exposure tertile to the nondetect group yielded statistically nonsignificant results (CitationWhyatt et al. 2004), and the mean birth length was similar for the highest exposure tertile compared to the other groups combined (CitationRauh et al. 2006). Furthermore, there were no material or statistically significant associations between personal air levels of CPF and birth length in the CCCEH cohort.
None of the reported associations between TCPy (CitationBerkowitz et al. 2004; CitationEskenazi et al. 2004), DEP or DAP (CitationEskenazi et al. 2004; CitationWolff et al. 2007), and birth length was statistically significant. The direction of the associations for the DEP and DAP metabolites was generally positive in the CHAMACOS cohort (CitationEskenazi et al. 2004) but mostly negative in the Mt. Sinai cohort (CitationWolff et al. 2007). Taken together, the evidence for an association between CPF and birth length is weak.
Ponderal Index and Abdominal Circumference
The associations between TCPy and/or DEP and DAP and ponderal index were evaluated in the CHAMACOS (CitationEskenazi et al. 2004) and Mt. Sinai cohorts (CitationWolff et al. 2007), with generally null findings. The association between CPF and abdominal circumference of the neonate was evaluated by the New Jersey cohort (CitationBarr et al. 2010). Mean abdominal circumference was approximately the same for participants categorized above or below the 75th percentile for CPF levels in maternal and umbilical cord sera (CitationBarr et al. 2010).
Potential Effect Modification by PON1 Status
Analyses based on PON1 polymorphisms and enzyme activity were included only in reports from the Mt. Sinai cohort study (CitationBerkowitz et al. 2004; CitationWolff et al. 2007). Briefly, CPF is metabolized to the toxic metabolite CPO, which is then rapidly hydrolyzed to TCPy by microsomal esterases including PON1 and CPF oxonase, or by nonenzymatic hydrolysis (CitationNeedham 2005). The authors were interested in evaluating if any effects of exposure to OP may be modified by PON1 enzyme activity level or genotype.
summarizes results relevant to the potential interaction of PON1 enzyme or PON1 genotype and CPF-related biomarkers of exposure that were presented in two reports from the Mt. Sinai Study (CitationBerkowitz et al. 2004; CitationWolff et al. 2007). CitationBerkowitz et al. (2004) stratified maternal PON1 enzyme activity into “low,” “medium,” and “high” based on the tertile distribution. The range of PON1 enzyme activity in the different PON1 tertiles was not described, but the cut points for PON1 enzyme activity (as measured in plasma using phenylacetate as a substrate) are assumed to be <96, 97–116.6, and 116.7–200 mg/m-ml for PON1 tertiles 1, 2 and 3, respectively, as reported for the same cohort by CitationWolf et al. (2007).
CitationBerkowitz et al. (2004) analyzed associations among TCPy; PON1 activity for each of five PON1 genotypes; and birth weight, birth length, or head circumference. The five different PON1 genotypes (Q192R, L55M, –909, –162, and –108) were measured in maternal blood samples obtained during the third trimester and cord blood samples were obtained at birth. CitationWolff et al. (2007) evaluated associations among DEPs, DMPs, or DAPs; maternal blood (third trimester) PON1 activity or PONQ192R genotype; and birth weight, birth length, or head circumference. CitationBerkowitz et al. (2004) considered mothers with genotype PON192 RR and PON192 QQ to possess the phenotype of higher and lower PON1 activity, respectively, based on previous research.
There were no statistically significant interactions between PON1 activity and TCPy levels for any of the birth outcomes (CitationBerkowitz et al. 2004). There was a statistically significant positive trend in head circumference across PON1 tertiles among those with TCPy levels above the limit of detection (LOD), but not below the LOD (). However, the magnitude of the head circumference for these infants was similar across PON1 activity tertiles for the TCPy < LOD groups (33.6, 33.7, and 34.1 cm) and TCPy > LOD group (33.3, 34, and 34.1 cm) (see ). Given the absence of a main effect for TCPy, and the similarity of patterns among those with TCPy levels above and below the LOD, it is not surprising that the test for interaction between PON1 activity and TCPy levels for head circumference was statistically nonsignificant. Furthermore, a significant trend was observed in head circumference when PON1 activity was considered alone, without level of TCPy, with adjusted means of 33.5, 33.9, and 34.1 cm for tertiles 1, 2, and 3, respectively. Thus, there is uncertainty about the contribution CPF exposure has on observed PON1 associations in the Mt. Sinai Study. At present, evidence that CPF may exert a detrimental effect on head circumference in infants of mothers who exhibit low PON1 activity is weak.
CitationWolff et al. (2007) reported “weak, nonsignificant inverse associations” with three birth outcomes (independent of PON1 status): DEPs with birth weight, DEPs with ponderal index, and DAPs with head circumference (CitationWolff et al. 2007). Based on these results, interactions between levels of DEPs or DAPs and PON1 status were evaluated. In addition, birth length was shorter by 0.68 cm among mothers with PON192RR (slower) than PON192QQ (faster) genotype independent of biomarker exposure (CitationWolff et al. 2007).
There was no statistically significant interaction among DEP level, PON1 activity level, and birth weight; however, there was a statistically significant 164 g (5%) decrease in birth weight of babies born to mothers with slow PON1 enzyme activity and DEP levels above the median compared to babies born to mothers with fast PON1 enzyme activity and DEP levels below the median (). A similar pattern was observed in the PONQ192R/DEP interaction analyses, in which babies born to women with the PON192RR genotype (slow) and DEP levels above the median weighed 199 g less (6% decrease) than babies born to women with the PON192QQ genotype (fast) and DEP levels below the median, but the test for interaction was not significant. In addition, the birth weight of babies born to women with the PON192QQ genotype who had DEP levels below the median was higher than for babies born to women with the PON192QQ (fast) genotype who had DEP levels above the median, but similar comparisons across heterozygotes and those with PON192RR (slow) genotypes were statistically nonsignificant. There was no statistically significant effect modification of PON1 activity by TCPy on birth weight for the same cohort (CitationBerkowitz et al. 2004); thus, the interpretation of the DEP-PON1 activity or genotype findings with respect to CPF is not clear. Only results of analyses of DEPs, PON1 status, and birth weight were reported by CitationWolff et al. (2007). CitationWolff et al. (2007) stated that after taking into account the interaction of DAPs with PON1, a “small effect” of DAPs on head circumference () was no longer present and there were no statistically significant associations with Ponderal Index.
