Abstract
The Texas Commission on Environmental Quality (TCEQ) conducted a chronic inhalation noncancer toxicity assessment for crotonaldehyde (CRO). Since there were limited toxicity data for CRO, a reference value (ReV) was derived using a relative potency factor (RPF) approach with acrolein as the index chemical. Both CRO and acrolein are α,β-unsaturated carbonyls and share common steps in their mode of action (MOA). Only studies that investigated the effects of CRO and acrolein in the same study were used to calculate a CRO:acrolein RPF. In vivo findings measuring both 50% respiratory depression in rats and two species of mice and subcutaneous 50% lethality in rats and mice were used to calculate an RPF of 3 (rounded to one significant figure). In vitro data were useful to compare the MOA of CRO and acrolein and to support the RPF determined using in vivo data. In vitro cell culture studies investigating cytotoxicity in normal human lung fibroblast cultures using the propidium iodide cytotoxicity assay and in mouse lymphocyte cultures using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay were used to calculate an in vitro RPF of 3, which supports the in vivo RPF. The chronic ReV for acrolein of 1.2 ppb derived by TCEQ was multiplied by the RPF of 3 to calculate the ReV for CRO of 3.6 ppb (10 μg/m3). The ReV for CRO was developed to protect the general public from adverse health effects from chronic exposure to CRO in ambient air.
Humans may be exposed to crotonaldehyde (CRO) from industrial and natural sources. CRO is used primarily for the production of sorbic acid and also utilized for synthesis of butyl alcohol, butyraldehyde, quinaldine, thiophenes, pyridenes, dyes, pesticides, pharmaceuticals, and rubber antioxidants. This compound was used in chemical warfare agents and as a warning agent in locating breaks and leaks in pipes (IARC Citation1995). CRO, acrolein, and other alkenals may be produced endogenously from lipid peroxidation, a process involving the oxidation of polyunsaturated fatty acids, basic components of biological membranes. The formation of these aldehydes may be causally related to oxidative stress (Ichihashi et al. Citation2001). Meat, many fruits and vegetables, bread, cheese, milk, beer, wine, and liquors contain CRO (IARC Citation1995). CRO might be emitted from volcanoes, is present in tobacco smoke, and is released in jet, gasoline, and diesel engine exhaust (IARC Citation1995). Pine and deciduous forests as well as the Chinese arbor vitae plant also emit CRO (Hazardous Substances Data Bank [HSDB] Citation2005).
The Texas Commission on Environmental Quality (TCEQ) recently developed inhalation toxicity factors for the evaluation of CRO air concentrations (TCEQ 2014a). The CRO Development Support Document (DSD) provides details on how acute and chronic toxicity values were derived. The reference value (ReV) is based on dose-response assessments of adverse health effects that were correlated with exposure to specific chemicals (TCEQ Citation2012).
There were adequate toxicity data for CRO to derive an acute ReV of 10 ppb (29 μg/m3), an estimation of an inhalation exposure for a 1-h duration protective for the human population, including susceptible subgroups (TCEQ Citation2014a). However, inhalation subchronic or chronic toxicity data were not available for CRO to derive an inhalation ReV, such that a relative potency approach was employed using acrolein as the index chemical (or reference chemical) to derive a chronic ReV based on procedures in the TCEQ Guidelines (Citation2012). A chronic ReV is an estimation of an inhalation exposure protective for the human population exposed for a lifetime. An index chemical is a structurally related chemical with adequate toxicity information, similar physical/chemical parameters, and mode(s) of action (MOA). The concept of relative potency was used to derive toxicity values for polycyclic aromatic hydrocarbons (PAH) with limited toxicity information based on data for benzo[a]pyrene, for which there is a wealth of information (Collins et al. Citation1998). Glass et al. (Citation1991) and Jones and Easterly (Citation1996) used a relative potency approach to evaluate the carcinogenic potential for different classes of chemicals.
Both in vivo and in vitro studies were considered in this derivation. Studies that evaluated CRO and acrolein in the same study were used to derive a relative potency factor (RPF) since relevant endpoints were determined using similar testing techniques, exposure durations, and species. Only endpoints that were closely tied to the expected critical effect and MOA for the index and limited toxicity data (LTD) chemical were considered.
The acrolein and CRO DSD underwent public comment periods (TCEQ Citation2014a; Citation2014b). The purpose of this study is to present the results of our review of the literature, as well as to demonstrate procedures used in the chronic noncarcinogenic toxicity assessment of CRO. This chronic assessment is an example of the use of in vivo data to calculate a RPF and in vitro data to support the in vivo RPF. In addition, in vitro data were also useful to compare the MOA of CRO and acrolein. This is consistent with the National Research Council Report “Toxicity Testing in the 21st Century: A Vision and a Strategy” (NRC Citation2007a; Krewski et al. Citation2010), which recommends the use of in vitro data in risk assessments.
METHODS
The TCEQ Guidelines (TCEQ Citation2012) employ the four-step risk assessment process formalized by the National Research Council (NRC Citation1983, Citation1994) and procedures recommended in numerous U.S. Environmental Protection Agency (EPA) risk assessment guidance documents and the scientific literature (U.S. EPA Citation1994; Citation2002; NRC Citation2001) for chemicals with adequate toxicity data. CRO has limited toxicity data for chronic and subchronic exposures, and is an LTD chemical. Therefore, a relative potency approach was followed to determine an ReV based on TCEQ Guidelines (Citation2012). Relative potency is defined as a procedure to estimate the “toxicity” of a LTD chemical in relation to a structurally similar reference or an index chemical(s) for which toxicity has been well defined.
Procedures for Calculating the RPF
The following rocedures outlined in TCEQ (Citation2012) are employed when similar chemical categories or an analog chemical approach is used: (1) Identify potential index chemical(s) where an index chemical is a structurally related chemical with similar physical/chemical parameters and MOA and for which toxicity factors have been developed; (2) gather data on physical, chemical, toxicological, and other relevant properties for the potential index chemical(s) and LTD chemical; (3) perform an MOA analysis and determine the relevant endpoints that might be used for an RPF approach—relevant endpoints need to be determined using similar testing techniques, exposure durations, and species; (4) construct a matrix of data on relevant endpoints for all chemicals; (5) evaluate data to determine if there was a correlation among chemicals and the endpoints by conducting a simple trend analysis to assess whether a predictable pattern exists amongst chemicals; and (6) calculate the RPF of the pertinent endpoint based on an MOA analysis of the index chemical to the pertinent endpoint of the LTD chemical.
Significant Figures and Rounding Procedures
For intermediate calculations of the RPF, several significant figures are provided. The median of all relevant RPF values is calculated and then the final RPF is rounded to one significant figure. The final ReV is rounded to two significant figures (TCEQ Citation2012). When rounding, if the number next to the significant figure to be rounded is a 5 or less, the number is rounded down, whereas if the number is 6 or more, the TCEQ rounds up.
