ABSTRACT
A quantitative weight of evidence (QWoE) methodology was used to assess higher tier studies on the effects of clothianidin (CTD) on honeybees. Assessment endpoints were population size and viability of commercially managed bees and quantity of hive products. A colony-level no-observed-adverse effect concentration (NOAEC) of 25 µg CTD/kg syrup, equivalent to an oral no-observed-adverse effect-dose (NOAED) of 7.3 ng/bee/d for all responses measured. Based on a NOAEC of 19.7 µg/kg pollen, the NOAED for honeybee larvae was 2.4 ng/bee larva/d. For exposures via dust, a no-observed-adverse effect rate of 4 g CTD/ha was used to assess relevance of exposures via deposition of dust. The overall weight of evidence suggested that there is minimal risk to honeybees from exposure to CTD from its use as a seed treatment. For exposures via dust, dust/seed and dust/foliar applications, there were no exposures greater than the NOAED for CTD in nectar and pollen, indicating a de minimis risk to honeybees when the route of exposure was via uptake in plants. Analysis of effect studies in the field indicated a consistent lack of relevant effects, regardless of the way CTD was applied. For exposures via dust, there were no adverse effects because of these applications and there were no exposures greater than the NOAED for CTD in nectar and pollen. The overall weight of evidence based on many studies indicated no adverse effects on colony viability or survival of the colony. Thus, the overall conclusion is that clothianidin, as currently used in good agricultural practices, does not present a significant risk to honeybees at the level of the colony.
Introduction and problem formulation
This paper is the third in a series of quantitative weight of evidence (QWoE) analyses on the effects and risks of the use of neonicotinoids as seed treatments on honeybees. The conceptual model for exposure, methodology, and use of the QWoE analysis is described in the first paper in this series (Solomon and Stephenson Citation2017) and this paper reports on a QWoE of the effects and risks of the neonicotinoid, clothianidin (CTD) in honeybees. Clothianidin was first registered in the USA in 2003 for use in maize, canola, grapes, pome fruit, rice, tobacco, turf, and ornamentals (Fairbrother et al. Citation2014). It is effective against turf and sucking insects, including flies, beetles, moths, and hemipterans (Fairbrother et al. Citation2014). The major use of CTD in areas without current restrictions (i.e., the EU) is as a seed dressing. Here, the product might be mixed with other pesticides, such as fungicides, and coated on the seed with binders and other materials that provide a hard coating that does not abrade and reduces release of the coating as a dust during handling and, more importantly, pneumatic seeding.
Physical, chemical, and toxicological properties of clothianidin
Clothianidin, CAS RN [210880–92-5], IUPAC Name (E)-1-(2-chloro-1,3-thiazol-5-ylmethyl)-3-methyl-2-nitroguanidine (), MW 249.67 g/M, is a solid at room temperature and has a low vapor pressure of 9.8 x10−10 mm Hg (25°C) (PubChem Citation2015). Because the vapor pressure is low, and Henry’s constant is small (2.9 x10−16 atm/m3/M), CTD is unlikely to be present in air at concentrations sufficiently large to be of toxicological concern. Solubility in water is 327 mg/L, the log KOW is 0.7 (25ºC), and the KOC is 60 (PubChem Citation2015) so it is relatively mobile in soil pore-water and can be readily taken up by the roots of plants. It is stable in soil with a range of half-lives (t½) between 148 and 1,555 d (PubChem Citation2015) and is efficacious as a soil-active insecticide. Generally, for a period after planting, CTD is readily available for uptake by plants and once in the plant, it can be subsequently translocated from the roots to other parts of the plant. CTD is metabolized in plants to two identified metabolites, TZNG (N-(2-chlorothiazol-5-ylmethyl)-N’-nitroguanidine; desmethyl clothianidin) and TZMU (N-(2-chlorothiazol-5-ylmethyl)-N’-methylurea; clothianidin urea) (Bayer CropScience 2014e). Degradation of CTD via hydrolysis in water is slow (t½ = 27 d, (PubChem Citation2015), thus CTD present in pollen, nectar and/or honeydew, and water carried to the hive is not expected to degrade rapidly. Thus, concentrations in these matrices are expected to be representative of those to which bees are exposed except for guttation fluid collected as “water.”
Clothianidin acts by binding to the insect nicotinic acetylcholine receptor (nAChR) and acts as an agonist (Jeschke et al. Citation2011). The oral toxicity of CTD in first-tier laboratory studies was summarized by EFSA and the acute oral LD50 for honeybees was reported as 3.8 ng active substance (a.s.) /bee and the contact LD50 as 27.5 ng/bee (EFSA Citation2013). The 10-d chronic no-adverse-effect concentration (NOAEC) was reported as 10 μg a.s./L (liquid diet) and is equivalent to 9.1 µg a.s./kg The NOAEC range for honeybee larvae was reported as 20–40 µg a.s./kg diet. These laboratory-derived toxicity values are appropriate for use as an initial screening value for characterizing risk from oral exposures but are less useful for assessing responses in higher tier field studies that measure apical endpoints directly.
Toxicity data for higher tier assessments were provided in whole-hive feeding-exposure studies with exposures via CTD-amended syrup and pollen conducted over a period of 41 d. One study (Bayer AG Crop Protection Citation2001b) exposed bees via sugar syrup and colony-relevant responses for survival and productivity were measured (see supplemental information; SI). As only one replicate (hive) was used per concentration, statistical analysis was not possible. No adverse effects were observed at a measured concentration of 25 µg/kg syrup. The NOAEC of 25 µg CTD/kg was equivalent to an oral no-observed-adverse effect-dose (NOAED; 7.3 ng/bee/day) for all responses measured. Unfortunately, a lowest-observed-adverse-effect-concentration (LOAEC) was not measured in the study and the NOAEC might be greater than the reported value of 25 µg CTD/kg.
In a similar study (Bayer AG Citation2001) with CTD-amended pollen, no adverse effects were observed at a measured concentration of 19.7 µg/kg pollen. Again, a LOAEC was not measured in the study and the NOAEC might be greater than the observed value. For exposures via pollen, the NOAEC of 19.7 µg CTD/kg was equivalent to an oral NOAED of 2.44 ng/bee larva/d. These toxicity values were used in this paper for assessing exposures of bees to CTD measured in higher tier field studies and are expressed in doses per honeybee to allow normalization from various sources of exposure via diet. The concentrations in the food in these studies were used to estimate doses in bees that are based on the assumptions of intake of food used by the USEPA (Citation2014) and water, see .
Table 1. Threshold concentration in food and water for toxicity values for CTD in honeybees.
The metabolites of CTD, TZNG, and TZMU were tested for toxicity in the laboratory and one colony-level study and have de minimis toxicity. TZMU was not biologically active in a whole-hive feeding study at measured concentrations up to 22 µg/kg (sugar syrup diet, equivalent to 6.4 ng/bee/day, see SI) (Bayer CropScience Citation2003). An acute oral feeding study with TZMU in the laboratory showed an oral acute NOAED of ≥113,000 ng/bee (USEPA Citation2003b). An acute oral feeding study with TZNG in the laboratory showed an oral acute NOAED of 890 ng/bee (USEPA Citation2003a). This is consistent with lack of effects of TZMU at the level of the colony and, for these reasons, the metabolites were not included in the assessment of residues of the parent CTD.
A tier-three study on the effects of dust abraded from seeds treated with CTD (Bayer CropScience AG Citation2010e) was used for assessing relevance of exposures via this route. The study was assessed for QWoE (see SI) and provided a no-adverse effect-rate (NOAER) for colony-relevant endpoints of 4 g CTD/ha. This value was used to determine relevance of exposures via dust as effects were seen on individual bees at rates of 0.5 to 4 g CTD/ha. This value (4 g CTD/ha) was used to determine relevance of exposures via dust.
The main null hypothesis being tested is that use of CTD as a seed treatment has no adverse impacts on colony viability or the survival of the colony. There are several sub-hypotheses addressed below:
Concentrations of CTD in bee-relevant matrices from seed-treated crops in the field do not exceed toxicity values derived from higher tier toxicity studies on colonies of bees.
Colonies of bees exposed under experimental conditions in the field to CTD from seed treatments do not show adverse effects.
Exposure of colonies of bees to measured concentrations of CTD resulting from use as a seed treatment in the field does not cause adverse effects.
Methods
Sources of information for this QWoE assessment
The source of studies, papers, and reports used in this QWoE analysis and the methods used to assess quality and relevance of the data are described in the first paper in the series (Solomon and Stephenson Citation2017). Reviews and papers lacking sufficient information to allow for assessment of quality and relevance were not included in the analysis (see discussion in Solomon and Stephenson Citation2017). Other than these, we included all papers and reports on higher tier studies conducted at the level of the colony and under realistic field conditions. Papers and reports were not excluded based on quality.