Acetylcholinesterase (AChE) and Fetal Growth
The CHAMACOS study (CitationEskenazi et al. 2004) measured AChE levels in maternal blood collected at the second pregnancy interview, in umbilical cord blood, and in predelivery maternal blood. None of these measures was statistically significantly associated with the three fetal growth measures of interest, specifically head circumference, birth weight, or birth length. There was no significant correlation between DAP levels and ChE levels collected at the second pregnancy interview (Pearson r = .02). Furthermore, somewhat surprisingly, there was a weakly positive but statistically significant correlation between DAPs measured in pregnancy urine and predelivery maternal blood (Pearson r = .11) and umbilical cord blood AChE levels (Pearson r = .13). CitationEskenazi et al. (2004) suggested that this deviation from the expected negative correlation may be a result of “substantial measurement error” in both variables, and/or because DAP metabolites are specific to OP, whereas AChE activity may also reflect exposure to n-methyl carbamate (CitationEskenazi et al. 2004). The Mt. Sinai study measured BuChE in maternal blood and observed no association between DEP or DAP exposures or birth outcomes with maternal BuChE in the Mt. Sinai Study (CitationWolff et al. 2007).
DISCUSSION OF EPIDEMIOLOGIC STUDIES
Our review of the data from four epidemiologic cohort studies identified a number of statistically significant results, several resulting from different analyses of the same exposure–outcome association in the same cohort study using different exposure modeling approaches or study population subgroups (e.g., CPF measured in cord plasma and birth weight in the CCCEH study, ). There were no notable or consistent patterns of association across the different cohort studies. One possible reason for the lack of consistency across the studies is, of course, that there is no “true” causal association between exposure to CPF and any of the fetal growth outcomes evaluated in these studies. Alternative explanations for these data should also be considered. For example, no more than two of the four cohort studies evaluated associations between the same biomarker of exposure and the same outcome. Thus, one needs to consider the studies in terms of the level of information they provide about CPF specifically (as opposed to information about OP, generally), as well as what is meant by “consistent findings” in the context of these four studies.
Two of the cohort studies (CCCEH and New Jersey) measured CPF in umbilical cord blood (plasma or serum). A strength of this approach is the use of a biomarker that is specific to CPF (CitationBarr et al. 2010; CitationPerera et al. 2003; CitationRauh et al. 2006; CitationWhyatt et al. 2004; Citation2005). Furthermore, the biomarker represents all sources of exposure (e.g., inhalation, ingestion, dermal). A limitation of this approach is that exposure and outcome information were measured at roughly the same point in time, making it difficult to establish a temporal relationship between exposures during gestation and outcome. The half-life of CPF in blood is relatively short, about 24 h (CitationBarr et al. 2010). If exposure is relatively constant over time, then cord blood levels at birth may represent exposure levels throughout pregnancy; however, there were no data available to validate this assumption. In the CCCEH study, personal air monitoring CPF levels measured during the third trimester of pregnancy were only weakly correlated with CPF levels in cord plasma (r = .19) or maternal plasma at delivery (r = .21) (CitationWhyatt et al. 2005).
There were no significant associations between personal air CPF levels measured during the third trimester on any of the birth outcomes (CitationWhyatt et al. 2004; Citation2005). These associations between personal air CPF levels and birth outcomes remained nonsignificant when analyses were stratified on year of delivery before versus after January 1, 2001 (CitationWhyatt et al. 2004). Indoor (collected continuously from 32nd week of pregnancy until delivery) and maternal personal air levels (collected over 48 h during 32nd week of pregnancy) of CPF were correlated (r = .85) and both declined four- to fivefold between 2001 and 2004 after the ban on residential uses (CitationWhyatt et al. 2007). Maternal personal air levels also correlated with indoor samples collected immediately (r = .86) and 8 wk after (r = .87) the maternal air sampling (CitationWhyatt et al. 2007). This suggests that maternal personal air levels of CPF may be reflective of inhalation exposures over 2 mo during the 3rd trimester of pregnancy that result from indoor residential uses. A limitation is that personal air samples may not reflect all possible routes of exposure. The extent to which noninhalation routes of exposure contributed to residential exposures is uncertain (CitationWhyatt et al. 2009). The Mt. Sinai and CHAMACOS cohort studies had the advantage of estimating exposure based on maternal urine samples collected during pregnancy; thus, exposure measurement clearly preceded measurement of fetal growth outcomes and may better represent a time period of critical growth and development. On the other hand, these cohorts used less specific biomarkers of CPF exposure, namely, TCPy, DEPs, and DAPs. As discussed previously, the metabolites in urine may reflect exposures other than, or in addition to, CPF. All of the studies suffered by relying on measurements collected at one (or at most, two) points in time. The extent to which the measures used in these analyses provided a valid estimate of exposure to CPF and/or other OP during the course of pregnancy is uncertain. For example, in a biomarker validation study (n = 102), conducted between 2001 and 2004, the CCCEH investigators (CitationWhyatt et al. 2009) reported no marked association between CPF in cord plasma and the following other exposure measures: personal and indoor air CPF levels, maternal self-reported pesticide use, and TCPy levels in maternal samples during pregnancy or after delivery. A limitation of this validation study was the relatively low proportion of subjects with detectable CPF cord blood levels (19–29% of cord blood samples in 2001–2002, and none of the samples collected in 2003–2004) (CitationWhyatt et al. 2009). A previous report from the CCCEH study indicated that even though CPF in maternal personal air samples also decreased from 2001 to 2004, CPF was still detectable in almost all of the air samples (CitationWhyatt et al. 2007).
Another possible explanation for the lack of consistent findings is different levels of exposure to CPF across the studies. Briefly, CPF exposure levels in the two New York cohorts have been estimated to be higher than exposures in the CHAMACOS cohort (CitationEaton et al. 2008; CitationEskenazi et al. 2004). The main source of exposure in both of the New York cohorts was home pesticide use, whereas in the CHAMACOS study, the likely sources included diet and agricultural use. CHAMACOS participants reported working in the fields (28%) or at other jobs in agriculture, including packing shed, nursery, and greenhouse work (14%). Furthermore, CitationEskenazi et al. (2004) reported that few of the products found in the homes of CHAMACOS cohort study participants contained CPF.