Chronic Reference Value
CRO is a reactive compound that is known to produce eye, skin, and respiratory irritation at low concentrations. When sufficient concentrations are inhaled for a sufficient duration, CRO produces a burning sensation in the nasal and upper respiratory tract, lacrimation, coughing, bronchoconstriction, pulmonary edema, and deep lung damage (NRC Citation2007b). Since CRO possesses potent odorous and irritant properties, exposure to higher concentrations may be limited, thereby avoiding other adverse effects (Henschler Citation1981).
There are no apparent human or animal subchronic or chronic inhalation studies appropriate for the development of a chronic ReV for CRO. A poorly reported study conducted by Voronin et al. (Citation1982) is described by the International Programme on Chemical Safety (IPCS Citation2008). Rats and mice (strain and number unknown) were treated with CRO for a continuous inhalation exposure for a period of 3 mo. Concentrations from 1.2 mg/m3 (0.419 ppm) led to alterations in hemoglobin content of blood, as well as in motor activity. The Voronin et al. (Citation1982) study was an abstract—no other information was available.
Ten male and female F344 rats and 10 male and female B6C3F1 mice per dose group were treated with CRO via oral gavage in corn oil at 2.5, 5, 10, 20, or 40 mg/kg body weight per day on 5 days per week for 13 wk (Wolfe et al. Citation1987; World Health Organization [WHO] Citation1995). At doses of 5 mg/kg per day (5 mg/kg-d) and above, compound-related mortality was observed in rats of both genders and acute inflammation of the nasal cavity was noted in females. At doses of 10 mg/kg-d, microscopic lesions (hyperplasia of the forestomach epithelia) were observed in the stomach in rats. At doses of 20 and 40 mg/kg-d, compound-related gross necropsy lesions (thickened forestomach or nodules) were noted in male and female rats and acute inflammation of the nasal cavity was found in male rats. At a dose of 40 mg/kg-d, mean body weights were significantly decreased for male rats at termination, and forestomach hyperkeratosis, ulcers, moderate necrosis, and acute inflammation were observed. Rats were more sensitive to CRO compared to mice. All mice survived to termination, and no compound-related gross necropsy lesions were noted. At a dose of 40 mg/kg-d, microscopic lesions (hyperplasia of the epithelial lining of the stomach) were noted in mice. Because CRO is a highly reactive compound and initiates point-of-entry effects, route-to-route extrapolation using the Wolfe et al. (Citation1987) study was not conducted (TCEQ Citation2012).
Chung, Tanaka, and Hecht (Citation1986) evaluated the carcinogenicity of CRO in a chronic study conducted for 113 wk. Male F344 rats were treated with control (0), or with CRO at 0.6 or 6 mM in drinking water. Starting at 8 wk, the high-dose group had approximately 10% lower body weight gain. Moderate to severe liver damage occurred in 10 of 23 high-dose group rats. The total incidence of both hepatic neoplastic nodules and hepatocellular carcinomas combined at control, 0.6, and 6 mM was reported as 0 of 23, 11 of 27, and 1 of 23, respectively. Carcinoma incidence alone was 0 of 23, 2 of 27, and 0 of 23, respectively. The incidence of enzyme-altered liver foci was 1 of 23 in control and significant with 23 of 27 and 13 of 23 at 0.6 and 6.0 mM, respectively. Enzyme-altered liver foci are considered to be precursors to neoplasms. There was a lack of a discernable dose-response trend for the observed incidence of neoplasms. Route-to-route extrapolation using the Chung, Tanaka, and Hecht (Citation1986) study was not considered (TCEQ Citation2012).
Index Chemical
The TCEQ identified potential index chemicals for CRO for which toxicity factors had been developed. Acrolein was selected as the index chemical for CRO. Both chemicals display similar physical/chemical properties, structures and reactivity since both are α,β-unsaturated carbonyl compounds. There are numerous studies that compared the toxicity of acrolein and CRO within the same study for relevant endpoints, although the health effects database for acrolein is more extensive than that for CRO. Most important, they display similar MOA and both produce similar adverse health effects. In humans, both CRO and acrolein produce sensory irritation to the eye and respiratory tract. Both CRO and acrolein produce respiratory-tract effects in animals (NRC Citation2007b; Citation2010). It is unknown whether chronic health effects for acrolein and CRO are similar because chronic inhalation studies for CRO are not available. However, similar chronic effects would be expected based on similar MOA.
The use of toxicity information for formaldehyde was initially considered, as the MOA for formaldehyde (TCEQ Citation2008) is similar to CRO, but there are more in vivo and in vitro studies that compared toxicity of CRO to acrolein within the same study than for formaldehyde. The chemical/physical parameters for formaldehyde are significantly different than CRO. Formaldehyde is an alkanal, whereas both acrolein and CRO are alkenals. Generally, alkenals are more reactive than alkanals. Therefore, formaldehyde was not identified as the index chemical for this RPF assessment.
Physical/Chemical Properties
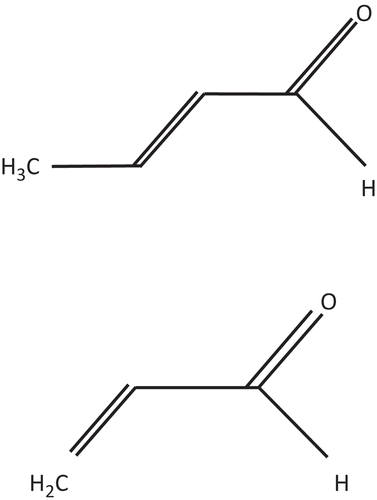
CRO is a white liquid that yellows on contact with air. CRO has a pungent, suffocating odor, which provides warning of hazardous concentrations (Agency for Toxic Substances and Disease Registry [ATSDR] Citation2002). CRO exists as a cis isomer (CASRN 15798–64-8) and a trans isomer (CASRN 123–73-9), or as a mixture of the two isomers (CASRN 4170–30-3). Commercial CRO (CASRN 4170–30-3) consists of >95% trans isomer and <5% cis isomer (O’Neil et al. Citation2006; IARC Citation1995). It is flammable and may polymerize violently. CRO is soluble in water, alcohol, ether, acetone, and benzene.
Chronic toxicity values were not developed separately for cis- and trans-CRO because no apparent studies were available on individual isomers. Acrolein (107–02-8) is a clear or yellow liquid with a piercing, disagreeable, “acrid” odor (ATSDR, Citation2007). Both acrolein and CRO are α,β-unsaturated carbonyls and are highly reactive (). CRO is similar to acrolein in physical/chemical properties (). Both acrolein and CRO are water soluble, volatile, and have a low n-octanol–water partition coefficient (Kow), which indicates bioaccumulation does not occur. The vapor pressure for CRO is lower than for acrolein.