The methods used to score the papers and reports for quality and relevance and how these scores were used to produce the QWoE diagrams in this paper are also provided in the text and supplemental information in Solomon and Stephenson (Citation2017). These methods are common to this and two other companion papers and are not discussed in detail here.
Characterization of exposures via surface water
There were several reports of concentrations of CTD in surface waters. These studies were from the open literature and were not included in the QWoE analysis because they did not address effects at the level of the colony; however, results are summarized here and the risks of the reported concentrations were assessed by comparison to the exposures that might be expected from guttation fluids (see and below). Results of the risk assessment are presented in .
Table 2. Estimated doses of CTD in adult honeybees collecting surface water from various sources.
The National Water Quality Monitoring Council (NWQMC) of the USA provides data on concentrations of pesticides measured in surface waters (NWQMC Citation2016). Processing of these data was as reported in Stephenson and Solomon (Citation2017). The method detection limit (MDL) was 0.025 μg/L and all non-detects were assigned a dummy value of zero. For samples of surface waters collected between 2011-06-15 and 2015-09-28, the 90th centile was 0.0 and the 99th centile 0.07 μg/L. Data from winter samples were not excluded as bees might be active in the southern US in winter.
Three studies on concentrations of CTD in samples collected in Saskatchewan from potholes in farmers’ fields where neonicotinoids were used as seed treatments have been reported (Main et al. Citation2017, Citation2014, Citation2016). The sampling and methods are reported in Stephenson and Solomon (Citation2017). In the first study (Main et al. Citation2014), mean values of samples collected in the summer from wetlands associated with fields planted with barley, canola, oats, peas, wheat, and grassland were 0.058, 0.142, 0.009, 0.010, 0.035, 0.001 μg/L, respectively. The second study (Main et al. Citation2016) used the same methods of analysis but sampled snow and meltwater in a subset of wetlands from the first study. The maximum concentration of CTD in snow, meltwater, and water from wetlands was 0.093 μg/L from 16 sites. Samples of snow were taken when bees would not be active and samples of water were taken from snow-melt until seeding (late May), also when bees would not be expected to be exposed. In the third study (Main et al. Citation2017), the maximum concentration of CTD detected was 0.037 µg/L and the level of quantitation (LOQ) was 0.005 µg/L; level of detection (LOD) was not reported. Raw data were not reported for any of the studies, so it was not possible to estimate centiles
In a study in S. Ontario, sources of water in or close to maize fields (puddles, ditches and drains ≤100 m from the edge of the field). Details of the study are in Stephenson and Solomon (Citation2017). LOQ for CTD in water was 0.04 μg/L. Median concentration of CTD in surface water potentially attractive to bees was 0.82 μg/L with a 90th centile of 2.3 μg/L (data from Schaafsma et al. Citation2015, SI ). In a study conducted in Ontario (Struger et al. Citation2017), CTD was detected in 13 of 15 drains, creeks, and rivers at frequencies of almost 100%. The sampling and methods are reported in Stephenson and Solomon (Citation2017). The overall median value was 0.006 µg/L (MDL = 0.0013 µg/L). The maximum value measured was 0.407 µg/L. The Ontario Ministry of Environment and Climate Change (OMECC) measured concentrations of CTD in surface waters in the major use-areas in S. Ontario (OMECC Citation2017). The sampling and methods are reported in Stephenson and Solomon (Citation2017). The MDL was 0.005 µg/L. The median for all sites was 0.045 µg/L and the maximum measured concentration was 0.52 µg/L, sampled during a precipitation event.
Results and discussion
Risks from exposures via surface waters
To assess risks, dose per honeybee was compared to the NOAED. In all cases (), the estimated dose of CTD was less than the chronic NOAED for water of 913 ng/bee and no acute risks were identified.
Risks resulting from exposures to honeybees from uptake and translocation of CTD from seed treatments
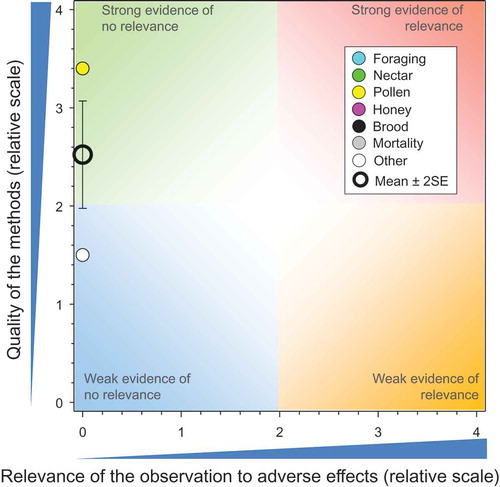
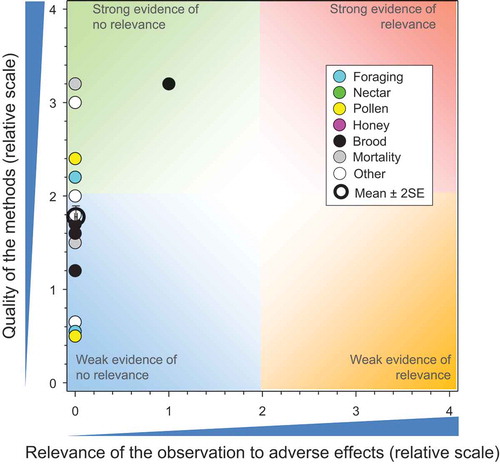
There were 45 studies (Bayer AG Crop Protection Citation2000a, Citation2000b, Citation2000c, Citation2001e, Citation2001f, Citation2001g; Bayer AG Crop Protection Citation2002b, Citation2002c; Bayer Corporation Citation2001; Bayer CropScience Citation2012, Citation2014c, Citation2014d, Citation2014a; Bayer CropScience Citation2015f, Citation2015g, Citation2015h, Citation2015i, Citation2015j, Citation2015k; Bayer CropScience Citation2015b, Citation2015c, Citation2015d; Bayer CropScience AG Citation2005a, Citation2005b, Citation2007a, Citation2009a, Citation2009k, Citation2009f, Citation2010a, Citation2011a, Citation2012a, Citation2012b, Citation2012c; Bayer CropScience AG Citation2014g, Citation2015c; Botías et al. Citation2015, Cutler et al. Citation2014; Kasiotis et al. Citation2014; Krupke et al. Citation2012; Kujawski and Namieśnik Citation2011; Marzaro et al. Citation2011; Pohorecka et al. Citation2012; Reetz et al. Citation2011; Rundlöf et al. Citation2015; Stewart et al. Citation2014) that reported 135 measures for concentrations of CTD in bee-relevant matrices from use of CTD-treated seeds (see SI). The result of the comparisons of exposures from bee-relevant matrices measured in these controlled field studies to toxicity values of CTD is shown in . There are multiple observations that have the same values for quality and relevance; these are separated for easier identification in the SI. The quality of some studies was better than others and the overall mean for quality was 2.71 ± SE of 0.8. The mean and SE for relevance was 0.36 ± 0.10, suggesting consistent lack of relevant effects in studies that were generally of high quality.
Figure 2. Quality and relevance of exposure values from controlled field studies with CTD-treated seeds. Symbols may obscure others, see SI for all responses, n = 135. There were no data points obscured by the legend. Points with a green border did not demonstrate adverse effects in field studies.
All but four (all from bee-collected pollen) of the reported concentrations of CTD in pollen (total = 68 bee-collected and also collected directly from plants) showed concentrations less than the NOAED for bees of 2.4 ng CTD/bee/d (Kasiotis et al. Citation2014; Krupke et al. Citation2012; Rundlöf et al. Citation2015). However, two of these studies in which higher concentrations were reported were problematic. In the one study (Krupke et al. Citation2012) with a score of 1.1 for quality of methods (QoM), concentrations of CTD in pollen collected by bees from maize before seeding an adjacent field with treated and untreated seeds were greater than those after seeding. This is counterintuitive and there is no obvious explanation other than differences in wind speed and wind direction, which were not reported. The concentrations of CTD measured by Kasiotis et al. (Citation2014) had a maximum value of 256 µg/kg. These values were not considered representative of typical exposure scenarios because the samples were not taken at random; the study focused on reported incidents from a survey of residues in pollen and honey from hives where deaths of bees had been reported in Greece (Kasiotis et al. 2014). The maximum value from Kasiotis et al. (2014) is equivalent to 3.07 ng/bee/day and it is unlikely that these residues in the pollen could have contributed to the reported bee loss. The probability that this peak concentration level would be sustained in combination with peak consumption rates for several days is very low. Estimated doses of CTD in pollen collected by bees from oil-seed rape flowers in Sweden were slightly greater than the NOAED (2.9 vs 2.4 ng/bee/day) but no effects on honeybees were reported (Rundlöf et al. Citation2015).