The New Jersey cohort was assembled after home use of CPF had been phased out (July, 2003–May, 2004), and the time span of the CCCEH study includes both pre- and post-ban periods (1998–2002). The median cord sera CPF level reported for the New Jersey cohort was 0.0007 ng/g (0.7 pg/g) (CitationBarr et al. 2010), which is lower than the mean cord plasma CPF level of 4 pg/g for the CCCEH cohort (CitationBarr et al. 2005; CitationWhyatt et al. 2004; Citation2005). CitationWhyatt et al. (2004; Citation2005) presented data from the CCCEH stratified on date of birth before and after January 1, 2001, in an effort to evaluate the potential impact of the cancellation of residential use of CPF. Inverse associations between cord blood CPF and birth weight and birth length observed among births prior to 2001 were no longer present after January 1, 2001. CitationWhyatt et al. (2004) reported that cord plasma CPF levels in the CCCEH study were significantly lower among newborns after January 1, 2001 (geometric means of 0.6 pg/g after January 1, 2001, compared to 2.5 pg/g prior to January 1, 2001). CitationWhyatt et al. (2004) also commented that few newborns had high CPF exposure levels after 2001, but did not report the number exposed at different levels. Furthermore, CitationRauh et al. (2006) observed no statistically significant difference in mean birth length among newborns with cord blood CPF levels in the upper versus lower exposure categories (). Thus, further evaluation of the pattern of associations for birth length (and birth weight) at different exposure levels (i.e., dose response), including a comparison of these dose-response associations during the two time periods, may be helpful. One would expect the magnitude of associations at given exposure levels to be approximately the same regardless of the time period. Further analyses to evaluate other factors that potentially changed over time and may influence fetal growth may also be helpful, because these factors may also explain some of the changes observed. Finally, it should be noted that CPF biomarker levels in the CCCEH study were variable as reported by CitationRauh et al. (2006). CitationRauh et al. (2006) reported the logarithmically transformed mean CPF level in cord blood for three time periods as follows: 0.92 pg/g (preban, before January 2000), 0.81 pg/g (midban, January 2000 to December 2000), and 0.90 pg/g (postban, January 2001 and later).
The potential role of uncontrolled or incompletely controlled confounding also needs to be considered as possible explanations for the weak to modest associations observed. The results from the New Jersey cohort study (CitationBarr et al. 2010) were initially consistent with those of the CCCEH study for birth weight and birth length until they adjusted for covariates. The New Jersey study adjusted for fewer covariates overall compared to the CCCEH study, but there were some differences. Specifically, the New Jersey study adjusted for maternal age, whereas the CCCEH study did not. The New Jersey study adjusted for prepregnancy body mass index (BMI), whereas the CCCEH study adjusted for prepregnancy weight and net pregnancy weight gain. The differences in adjustment for these variables may or may not have changed the results.
Study design features resulted in few or no preterm births being included in the New York and New Jersey cohorts. CitationEskenazi et al. (2004) observed a lower rate of preterm births, a major cause of low birth weight, in the CHAMACOS cohort (6.4%) compared with Mexican-born women in the United States (10%). Relatively few women in any of the four cohort studies reported smoking during pregnancy. Specifically, approximately 6%, 5%, and 4% of the women in the CHAMACOS study, Mt. Sinai Study, and New Jersey study, respectively, reported smoking during pregnancy (CitationBarr et al. 2010; CitationEskenazi et al. 2004; CitationWolff et al. 2007). Women were excluded from the CCCEH study if they reported any smoking during pregnancy or if cotinine levels in blood collected at delivery were above 15 ng/mL (CitationWhyatt et al. 2004). Despite this restriction on maternal smoking, 38% of the women in the CCCEH study reported a smoker in the home (CitationWhyatt et al. 2004), whereas only 8.3% of women in the CHAMACOS study reported living with a smoker during pregnancy (CitationEskenazi et al. 2007). Only the CCCEH study reported results after adjustment for either environmental tobacco smoke or cotinine. Investigators from the CHAMACOS study indicated that results were similar after adjustment for either maternal smoking or environmental tobacco smoke, and that they did not include maternal smoking in the final model in part because of small numbers (CitationEskenazi et al. 2004).
Regression modeling methods were used to evaluate the association between these metabolite levels and fetal growth outcomes, particularly head circumference, birth weight, and birth length. The regression models reported varied within and across the studies by several key factors, including the metabolite measured, the timing of exposure measurement (pregnancy or delivery), timing of data collection (before or after home use of CPF was banned), and the outcome. In addition, modeling choices such as using continuous versus categorical variables, performing logarithmic transformations on continuous variables, and inclusion of additional covariates varied across the studies. These differences need to be considered when evaluating the findings across studies. In addition, linear regression models were used for many analyses, but most studies did not describe models that tested this assumption of linearity or that formally evaluated nonlinearity. As one example, it would have been helpful if CCCEH investigators had offered guidance in interpreting the validity of the linear regression models in view of the results of the analyses of the categorical analyses, which indicated a possible nonlinear component to the association between CPF and birth weight (CitationWhyatt et al. 2004).
Measures of fetal growth (birth weight, birth length, and head circumference) may be considered more objective than measures of cognitive or psychomotor development, but these endpoints are also subject to a number of sources of variability. These include genetic variables such as height and weight of the parents, nutrition, and uncertainties around dating of gestational age at birth (i.e., time from conception), as well as measurement error.
Considered together, the results from the four cohort studies have not indicated a consistent, strong association between biomarkers of CPF exposure and measures of fetal growth across the different cohort studies. A clear pattern of associations did not emerge when considering the relative specificity of the different biomarkers used in the studies, because essentially null results were observed for the New Jersey cohort study, which used CPF in cord serum as a biomarker, or for the CHAMACOS study, which used the biomarker TCPy (in addition to the less specific biomarkers DEP and DAP). It should be noted, however, that CPF measures in the New Jersey cohort study indicated very low exposure levels, which is not surprising, given that the study was initiated after home use of CPF was banned in the United States. The CCCEH and Mt. Sinai cohort studies observed statistically significant results for different biomarker-outcome associations, and there was no corroboration of any finding across studies. Thus, it would be premature to draw causal conclusions based on these inconsistent, uncorroborated findings.