TABLE 1. Physical Chemical Parameters for Acrolein and CRO
IN VIVO STUDIES
The only available in vivo toxicity studies that evaluated CRO and acrolein in the same study using similar methods are acute studies that determined 50% odor detection thresholds, 50% inhalation concentration lethality data (LC50), inhalation concentration for 50% respiratory depression (RD50), and 50% lethality data for subcutaneous dose (LD50) (). Acrolein was consistently more toxic than CRO.
TABLE 2. Comparison of Acute Sensory and Lethality Data
Skog (Citation1950) determined 30-min LC50 data in rats for CRO of 1400 ppm and for acrolein of 131 ppm (nominal concentrations), a CRO-to-acrolein ratio of 13.3. Rinehart (Citation1967) determined a 30-min LC50 in rats for CRO of 593 ppm (analytical concentrations). The Rinehart (Citation1967) LC50 data for CRO were approximately twofold lower than data obtained by Skog (Citation1950). Rinehart (Citation1967) suggested this difference may have been due to a loss of CRO between point of vapor generation and animal breathing zone in the Skog (Citation1950) study. There are other LC50 studies in rats available for acrolein and CRO for similar exposure durations (i.e., 10 min and 4 h), but these LC50 values were determined by different researchers so were not used to calculate an RPF:
The 10-min LC50 for CRO was 1480 ppm (Rinehart Citation1967) and for acrolein it was 374 ppm (Catalina, Thieblot, and Champeix Citation1966), a ratio of CRO to acrolein of 3.95.
The 4-h LC50 for CRO was 70 ppm (Voronin et al. Citation1982) to 88 ppm (Rinehart Citation1967), whereas the 4-h LC50 for acrolein was 8 ppm (Carpenter, Smyth, and Pozzani Citation1949). The ratio of CRO to acrolein ranged from 8.75 to 11.
Rinehart (Citation1967) was a high-quality study that reported analytical concentrations. The other LC50 studies reported nominal concentrations or were poorly described. Therefore, LC50 data were not used to determine a ratio of CRO to acrolein for the RPF approach.
RD50 investigations were conducted at lower, more relevant concentrations, and reported analytical concentrations. RD50 data for CRO ranged from 3.43- to 3.87-fold higher than acrolein in rats (Babiuk, Steinhagen, and Barrow Citation1985) and mice (Steinhagen and Barrow Citation1984) (). Rodents likely experienced cellular damage in the respiratory tract at concentrations used to determine RD50 values.
For acrolein, the RD50 value is 1.03 ppm in Swiss-Webster mice (Steinhagen and Barrow Citation1984) (). Buckley et al. (Citation1984) exposed groups of 16–24 male Swiss-Webster mice to 1.7 ppm acrolein 6 h/d for 5 d. Acrolein-exposed mice exhibited lesions in the nasal region. There was minimal to moderate recovery after 72 h.
For acrolein, the RD50 in rats was 6 ppm (Babiuk, Steinhagen, and Barrow Citation1985). Acrolein produced respiratory damage at 1.8 ppm after treatment of rats for 6 h/d for 4 d (Dorman et al. Citation2008; TCEQ Citation2014b)
For CRO, the RD50 in rats and mice ranged from 3.53 to 23.2 ppm (refer to ). Trofimov (Citation1962) reported irritation to the mucosa of rabbits at 17.5 ppm CRO, and the threshold concentration irritating to the mucosa of cats was 3.15 ppm CRO.
LD50 data were determined by Skog (Citation1950) via the subcutaneous route. Approximately 8 animals/group were injected with acrolein at a concentration range of 20–80 mg/kg for mice and 40–60 mg/kg for rats. For CRO, mice were injected at a concentration range of 120–260 mg/kg and rats were injected at a concentration range of 100–180 mg/kg. Rodents were observed for up to 3 wk. Histological examinations of lungs, heart, liver, spleen, kidneys, and brain were performed for each aldehyde from at least four animals. For acrolein, the animals experienced moderate anesthesia, with general convulsions of short duration being noted in some animals. Mice seemed to have respiratory trouble more than rats. In the lungs, the following effects were observed: intra-alveolar and perivascular edema, especially perivenously with insignificant hemorrhages. Hyperemia and slight fatty degeneration occurred in the liver, whereas focal inflammation changes were observed in the kidney. The subcutaneous (sc) LD50 for acrolein was 30 mg/kg in mice and 50 mg/kg in rats (). For CRO, the animals experienced an intense excitation lasting 10–15 min, during which the animals showed signs of distress. The nose, ears, and feet became strongly reddened during the same excitation stage. At higher doses, death occurred during or close to the excitation stage. In lungs, the following effects were observed: hyperemia, hemorrhages, and perivascular edema with slight peribronchial pneumonic changes. Hyperemia was observed in the heart, liver, and kidneys. The sc LD50 for CRO was 160 mg/kg in mice and 140 mg/kg in rats (). The sc LD50 data were considered relevant for deriving the CRO-to-acrolein RPF ratio ().
IN VITRO STUDIES
Meacher and Menzel (Citation1999)
Meacher and Menzel (Citation1999) conducted in vitro studies in adult rat lung cells to compare the effective aldehyde concentration that reduced intracellular glutathione (GSH) by 50% (EC50). Cells were treated for 20 min with a range of aldehyde concentrations and then GSH levels were evaluated using GSH-monochlorobimane fluorescence intensity measured using laser cytometry. Results were reported for aldehyde concentrations that produced no marked changes in cell morphology as observed by phase-contrast microscopy. One of the proposed MOA for aldehydes, especially acrolein and CRO, is depletion of cellular GSH, leading to oxidative stress and cellular damage, as discussed later. An in vitro assay that ranks GSH reduction may be used to rank the potency of aldehydes.
The EC50s for the n-alkanals (formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde) ranged from 110 to 400 mmol/L, a factor of approximately 1000-fold less potent when compared to the 2-alkenals, acrolein and CRO. Acrolein was the most potent 2-alkenal studied, as it had the lowest EC50 of 2 μmol/L followed by CRO at 130 μmol/L. The ratio of EC50s for GSH depletion for CRO compared to acrolein was 65.
Moretto et al. (Citation2009)
Moretto et al. (Citation2009) examined the acute effects of aqueous cigarette smoke extract (CSE) and of two α,β-unsaturated aldehydes (acrolein and CRO) contained in CSE in cultured normal human lung fibroblasts (NHLF) and small airway epithelial cells (SAEC). By examining a panel of 19 cytokines and chemokines, data showed that interleukin (IL)-8 release was elevated by CSE. Acrolein and CRO concentrations mimicked the CSE-evoked IL-8 release. Cultured cells were treated with 0, 3, 10, 30, and 60 μM acrolein or CRO. Acrolein and CRO stimulated the release of IL-8 from both SAEC and NHLF in a concentration-dependent manner. In SAEC cultures, acrolein (171.7 ± 5.2% of basal release, n = 4) and CRO (195.5 ± 6.2% of basal release, n = 4) elicited their maximal effect at 30 μM. In NHLF cells, acrolein elicited its maximal effect at 10 μM (258.4 ± 23.5% of basal release, n = 4) and CRO at 30 μM (202.1 ± 13.6% of basal release, n = 4).