Of the concentrations of CTD measured in samples from 20 studies of guttation fluid, four of the estimated exposures were >NOAED (Bayer CropScience Citation2012, Citation2014a; Bayer CropScience AG Citation2009f, Citation2014g), suggest-ing possible adverse effects based on consumption of water from these sources only. Three of these studies (Bayer CropScience Citation2012, Citation2014a, Bayer CropScience AG Citation2014g) were part of larger studies where effects at the level of the colony were evaluated and adverse effects were not observed (shown in as points with a green border). The study by Reetz et al. (Citation2011) did not include an evaluation of effects; however, they did observe a rapid decline in concentrations over time. Overall, the lack of effects observed at the level of the colony, even in studies where large concentrations of CTD were observed in guttation fluids (such as Bayer CropScience AG Citation2009f) supports the conclusion that this is not a relevant route of exposure to CTD for honeybees.
None of the concentrations of CTD measured in honey or nectar (39 observations) would have resulted in doses above the chronic NOAED for honeybees. This is consistent with the lack of effects observed in field studies. Seven observations of concentrations of CTD in and/or on dead honeybees were reported but only two of these exceeded the NOAED (Bayer CropScience AG Citation2009f), a study in which adverse effects at the level of the colony were not reported (Bayer CropScience AG Citation2010d).
The overall weight of evidence for concentrations in bee-relevant matrices (honey, nectar, pollen, bee-bread, and guttation fluid) suggests that there is little or no risk for bees from exposure to CTD from its use as a seed treatment.
Risks to honeybees from exposures to CTD via deposition of dusts from treated seeds
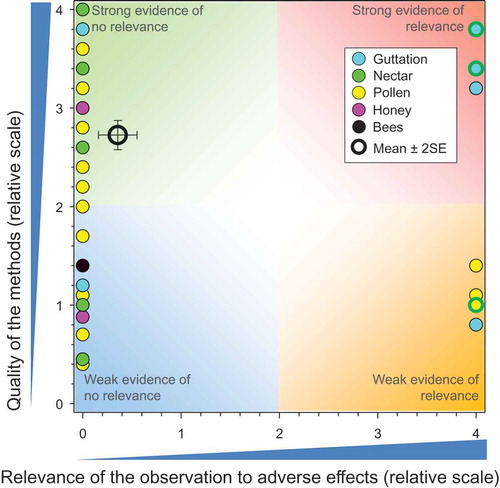
There were six studies on deposition of CTD from dust during sowing of seeds treated with CTD and these included 19 responses (see SI). These studies (Bayer CropScience AG Citation2005d, Citation2008c, Citation2008d, Citation2009h, Citation2009i; Biocca et al. Citation2015) () showed that the rates of deposition were smaller than the NOAER for adverse effects at the level of the colony.
Figure 3. Quality and relevance of exposure values from controlled field studies on deposition of dust during sowing of seeds treated with CTD, n = 19. Symbols may obscure others, see SI for all responses. There were no data points obscured by the legend.
In general, greater rates of deposition were observed for pneumatic seeders than mechanicals seeders. Two studies measured deposition of CTD on bees (Girolami et al. Citation2013, Citation2012) but the amounts of CTD in the body and on surface of the bee were not determined so it was not possible to compare these values to toxicity data. The QWoEs for these studies are presented in the SI but not included in .
As for seed treatments, the quality of some studies was better than others and the overall mean for score for QoM was 3.43 ± SE of 0.12. The mean and SE for relevance was 0 (n = 19). These results are consistent with the lack of effects observed in experimental field studies where bees were exposed to dust particles from treated seeds. One weaker study (Marzaro et al. Citation2011, SOM = 1.2) assessed concentrations of CTD in guttation fluid from plants exposed to dust during seeding of seeds treated with CTD and reported exposure concentrations less than the NOAED. Overall there is no evidence to suggest that biologically relevant exposures would result from dust released from seeders operated under conditions of good agricultural practice.
Experimental studies (higher tier semi-field and field)
The studies for which effects of CTD to honeybees were evaluated resulted from evaluation of different types of applications which included predominantly studies where seeds were treated with CTD and potential exposure of honeybees occurred while bees were foraging for pollen and nectar or guttation fluid from plants with systemic residues (Bayer AG Crop Protection Citation2000d, Citation2000b, Citation2000c, Citation2001e, Citation2001f, Citation2001h, Citation2001d; Bayer Corporation Citation2001; Bayer CropScience Citation2006, Citation2015d, Citation2015h, Citation2015g; Bayer CropScience AG Citation2009e, Citation2009j, Citation2014i; Cutler et al. Citation2014; Pohorecka et al. Citation2012, Citation2013; Schneider et al. Citation2012). Field or semi-field studies whereby bees were exposed via dietary amendments were also evaluated (Bayer AG Citation2001; Bayer AG Crop Protection Citation2001a, Citation2001b, Citation2001c; Bayer AG Crop Protection Citation2002a; Bayer CropScience Citation2003). A third group involved studies whereby CTD was applied as a foliar spray or seed treatment in combination with dust drift (Bayer CropScience AG Citation2011d, Citation2010e). A few studies incorporated exposure to soil residues of CTD from historical use patterns (Bayer CropScience Citation2015i; Bayer CropScience AG Citation2014j; Jones, Harrington, and Turnbull Citation2014). One controlled study combined exposure of honeybees in tents to formulated dust residues and to a formulated spray application in order to assess how these different routes of exposure would affect foraging honeybees (Bayer CropScience AG Citation2010e).
Effects on honeybees from exposures via crops grown from seeds treated with CTD
A total of 235 responses were included in the effects assessment; data from 33 studies were used to assess the relationship between quality and relevance of potential effects of CTD to honeybees exposed to systemic residues potentially in plants growing from seeds treated with the chemical. To mitigate the potential for a Type 2 error, studies in which honeybees were exposed to CTD in combination with other neonicotinoids were included only when exposure resulted in no effects. Most of the studies were intentionally treated field studies conducted under good laboratory practice guidelines. However, the quality of many of these was diminished by insufficient replication, failure to measure any detectable or quantifiable concentrations in field-collected matrices, failure to adequately characterize effects of exposures ≤20 µg/kg, or failure to apply statistical procedures to the data, presumably because there were no obvious effects observed. The score for QoM of the studies on treated-seed (including mixtures with CTD in combination with other neonicotinoids) was 1.87 ± SE of 0.06 and the values ranged from 0.5 to 3.4 ().
Figure 4. Strength and relevance of effects in honeybees exposed in controlled field studies via crops treated with CTD as seed dressings, n = 138. Symbols may obscure others, see SI for all responses. There were no data points obscured by the legend.
When data for mixtures were excluded, the QoM was similar at 1.78 ± SE of 0.06. The relevance of responses were similar regardless of whether the study was weak or of moderate quality because: essentially, most studies indicated that exposure of honeybees to plants that had been sown with seed treatments of CTD or CTD in combination with other neonicotinoids resulted in no adverse effects on a multitude of measurement endpoints that included mortality (adults, workers, drones, pupae, larvae, queen); colony strength (hive weights, number of workers, total number of adult bees, overwintering performance, food consumption rates etc.); colony development (queen development, brood development, numbers of eggs, larvae, capped cells, pupae, honey, nectar, and pollen stores etc.); colony health (infestation with Varroa mite, viruses, or disease); foraging intensity and activity; flight dynamics (intensity and activity); behavior (trembling, agitation, immobilization, incoordination, hyper- or hypo-responsiveness etc.); and hive productivity (hive weights, honey production etc.). Combinations of these metrics were generally measured over time (repeated measures) with multiple colony condition assessments (CCAs) conducted periodically (4 to 9 per study) for multi-seasonal studies.
Most of the earlier studies included in the QWoE were designed to assess exposure and, in these studies, honeybees were used as a tool to collect some of the exposure data. The effects data associated with these earlier studies were generally qualitative observations collected over relatively short periods of time. Since the observational metrics of the bees were incidental to the study rather than the direct focus, it is not surprising that these studies indicated that CTD did not present a risk to honeybees. Results of the more recent field and semi-field studies that have focused on the effects on honeybees indicate that the exposure concentrations in nectar, pollen, and guttation fluid were either insufficient to cause effects on apical endpoints or minimal exposure of bees was occurring. Small doses of CTD can likely be readily metabolized by honeybees and cleared from their bodies so exposure might be occurring with rapid recovery. Residues in honeybees ranged from 0 to 32 µg/kg which, for the most part, have been demonstrated to be below the threshold of effects (see SI with QWoE for exposure). CTD residues measured in dead honeybees associated with known poisonings were reported to be above the NOAED of 7.3 ng/bee/day (e.g., 9.6 ng/bee; (Bayer CropScience AG Citation2009f); however, bees were not rinsed before analysis and the residues in the body of the bee could not be estimated. The absence of effects and preponderance of no-effects was attributed, in part, to resilience that has evolved in the social system of honeybees where the colony functions as an “organism” with highly conserved traits (Henry et al. Citation2015). The QWoE (see SI) clearly demonstrates that environmentally realistic exposures to CTD result in no adverse effects to honeybees at the colony level of biological organization.