ANIMAL STUDY RESULTS
Five research papers met our inclusion criteria of robust studies with data on birth weight, growth, and fetal crown–rump length after gestational and early postnatal exposure to CPF (). Two papers included results of two separate animal studies in mice (CitationDeacon et al. 1980) and rats (CitationBreslin et al. 1996). One of the papers was a developmental neurotoxicity study in rats (CitationMaurissen et al. 2000), but because this review focuses on pup birth growth measures, only those endpoints are discussed here. Brief summaries of other general developmental and maternal toxicity are also discussed relative to the primary outcomes of interest. One additional paper found that did not meet our inclusion criteria but looked at birth and early postnatal weight (CitationLassiter and Brimijoin 2008) is also summarized in .
TABLE 5. Birth outcomes and other related measures in animal CPF studies
In the developmental toxicity study in Fischer 344 (F344) rats reported by CitationBreslin et al. (1996), fetal weight was increased at all doses above controls, with statistically significant increases of 5.6% and 4.2% at 3 and 15 mg/kg-d, respectively (). CitationBreslin et al. (1996) did not consider the increased fetal body weight to be treatment related, because there was no clear dose response and the weight rise was within the range of historical control data. There was a statistically nonsignificant elevation in fetal crown-rump length at 3 but not at 0.1 or 15 mg/kg-d, and it was not considered to be treatment related. The highest CPF dose level produced clear signs of maternal toxicity, including tremors, vaginal bleeding, excessive salivation, and decreased body weights.
In the two-generation reproduction study in Sprague-Dawley rats (CitationBreslin et al. 1996), the most relevant findings for birth outcomes were those noted at birth. No developmental effects were seen at birth except for reduced pup weight at postnatal day (PND) 1 (day of birth; not statistically significant). During lactation, there were statistically significant decreased pup weights at PND 4 and 21 and increased pup mortality in F1 pups at 5 mg/kg-d on PND 14 and 21.These changes were not seen in F2 pups, although there was an increase in pup mortality due to entire litter losses at 5 mg/kg-d that was not considered treatment-related (5 litter losses at 5 mg/kg-d vs. 3 in controls). Histologic alterations in the zona fasciculata of the adrenal gland in P1 and P2 males and females were observed at the 5-mg/kg-d dose level. These effects during lactation indicate that the 5-mg/kg-d dose level was maternally toxic. In addition, significant RBC AChE inhibition was measured in P1 and P2 adults at 1 (67–69% inhibition) and 5 (70–75% inhibition) mg/kg-d. Brain AChE inhibition of 48% was measured in P1 and P2 adults at 5 mg/kg-d. At 0.1 mg/kg-d, AChE inhibition measured in P2 males was considered spurious because there was no marked effect in either the P1 males or females or the P2 females.
CitationMaurissen et al. (2000) reported significantly reduced pup body weights at birth and 4 d later prior to culling in Sprague-Dawley rats exposed to 5 but not to 0.3 or 1 mg CPF/kg-d. There was increased mortality in pups (more than half cannibalized) in the high-dose (5 mg/kg-d) group with a resultant decrease in live litter size before litter size standardization on PND 4. In addition, delays in vaginal opening, pinna detachment, and preputial separation were noted at 5 mg/kg-d only, although the latter two were not statistically significant. Clinical signs of toxicity, including muscle fascilitations and decreased body weight gains, were noted in dams at 5 mg/kg-d. At GD20, there was significant (90%) brain AChE inhibition at 5 mg/kg-d, and substantial RBC ChE inhibition at 5, 1, and 0.3 mg/kg-d.
CitationFarag et al. (2003) observed effects of CPF on fetal body weight in the F344 strain of rats at 25 but not 5 and 15 mg/kg-d. Signs of developmental toxicity consisting of decreased fetal weight and viability as well as increased fetal death and early resorption, and increased fetal variations were also seen at 25 mg/kg-d but not at 5 or 15 mg/kg-d. Maternal toxicity in the form of depressed body weight and AChE inhibition was observed at 15 and 25 mg/kg-d, with significant ChE inhibition (31% and 49%, respectively) measured 6 d after the last CPF dose (PND 21).
CitationAkhtar et al. (2006b) reported decreased fetal weight, increased fetal death, and “minor insignificant” malformations in Wistar rats at 15 mg/kg-d but not at 9.6 and 12 mg/kg-d. Clinical signs of AChE inhibition toxicity and decreased food consumption were observed in dams at 15 mg/kg-d. A significant decrease in maternal body weight gain was measured at 12 and 15 mg/kg-d.
In CF-1 mice exposed to 1, 10, and 25 mg/kg-d during organogenesis, fetal weight and crown–rump length were reduced only at 25 mg/kg-d (CitationDeacon et al. 1980). In addition, there were significant reductions in skeletal ossification of the skull bones, delayed ossification of the sternebrae, and unfused sternebrae in the 25 mg/kg-d fetuses, all indicating delayed growth and bone development. Maternal plasma and RBC AChE levels measured in a separate group of animals treated on gestation days (GD) 6–10 or 6–15 were significantly reduced at all dose levels when measured 5 h after the last dose, as were levels in a fetal homogenate from the 10- and 25-mg/kg-d groups. Severe maternal toxicity was seen at 25 mg/kg-d and included increased maternal deaths, severe symptoms of AChE inhibition, and reduced body weight gain. In mice exposed to 0, 0.1, 1, or 10 mg/kg/d on GD 6–15, no fetal toxicity was seen, except for a significant reduction in the incidence of delayed skull bone ossification at 1 and 10 mg/kg-d and a significant reduction in delayed ossification of the sternebrae at 10 mg/kg-d, suggesting that these animals were somewhat advanced compared to controls. These changes occurred in the presence of numerically larger size (body weight and crown–rump length) of fetuses in the two higher dose groups (statistically non significant), and are not considered toxicologically significant. Additional animals were treated at GD 6–10 or GD 6–15 and sacrificed 5 h after final dosing for AChE levels. Maternal RBC AChE and fetal homogenate AChE levels were reduced at 1 and 10 mg/kg-d.
CitationLassiter et al. (2008) was a smaller study (n = 9–10; Long-Evans rats) that did not meet the inclusion criteria for animal studies but was evaluated because the primary focus of the study was on body weights. There was no effect of CPF on neonatal body weight prior to weaning in the one dose level (2.5 mg/kg-d) tested.