Moretto et al. (Citation2009) also evaluated the cytotoxicity of acrolein and CRO using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test in SAEC and NHLF cells. There were no statistical differences in cell viability after treatment with acrolein and CRO compared to control SAEC cells (no statistical differences at concentrations of 3, 10, 30, or 60 μM). However, in NHLF cells, acrolein significantly decreased cell viability at 60 μM, whereas a numerical lower trend was observed for CRO (). Cell viability was evaluated by percent reduction of MTT absorbance of treated cells compared to controls. The ratio for CRO compared to acrolein at 60 μM (a concentration where acrolein produced a significant fall in cell viability) was 3.64 (i.e., 91% reduction/25% decrease) (Moretto et al. Citation2009).
TABLE 3. Acrolein and CRO Percent Viability Evaluated in NHLF Cells (MTT Testa)
Another method to calculate a RPF value from the Moretto et al. (Citation2009) study is to compare the concentration of CRO and acrolein that produce the same reduction in cell viability. The concentration that resulted in a 91% decrease in absorbance for CRO was 60 μM. The concentration corresponding to a 91% reduction in MTT absorbance for acrolein was not provided, but can be estimated using a linear interpolation between 10 μM (99% reduction in absorbance) and 30 μM (76% decrease in absorbance). Based on this interpolation, the concentration of acrolein projected to result in a 91% reduction in cell viability is 17 μM. The RPF for the CRO concentration of 60 μM (91% decrease) to the acrolein concentration of 17 μM (91% reduction) is 3.53. This supports the RPF of 3.64 already calculated.
Poirier et al. (Citation2002)
Poirier et al. (Citation2002) assessed 13 chemicals present in tobacco smoke, including acrolein and CRO, for their effect on viability and proliferation of mouse lymphocytes in vitro. Lymphocytes were obtained from the spleen and were referred to as splenocytes. Cell viability was assessed with propidium iodide (PI), with subsequent analyses by flow cytometry. For cell proliferation, control and treated cells were exposed to concanavalin A (ConA), a T-cell mitogen, and lipopolysaccharide (LPS), a B-cell mitogen. After a 48-h incubation period, 0.5 μCi [3H]methylthymidine was added to each well. The incubation was resumed for another 18 h under the same conditions. Cells were then collected on filters and counted in a β counter. Only acrolein and CRO induced a cytotoxic effect in the viability assay. The other 11 compounds produced no cytotoxic effects on splenocytes. Both aldehydes produced a concentration- and time-dependent significant effect on splenocyte viability as determined by PI dye exclusion. At concentrations of 10−5 M and higher, the significant suppressive effect was already observed after 3 h of exposure. A longer incubation period with acrolein and CRO at the highest concentrations resulted in death of almost all cells. The concentrations inducing 50% inhibition (IC50) for viability and the mitogenic assay after a 3-h exposure are shown in . Acrolein and CRO inhibited both T-cell and B-cell proliferation (). The antiproliferative effect of CRO and acrolein may partly be attributed to their cytotoxic effects, with IC50 values for viability and mitogenic assays being within the same range. The ratio of IC50 values for cell viability for CRO compared to acrolein was 1.58.
TABLE 4. Comparison of IC50 Values for Acrolein and CRO (3-h Exposure)
MOA ANALYSIS
An MOA analysis was performed to determine the relevant endpoints that might be used for an RPF approach. Relevant endpoints for both acrolein and CRO need to be closely tied to the expected critical effect for the index chemical and LTD chemical and need to be determined using similar testing techniques, exposure durations, and species. The critical effects are noncarcinogenic and the toxicity of each effect was assumed to have a threshold exposure associated with its MOA (threshold MOA).
CRO MOA
Because CRO is an α,β-unsaturated carbonyl, it is highly reactive with cellular components and forms protein adducts and histone–DNA cross-links (Kurtz and Lloyd Citation2003). The general metabolic pathway for aldehydes is oxidation by aldehyde dehydrogenase (ADH). However, the major detoxification pathway of CRO is with GSH to form GSH conjugates.
Liu et al. (Citation2010a) investigated the MOA for cell death in a normal human bronchial epithelial cell line (BEAS-2B cells) after exposure to CRO. CRO induced cytotoxicity through induction of cellular oxidative stress with depletion of intracellular GSH and increase in reactive oxygen species (ROS). CRO induced both apoptosis and necrosis, and there was a transition from apoptosis to necrosis with increasing CRO concentrations (Liu et al. Citation2010a). This transition was dependent on decreasing ATP levels, reduction in mitochondrial membrane potential, opening of the mitochondrial permeability transition pore (a critical event), and cytochrome c release from the mitochondria to the cytosol. Apoptosis was mediated via cytochrome c release and caspases cascade (caspase-9 increased, but diminished after prolonged exposure; caspase-3/7 was elevated at higher concentrations). Liu et al. (Citation2010a) could not rule out the possibility that CRO might induce apoptosis through another caspase-independent pathway, such as apoptosis-inducing factor.
In a later study, Liu et al. (Citation2010b) used microarray analysis to examine the gene expression profile of BEAS-2B cells after exposure to increasing concentrations of CRO. Cell cycle arrest was also investigated in the study. A large number of inflammation responsive genes were suppressed by CRO. HMOX1 (antioxidant response) and ALDH1A3 (ADH metabolism) were induced at three different increasing concentrations. Although some cell cycle genes were upregulated, several were down regulated; overall, CRO produced cell cycle arrest in S and G2M phase. Heat-shock response-related genes were strongly upregulated. Taken into account HMOX1 mediating cellular pathways and ALDH1A3 detoxifying toxicants, HMOX1 and ALDH1A3 were considered as novel transcriptional markers for CRO toxicity.
Both Moretto et al. (Citation2009) and Yang et al. (Citation2013) investigated inflammatory mechanisms after exposure of cultured cells to CRO. Moretto et al. (Citation2009) demonstrated CRO increased IL-8 release in cultured normal human lung fibroblasts and small airway epithelial cells. Phosphorylation of both ERK1/2 (extracellularly regulated kinase-1 and -2) and p38 (38-kD mitogen-activated protein kinase) underlies the IL-8 release. Yang et al. (Citation2013) showed that CRO treatment is capable of directly stimulating the production of IL-8 in both macrophages and airway epithelial cells (BEAS-2B and A549 cells). In addition, conditioned media from THP-1 cells stimulated after CRO exposure elevated IL-8 production, enhanced nuclear factor (NF)-κB and activator protein (AP)-1 DNA-binding activity in BEAS-2B and A549 cells. CRO-stimulated macrophages also amplify the inflammatory response by enhancing IL-8 release from airway epithelial cells and produce lung inflammatory response via multiple mechanisms that result in chronic airway inflammation in smokers.
Acrolein MOA
Similar to CRO, acrolein is highly reactive and rapidly forms conjugates with cellular GSH, cysteine, N-acetylcysteine, and/or thioredoxin (Moghe et al. Citation2015). Acrolein was found to be cytotoxic to various cells in vivo and in vitro (Li et al. Citation1997). Many of the effects of acrolein may be due to saturation of protective cellular mechanisms (e.g., GSH) and reactions with critical sulfhydryl groups in proteins and peptides (WHO Citation2002). The effects following inhalation exposure to acrolein are qualitatively similar to those of other aldehydes, although acrolein is the most irritating (NRC Citation2010). The respiratory irritancy of acrolein may be due to reactivity toward sulfhydryl groups in receptor proteins in the nasal mucosa (Beauchamp et al. Citation1985). Acrolein was also shown to suppress defenses against infections. In order to study how acrolein may decrease host defense, Li et al. (Citation1997) studied human alveolar macrophage function and response after exposure to acrolein. Macrophages treated with varying concentrations of acrolein displayed a concentration-dependent inhibition in release of IL-1β, IL-12, and tumor necrosis factor (TNF)-α. Treatment of alveolar macrophages by acrolein also induced concentration-dependent necrosis and apoptosis after 24 h.
Comparison of the MOA for Acrolein and CRO
There are differences between the MOA of acrolein and CRO involving mechanisms affecting apoptosis and necrosis, as well as differences in gene expression profiles, as described by Liu et al. (Citation2010a, Citation2010b). However, the primary mechanisms of toxicity are similar. Both acrolein and CRO release IL-8 (Moretto et al. Citation2009) and result in a decrease in T-cell and B-cell stimulated proliferation (Poirier et al. Citation2002). Both CRO and acrolein are highly reactive and induce toxicity in a variety of ways. An increase in ROS resulting from reaction with and depletion of GSH is considered to be the primary mechanism underlying toxicity (Meacher and Menzel Citation1999).
Matrix of Data and Pattern of Relative Toxicity
The next step in developing a toxicity factor for CRO is to construct a comparison of CRO to acrolein for relevant endpoints. Cytotoxicity and cellular damage would be the most relevant endpoints to evaluate chronic exposure based upon MOA. shows the endpoints considered relevant. Subcutaneous LD50 data (Skog Citation1950) determined for both acrolein and CRO in one study were considered relevant for inhalation exposure (Collins et al. Citation1998; Glass et al. Citation1991). RD50 values, although a measure of sensory irritation, were considered relevant for both acrolein and CRO because rodents likely experienced respiratory tissue damage at concentrations used to calculate RD50 values as demonstrated by Buckley et al. (Citation1984) (TCEQ Citation2014a; Citation2014b). The quality of the RD50 studies was high and results were available in both rats and two species of mice. In vitro results evaluating cell viability or cytotoxicity ( and ) were also deemed to be relevant as supporting information. The RPF of 3.64 from the Moretto et al. (Citation2009) study is informative because it is based on responses from cultured normal human lung cells. Data were evaluated to determine if there was a correlation among chemicals and endpoints to assess whether a predictable pattern exists among the chemicals. There was a definite pattern for relevant endpoints (). In all cases, acrolein was more toxic than CRO.
TABLE 5. Comparison of Relevant Endpoints for Acrolein and CRO
The following endpoints were not considered relevant to calculate a RPF. Odor potential was not considered to be predictive of chronic adverse effects. Depletion of GSH as evaluated by Meacher and Menzel (Citation1999) is an early event in the MOA of aldehydes and may not lead to cytotoxicity, so this endpoint was not considered relevant. LC50 data would be a relevant endpoint because the primary effect observed in animals in inhalation lethality studies was respiratory failure. However, LC50 data were not used due to study quality issues.
Relevant endpoints in were determined using similar researchers, testing techniques, exposure durations, and species. The RPF of the pertinent endpoints based on MOA analysis of the index chemical (acrolein) to the pertinent endpoint of the LTD chemical (CRO) was calculated as follows The RPF of the pertinent endpoints based on MOA analysis of the LTD chemical (CRO) to the index chemical (acrolein) was calculated as follows:
If multiple RPF values, based on the same or different relevant endpoints, are available, a median of the RPF is calculated. The median value is the most appropriate summary statistic of the central biologic tendency (Glass et al. Citation1991; Jones and Easterly Citation1996). The median of applicable RPF values for in vivo endpoints was 3.46 (n = 5). In contrast, the median of applicable RPF values for in vitro endpoints was 2.61 (n = 2), which is less than a factor of 2 compared to the in vivo RPF. When rounded to one significant figure, the in vivo RPF of 3 and in vitro RPF of 3 are identical. The most relevant RPF is based on in vivo data since they best represent the response in the intact organism.
Calculation of the Chronic ReV for CRO
The chronic ReV for the LTD chemical can then be calculated by multiplying the median in vivo RPF of 3 by the ReV of the structurally similar index chemical. shows a summary of the derivation of the acrolein chronic noncarcinogenic ReV assuming a threshold MOA (TCEQ Citation2014b). The acrolein chronic ReV of 1.2 ppb was based on the Dorman et al. (Citation2008) study conducted in rats (Supplemental Material). The animal-to-human dosimetric adjustments for acrolein are relevant to CRO since both aldehydes are water soluble with low Kows and are expected to produce respiratory damage in the extrathoracic region (U.S. EPA, Citation1994; 2012). The duration adjustments for acrolein are applicable to CRO since respiratory damage is assumed to be concentration and duration dependent. The index chemical’s chronic ReV of 1.2 ppb is multiplied by the in vivo RPF of 3 to calculate the chronic ReV for CRO of 3.6 ppb (10 μg/m3), rounded to two significant figures (TCEQ Citation2012).
TABLE 6. Derivation of the Chronic ReV for CRO Based on Relative Potency
DISCUSSION
The National Research Council Report “Toxicity Testing in the 21st Century: A Vision and a Strategy” (NRC Citation2007a; Krewski et al. Citation2010) recommends the use of in vitro data in risk assessments. This approach reduces animal use and costs, while still allowing for sound risk-management decisions without the need for in vivo testing. The chronic toxicity factor approach used for CRO was an example of using robust in vivo data for acrolein, and MOA information for CRO and acrolein from both in vivo and in vitro studies to justify use of acrolein as an adequate index chemical. An RPF approach was used to develop an inhalation chronic toxicity factor for CRO. This RPF approach employed structural information, as well as in vivo and in vitro data, as suggested by NRC (Citation2007a) and Krewski et al. (Citation2010). In vivo endpoints were preferred to calculate the final RPF because these endpoints are more appropriate for observing the overall effects on the whole organism. In vitro cytotoxicity data were used to support the in vivo RPF. The in vitro RPF of 3 (rounded to one significant figure) based on decreases in cell viability in cultured normal human lung cells and mouse lymphocytes is the same as the in vivo RPF.