The major limitations of the field studies conducted to date is the lack of statistical rigor and power in the experimental designs because of insufficient independent replication and the prevalence of pseudo-replication in the experimental designs of these studies. Statistical procedures are available that can address complex issues that arise when dealing with multiple measures collected over time from multiple sites (independent) or reference plots and these tools were used in only a few (n = 3) studies. There currently are no standard methods for assessing impacts at higher tiers and such methods when applied appropriately would eliminate or reduce much of the uncertainty that arises when deciding if the effect is the result of the neonicotinoid or attributable to other non-contaminant variables.
Recent reviews examining the potential impacts of neonicotinoids, in particular CTD, on honeybees (Blacquière et al. Citation2012; Schneider et al. Citation2012) suggest that effects on individual honeybee behavior (i.e., feeding and foraging behaviors) might be occurring but there is no quantifiable consequence to the function of the colony. Most studies assessed herein demonstrated a low relevance associated with these endpoints. Very recent research has demonstrated that neonicotinoids might function as an immunosuppressant with the implication that exposure of honeybees to these chemicals renders them more susceptible to pests and pathogens (Di Prisco et al. Citation2013). Neither of these adverse effects have been observed in field studies; however, the molecular mechanisms have been proposed and demonstrated.
Effects on honeybees from exposures via crops grown from seeds treated with CTD in combination with other neonicotinoids
A total of five studies with 32 measurement endpoints were included in the effects assessment for exposure to CTD in combination with other neonicotinoids. These data were used to assess the relationship between quality and relevance of potential effects of CTD to honeybees exposed while foraging on plants grown from CTD-treated seed. The mean score for quality of these studies (2.28 ± SE of 0.18; ) was, on average, higher than the mean score quality of the seed-treatment studies (). Inclusion of the data for CTD in mixtures assumed that, if the mixture was not toxic to honeybees, then the concentration of the CTD in the mixture was also non-toxic.
Figure 5. Quality and relevance of effects observed in honeybees exposed in controlled field studies to seeds treated with CTD and other neonicotinoids, n = 32. Symbols may obscure others, see SI for all responses. There were no data points obscured by the legend.
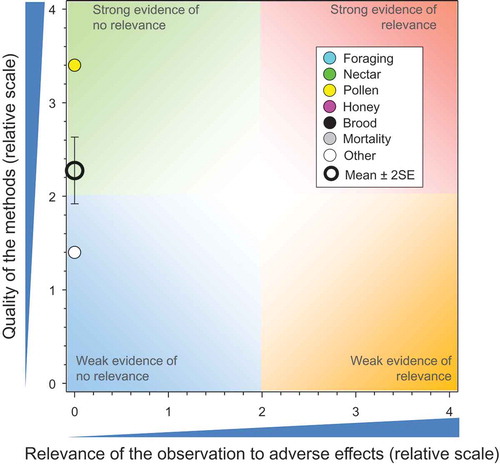
The seed treated with CTD in combination with other neonicotinoids included two studies with winter barley, two with sugar beet, and one with winter wheat. For these studies (Bayer CropScience Citation2012; Citation2014a; Bayer CropScience AG Citation2014a, Citation2014g; Bayer CropScience Citation2014d), the concentration in guttation fluid of plants grown from treated-seed was measured. Most concentrations of CTD were either below the level of detection (LOD) or the LOQ. Of the 29 comparisons (n > 1000 samples) for which concentrations in guttation fluid were >LOD, and using either the 90th centile or median, only four had concentrations of CTD that might have resulted in exposures above the NOAED of 7.3 ng/bee/d for honeybees. The 90th centile concentrations of CTD in the guttation fluid for these four comparisons ranged from 1,063 to 11,300 µg/L or the equivalent of 9 to 90 ng/bee (see SI for QWoEs for exposure). The relevance score for effects in all comparisons was low (e.g., equal to zero) because there were no adverse effects associated with the 32 measurement endpoints used in this assessment.
When these effects data were combined with the data for CTD-treated seeds, only 1 of 170 measurement endpoints was greater than 0 for relevance. Clearly, the risk to honeybees from exposure to CTD in plants grown from seed treated either as a single chemical or as a component in a nicotinoid mixture is minimal.
Effects on honeybees resulting from exposures to dust during sowing of seeds treated with CTD
Other forms of application potentially resulting in exposure of honeybees included application of the chemical as residues in dust formulated to mimic that which would arise during seeding of CTD-treated maize seed in combination with either foliar application (Bayer CropScience AG Citation2010e) or CTD-treated seed (Bayer CropScience AG Citation2011d). Two studies examined the potential effects in honeybees exposed via drift of dust with residues during and immediately following sowing of treated seed. The impact of dust residues on honeybees either foraging and flying in adjacent fields with flowering plants or in tunnels with the spray regime mimicked (Bayer CropScience Citation2015a) indicated that there were no persistent impacts on honeybees. For the first study, mortality in the dead bee traps (DBTs) was significantly higher for all treatments with abraded dust (p ≤ 0.001) but not for the spray application (Bayer CropScience AG Citation2010e). Similar impacts were observed for foraging activity. In the post-exposure phase, there were no significant differences (p = 0.115) observed between the CTD-treated and control groups for either foraging or mortality. For the second study, when comparing the performance of the honeybees in the treatment group with that in the control group, there were neither distinct differences in mortality of honeybees, as determined by daily emptying of dead bee traps throughout three consecutive weeks after maize sowing, nor distinct differences in strength and development of the colony, as determined from regular assessments. The observed overwintering performance in the CTD treatment and control, respectively, was similar and within the normal range of that for a long winter, with no distinct differences between treatment and control (Bayer CropScience AG Citation2011d).
The QoM for the studies on exposure via dust was 2.52 ± SE of 0.27 (n = 13), which was higher than that for the other exposure pathways, a likely reflection of greater rigor in experimental design and analyses or in reporting of the data and information. Because there was no persistent adverse impact on apical endpoints, the relevance of the responses remained low ().
Ecoepidemiology studies (higher tier observational studies) of effects of CTD on honeybees in the field
Seven higher-tier observational studies were conducted in the field with CTD in honeybees. Most of the studies involving CTD were conducted in response to incident reports of honeybee colony deaths. The nature of the data collection and analyses provided qualitative observations that were challenging to quantify. Therefore, the approach taken for the above effects assessment could not consistently be applied. That said, some studies were evaluated using the established criteria for the ecoepidemiology assessment (SI Table 3). Only three of the six studies (Chauzat et al. Citation2010; Pohorecka et al. Citation2012; Rundlöf et al. Citation2015) were scored and the QoMs were either relatively weak (0.3) or of good quality (2.4, 2.6), respectively. The former was based on a three-page summary of residue analyses of samples collected haphazardly; the uncertainty regarding data quality was high. One of the studies associated with the good QoMs (Pohorecka et al. Citation2012) demonstrated no observed adverse effects of CTD to honeybee mortality, brood development, colony strength or health, or honey yield. This was not surprising given that no exposure matrices (e.g., bee bread, nectar collected by bees, nectar from plants, hive-honey or hive-pollen) had measured residues of CTD >NOAED for adult honeybees of 7.3 ng/bee/d. The second paper with a good QoM (Rundlöf et al. Citation2015) reported no adverse effects on numbers of honeybees in hives located near and foraging on pollen from oilseed rape grown from seeds treated with CTD.
Two studies examined the potential relationship between the growing of various varieties of CTD-treated seed for different crops and reported damage to colonies of honeybees on a regional scale (Bayer CropScience AG Citation2008a, Citation2008b). For the Rhine Valley region, correlation analyses using reported sales of treated seed and data from reports of damage to honeybee colonies suggested that this damage was associated with the use of specific varieties of CTD-treated seed. Further investigation involving the application of abrasion testing demonstrated that, for the three varieties implicated in colony deaths, the adhesion quality of the treatment was poor. It was thought that the poor adhesion resulted in the inadvertent exposure of honeybees to dust with CTD residues. Subsequently, the adhesion of CTD to seed has improved and, when coupled with improved sowing methods designed to mitigate release of dust, the incidence of damage attributed to dust exposure has diminished. Additionally, Liebig, Kustermann, and De Craigher (Citation2008) concluded that the initial impact of CTD on honeybee colonies exposed during sowing of CTD-treated maize was mitigated within a relatively brief period of time which would explain, in part, why adverse effects to apical endpoints are rarely observed in field studies. For the Lake Constance region, the number and extent of damage to bees was insufficient to conduct a similar analysis. There was only one incident of damage to honeybee colonies and the seed variety linked to high abrasion was grown in the municipality where the damage was reported.