Discussion of Animal Studies In Terms of Biological Plausibility for Epidemiologic Studies
The issue of biological plausibility for epidemiologic studies includes the question of whether the findings are consistent with comparable birth outcomes from animal studies (). In general, most chemicals will produce effects on fetal or pup body weight in developmental or reproductive toxicity animal studies because regulatory guidelines for toxicology studies require that the highest dose level produce some level of toxicity. Treatment-related decreases in fetal weight were generally seen at doses of 15–25 mg/kg-d (no-observed-adverse-effect level [NOAEL] = 10 mg/kg-d) in rats and mice following gestational exposures (Akhtar et al. 2006a ; CitationBreslin et al. 1996; CitationDeacon et al. 1980; CitationFarag et al. 2003). Following exposures of the dams throughout both gestation and lactation, decreases in birth weight or pup weight prior to weaning occurred more consistently at doses of 5 mg/kg-d (NOAEL = 1 mg/kg-d) (CitationBreslin et al. 1996; CitationMaurissen et al. 2000).
The developmental effects reported in offspring in these studies occur at doses that produce clear signs of maternal toxicity based on clinical signs and/or AChE inhibition (). Most of these effects are related to delayed growth and development, particularly of the skeleton, which is forming around the time of examination. The growth delays in animal studies are likely to be secondary to maternal toxicity, although potential direct effects on the fetus cannot be ruled out. During gestation, the maternal mammal is the sole source of nutrients, electrolytes, and oxygen, controls homeostatic mechanisms, regulates fluids and temperature, and provides means for the elimination of metabolic wastes (DeSesso 1987; DeSesso et al. 2009). Environmental insults that produce maternal toxicity (e.g., reductions in body weight, clinical symptoms) can lead to induction of an acute-phase response and changes in maternal metabolism that result in fetal or pup toxicity (DeSesso 1987; Keen et al. 2003a; 2003b).
Based on comparison with the NOAEL for developmental toxicity, RBC AChE inhibition in dams is a much more sensitive endpoint than effects on fetal or pup birth weight or length. The lowest dose at which RBC AChE inhibition was reported was 0.3 mg/kg-d (CitationMaurissen et al. 2000), and two studies reported no RBC AChE inhibition at 0.1 mg/kg-d (CitationBreslin et al. 1996; CitationDeacon et al. 1980). Thus, 0.1 mg/kg-d may be regarded as a NOAEL for RBC AChE inhibition in dams, based on animal studies included in this review. These animal studies suggest that risk assessments based on RBC AChE inhibition in adults or offspring animals will be protective of effects on fetal weight, pup growth, and other general developmental effects. This is also true for specialized neurodevelopmental animal studies, the majority of which were conducted at doses between 1 and 5 mg/kg-d, which produce substantial RBC AChE inhibition in dams (CitationEaton et al. 2008; CitationMattsson et al. 2000; CitationMaurissen et al. 2000; CitationLi et al. 2012).
The Mt. Sinai cohort study evaluated the potential interaction between biomarkers of OP exposure and PON1 enzyme activity or PON 1 genotype. The animal data suggest that the overall balance between decreased PON1 detoxification and CYP450 activation/ detoxification, as well as higher rates of ChE enzyme synthesis in the preweanling rat (CitationMoser et al. 1998; CitationPope et al. 1991), contributes to age-dependent and individual susceptibility to CPF toxicity (CitationTimchalk et al. 2006). These experiments tend to be conducted under more extreme conditions using knockout mice (with PON1 status replacement) and higher acute doses of CPF. The extent to which these differences are of importance to humans may be dependent on exposure levels, with greater differences in susceptibility at higher acute than at low exposure levels (CitationEaton et al. 2008). CitationCole et al. (2005) reported that PBPK model simulations predict that PON1 Q192R polymorphism might exert the greatest impact on CPF metabolism and detoxification at dose levels greater than 0.5 mg/kg. At lower exposure levels, other esterase detoxification pathways are predicted to be capable of compensating for the interindividual differences in CPOase activity due to the PON1 Q192R polymorphism (CitationCole et al. 2005). Together, these animal data weaken the biological plausibility of interactions between CPF and PON1 activity on birth outcomes in human studies at lower human exposure levels.
Indeed, as discussed in greater detail earlier in this article, the evidence for PON1 enzyme activity or genotype modifying CPF effects on birth outcomes in the Mt. Sinai cohort is weak. Of the biomarkers measured, TCPy was the most specific metabolite of CPF measured, and there were no statistically significant interactions between PON1 and TCPy for any of the birth outcomes. Even though there was a statistically significant trend in head circumference across PON1 tertiles among participants with TCPy greater than the LOD (but not among those with TCPy below the LOD), the head circumference mean values for each PON1 tertile were similar for the two TCPy groups (see ).
Estimates of exposure to pregnant women in the epidemiologic papers that meet our inclusion criteria were generally not expressed in units that easily allow direct comparisons with the animal data. CitationZhao et al. (2005) compared internal dose concentrations of CPF reported in human and animal studies and concluded that the CitationWhyatt et al. (2004) estimate of 2.5 pg/g in human umbilical cord blood (before January 1, 2001) is approximately 1/400 the 1-ng/g CPF level found in the blood from rat fetuses exposed at 1 mg/kg-d (animal NOAEL for fetal RBC AChE inhibition) (CitationMattsson et al. 2000). CitationZhao et al. (2005) suggested that based on this large difference in internal exposure levels, there is low biological plausibility that participants in the CCCEH study were exposed to CPF at levels that produced AChE inhibition or inhibited fetal growth. Such comparisons of animal and human exposure data are limited by differences in anatomy and physiology, including differences in respiratory, heart and metabolic rates, as well as potential differences in target tissue distribution and metabolic capacity.
CitationEaton et al. (2008) did a comprehensive review of the literature on human and animal toxicity and exposure data and estimated average daily combined CPF inhalation and dietary exposures in the CCCEH and CHAMACOS cohorts to be 0.008 and 0.007 μg/kg-d, respectively. CitationMcKone et al. (2007) estimated total CPF exposures (dietary, inhalation, dermal, nondietary ingestion) among CHAMACOS pregnant women to be 1.43 to 6.73 nmol/d, which is equivalent to 0.007 to 0.031μg/kg-d assuming a 75.8-kg pregnant female (CitationU.S. EPA 2011a, from NHANES). Using a physiologically based pharmacokinetic (PBPK) model, CitationLowe et al. (2009) estimated that daily doses of 0.15 μg/kg-d would result in maternal and fetal blood CPF levels within the range of the mean maternal and cord blood concentrations reported by CitationWhyatt et al. (2005) in the CCCEH study. Despite possible differences in physiology and metabolism, these levels in humans are several orders of magnitude below the 0.1-mg/kg-d NOAEL for laboratory animals based on RBC AChE inhibition in the developmental and reproduction studies included in this review and would not be expected to affect growth and development in humans.
SUMMARY CONCLUSIONS AND IMPLICATIONS FOR RISK ASSESSMENT
This review presents a comprehensive analysis that compares and contrasts results across human and animal studies which evaluated the hypothesis that CPF adversely affects birth outcomes related to fetal growth. Using guidance recommended by CitationHill (1965), an assessment was made of the evidence for and against a causal relation between possible prenatal CPF, as measured in umbilical cord plasma or serum or estimated based on levels of urinary metabolites, and these growth outcomes. Alternative explanations for the findings were considered, such as the presence of bias in the implementation of the study or during the analysis.
This review of the epidemiologic literature did not identify any strong associations exhibiting an exposure-response pattern that were observed in more than one of the four cohort studies evaluated. The adverse associations that were reported were neither particularly strong nor precise. This can be seen by general patterns of similar mean values of the birth outcome measures at different exposure levels, beta coefficients that are close to the null value of zero, and the relatively high standard errors in some of the results.
The CCCEH study is the only study that measured the parent compound CPF levels prior to cancelation of residential CPF use. Despite consistent statistically significant inverse associations between CPF in cord plasma and weight and length at birth in the CCCEH study, no marked associations were reported between CPF in air samples taken from participants in the CCCEH study during the third trimester of pregnancy and any of the birth outcomes. In addition, the statistically significant associations with CPF reported in the CCCEH studies were not corroborated by the Mt. Sinai study (TCPy, DEPs), even though there was evidence of higher residential applications of CPF among these two New York study populations.
Specifically, there were no statistically significant associations between birth length or birth weight and CPF, TCPy, or DEPs in the New Jersey, Mt. Sinai, or CHAMACOS studies. As noted previously, a majority of the cord serum CPF values was very near the limit of detection in the New Jersey Study cohort (CitationBarr et al. 2010). No material or statistically significant associations were observed between CPF in cord blood and head circumference in the CCCEH or New Jersey cohort studies. The Mt. Sinai and CHAMACOS studies observed statistically significant associations between head circumference and DAPs, but not with DEPs or TCPy. However, these associations were in opposite directions. The Mt. Sinai study reported statistically significant trends and other group differences between PON1 activity or PON192 genotype and DEPs, DAPs, or TCPy on birth outcomes. As discussed earlier in detail, these results are inconsistent preliminary results.
Considered together, the data from the four epidemiologic cohort studies do not support a causal association between CPF exposures during pregnancy and measures of fetal growth. At present, there is insufficient scientific evidence to support the use the epidemiologic data on birth outcomes to derive a point of departure or require additional uncertainty factors for risk assessment purposes.
Challenges to future epidemiologic research on CPF exposure and fetal growth outcome measures include limited exposure among most individuals, difficulties in estimating exposure during critical periods of development, and consistency in biomarkers measured across multiple studies to allow comparisons. Although measuring the parent compound in umbilical cord blood has many clear advantages, it may be less feasible than collecting urine samples in many populations. Furthermore, it represents only one point in time. Collection of maternal blood at additional, earlier times in pregnancy would greatly enhance the information about exposure during pregnancy in future studies. Additional analyses from the existing cohorts including repeated measures of growth outcomes and patterns (e.g., height, weight, BMI), correlations among all available exposure measures (self-report, biomarker, air monitoring data), and correlations among fetal growth outcomes and neurobehavioral outcomes in infancy and early childhood may also be informative.
There is strong evidence from robust animal studies in the published literature that effects on fetal and birth weight occur at doses that are several orders of magnitude higher than those estimated in the human studies, and only at levels that produce substantial maternal RBC AChE inhibition and other signs of maternal toxicity, including reduced body weight or weight gain, or death in the most severe cases. The lowest dose level producing treatment-related decreases in fetal or birth weight is 5 mg/kg-d based on statistically nonsignificant decreases in body weight on the day of birth (CitationMaurissen et al. 2000). In this study, there was substantial (>40%) maternal RBC AChE inhibition at 0.3 mg/kg-d, the lowest dose level tested (). Indeed, using a response level of 10% RBC AChE inhibition compared to background, the U.S. EPA estimated the lower confidence bound on the benchmark dose (BMDL10) to be 0.03 mg/kg-d in rat dams following GD6-20 exposures (CitationU.S. EPA 2011b). Thus, based on consideration of both the epidemiologic and animal data, RBC AChE inhibition is a more sensitive endpoint for risk assessment than somatic developmental effects reviewed in this article.
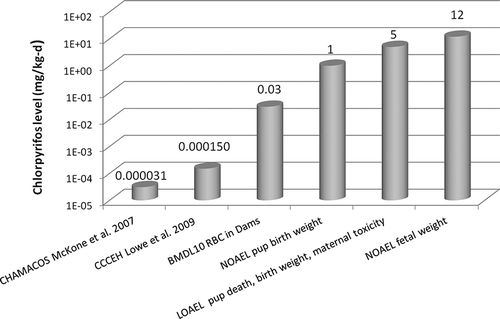
FIGURE 2. Comparison of NOAELs and LOAELs for fetal weight, birth weight, and maternal toxicity from animal studies with human exposure estimates. Human exposure estimates were calculated from McCone et al. (2007) based on CHAMACOS study. CitationMcKone et al. (2007) estimated total CPF exposures (dietary, inhalation, dermal, nondietary ingestion) among pregnant women in the CHAMACOS cohort to be 1.43 to 6.73 nmol/d, which is equivalent to 0.007 to 0.031μg/kg-d, assuming a 75.8-kg pregnant female (CitationU.S. EPA 2011a, from NHANES). CitationLowe et al. (2009) estimated exposures based on umbilical cord blood levels from the CCCEH cohort (CitationWhyatt et al. 2005). The NOAEL of 1 mg/kg-d for pup birth weight is based on non-statistically significant treatment-related decreases at 5 mg/kg-d CitationBreslin et al. (2006) and CitationMaurissen et al. (2000) (see ). The LOAEL of 5 mg/kg-d for maternal toxicity (e.g., tremors) and pup death is based on CitationMaurissen et al. (2000). The NOAEL of 12 mg/kg-d for fetal birth weight is the highest NOAEL for effects in multiple studies as summarized in .
Acknowledgments
The authors are grateful to Dow AgroSciences LLC for funding support. The analyses, conclusions, and opinions expressed in this article are solely those of the authors and may not represent those of Dow AgroSciences LLC. The authors also acknowledge editorial support from Rebecca Edwards, and scientific technical support from Drs. Kimberly Lowe and Laura McIntosh.
REFERENCES
- Akhtar , N. , Srivastava , M. K. and Raizada , R. B. 2006b . Transplacental disposition and teratogenic effects of chlorpyrifos in rats . J. Toxicol. Sci , 31 : 521 – 27 .
- Barr , D. B. , Allen , R. , Olsson , A. O. , Bravo , R. , Caltabiano , L. M. , Montesano , A. , Nguyen , J. , Udunka , S. , Walden , D. , Walker , R. D. , Weerasekera , G. , Whitehead , R. D. Jr. , Schober , S. E. and Needham , L. L. 2005 . Concentrations of selective metabolites of organophosphorus pesticides in the United States population . Environ. Res , 99 : 314 – 26 .
- Barr , D. B. , Ananth , C. V. , Yan , X. , Lashley , S. , Smulian , J. C. , Ledoux , T. A. , Hore , P. and Robson , M. G. 2010 . Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey . Sci. Total Environ , 408 : 790 – 95 .
- Barr , D. B. and Angerer , J. 2006 . Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion . Environ. Health Perspect , 114 : 1763 – 69 .
- Berkowitz , G. S. , Wetmur , J. G. , Birman-Deych , E. , Obel , J. , Lapinski , R. H. , Godbold , J. H. , Holzman , I. R. and Wolff , M. S. 2004 . In utero pesticide exposure, maternal paraoxonase activity, and head circumference . Environ. Health Perspect , 112 : 388 – 91 .
- Bravo , R. , Caltabiano , L. M. , Weerasekera , G. , Whitehead , R. D. , Fernandez , C. , Needham , L. L. , Bradman , A. and Barr , D. B. 2004 . Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification . J. Expos. Anal. Environ. Epidemiol , 14 : 249 – 59 .
- Breslin , W. J. , Liberacki , A. B. , Dittenber , D. A. and Quast , J. F. 1996 . Evaluation of the developmental and reproductive toxicity of chlorpyrifos in the rat . Fundam. Appl. Toxicol , 29 : 119 – 30 .
- Clegg , D. J. and van Gemert , M. 1999a . Determination of the reference dose for chlorpyrifos: Proceedings of an expert panel . J. Toxicol. Environ. Health B Crit. Rev , 2 : 211 – 55 .
- Clegg , D. J. and van Gemert , M. 1999b . Expert panel report of human studies on chlorpyrifos and/or other organophosphate exposures . J. Toxicol. Environ. Health B Crit. Rev , 2 : 257 – 79 .
- Cole , T. B. , Walter , B. J. , Shih , D. M. , Tward , A. D. , Lusis , A. J. , Timchalk , C. , Richter , R. J. , Costa , L. G. and Furlong , C. E. 2005 . Toxicity of chlorpyrifos and chlorpyrifos oxon in a transgenic mouse model of the human paraoxonase (PON1) Q192R polymorphism . Pharmacogenet. Genom , 15 : 589 – 98 .
- Deacon , M. M. , Murray , J. S. , Pilny , M. K. , Rao , K. S. , Dittenber , D. A. , Hanley , T. R. Jr. and John , J. A. 1980 . Embryotoxicity and fetotoxicity of orally administered chlorpyrifos in mice . Toxicol. Appl. Pharmacol , 54 : 31 – 40 .
- Eaton , D. L. , Daroff , R. B. , Autrup , H. , Bridges , J. , Buffler , P. , Costa , L. G. , Coyle , J. , McKhann , G. , Mobley , W. C. , Nadel , L. , Neubert , D. , Schulte-Hermann , R. and Spencer , P. S. 2008 . Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment . Crit. Rev. Toxicol , 38 ( suppl. 2 ) : 1 – 125 .
- Eskenazi , B. , Harley , K. , Bradman , A. , Weltzien , E. , Jewell , N. P. , Barr , D. B. , Furlong , C. E. and Holland , N. T. 2004 . Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population . Environ. Health Perspect , 112 : 1116 – 24 .
- Eskenazi , B. , Marks , A. R. , Bradman , A. , Harley , K. , Barr , D. B. , Johnson , C. , Morga , N. and Jewell , N. P. 2007 . Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children . Environ. Health Perspect , 115 : 792
- Farag , A. T. , El Okazy , A. M. and El-Aswed , A. F. 2003 . Developmental toxicity study of chlorpyrifos in rats . Reprod. Toxicol , 17 : 203 – 08 .
- Hill , A. B. 1965 . The Environment and Disease: Association or Causation? . Proc. R. Soc. Med , 58 : 295 – 300 .
- Lassiter , T. L. and Brimijoin , S. 2008 . Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos . Neurotoxicol. Teratology , 30 : 125 – 30 .
- Li , A. A. , Lowe , K. A. , McInstosh , L. J. and Mink , P. J. 2012 . Evaluation of epidemiology and animal data for risk assessment: Chlorpyrifos developmental neurobehavioral outcomes . J. Toxicol. Environ. Health B Crit. Rev , 15: 109–184
- Liu , J. and Pope , C. N. 1998 . Comparative presynaptic neurochemical changes in rat striatum following exposure to chlorpyrifos or parathion . J. Toxicol. Environ. Health A , 53 : 531 – 44 .
- Lowe , E. R. , Poet , T. S. , Rick , D. L. , Marty , M. S. , Mattsson , J. L. , Timchalk , C. and Bartels , M. J. 2009 . The effect of plasma lipids on the pharmacokinetics of chlorpyrifos and the impact on interpretation of blood biomonitoring data . Toxicol. Sci , 108 : 258 – 72 .