The RPFs from in vivo and in vitro endpoints ranged from 1.58 to 5.33, a threefold difference. A potential reason the RPF values are consistent was that only studies that evaluated CRO and acrolein in the same study using similar testing techniques, exposure durations, and species were used. Only endpoints that were closely tied to the expected critical effect and MOA for the index and LTD chemical were considered. RD50 studies in rodents (Babiuk, Steinhagen, and Barrow Citation1985; Steinhagen and Barrow Citation1984) exposed to concentrations of CRO and acrolein that produce respiratory-tract damage (Buckley et al. Citation1984; TCEQ Citation2014a; Citation2014b) were indicative of reactivity and ability to cause cellular damage. Subcutaneous (sc) LD50 data (Skog Citation1950) compared lethality for CRO and acrolein. Although LC50 studies were preferred to predict toxicity through the inhalation route, sc LD50 studies can be used to calculate RPF values applicable to the inhalation route (Collins et al. Citation1998; Glass et al. Citation1991). The TCEQ did not elect to use early precursor events, such as GSH depletion (Meacher and Menzel Citation1999), to calculate an RPF, but instead selected more apical endpoints such as decrease in cell viability in different cell lines determined with cytotoxicity assays (Poirier et al. Citation2002; Moretto et al. Citation2009).
Even though in vivo tests were short-term tests, they are useful to calculate a chronic CRO-to-acrolein RPF. Jones and Easterly (Citation1996) used numerous short-term tests to evaluate carcinogenic potential of chemicals. In addition, they stated, “It is desirable for the reference compounds to have been tested extensively in various bioassays so that several relative potency values can be computed for each new compound of interest.”
The TCEQ developed a chronic ReV for CRO for evaluating ambient air monitoring data. In 1992, the TCEQ established the Community Air Toxics Monitoring Network, which has grown into the largest ambient air monitoring network in the country (Capobianco et al. Citation2013). These air monitors provide information on ambient CRO concentrations in Texas. There are 6 locations in Texas that monitor for CRO using 24-h canister samplers that collect samples every sixth day. The 2014 annual average concentration for CRO at these sites ranged from 0.007 to 0.03 ppb, well below the chronic ReV of 3.6 ppb (10 μg/m3).
The TCEQ also developed an acute ReV of 10 ppb (29 μg/m3) for CRO for evaluation of 1-h data (TCEQ Citation2014a). The acute ReV was based on a National Institute for Occupational Safety and Health (NIOSH) occupational study reported by Fannick (Citation1982). The critical effect was minor sensory eye irritation reported by occupational workers exposed to an average CRO concentration of 0.56 ppm (range from <0.35 to 1.1 ppm) measured in general air samples. Uncertainty factors totaling 54 were applied to the human point of departure to calculate the CRO acute ReV of 10 ppb (29 μg/m3). The CRO acute ReV was 2.1-fold higher than acrolein’s acute ReV of 4.8 ppb (11 μg/m3) based on eye, nose, and throat irritation and diminished respiratory rate in human volunteers (TCEQ Citation2014b). This ratio is similar to the chronic median in vivo RPF of 3.
A unit risk factor has not been developed for acrolein. Therefore, the RPF used to develop a chronic inhalation ReV for CRO based on the acrolein ReV is applicable for noncarcinogenic effects only. The potential carcinogenicity of acrolein cannot be determined because of inadequate data to assess the human carcinogenic potential for either the oral or inhalation route of exposure (U.S. EPA Citation2003). Acrolein has induced DNA adducts in vitro in a variety of cell types and mutagenesis under certain conditions, but there is only limited information on its ability to induce mutations in normal mammalian cells. Because acrolein is highly reactive and not distributed systemically, acrolein is unlikely to reach potential target sites at a concentration sufficient to initiate a carcinogenic response (U.S. EPA Citation2003). The U.S. EPA classified CRO as a possible human carcinogen (Class C) (U.S. EPA Citation2005a) based on an absence of human data and an increased incidence of hepatocellular carcinomas and hepatic neoplastic nodules in male rats (Chung, Tanaka, and Hecht Citation1986). There was a lack of a discernable dose-response trend for CRO for the observed incidence of neoplasms. Information supporting the possible carcinogenicity of CRO includes its genotoxic activity and that it is a suspected metabolite of N-nitrosopyrrolidine, a probable human carcinogen. Based on the recent Guidelines for Carcinogen Risk Assessment (U.S. EPA Citation2005b), the cancer classification descriptor developed by the TCEQ for CRO would be suggestive evidence of carcinogenicity via the oral pathway, but not sufficient to assess human carcinogenic potential via inhalation exposure (TCEQ Citation2012).
The chronic ReV for CRO will be used as an air monitoring comparison value (AMCV) during health effects evaluation of annual-averaged ambient air monitoring data. For air permitting, the chronic ReV is reduced by 70% to calculate the long-term effects screening level (ESL). The ESL used in air permitting is lower than the ReV to account for cumulative and aggregate exposure during the air permit review process (Capobianco et al. Citation2013; TCEQ Citation2012). The long-term ESL for CRO of 1.1 ppb (3.2 μg/m3) will be used during health effects evaluation of modeled annual-averaged ambient air data to assess the protectiveness of substance-specific emission rate limits for facilities undergoing air permit reviews. If the chronic maximum ground-level concentration (GLCmax), a worst-case modeled concentration resulting from a worst case emission rate, is below the long-term ESL, the substance can be judged, with reasonable confidence, to present a low probability of risk. If inhalation subchronic or chronic toxicity data become available in the future, the TCEQ will update its chronic toxicity assessment for CRO.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/10937404.2015.1081574
Supplemental_Material
Download PDF (108.5 KB)REFERENCES
- Agency for Toxic Substances and Disease Registry. 2002. ToxFAQs™ for crotonaldehyde. http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=948&tid=197 ( accessed April 25, 2013).
- Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for acrolein. U.S. Department of Health and Human Services. http://www.atsdr.cdc.gov/toxprofiles/tp124.pdf ( accessed April 25, 2013).
- Babiuk, C., W. H. Steinhagen, and C. S. Barrow. 1985. Sensory irritation response to inhaled aldehydes after formaldehyde pretreatment. Toxicology and Applied Pharmacology 79:143–49. doi:10.1016/0041-008X(85)90376-X.