Overall, superior quality ecoepidemiology studies are lacking for CTD. The weight of evidence does not support a causal relationship between exposure to CTD and adverse effects in bees because there is no consistent weight of evidence for ecoepidemiology. Even the data linking crop varieties with seeds that have a high abrasion index with honeybee colony damage are regionally inconsistent, suggesting that other confounding variables might be wholly or partially responsible.
Conclusions
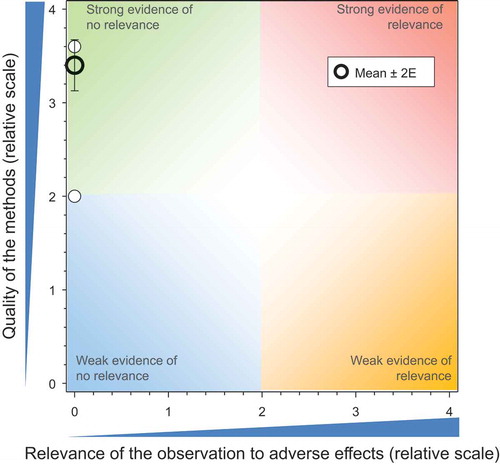
The overlay of the mean and 2 x SE values from the above five major groupings of studies () provides an overall summary of the weights of evidence of all the studies included in this assessment. These results indicate that that CDT, as currently used in good agricultural practices as a seed-treatment, does not present a significant risk to honeybees at the level of the colony.
Figure 7. Overview of the conclusions of the QWoE for the effects of CTD on honeybees at the level of the colony.
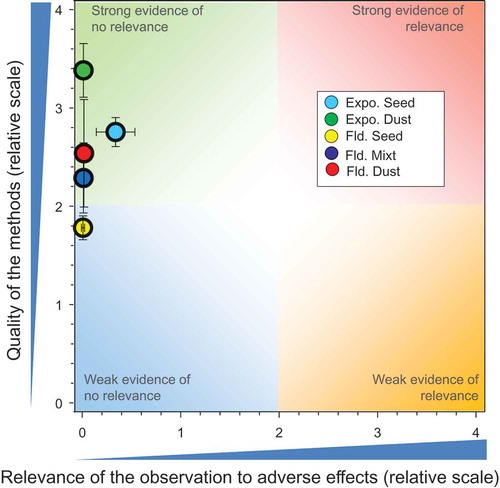
As pointed out above, the effects estimated from exposure of honeybees to CTD via nectar, guttation fluid, and pollen are based on a conservative assumption that honeybees are exposed exclusively to these sources and obtain all food via these routes. All the field studies where honeybees were exposed under more realistic conditions, whether from treated seed, artificial diet, or other routes of exposure, show very little relevance for adverse effects on the colony. Unfortunately, the ecoepidemiology studies contributed little to reducing the uncertainty associated with potential adverse effects that might occur under realistic use conditions where good agricultural practices further mitigate against adverse effects.
Quality of the studies in the QWoE assessments was variable but the results of the studies are consistent and point to the same conclusion. The overall weight of evidence based on many studies thus does not falsify the main null hypothesis being tested, i.e., that CTD has no negative impacts on colony viability and survival of the colony.
Declaration of interest
No potential conflicts of interest were reported by the authors.
Paper-3-CTD-WoE-SI-2017-07-29.pdf
Download PDF (6.3 MB)Acknowledgments
The authors wish to thank Ms. Jennifer Miller (Miller Environmental Science Inc.) for QA services and Mr. Spencer West for assistance with literature searches and obtaining the references and associated SI. The authors wish to thank Bayer Crop Science for providing access to the unpublished reports and for funding the study. The decision to publish this assessment was the authors’ and they were solely responsible for the content and the opinions herein. We are indebted to Dr EJ Marshall and Prof Keith Walters for editing these papers and to the reviewers for the helpful and constructive comments.
Supplemental data
Supplemental data for this article can be accessed here.
References
- [NWQMC] National Water Quality Monitoring Council. 2016. Water Quality Data. NWQMC. Accessed January 2016. http://waterqualitydata.us/portal/.
- [OMECC] Ontario Ministry of Environment and Climate Change. 2017. Stream neonicotinoid monitoring study OMECC. Accessed January 25, 2017. https://www.ontario.ca/search/data-catalogue?sort=asc&query=Neonicotinoid.
- [PubChem] PubChem. 2015. Clothianidin. PubChem. Accessed November 2015. http://pubchem.ncbi.nlm.nih.gov/compound/Clothianidin#section=Top.
- Bayer AG. 2001. Effects of TI 435 techn. Residues in pollen on the development of small bee colonies and on behavior and mortality of honey bees. Report R-318040-01-1, Bayer AG (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2000a. Residues of TI-435 in nectar, blossoms, pollen and honey bees sampled from a summer rape field in Sweden and effects of these residues on foraging honeybees. Report M-027785-01-1. Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2000b. Residues of TI-435 in nectar, blossoms, pollen and honey bees sampled from a british summer rape field and effects of these residues on foraging honeybees. Report M-023456-01-1, Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2000c. Residues of TI-435 in nectar, blossoms, pollen and honey bees sampled from a summer rape field in sweden and effects of these residues on foraging honeybees. Report M-027785-01-1, BAYER AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2000d. Residues of TI-435 in nectar, blossoms, pollen and honey bees sampled from a French summer rape field and effects of these residues on foraging honeybees. Report M-023191-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001a. Effects of TI 435 techn. Residues in sunflower honey on the development of small bee colonies and on behavior and mortality of honey bees. Report M-031695-01-1, Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001b. Effects of Diet (sugar solution) Spiked with TI 435 techn. on behavior and mortality of honey bees (Apis mellifera) and on the weight development of bee colonies under field conditions. Report M-031717-01-1, Bayer AG Crop Protection (Unpublihed Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001c. Effects of diet (sugar solution) spiked with the TI 435 metabolite TZMU on behavior and mortality of honey bees (Apis mellifera) and on the weight development of bee colonies under field conditions. Report M-031721-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001d. Residue levels of TI 435 FS 600 and its relevant metabolites in nectar, blossoms and pollen of sunflowers from dressed seeds and effects of these residues on foraging honeybees. Test Location: Farmland “Höfchen”. Report M-031715-01-1, Bayer AG Crop Protection (unpublished report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001e. Residue levels of TI 435 FS 600 and its relevant metabolites in nectar, blossoms and pollen of summer rape from dressed seeds and effects of these residues on foraging honeybees. Test location: Farmland “Laacher Hof’. Report M-031706-01-1, Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001f. Residue Levels of TI 435 FS 600 and its relevant metabolites in nectar, blossoms and pollen of sunflowers from dressed seeds and effects of these residues on foraging honeybees. Test location: “Laacher Hof”. Report M-031709-01-1, Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001g. Residue Levels of TI 435 FS 600 and its relevant metabolites in pollen of maize plants from dressed seeds. Test location: Farmland “Höfchen”. Report M-032379-01-1, Bayer AG Crop Protection (Unpublished Report) Leverkusen, Germany.
- Bayer AG Crop Protection. 2001h. Residue Levels of TI 435 FS 600 and its Relevant metabolites in nectar, blossoms and pollen of summer rape from dressed seeds and effects of these residues on foraging honeybees. Test Location: Farmland “Höfchen”. Report M-031711-01-1, Bayer AG Crop Protection (unpublished reports), Leverkusen, Germany.
- Bayer AG Crop Protection. 2001i. Residue Levels of TI 435 FS 600 and its relevant metabolites in pollen of maize plants from dressed seeds. Test location: Farmland “Laacher Hof”. Report M-032370-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2002a. Evaluation of the effects of residues of Tl 435 in Maize pollen from dressed seeds on honeybees (Apis mellifera) in the Semifield. Appendix XIII Containing Data from Report MR-581/01. Report M-043264-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2002b. Determination of the residue levels of Tl 435 and its relevant metabolites in nectar and pollen of winter rape from dressed seeds. Test location: Farmland “Laacher Hof”. Report M-058143-01-1, Bayer AG Crop Protection (Unpublished Report) Leverkusen, Germany.
- Bayer AG Crop Protection. 2002c. Residue levels of TI435 and its relevant metabolites in pollen of maize plants from dressed seeds, test location farmland “Höfchen”. Report M-067021-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer AG Crop Protection. 2002d. Residue levels of tl 435 and its relevant metabolites in pollen of maize plants from dressed seeds. Test location: Farmland “Laacher Hof”. Report M-066621-01-1, Bayer AG Crop Protection (Unpublished Report), Leverkusen, Germany.
- Bayer Corporation. 2001. The impact of GAUCHO® and TI-435 seed-treated canola on honey bees, Apis mellifera L. Guelph, Canada, St. Paul, United States. Report M-084721-01-1, University of Guelph (Biological), University of Minnesota (Biological), Enviro-Test Laboratories (Analytical) (Unpublished Report), Edmonton, Canada.