- Mattsson , J. L. , Maurissen , J. P. , Nolan , R. J. and Brzak , K. A. 2000 . Lack of differential sensitivity to cholinesterase inhibition in fetuses and neonates compared to dams treated perinatally with chlorpyrifos . Toxicol Sci , 53 : 438 – 46 .
- Maurissen , J. P. , Hoberman , A. M. , Garman , R. H. and Hanley , T. R. Jr. 2000 . Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos . Toxicol. Sci , 57 : 250 – 63 .
- McKone , T.E. , Castorina , R. , Harnly , M.E. , Kuwabara , Y. , Eskenazi , B. and Bradman , A. 2007 . Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California . Environ. Sci. Technol , 41 : 3233 – 40 .
- Moser , V. C. , Chanda , S. M. , Mortensen , S. R. and Padilla , S. 1998 . Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities . Toxicol. Sci , 46 : 211 – 22 .
- Needham , L. L. 2005 . Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes . Environ. Health Perspect , 113 : 494 – 98 .
- Perera , F. P. , Rauh , V. , Tsai , W. Y. , Kinney , P. , Camann , D. , Barr , D. , Bernert , T. , Garfinkel , R. , Tu , Y. H. , Diaz , D. , Dietrich , J. and Whyatt , R. M. 2003 . Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population . Environ. Health Perspect , 111 : 201 – 5 .
- Pope , C. N. 1999 . Organophosphorus pesticides: Do they all have the same mechanism of toxicity? . J. Toxicol. Environ. Health B Crit. Rev , 2 : 161 – 81 .
- Pope , C. N. , Chakraborti , T. K. , Chapman , M. L. , Farrar , J. D. and Arthun , D. 1991 . Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides . Toxicology , 68 : 51 – 61 .
- Rauh , V. A. , Garfinkel , R. , Perera , F. P. , Andrews , H. F. , Hoepner , L. , Barr , D. B. , Whitehead , R. , Tang , D. and Whyatt , R. W. 2006 . Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children . Pediatrics , 118 : e1845 – 59 .
- Slotkin , T. A. and Seidler , F. J. 2007 . Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects . Reprod. Toxicol , 23 : 421 – 27 .
- Timchalk , C. , Poet , T. S. and Kousba , A. A. 2006 . Age-dependent pharmacokinetic and pharmacodynamic response in preweanling rats following oral exposure to the organophosphorus insecticide chlorpyrifos . Toxicology , 220 : 13 – 25 .
- Udarbe Zamora , E. M. , Liu , J. and Pope , C. N. 2008 . Effects of chlorpyrifos oxon on M2 muscarinic receptor internalization in different cell types . J. Toxicol. Environ. Health A , 71 : 1440 – 47 .
- U.S. Environmental Protection Agency . 1991 . Guidelines for developmental toxicity risk assessment . Fed. Reg , 56: 63798-63826.
- U.S. Environmental Protection Agency . 2002 . Interim reregistration eligibility decision: Chlorpyrifos , Washington , DC : U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances .
- U.S. Environmental Protection Agency . 2006 . Internal memorandum from D. Edwards to J. Jones, Director, Office of Pesticide Programs, regarding finalization of interim reregistration eligibility decisions (IREDs) and Interim tolerance reassessment and risk management decisions (TREDs) for the organophosphate pesticides, and completion of the tolerance reassessment and reregistration eligibility process for the organophosphate pesticides , Washington , DC : U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances . July 31, 2006
- U.S. Environmental Protection Agency. 2011a. Chlorpyrifos: Preliminary human health risk assessment for registration. U.S. Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention. Available fromaccessed August 9, 2011 http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2008-0850-0025 (http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2008-0850-0025)
- U.S. Environmental Protection Agency. 2011b. Exposure factors handbook. U.S. Environmental Protection Agency Office of Research and Development National Center for Environmental Assessment. Available at http://www.epa.gov/ncea/efh/pdfs/efh-chapter08.pdf (http://www.epa.gov/ncea/efh/pdfs/efh-chapter08.pdf)
- Weselak , M. , Arbuckle , T. E. and Foster , W. 2007 . Pesticide exposures and developmental outcomes: the epidemiological evidence . J. Toxicol. Environ. Health B Crit. Rev , 10 : 41 – 80 .
- Whyatt , R. M. , Camann , D. , Perera , F. P. , Rauh , V. A. , Tang , D. , Kinney , P. L. , Garfinkel , R. , Andrews , H. , Hoepner , L. and Barr , D. B. 2005 . Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth . Toxicol. Appl. Pharmacol , 206 : 246 – 54 .
- Whyatt , R. M. , Garfinkel , R. , Hoepner , L. A. , Andrews , H. , Holmes , D. , Williams , M. K. , Reyes , A. , Diaz , D. , Perera , F. P. , Camann , D. E. and Barr , D. B. 2009 . A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy . Environ. Health Perspect , 117 : 559 – 67 .
- Whyatt , R. M. , Garfinkel , R. , Hoepner , L. A. , Holmes , D. , Borjas , M. , Williams , M. K. , Reyes , A. , Rauh , V. , Perera , F. P. and Camann , D. E. 2007 . Within- and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City . Environ Health Perspect , 115 ( 3 ) : 383 – 9 . Epub December 11, 2006. PubMed PMID: 17431487; PubMed Central PMCID: PMC1849946
- Whyatt , R. M. , Rauh , V. , Barr , D. B. , Camann , D. E. , Andrews , H. F. , Garfinkel , R. , Hoepner , L. A. , Diaz , D. , Dietrich , J. , Reyes , A. , Tang , D. , Kinney , P. L. and Perera , F. P. 2004 . Prenatal insecticide exposures and birth weight and length among an urban minority cohort . Environ. Health Perspect , 112 : 1125 – 32 .
- Wolff , M. S. , Engel , S. , Berkowitz , G. , Teitelbaum , S. , Siskind , J. , Barr , D. B. and Wetmur , J. 2007 . Prenatal pesticide and PCB exposures and birth outcomes . Pediatr. Res , 61 : 243 – 50 .
- Zhao , Q. , Dourson , M. and Gadagbui , B. 2006 . A review of the reference dose for chlorpyrifos . Regul. Toxicol. Pharmacol , 44 : 111 – 24 .
- Zhao , Q. , Gadagbui , B. and Dourson , M. 2005 . Lower birth weight as a critical effect of chlorpyrifos: A comparison of human and animal data . Regul. Toxicol. Pharmacol , 42 : 55 – 63 .