- Beauchamp, R. O., Jr., D. A. Andjelkovich, A. D. Kligerman, K. T. Morgan, and H. D. Heck. 1985. A critical review of the literature on acrolein toxicity. Critical Reviews in Toxicology 14:309–80. doi:10.3109/10408448509037461.
- Buckley, L. A., X. Z. Jiang, R. A. James, K. T. Morgan, and C. S. Barrow. 1984. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicology and Applied Pharmacology 74:417–29. doi:10.1016/0041-008X(84)90295-3.
- Capobianco, T., S. M. Hildebrand, M. Honeycutt, J.-S. Lee, D. McCant, and R. L. Grant. 2013. Impact of three interactive Texas state regulatory programs to decrease ambient air toxic levels. Journal of the Air & Waste Management Association 63:507–20. doi:10.1080/10962247.2013.763868.
- Carpenter, C. P., H. F. Smyth, and U. C. Pozzani. 1949. The assay of acute vapor toxicity, and the grading and interpretation of results on 96 chemical compounds. Journal of Industrial Hygiene and Toxicology 31:343–46.
- Catalina, P., L. Thieblot, and J. Champeix. 1966. Experimental respiratory lesions by inhalation of acrolein in the rat [in French]. Arch Mal Prof 27:857–67. As cited in National Research Council. 2010. Acrolein, 13–48. National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. Volume 8. Committee on Acute Exposure Guideline Levels, Committee on Toxicology. Washington DC: The National Academy Press
- ChemIDPlus. 2015. A Toxnet database. http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?chemidheavy ( accessed April 23, 2015).
- Chung, F., T. Tanaka, and S. S. Hecht. 1986. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Research 46:1285–89.
- Collins, J. F., J. P. Brown, G. V. Alexeeff, and A. G. Salmon. 1998. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regulatory Toxicology and Pharmacology 28:45–54. doi:10.1006/rtph.1998.1235.
- Dorman, D. C., M. F. Struve, B. A. Wong, M. W. Marshall, E. A. Gross, and G. A. Willson. 2008. Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhalation Toxicology 20:205–16. doi:10.1080/08958370701864151.
- Fannick, N. 1982. Health hazard evaluation report: Sandoz colors and chemicals, East Hanover, New Jersey. HETA-81-102-1244. Cincinnati, OH: National Institute for Occupational Safety and Health.
- Glass, L. R., C. E. Easterly, T. D. Jones, and P. J. Walsh. 1991. Ranking of carcinogenic potency using a relative potency approach. Archives of Environmental Contamination and Toxicology 21:169–76. doi:10.1007/BF01055333.
- Hazardous Substances Data Bank. 2005. Crotonaldehyde (CASRN 4170-30-3). http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB ( accessed July 25, 2007).
- Henschler, D. 1981. Gesundheitsschädliche Arbeitsstoffe, Toxikologisch-arbeits-medizinische Begründungen von MAK-Werten, Loseblattsammlung, 8. Lfg. DFG, Deutsche Forschungsgemeinschaft, VCH Verlag Weinheim. (cited in Scientific Committee on Occupational Exposure Limits (SCOEL). 2013. Recommendation from the Scientific Committee on Occupational Exposure Limits for 2-butenal. European Commission. SCOEL/SUM/180)
- Ichihashi, K., T. Osawa, S. Toyokuni, and K. Uchida. 2001. Endogenous formation of protein adducts with carcinogenic aldehydes: Implications for oxidative stress. Journal of Biological Chemistry 276:23903–13. doi:10.1074/jbc.M101947200.
- International Agency for Research on Cancer. 1995. Crotonaldehyde. In Dry cleaning, some chlorinated solvents and other industrial chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 63:373–91.
- International Programme on Chemical Safety. 2008. Concise international chemical assessment document 74. Geneva, Switzerland: World Health Organization. http://www.who.int/ipcs/publications/cicad/cicad74.pdf ( accessed March 26, 2013).
- Jones, T. D., and C. E. Easterly. 1996. A RASH analysis of national toxicology program data: Predictions for 30 compounds to be tested in rodent carcinogenesis experiments. Environmental Health Perspectives 104:1017–30. doi:10.1289/ehp.96104s51017.
- Krewski, D., D. Acosta Jr, M. Andersen, H. Anderson, J. C. Bailar 3rd, K. Boekelheide, R. Brent, G. Charnley, V. G. Cheung, S. Green Jr, K. T. Kelsey, N. I. Kerkvliet, A. A. Li, L. McCray, O. Meyer, R. D. Patterson, W. Pennie, R. A. Scala, G. M. Solomon, M. Stephens, J. Yager, L. Zeise, and S. of Committee on Toxicity Test. 2010. Toxicity testing in the 21st century: A vision and a strategy. Journal of Toxicology and Environmental Health, Part B 13:51–138. doi:10.1080/10937404.2010.483176.
- Kurtz, A. J., and R. S. Lloyd. 2003. 1,N2-Deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. Journal of Biological Chemistry 278:5970–76. doi:10.1074/jbc.M212012200.
- Li, L., R. F. Hamilton, D. E. Taylor, and A. Holian. 1997. Acrolein-induced cell death in human alveolar macrophages. Toxicology and Applied Pharmacology 145:331–39. doi:10.1006/taap.1997.8189.
- Liu, X. Y., Z. H. Yang, X. J. Pan, M. X. Zhu, and J. P. Xie. 2010a. Crotonaldehyde induces oxidative stress and caspase-dependent apoptosis in human bronchial epithelial cells. Toxicology Letters 195:90–98. doi:10.1016/j.toxlet.2010.02.004.
- Liu, X. Y., Z. H. Yang, X. J. Pan, M. X. Zhu, and J. P. Xie. 2010b. Gene expression profile and cytotoxicity of human bronchial epithelial cells exposed to crotonaldehyde. Toxicology Letters 197:113–22. doi:10.1016/j.toxlet.2010.05.005.
- Meacher, D. M., and D. B. Menzel. 1999. Glutathione depletion in lung cells by low-molecular-weight aldehydes. Cell Biology and Toxicology 15:163–71. doi:10.1023/A:1007633519962.
- Moghe, A., S. Ghare, B. Lamoreau, M. Mohammad, S. Barve, C. McClain, and S. Joshi-Barve. 2015. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicological Sciences 143:242–55. doi:10.1093/toxsci/kfu233.
- Moretto, N., F. Facchinetti, T. Southworth, M. Civelli, D. Singh, and R. Patacchini. 2009. α,β-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. AJP: Lung Cellular and Molecular Physiology 296:L839–L848. doi:10.1152/ajplung.90570.2008.
- Nagata, Y. 2003. Measurement of odor threshold by triangle odor bag method. Odor Measurement Review, 118–27. Japan Ministry of the Environment. http://www.env.go.jp/en/air/odor/measure/02_3_2.pdf ( accessed July 22, 2015).
- National Research Council. 1983. Risk assessment in the federal government. Washington, DC: National Research Council, National Academy Press.
- National Research Council. 1994. Science and judgment in risk assessment. Washington, DC: National Research Council, National Academy Press.
- National Research Council. 2001. Standing operating procedures for developing acute exposure guideline levels for hazardous chemicals. Washington, DC: National Research Council, National Academy Press.
- National Research Council. 2007a. Toxicity testing in the 21st century, A vision and a strategy. Washington, DC: National Academies Press. The National Academy Press
- National Research Council. 2007b. Crotonaldehyde, trans and cis + trans. National Research Council. Acute exposure guideline levels for selected airborne chemicals. In Committee on Acute Exposure Guideline Levels, Committee on Toxicology, vol. 6. 123–72. Washington DC: The National Academy Press
- National Research Council. 2010. Acrolein. National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. In Committee on Acute Exposure Guideline Levels, Committee on Toxicology, vol. 8, 13–48. Washington DC: The National Academy Press
- O’Neil, M. J., P. E. Heckelman, C. Koch, and K. Roman, eds. 2006. P. 2595. In The Merck Index: An encyclopedia of chemicals, drugs, and biologicals, 14th ed. Hoboken, NJ: John Wiley & Sons.
- Poirier, M., M. Fournier, P. Brousseau, and A. Morin. 2002. Effects of volatile aromatics, aldehydes, and phenols in tobacco smoke on viability and proliferation of mouse lymphocytes. Journal of Toxicology and Environmental Health, Part A 65:1437–51. doi:10.1080/00984100290071342.
- Rinehart, W. E. 1967. The effect on rats of single exposures to crotonaldehyde vapor. American Industrial Hygiene Association Journal 28:561–66. doi:10.1080/00028896709342685.
- Skog, E. 1950. A toxicological investigation of lower aliphatic aldehydes: I. Toxicity of formaldehyde, acetaldehyde; propionaldehyde and butyraldehyde; as well as of acrolein and crotonaldehyde. Acta Pharmacologica et Toxicologica 6:299–318. doi:10.1111/(ISSN)1600-0773.
- Steinhagen, W. H., and C. S. Barrow. 1984. Sensory irritation structure–activity study of inhaled aldehydes in B6C3F1 and Swiss-Webster mice. Toxicology and Applied Pharmacology 72:495–503. doi:10.1016/0041-008X(84)90126-1.
- Texas Commission on Environmental Quality. 2008. Development support document Formaldehyde, CAS registry number: 50-00-0. Toxicology Division, Chief Engineer’s Office. http://www.tceq.state.tx.us/implementation/tox/dsd/final.html
- Texas Commission on Environmental Quality. 2012. TCEQ guidelines to develop toxicity factors (Revised RG-442). Office of the Executive Director.
- Texas Commission on Environmental Quality. 2014a. Development support document: Crotonaldehyde (Cis and Trans), CAS registry number: 4170-30-3 Trans-Crotonaldehdye, CAS registry number: 123-73-9; Cis-Crotonaldehyde, CAS registry number: 15798-64-8. Toxicology Division, Office of the Executive Director. http://www.tceq.state.tx.us/implementation/tox/dsd/final.html
- Texas Commission on Environmental Quality. 2014b. Development support document: Acrolein, CAS registry number: 107-02-8. Toxicology Division, Office of the Executive Director. http://www.tceq.state.tx.us/implementation/tox/dsd/final.html
- Texas Risk Reduction Program. 2009. Chemical/physical properties table. http://www.tceq.state.tx.us/remediation/trrp/trrppcls.html ( accessed April 25, 2013).
- Trofimov, L. V. 1962. Comparative toxic action of crotonaldehyde and butyraldehyde [in Russian]. Gigiena truda i professional’nye zabolevaniia 6:34–40. As cited in National Research Council (NRC). 2007b. Crotonaldehyde, trans and cis + trans. National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals. Volume 6, 123–72. Committee on Acute Exposure Guideline Levels, Committee on Toxicology.
- U.S. Environmental Protection Agency. 1994. Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. EPA/600/8–90/066F. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development.
- U.S. Environmental Protection Agency. 2002. A review of the reference dose and reference concentration processes. EPA/630/P-02/002F. Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum.
- U.S. Environmental Protection Agency. 2005b. Guidelines for carcinogen risk assessment. Washington, DC: Risk Assessment Forum. EPA/630/P-03/001B.
- U.S. Environmental Protection Agency. 2012. Advances in inhalation gas dosimetry for derivation of a reference concentration (RfC) and use in risk assessment. EPA/600/R-12/044. Washington, DC: U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 2003. Acrolein [CAS No. 107-02-8]. Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency [online]. http://www.epa.gov/iris/subst/0364.htm ( accessed August 5, 2015).
- U.S. Environmental Protection Agency. 2005a. Crotonaldehyde [CAS No. 123-73-9]. Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency [online]. http://www.epa.gov/iris/subst/0464.htm ( accessed August 5, 2015).
- Voronin, V., I. Bel’gova, L. Voronkova, O. Grigor’ev, V. Zhdanov, M. Nezhentsev, N. Antelava, V. Gusel, and A. Rotleder. 1982a. Korrektur der Toxizitätsbefunde über Crotonaldehyde (CA; Technische Vorschriften TU 6-09-3667-74). Gigiena Truda I Professional’nye Zabolevaniia 26:53–54 (abstract). As cited in International Programme on Chemical Safety (IPCS). 2008. Concise International Chemical Assessment Document 74. World Health Organization, Geneva, Switzerland. http://www.who.int/ipcs/publications/cicad/cicad74.pdf ( accessed March 26, 2013).
- Wolfe, G., M. Rodwin, J. French, and G. Parker. 1987. Thirteen week subchronic toxicity study of crotonaldehyde (CA) in F344 rats and B6C3F1 mice. Toxicologist 7:209.
- World Health Organization. 1995. Dry cleaning, some chlorinated solvents and other industrial chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 63, 7–14 February. http://monographs.iarc.fr/ENG/Monographs/vol63/mono63.pdf ( accessed July 27, 2015).
- World Health Organization. 2002. Acrolein. Concise international chemical assessment document 43. Geneva, Switzerland: International Programme on Chemical Safety (IPCS). http://www.inchem.org/documents/cicads/cicads/cicad43.htm accessed April 22, 2015.
- Yang, B. C., Z. H. Yang, X. J. Pan, F. J. Xiao, X. Y. Liu, M. X. Zhu, and J. P. Xie. 2013. Crotonaldehyde-exposed macrophages induce IL-8 release from airway epithelial cells through NF-κB and AP-1 pathways. Toxicology Letters 219:26–34. doi:10.1016/j.toxlet.2013.02.018.