- Bayer CropScience. 2001. Effects of TI 435 techn. Residues in Pollen on the Development of Small Bee Colonies and on Behavior and Mortality of Honey Bees. Report M-031689-03-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2003. Effects of diet (sugar solution) spiked with the clothianidin metabolite TZMU on behaviour and mortality of honeybees (Apis mellifera) and on the weight development of bee colonies under field conditions. Report M-076068-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2006. An investigation of the potential long-term impact of clothianidin seed treated canola on honey bees, Apis mellifera L. Report M-289165-01-2, Bayer CropScience (Unpublished Report), Guelph, Canada.
- Bayer CropScience. 2012. Final Report (Non-GLP) field study to monitor potential effects on honey bees from exposure to guttation fluid of Winter Barley (W-BAR), seed-treated either with an imidacloprid or a clothianidin combi-product. Report M-498922-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2014a. Field study to monitor potential effects on honey bees from exposure to guttation fluid of Winter Barley (W-BAR), seed-treated with the insecticidal seed-treatment product clothianidin + imidacloprid FS 100 + 175 G in Germany in 2011/2012. Report M-501261-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2014b. Final report assessment of potential impacts on honeybee colony development, their hibernation performance and concurrent monitoring of aerial dust drift during the sowing operation of poncho beta plus - treated sugar beet pills with typical commercial vacuum-pneumatic sowing technology, directly adjacent to full-flowering Phacelia tanacetifolia in Germany. Report M-504065-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2014c. Clothianidin plant bioavailability and soil accumulation study. Report M-498438-01-1, Bayer CropScience (Unpublished Report), Research Triangle Park, North Carolina, USA.
- Bayer CropScience. 2014d. A long-term field study to monitor potential effects on the honeybee (Apis mellifera L.) from exposure to guttation fluid of sugar beets, seed-treated with the insecticides clothianidin + imidacloprid + beta-cyfluthrin in Southern Germany in 2013 and 2014. Report M-500724-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015a. Assessment of potential impacts on honeybee colony development, their hibernation performance and concurrent monitoring of aerial dust drift during the sowing operation of imidacloprid FS 350A G - treated winter barley with typical commercial pneumatic sowing technology, directly adjacent to full-flowering phacelia tanacetifolia in United Kingdom. Report M-504522-02-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015b. Determination of residues of imidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of cotton after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or foliar application with Provado® 200 SC (Imidacloprid 200 SC) in a Semi-Field Study in Brazil. Report M-525745-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015c. Determination of residues of imidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of soybean after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or Foliar application with Connect® Imidacloprid & Beta-Cyfluthrin 112.5 SC) in a Semi-Field Study in Brazil. Report M-525757-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015d. Assessment of side effects of maize grown from seeds treated with clothianidin FS 600B G on the Honeybee (Apis mellifera L.) in a long-term field study in Aquitaine (France). Report M-533855-02-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015e. Determination of residues of imidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of cotton after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or Foliar Application with Provado® 200 SC (Imidacloprid 200 SC) in a Semi-Field Study in Brazil. Report M-525732-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015f. Assessment of side effects of clothianidin FS 600B G treated maize seed on the honeybee (Apis mellifera L.) in a long-term field study in Languedoc-Roussillon (France). Report M-347416-03-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015g. Assessment of Side Effects of Clothianidin FS 600B G treated maize seed on the honeybee (Apis mellifera L.) in a long-term field study in Champagne (France). Report M-347482-03-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015h. Assessment of Side Effects of Clothianidin FS 600B G treated maize seed on the honeybee (Apis mellifera L.) in a Long-Term Field Study in Alsace (France). Report M-347491-03-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015i. Determination of clothianidin residues in bee relevant matrices, collected in a succeeding crop scenario with natural aged clothianidin residues – Field phase conducted with phacelia and maize in the UK (Goole, East Yorkshire). Report M-504590-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015j. Determination of the residues of clothianidin in bee relevant matrices collected from succeeding crops following application of CLOTHIANIDIN FS 600B G via soil incorporation to plateau concentration and sowing of clothianidin-treated winter barley seeds. Field phase conducted in Southern France. Report M-504814-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience. 2015k. Residues of clothianidin in nectar and pollen of flowering rotational crops in Western Germany. Report M-504884-01-1, Bayer CropScience (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2003. Effects of TI 435 techn. residues in pollen on the development of small bee colonies and on behavior and mortality of honey bees. Report R-319745-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2005a. Determination of residue levels of clothianidin, TZMU and TZNG in maize pollen in a succeeding crop scenario at bayer cropscience AG experimental farm “Laacher Hof”, Germany. Report M-256474-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2005b. Determination of residue levels of clothianidin, TZMU and TZNG in bee-relevant matrices of summer rape in a succeeding crop scenario at bayer cropscience AG experimental farm “Höfchen”, Germany. Report M-256718-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2005c. Determination of residue levels of clothianidin, TZMU and TZNG in maize pollen in a succeeding crop scenario at bayer cropscience AG experimental farm “Höfchen”, Germany. Report M-256564-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2005d. Deposition of clothianidin, emitted during sowing of dressed maize seeds with pneumatic sowing machines. Report M-256656-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2007a. Determination of residue levels of clothianidin, TZMU and TZNG in bee-relevant matricies of winter rape in a cereal succeeding crop scenario at bayer cropscience AG experimental farm “Laacher Hof”, Germany. Report M-291947-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2007b. Determination of residue levels of clothianidin, TMZU, And TZNG in bee-relevant matrices of winter rape in a cereal succeeding crop scenario at bayer cropscience AG experimental farm “Höfchen”, Germany. Report M-291950-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2008a. Addendum to Report MEF-08/453 - Investigations on the relationship between bee damage reports in the lake constance region in 2008 and the use of PonchoPro in maize seed treatment. Report M-309949-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2008b. Investigations on the relationship between bee damage reports in the Upper Rhine Valley in 2008 and the use of PonchoPro in maize seed treatment. Report M-309211-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2008c. Monitoring of dust drift deposits during and after the Drilling of Oilseed Rape (OSR), Dressed with ELADO® in Germany. Report M-313052-01-1, Bayer CropScience AG (Unpublished Report), Heidelberg, Germany.
- Bayer CropScience AG. 2008d. Drift deposition pattern of seed treatment particles abraded from Smaragd Forte® Dressed barley seeds and emitted by a typical pneumatic and mechanical sowing machine. Report M-311825-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2008e. Determination of the residues of clothianidin and its metabolites TZMU and TZNG in pollen, harvested from maize plants, grown in commercial practice from Poncho Pro® Dressed Seeds (Nominally 1.25 mg Clothianidin/Seed) in the Upper Rhine Valley in Germany. Analytical Phase Report of Report M-309823-02-1. Report M-309799-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2009a. Determination of residue levels of clothianidin and its metabolites TZMU and TZNG in pollen harvested from maize plants grown in commercial practice from Poncho Pro® Dressed Seeds (Nominally 1.25 mg Clothianidin/Seed) in the Upper Rhine Valley in Germany. Report M-309823-02-1, Bayer CropScience AG (Unpublished Report), Heidelberg, Germany.
- Bayer CropScience AG. 2009b. Clothianidin FS 600B G: A residue study with Clothianidin FS 600B G treated maize seed, investigating residues in crop, soil and honeybee products in Champagne (France). Niefern-Öschelbronn, Germany, Bayer CropScience AG (Unpublished Report). Report M-347748-01-1.
- Bayer CropScience AG. 2009c. Monitoring of dust drift deposits during the sowing of maize seeds, treated with Poncho® (Clothianidin FS 600) on bee health study plots in France. Report M-352132-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2009d. Assessment of side effects of clothianidin FS 600B G treated maize seed on the honeybee {Apis mellifera L.) in a long-term field study in Languedoc-Roussillon (France). Niefern-Öschelbronn, Germany, Bayer CropScience AG (Unpublished Report). Report M-351028-01-1.
- Bayer CropScience AG. 2009e. Assessment of side effects of clothianidin FS 600B G treated maize seed on the honeybee (Apis mellifera L.) in a long-term field study in Languedoc-Roussillon (France). Report M-347416-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Oschelbronn, Germany.
- Bayer CropScience AG. 2009f. Guttation monitoring of maize seedlings under agronomic use conditions in Austria and assessment of the relevance of guttation for honeybees. Report M-355004-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2009g. Clothianidin FS 600B G: A residue study with clothianidin FS 600B G treated maize seed, investigating residues in crop, soil and honeybee products in Languedoc-Roussillon (France), Interim report. Report M-347742-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Öschelbronn, Germany.
- Bayer CropScience AG. 2009h. Drift deposition pattern of seed treatment particles abraded from Elado® Dressed OSR seeds and emitted by a typical pneumatic and mechanical sowing machine, amendment no. 1 to report NAX/SP04-2008. Report M-311822-03-1, Monheim, Germany, Bayer CropScience AG (Unpublished Report).
- Bayer CropScience AG. 2009i. Monitoring of dust drift deposits during the sowing of maize seeds, treated with Poncho® (Clothianidin FS 600) on bee health study plots in France. Report M-355589-01-1, Bayer CropScience AG (Unpublished Report), Monheim am Rhein, Germany.
- Bayer CropScience AG. 2009j. Assessment of Side Effects of Clothianidin FS 600B G Treated Maize Seed on the Honeybees (Apis mellifera L.) in a Long-Term Field Study in Alsace (France). Report M-347491-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Oschelbronn, Germany.
- Bayer CropScience AG. 2009k. Clothianidin FS 600B G: A residue study with clothianidin FS 600B G treated maize seed, investigating residues in crop, soil and honeybee products in Alsace (France). Niefern-Öschelbronn, Germany, Bayer CropScience AG (Unpublished Report). Report M-347727-01-1.
- Bayer CropScience AG. 2010a. Determination of Residues of Clothianidin and Imidacloprid and their Metabolites in Melon Following an Application of Clothianidin & Imidacloprid WS 56.25 + 18.75 as Seed Treatment. Report M-361798-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Öschelbronn, Germany.
- Bayer CropScience AG. 2010b. Determination of residues of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of spring rape at Bayer CropScience AG Experimental Farm “Höfchen”, Germany. Report M-397561-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2010c. Assessment of potential impacts on honeybee colony development and monitoring of aerial dust drift during the sowing of clothianidin fs 600 treated maize seeds with modified seeding technology directly adjacent to full-flowering winter oil seed rape in Austria Amendment No. 2 to Report. Report M-393055-03-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2010d. Field survey on guttation of maize seedlings under agronomic use conditions in Austria and assessment of the relevance of guttation fluid for honeybees. Report M-355018-03-2, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2010e. Exposure of bees under semi-field conditions to dust abraded from maize seeds dressed with the seed dressing product Clothianidin FS 600 and to Clothianidin FS 600 when applied as liquid formulation to flowering Phacelia crop. Report M-362814-01-1, Bayer CropScience AG (Unpublished Report), Amsterdam, The Netherlands.
- Bayer CropScience AG. 2010f. Determination of residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of winter rape at Bayer CropScience AG Experimental Farm “Laacher Hof”, Germany. Report M-397570-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2011a. Determination of the residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of winter rape at Bayer CropScience AG Experimental Farm “Laacher Hof”, Germany. Report M-412082-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2011b. Determination of residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of spring rape at Bayer CropScience AG experimental farm “Laacher Hof”, Germany. Report M-400327-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2011c. Determination of residues of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of winter rape at bayer cropscience AG experimental farm “höfchen”, germany amendment no. 1 to analytical phase report. Report M-400814-02-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2011d. Assessment of potential impacts on honey bee colony development and monitoring of aerial dust drift during the sowing of clothianidin FS 600 treated maize seeds with modified seeding technology directly adjacent to full- flowering winter oil-seed rape in Austria. Report M-394653-02-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2012a. Determination of the residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of winter rape at Bayer CropScience AG Experimental Farm “Höfchen”, Germany. Report M-421561-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2012b. Determination of the residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of spring rape at Bayer CropScience AG Experimental Farm “Höfchen”, Germany. Report M-421571-01-1, Bayer CropScience AG (Unpublished Report), Monheim am Rhein, Germany.
- Bayer CropScience AG. 2012c. Determination of the residue levels of clothianidin, TZNG and TZMU in bee relevant matrices of two different varieties of spring rape at Bayer CropScience AG Experimental Farm “Laacher Hof”, Germany. Report M-421580-01-2, Bayer CropScience AG (Unpublished Report), Monheim am Rein, Germany.
- Bayer CropScience AG. 2014a. Assessment of potential impacts on honeybee colony development, their hibernation performance and concurrent monitoring of aerial dust drift during the sowing operation of poncho beta plus - treated sugar beet pills with typical commercial Vacuum-Pneumatic sowing technology, directly adjacent to full-flowering Phacelia tanacetifolia in Germany. Report M-504065-01-2, Bayer CropScience AG (Unpublished Report), Hirschberg, Germany.
- Bayer CropScience AG. 2014b. Large-scale monitoring of long-term effects of Elado (10 g Clothianidin & 2 g Beta-Cyfluthrin/kg seed) dressed oilseed rape on pollinating insects in Mecklenburg-Vorpommern, Germany: VI residues of clothianidin in nectar and pollen collected by honey bees in tunnel tents. Report M-504416-01-1, Bayer CropScience AG (Unpublished Report), Leverkusen, Germany.
- Bayer CropScience AG. 2014c. Large-scale monitoring of long-term effects of elado (10 g clothianidin & 2 g beta-cyfluthrin/kg seed) dressed oilseed rape on pollinating insects in Mecklenburg-Vorpommern, Germany: V seed characterisation, drilling and growth of oilseed rape. Report M-504076-01-1, Bayer CropScience AG (Unpublished Report), Leverkusen, Germany.
- Bayer CropScience AG. 2014d. A long-term field study to monitor potential effects on the honeybee (Apis mellifera L.) from exposure to guttation fluid of sugar beets, seed-treated with the insecticides Clothianidin + Imidacloprid + Beta-Cyfluthrin in Southern Germany in 2013 and 2014. Report M-500734-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2014e. Large-scale monitoring of long-term effects of Elado (10 g Clothianidin & 2 g Beta-Cyfluthrin/kg seed) dressed oilseed rape on pollinating insects in Mecklenburg-Vorpommern, Germany: IV residues of clothianidin in soil before drilling and soil characterisation. Report M-503397-01-1, Bayer CropScience AG (Unpublished Report), Leverkusen, Germany.
- Bayer CropScience AG. 2014f. Large-scale monitoring of long-term effects of Elado (10 g Clothianidin & 2 g Beta-Cyfluthrin/kg seed) dressed oilseed rape on pollinating insects in Mecklenburg-Vorpommern, Germany: II Project area and study fields characterisation. Report M-503370-01-1, Bayer CropScience AG (Unpublished Report), Monheim am Rhein, Germany.
- Bayer CropScience AG. 2014g. Field study to monitor potential effects on honey bees from exposure to guttation fluid of winter wheat (W-WHT), seed-treated either with an imidacloprid or a clothianidin combi-product. Report M-498939-01-1, Bayer CropScience AG (Unpublished Report), Monheim, Germany.
- Bayer CropScience AG. 2014h. Residues of clothianidin in nectar and pollen of flowering rotational crops in Western Germany. Report M-501707-01-1, Bayer CropScience AG (Unpublished Report), Leverkusen, Germany.
- Bayer CropScience AG. 2014i. Large-scale monitoring of long-term effects of Elado (10 g Clothianidin & 2 g Beta-Cyfluthrin/kg seed) dressed oilseed rape on pollinating insects in Mecklenburg-Vorpommern, Germany: VII Effects on honey bees (Apis mellifera). Report M-503572-01-1, Bayer CropScience AG (Unpublished Report), Leverkusen, Germany.
- Bayer CropScience AG. 2014j. Determination of Clothianidin residues in bee relevant matrices, collected in a succeeding crop scenario with natural aged clothianidin residues - Field phase conducted with Phacelia and Maize in the UK (Goole, East Yorkshire). Report M-502462-01-1, Bayer CropScience AG (Unpublished Report), North Yorkshire, UK.
- Bayer CropScience AG. 2015a. 1Determination of residues of lmidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of soybean after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or Foliar Application with Connect® (Imidacloprid & Beta-Cyfluthrin 112.5 SC) in a semi-field study in Brazil. Report M-515291-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Oeschelbronn, Germany.
- Bayer CropScience AG. 2015b. Determination of residues of lmidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of cotton after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or Foliar Application with Provado® 200 SC (Imidacloprid 200 SC) in a semi-field study in Brazil. Report M-519517-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Oeschelbronn, Germany.
- Bayer CropScience AG. 2015c. Determination of residues of imidacloprid and clothianidin in flowers, leaves, soil, nectar and pollen of cotton after seed treatment with Gaucho® FS (Imidacloprid 600 FS) or Poncho® (Clothianidin 600 FS), or Foliar Application with Provado® 200 SC (Imidacloprid 200 SC) in a semi-field study in Brazil. Report M-512861-01-1, Bayer CropScience AG (Unpublished Report), Niefern-Oeschelbronn, Germany.
- Bayer CropScience L. 2014e. Clothianidin – overview of accumulation in soil and bioavailability for uptake into crops, pollen and nectar. Report M-497930-01-1, Bayer CropScience LP (Unpublished Report), Research Triangle Park, NC, USA.
- Biocca, M., R. Fanigliulo, P. Gallo, P. Pulcini, and D. Pochi. 2015. The assessment of dust drift from pneumatic drills using static tests and in-field validation. Crop Protection 71:109–15. doi:10.1016/j.cropro.2015.02.006.
- Blacquière, T., G. Smagghe, CAM Van Gestel, and V. Mommaerts. 2012. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 21:973–92. doi:10.1007/s10646-012-0863-x.
- Botias, C., A. David, J. Horwood, A. Abdul-Sada, E. Nicholls, E. Hill, and D. Goulson. 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environmental Science & Technology 49:12731–40. doi:10.1021/acs.est.5b03459.
- Chauzat, M.-P., A.-C. Martel, P. Blanchard, M.-C. Clément, F. Schurr, C. Lair, M. Ribière, K. Klaus, P. Rosenkranz, and J.-P. Faucon. 2010. A case report of a honey bee colony poisoning incident in France. Journal of Apicultural Research 49:113–15. doi:10.3896/IBRA.1.49.1.22.
- Cutler, G. C., and C. D. Scott-Dupree. 2007. Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. Journal of Economic Entomology 100:765–72. doi:10.1603/0022-0493(2007)100[765:ETCSCH]2.0.CO;2.
- Cutler, G. C., C. D. Scott-Dupree, M. Sultan, A. D. McFarlane, and L. Brewer. 2014. A large-scale field study examining effects of exposure to clothianidin seed-treated canola on honey bee colony health, development, and overwintering success. Peer Journal 2:e652. doi:10.7717/peerj.652.
- Di Prisco, G., V. Cavaliere, D. Annoscia, P. Varricchio, E. Caprio, F. Nazzi, G. Gargiulo, and F. Pennacchio. 2013. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences of the USA 110:18466–71. doi:10.1073/pnas.1314923110.
- EFSA. 2013. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance clothianidin. EFSA Journal 11–3066:58.
- Fairbrother, A., J. Purdy, T. Anderson, and R. Fell. 2014. Risks of neonicotinoid insecticides to honeybees. Environmental Toxicology and Chemistry 33:719–31.
- Girolami, V., M. Marzaro, L. Vivan, L. Mazzon, C. Giorio, D. Marton, and A. Tapparo. 2013. Aerial powdering of bees inside mobile cages and the extent of neonicotinoid cloud surrounding corn drillers. Journal of Applied Entomology 137:35–44. doi:10.1111/jen.2012.137.issue-1-2.
- Girolami, V., M. Marzaro, L. Vivan, L. Mazzon, M. Greatti, C. Giorio, D. Marton, and A. Tapparo. 2012. Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. Journal of Applied Entomology 136:17–26. doi:10.1111/jen.2011.136.issue-1-2.
- Henry, M., N. Cerrutti, P. Aupinel, A. Decourtye, M. Gayrard, J.-F. Odoux, A. Pissard, C. Rüger, and V. Bretagnolle. 2015. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proceedings of the Royal Society B: Biological Sciences 282:20152110. doi:10.1098/rspb.2015.2110.
- Jeschke, P., R. Nauen, M. Schindler, and A. Elbert. 2011. Overview of the status and global strategy for neonicotinoids. Journal of Agricultural and Food Chemistry 59:2897–908. doi:10.1021/jf101303g.
- Jones, A., P. Harrington, and G. Turnbull. 2014. Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Management Science 70:1780–84. doi:10.1002/ps.2014.70.issue-12.
- Kasiotis, K. M., C. Anagnostopoulos, P. Anastasiadou, and K. Machera. 2014. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Science of the Total Environment 485–486:633–42. doi:10.1016/j.scitotenv.2014.03.042.
- Krupke, C. H., G. J. Hunt, B. D. Eitzer, G. Andino, and, K. Given. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7:e29268. doi:10.1371/journal.pone.0029268.
- Kujawski, M. W., and J. Namieśnik. 2011. Levels of 13 multi-class pesticide residues in Polish honeys determined by LC-ESI-MS/MS. Food Control 22:914–19. doi:10.1016/j.foodcont.2010.11.024.
- Liebig, G., T. Kustermann, and D. De Craigher 2008. Bee monitoring during and after the 2008 maize flowering season in the Rhine Valley. Report M-328613-01-4, Germany Universitat Hohenheim (Unpublished Report), Stuttgart.
- Main, A. R., J. Fehr, K. Liber, J. V. Headley, K. M. Peru, and C. A. Morrissey. 2017. Reduction of neonicotinoid insecticide residues in Prairie wetlands by common wetland plants. Science of the Total Environment 579:1193–202. doi:10.1016/j.scitotenv.2016.11.102.
- Main, A. R., J. V. Headley, K. M. Peru, N. L. Michel, A. J. Cessna, and C. A. Morrissey. 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE 9:e92821. doi:10.1371/journal.pone.0092821.
- Main, A. R., N. L. Michel, M. C. Cavallaro, J. V. Headley, K. M. Peru, and C. A. Morrissey. 2016. Snowmelt transport of neonicotinoid insecticides to Canadian Prairie wetlands. Agriculture, Ecosystems & Environment 215:76–84. doi:10.1016/j.agee.2015.09.011.
- Marzaro, M., L. Vivan, A. Targa, L. Mazzon, N. Mori, M. Greatti, E. P. Toffolo, A. Di Bernardo, C. Giorio, D. Marton, A. Tapparo, and V. Girolami. 2011. Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bulletin of Insectology 64:119–26.
- Pohorecka, K., P. Skubida, A. Miszczak, P. Semkiw, P. Sikorski, K. Zagibajło, D. Teper, Z. Kołtowski, M. Skubida, D. Zdańska, and A. Bober. 2012. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. Journal of Apicultural Science 56:115–34.
- Pohorecka, K., P. Skubida, P. Semkiw, A. Miszczak, D. Teper, P. Sikorski, K. Zagibajło, M. Skubida, D. Zdańska, and A. Bober. 2013. Effects of exposure of honey bee colonies to neonicotinoid seed–treated maize crops. Journal of Apicultural Science 57:199–208.
- Reetz, J. E., S. Zühlke, M. Spiteller, and K. Wallner. 2011. Neonicotinoid insecticides translocated in guttated droplets of seed-treated maize and wheat: A threat to honeybees? Apidologie 42:596–606. doi:10.1007/s13592-011-0049-1.
- Rundlöf, M., GK Andersson, R. Bommarco, I. Fries, V. Hederström, L. Herbertsson, O. Jonsson, B. K. Klatt, T. R. Pedersen, J. Yourstone, and H. G. Smith. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80. doi:10.1038/nature14420.
- Schaafsma, A., V. Limay-Rios, T. Baute, J. Smith, and Y. Xue. 2015. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in Southwestern Ontario. PLoS ONE 10:e0118139. doi:10.1371/journal.pone.0118139.
- Schneider, C. W., J. Tautz, B. Grunewald, and S. Fuchs. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7:e30023. doi:10.1371/journal.pone.0030023.
- Solomon, K. R., and G. L. Stephenson. 2017. Quantitative weight of evidence assessment of higher tier studies on the toxicity and risks of neonicotinoid insecticides in honeybees 1: Methods. Journal Toxicogical Environment Health B This Issue.
- Stephenson, G. L., and K. R. Solomon. 2017. Quantitative weight of evidence assessment of higher tier studies on the toxicity and risks of neonicotinoid insecticides in honeybees 2: Imidacloprid. Journal Toxicogical Environment Health B This issue.
- Stewart, S. D., G. M. Lorenz, A. L. Catchot, J. Gore, D. Cook, J. Skinner, T. C. Mueller, D. R. Johnson, J. Zawislak, and J. Barber. 2014. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the Mid-Southern United States. Environmental Science & Technology 48:9762–69. doi:10.1021/es501657w.
- Struger, J., J. Grabuski, S. Cagampan, E. Sverko, D. McGoldrick, and C. H. Marvin. 2017. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 169:516–23. doi:10.1016/j.chemosphere.2016.11.036.
- Tapparo, A., C. Giorio, M. Marzaro, D. Marton, L. Solda, and V. Girolami. 2011. Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. Journal of Environmental Monitoring 13:1564–68. doi:10.1039/c1em10085h.
- USEPA. 2003a. Data evaluation record honey bee - acute oral LD50 Test TI-435 metabolite TZNG: Acute oral toxicity to honey bees (Apis mellifera).Report MRID No.: 45422430, National Bee Unit, Central Science Laboratory, York, UK.
- USEPA. 2003b. Data evaluation record honey bee - acute oral LD50 test TI-435 metabolite TZMU: Acute oral toxicity to honey bees (Apis mellifera). Report MRID No.: 45422429, National Bee Unit, Central Science Laboratory,York, UK.
- USEPA. 2014. Guidance for assessing pesticide risks to bees. Report, Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs. United States Environmental Protection Agency, Washington, DC. https://www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf.