ABSTRACT
A quantitative weight of evidence (QWoE) methodology was used to assess several higher-tier studies on the effects of thiamethoxam (TMX) on honeybees. Assessment endpoints were population size and viability of commercially managed honeybee colonies and quantity of hive products. A higher-tier field toxicology study indicated a no-observed-adverse effect concentration (NOAEC) of 29.5 µg TMX/kg syrup, equivalent to an oral no-observed-adverse-effect-dose (NOAED) of 8.6 ng/bee/day for all responses measured. For exposures via deposition of dust, a conservative no-observed-adverse-effect-rate at the level of the colony was 0.1 g TMX/ha. There was minimal risk to honeybees from exposure to TMX via nectar and pollen from its use as a seed-treatment. For exposures via dust and dust/seed applications, there were no concentrations above the risk values for TMX in nectar and pollen. Although some risks were identified for potential exposures via guttation fluid, this route of exposure is incomplete; no apparent adverse effects were observed in field studies. For exposures via dust/seed and dust/foliar applications, few adverse effects were observed. Considering all lines of evidence, the quality of the studies included in this analysis was variable. However, the results of the studies were consistent and point to the same conclusion. The overall weight of evidence based on many studies indicates that TMX has no adverse effects on viability or survival of the colony. Thus, the overall conclusion is that the treatment of seeds with thiamethoxam, as currently used in good agricultural practices, does not present a significant risk to honeybees at the level of the colony.
Introduction and problem formulation
This paper is the fourth in a series of quantitative weight of evidence (QWoE) analyses on the effects and risks to honeybees from neonicotinoids used as seed treatments. The methodology used for the QWoE analysis is described in the first paper in this series (Solomon and Stephenson Citation2017a). Moreover, this paper reports on a QWoE of the effects and risks of the neonicotinoid, thiamethoxam (TMX), in honeybees. Thiamethoxam was first registered in the market in 1997 (BCPC Citation2015). The major crops for which TMX is used include Brassica spp., leafy and fruity vegetables, potatoes, rice, cotton, deciduous fruit, citrus, tobacco, and soya beans. For seed treatment, the major crops are maize, sorghum, cereals, sugar beet, oilseed rape (canola), cotton, peas, beans, sunflowers, soya beans, rice, and potatoes (BCPC Citation2015). Although subject to changes in regulations in several jurisdictions (i.e., the EU), the major current use of TMX in areas without current restrictions is as a seed treatment. Here, the product might be mixed with other pesticides, such as fungicides, and coated on the seed with binders and other materials that provide a hard coating that minimizes abrasion and reduces release of the coating as a dust during handling and, more importantly, during planting with a pneumatic seeder (drill).
Physical, chemical, and toxicological properties of thiamethoxam
Thiamethoxam, CAS RN [153719–23-4], IUPAC Name, 3-(2-chloro-1,3-thiazol-5-ylmethyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene(nitro)amine (), MW 291.7 g/M (BCPC Citation2015), is a solid at room temperature and has a low vapor pressure of 4.95 × 10–11 mm Hg (25°C) (PubChem 2016). Because the vapor pressure is low and Henry’s constant (Hc) is small (4.70 × 10–10 Pa m3 mol−1), TMX is unlikely to be present in air at concentrations sufficiently large to be of toxicological concern. Solubility in water is 4.1 g/L, the log KOW is −0.13 (25°C), and the KOC is 68.4 (PubChem 2016). Therefore, it is relatively mobile in soil pore-water and can be readily taken up by the roots of plants. In water, hydrolysis half-lives of 42.8, 4.6, and 2.2 d were reported at pH 8, 9, and 10, respectively. (PubChem 2016) and a median half-life in soil of 31 d was reported (Hilton, Jarvis, and Ricketts Citation2016). Generally, for a period after planting, TMX is readily available for uptake by plants and once in the plant, it can be translocated from the roots to other parts of the plant via the xylem. TMX is metabolized in plants and the environment to clothianidin, also registered as an insecticide by another company and subjected to a separate QWoE (Solomon and Stephenson Citation2017b). This factor complicates the characterization of field exposure for risk assessment as there are two possible sources of clothianidin in the environment: direct use as an insecticide and as a breakdown product of TMX. This is not an issue for controlled exposure studies; however, this transformation was considered in the QWoE.
As for all neonicotinoid insecticides, TMX acts as an agonist at the insect nicotinic acetylcholine receptor (nAChR) (Jeschke et al. Citation2011). The companion paper (Solomon and Stephenson Citation2017a) presents a generic conceptual model for exposure of honeybees to neonicotinoids. This assessment of TMX was based on that conceptual model.
The oral toxicity of TMX in first-tier laboratory studies was summarized by European Food Safety Authority (EFSA). The acute oral LD50 for honeybees was reported as 5 ng a.s./bee and the contact LD50 as 24 ng active substance (a.s.)/bee (EFSA Citation2014). These values are similar to those for the toxic metabolite, clothianidin, reported as 3.8 ng active substance (a.s.)/bee via the oral route and 28 ng a.s./bee via contact (EFSA Citation2014). Because of reduced penetration of neonicotinoids through the cuticle of insects, the contact toxicity of TMX is less likely to be relevant to honeybees when used as a seed treatment.
Toxicity tests at the level of the colony integrate lethal and sublethal effects at the apical endpoints (see Solomon and Stephenson Citation2017a) and data for higher-tier assessments were provided in whole-hive feeding- and dust-exposure studies conducted over an extended period of time (Syngenta Citation2010a). These reports were reviewed using the guidelines for effect-studies as described in the supplemental information, SI of Solomon and Stephenson (Citation2017a). The feeding study (Syngenta Citation2015) exposed honeybees via sugar syrup and colony-relevant responses for survival and productivity were measured over 42 d with a follow-up the next spring. The score for quality of the methods (QoM) was 4.0, and a detailed summary of the study is provided in the SI. The study was robust with 24 replicates for the controls and 12 for each exposure concentration; nominal exposure concentrations ranged from 12.5 to 100 μg a.s./L of syrup. The lowest measured concentration at which no adverse effects were observed was 29.5 µg/kg syrup with the endpoint of area of brood with pupae in the colony (Response 7 of (Syngenta Citation2015) in the SI). This concentration was considered to accurately reflect the no-observed-adverse effect concentration (NOAEC).Footnote1 Concentrations of the toxic metabolite, clothianidin, were also measured in the syrup and were less than the level of detection (LOD = 0.25 µg/L). In the same study (Syngenta Citation2015), the measured concentrations in bee bread and nectar and/or honey in the hives were between 10.4 and 24 µg/kg and 0 and 28 µg/kg, respectively, for the 37.5 μg/L treatment. The measured concentration in the nominal 50 μg/L treatment was 39.7 μg/kg, which was taken as the lowest observed adverse effect concentration (LOAEC).
Table 1. Threshold concentration in food and water for toxicity values for TMX in honeybees.
Table 2. Estimated doses of TMX in adult honeybees collecting surface water from various sources.
The study with amended syrup provided a conservative NOAEC of 29.5 µg TMX/kg, equivalent to an oral no-observed-adverse effect dose (NOAED) of 8.6 ng/bee/day for all responses measured (assuming a daily intake of food of 292 mg/d/bee, USEPA Citation2014). The same NOAED for exposure from syrup (8.6 ng/bee/day) was used for exposure via pollen and bee bread. These toxicity values were used in this paper for assessing the exposures of honeybees to TMX measured in higher-tier field studies and are expressed in doses per honeybee to allow normalization from various sources of exposure. The lowest observed adverse effect dose (LOAED) was equivalent to 11.6 ng/bee/day.
The concentrations in the food in these studies can be used to estimate doses in honeybees that are based on the assumptions of intake of food used by the USEPA (Citation2014), see .
The assessment of exposures to dust from treated seed was based on a semi-field study where honeybees were exposed to dust released from treated maize seeds in tunnel studies (Syngenta Citation2010a) (see the SI for a detailed summary of this study). Two concentrations of dust collected from treated maize seeds in three replicates were used in the study: 13.8 and 69 g dust/ha, equivalent to an application rate of 1 and 5 g TMX/ha (based on measured concentrations in the dust), respectively. The contaminated dust was mixed with a carrier (“Schmelzflocken”, made from finely ground baby food) to maximize dispersion of the material within the tunnels and applied to the leaves of Phacelia tanacetifolia as a dust. There were signs of intoxication of bees within the 7-d exposure period at both exposures. There were significant effects on mortality and foraging intensity at 1 and 5 g TMX/ha (assessed daily from d-4 to d + 21). No adverse effects on bee brood development were attributed to the exposure of the hives to the test item treated crop. A no observed adverse effect rate (NOAER) for behavior and mortality could not be determined, because the effect range was unbounded. This level of toxicity is unexpected; however, it might be related to increased bioavailability of TMX from dust because of the Schmelzflocken carrier used to achieve a uniform distribution of dust on the plants. It is possible that the Schmelzflocken–TMX dust mixture was more attractive to the honeybees because it was gathered and consumed by honeybees as if it were pollen. The increase in mortality and other effects might have been an experimental artifact, so an uncertainty factor was applied to the data. A tentative and conservative NOAER was derived from the lowest rate applied with an uncertainty factor of 10. This provided a NOAER, 0.1 g TMX/ha, which was used to assess the risk from deposition of dust generated from treated seeds during seeding.
The main null hypothesis tested in this analysis was that TMX, used as a seed treatment, has no negative impacts on colony viability and no adverse effects on survival of the colony. There are several sub-hypotheses addressed below, and these also consider the toxic metabolite clothianidin (where it was measured in the relevant matrix; see SI):
Concentrations of TMX (as the sum of TMX and the toxic metabolite, clothianidin) in bee-relevant matrices from seed-treated crops in the field do not exceed the toxicity values derived from higher-tier toxicity studies on colonies of honeybees.
Colonies of honeybees exposed under experimental conditions in the field to TMX from seed-treated crops do not show adverse effects.
Exposure of colonies of honeybees to TMX and toxic metabolites resulting from use as a seed treatment in open agricultural fields do not cause adverse effects.
Methods
Sources of information and methods used in this QWoE assessment
The source of studies, papers, and reports used in this QWoE analysis and the methods used to assess quality and relevance of the data are described in the first paper in the series Solomon and Stephenson (Citation2017a). Reviews and papers lacking sufficient information to allow for the assessment of quality and relevance were not included in the analysis (see discussion in Solomon and Stephenson Citation2017a). Other than these, we included all available papers and reports on higher-tier studies conducted at the level of the colony and under realistic field conditions. Papers and reports were not excluded based on quality.
The methods used to score the papers and reports for quality and relevance and how these scores were used to produce the QWoE diagrams in this paper are also provided in the text and supplemental information in Solomon and Stephenson (Citation2017a). These methods are common to this and two other companion papers (Solomon and Stephenson Citation2017b; Stephenson and Solomon Citation2017) and are not discussed in detail here.
Characterization of exposures via surface water
There were several reports of concentrations of TMX measured in surface waters. These were monitoring or survey studies and were not coupled to higher-tier assessment of effects. These studies were from the open literature and were not specifically included in the QWoE analysis; however, the results are summarized here, and the risks of the reported concentrations were assessed using the same procedure as for guttation fluids (see below).
The National Water Quality Monitoring Council (NWQMC) of the United States provides data on concentrations of pesticides measured in surface waters (NWQMC 2016). This database was queried for concentrations of TMX and data processed as described in Stephenson and Solomon (Citation2017). The method detection limit ranged from 0.010 to 0.025 μg/L, and all non-detects were assigned a dummy value of zero. For samples of surface waters collected and analyzed between 2009–07-21 and 2015–09-28, the 90th centile was 0.0 and the 99th centile was 0.088 μg/L.
In a study in Japan, samples of water from rivers and estuaries in Osaka were analyzed for TMX (Yamamoto et al. Citation2012). The paper described an analytical method for neonicotinoids based on atmospheric pressure photoionization (APPI) source for liquid chromatography/mass spectrometry (LC/MS) and reported a LOD for TMX of 0.0006 μg/L with recoveries (relative standard deviation—RSD) ranging from 70% (10) to 117% (6). The use of transport and storage spikes and storage conditions were not reported. The 50th centile and maximum concentrations were 0.0015 and 0.0032 μg/L in 24 samples collected in 2009 and 3.8 and 11 μg/L in 21 samples collected in 2010, respectively.
In a study conducted in the Southern High Plains of Texas in 2005, samples of water were collected weekly from all wet playas from 18 May until 27 July and then bi-weekly until 22 September (Anderson et al. Citation2013). Water samples were stored at −20°C until analyzed. Pesticides were extracted from water samples with a C18 cartridge and then analyzed by gas-liquid chromatorgaphy (GLC) with electron capture detection using a published method (Zhang et al. Citation2008). The LOD was 1 μg/L but recovery was not reported. Field spikes and blanks were not reported. Raw data were not provided but the median concentration of TMX was 0.1 μg/L and the maximum concentration was 225 μg/L. The actual number of samples analyzed per playa was not reported but, from the dates of sampling, appears to be 15 per playa with a total of 180 (n = 180).
Three studies on concentrations of TMX in samples collected in Saskatchewan from potholes in farmers’ fields have been reported (Main et al. Citation2017, Citation2014, Citation2016). The sampling and methods are reported in Stephenson and Solomon (Citation2017). In the first study (Main et al. Citation2014), the maximum concentration of TMX was 1.5 μg/L (level of quantitation (LOQ) = 0.0009 μg/L), detected during the summer growing season when honeybees are active. The mean values of samples collected in the summer from wetlands associated with fields planted with barley, canola, oats, peas, wheat, and grassland were 0.019, 0.04, 0.121, <0.0009, 0.023, and <0.0009 μg/L, respectively. The second study (Main et al. Citation2016) used the same methods of analysis but sampled snow and meltwater in a subset of wetlands from the first study. The maximum concentration of TMX in snow, meltwater, and water from wetlands was 0.335 μg/L from 16 sites. The samples of snow were taken when bees would not be active, and the samples of water were taken from snow-melt until seeding (late May) when bees would not be expected to be exposed. In the third study (Main et al. Citation2017), the maximum concentration of TMX detected was 0.425 µg/L and the LOQ was 0.005 µg/L. The raw data were not reported for any of the studies. Therefore, it was not possible to estimate the centiles.
In a study in southern (S) Ontario, the sources of water in or close to maize fields (puddles, ditches, and drains ≤100 m from the edge of the field) that might be attractive to nearby bee hives were surveyed (Schaafsma et al. Citation2015). The details of the study are in Stephenson and Solomon (Citation2017). The raw data were provided, and the centiles could be calculated. The median concentration of TMX in surface water potentially attractive to honeybees was 0.61 μg/L with a 90th centile of 2.3 μg/L (data from Schaafsma et al. Citation2015, SI ). In a study conducted in S. Ontario (Struger et al. Citation2017), TMX was detected in drains, creeks, and rivers. The sampling and methods are reported in Stephenson and Solomon (Citation2017). The overall median value was 0.006 µg/L (method detection limit; MDL = 0.0014 µg/L). The maximum value measured was 1.34 µg/L. The Ontario Ministry of Environment and Climate Change (OMECC) measured concentrations of TMX in surface waters in the major use-areas in S. Ontario (OMECC 2017). The sampling and methods are reported in Stephenson and Solomon (Citation2017). The MDL was 0.005 µg/L. The median for all sites was 0.027 µg/L and the maximum measured concentration was 2.7 µg/L, sampled during a precipitation event.
Another paper reported on concentrations of neonicotinoids in surface waters in the United States (Hladik and Kolpin Citation2016). The report was based on one-time samples collected in 38 streams across 24 US states and Puerto Rico between November 2012 and June 2014. The analysis followed the United States Geological Survey (USGS) protocols with appropriate blanks and control samples and surrogate standards. TMX was detected above the method detection limits (MDL = 0.004 to 0.006 µg/L) in 21% of the samples, and the maximum concentration was 0.19 µg/L. The temporal studies in selected streams showed peak concentrations in the spring and summer months, but the maximum concentrations observed were less than those listed in .
Results and discussion
Risks from exposures via surface waters
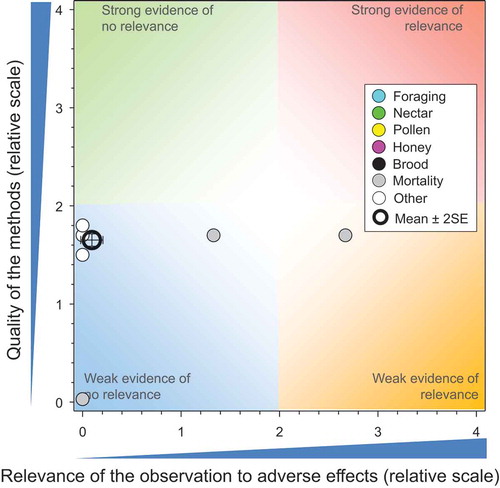
To assess the risks from these sources of water, the dose per honeybee was estimated as described for guttation fluid (Solomon and Stephenson Citation2017a) and compared to the chronic NOAED of 8.6 ng/adult bee/day (). The results are shown in . In all cases, the estimated dose of TMX was less than the chronic NOAED indicating de minimis risk.
Risks to honeybees from exposures resulting from uptake and translocation of TMX from seed treatments
There were 42 studies (Bargańska, Ślebioda, and Namieśnik Citation2013; Botias et al. Citation2015; Codling et al. Citation2016; David et al. Citation2016; Dively and Kamel Citation2012; Henry et al. Citation2015; Jones and Turnbull Citation2016; Kasiotis et al. Citation2014; Krupke et al. Citation2012; Mullin et al. Citation2010; Pacífico Da Silva et al. Citation2015; Pohorecka et al. Citation2012; Stewart et al. Citation2014; Stoner and Eitzer Citation2012; Syngenta Citation2000a, Citation2000e, Citation2000c, Citation2001b, Citation2001c, Citation2001d, Citation2002, Citation2003a, Citation2007b, Citation2007c, Citation2007d, Citation2007a, Citation2007f, Citation2007g, Citation2007h, Citation2007i, Citation2007j, Citation2010l, Citation2010k, Citation2010j, Citation2010b, Citation2010i, Citation2011b, Citation2012d, Citation2012a, Citation2012b, Citation2013b, Citation2014) that reported on the concentrations of TMX in bee-relevant matrices from the use of TMX-treated seeds, and these included 158 independent measures of concentrations in bee-relevant matrices (see SI). The result of the comparisons of exposures from bee-relevant matrices measured in these controlled field studies to toxicity values of TMX is shown in . There are multiple observations that have the same values for quality and relevance; these are separated for easier identification in the SI. Some studies were of better quality than others and the overall mean for quality was 2.76 ± SE of 0.07. The mean and SE for relevance was 0.16 ± 0.06, suggesting consistent lack of relevant effects in studies that were generally strong.
Figure 2. Quality and relevance of exposure values from controlled field studies on plants grown from seeds treated with TMX. Symbols might obscure others, see SI for all responses, n = 158. There were no data points obscured by the legend. Points with a heavy green border did not demonstrate adverse effects in field studies.
All but one of the reported concentrations of TMX in pollen and bee-bread (total = 75) resulted in exposure values less than the NOAED for honeybees of 8.6 ng TMX/bee/day. In the study by Codling et al. (Citation2016), the maximum concentration of ΣTMX measured in pollen resulted in exposure values three-times the NOAED. Most of the residue was TMX, so this was likely not a case of combined use of clothianidin and TMX. Unfortunately, information on which neonicotinoids had been used and in what quantity in the specific locations where samples were collected was not reported.
None of the concentrations of TMX measured in nectar (41 observations) would have resulted in doses above the NOAED for honeybees. This is consistent with the lack of effects observed in field studies (see below). Among the 21 measurements of TMX in honey, only one was more than the NOAED for honeybees. In this study (Codling et al. Citation2016), the maximum concentration of ΣTMX measured in honey was ca. 2.5-times the NOAED; however, as for pollen, clothianidin could have been used in the fields as well as TMX, which would have increased the apparent exposure (half the residue was clothianidin).
Among the six studies that measured concentrations of ΣTMX in samples of guttation fluid, four reported large concentrations that would have resulted in exposures >LOAED, suggesting possible adverse effects based on consumption of water from these sources only. One study (Syngenta Citation2010l) showed a 90th centile concentration equivalent to a dose between the NOAED and LOAED. However, it should be noted that concentrations in guttation fluid declined rapidly over time (Syngenta Citation2010i, Citation2012d, Citation2012a, Citation2012b). Three of these studies (shown in as points with a heavy green border) (Syngenta Citation2012d, Citation2012a, Citation2012b) were part of larger studies where effects at the level of the colony were evaluated (see below) and adverse effects were not observed for 12 of 13 endpoints and the effects on mortality during the exposure period were inconsistent and transient. These estimated exceedances of the NOAED did not result in the observation of adverse effects and indicate that exposure, if any, was less than the conservative assumption used in this QWoE analysis. Overall, the lack of effects observed at the level of the colony, even in studies where large concentrations of TMX were observed in guttation fluids, supports the conclusion that this is not a relevant route of exposure for honeybees. The potential exceedances here are likely the result of conservative assumptions about absorption of water from the honey-stomach, the attractiveness of guttation fluid to bees, and/or the co-occurrence of guttation events with the need for collection of water.
The overall weight of evidence of concentrations in bee-relevant matrices (honey, nectar, pollen, bee-bread, and guttation fluid) suggests that there is little or no risk for honeybees from exposure to TMX when used as a seed treatment.
Risks to honeybees resulting from exposure to dusts containing TMX
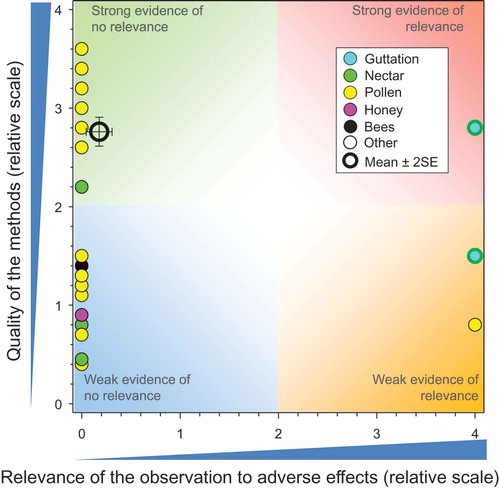
There were 12 studies on deposition of TMX from dust during seeding of seeds treated with TMX, and these included 33 responses (see SI). These studies (Biocca et al. Citation2015; Syngenta Citation2007e, Citation2008b, Citation2008a, Citation2010g, Citation2010f, Citation2010e, Citation2010i, Citation2011c, Citation2011a, Citation2012c, Citation2012e) showed that the rates of deposition were smaller than the NOAER for adverse effects at the level of the colony ().
Figure 3. Quality and relevance of exposure values from controlled field studies on deposition of dust during drilling of seeds treated with TMX, n = 33. Symbols may obscure others, see SI for all responses. There were no data points obscured by the legend.
As for seed treatments, some studies were stronger than others and the overall mean for the score for QoM was 3.28 ± SE of 0.10. The mean and SE for relevance was 0.73 ± SE of 0.27 (n = 33). Some of these results suggested risks from deposition of dust, which was inconsistent with the lack of effects observed in experimental field studies where honeybees were exposed to dust particles from treated seeds (see below). Identification of these risks might have been the result of the conservative NOAER used to characterize the risks from deposition of dust. There were 17 measurements of deposition of dust from seeders equipped with deflectors and 16 from seeders without deflectors. As a general observation, deposition was reduced by the use of a deflector and was further reduced when a mechanical rather than a pneumatic seeder was used. One study specifically measured deposition of dust downwind from hoppers on the seeder during loading (Syngenta Citation2010f) and reported greater deposition of dust 1 m from the seeder than from any of the seeding operations in the same study. This suggests that precautions during filling of hoppers or closed-loading systems would further reduce exposures.
Several papers from the literature were not included for the following reasons. The paper by Pilling et al. (Citation2013) was a compilation of reports by the registrant of TMX; the individual reports were assessed here, and the paper was omitted to avoid double-counting. The paper by Hladik, Vandever, and Smalling (Citation2016) was not used, because only aggregate data were provided. The paper by Sanchez-Hernandez et al. (Citation2016) described the development of an analytical method and provided no exposure data. The paper by Lawrence et al. (Citation2016) did not provide raw data, only risk ratios, which could not be incorporated into the QWoE.
Effects of TMX on honeybees resulting from exposures via crops grown from treated seeds
A total of 267 responses were included in the effects assessment; data from 29 studies were used to assess the relationship between quality and relevance of potential effects of TMX to honeybees exposed to systemic residues potentially in plants that were grown from seeds treated with the chemical (Henry et al. Citation2015; Pilling et al. Citation2013; Pohorecka et al. Citation2012; Syngenta Citation1998b, Citation1998a, Citation1999, Citation2000b, Citation2000g, Citation2001g, Citation2001h, Citation2001i, Citation2001j, Citation2001k, Citation2001l, Citation2001m, Citation2001n, Citation2001a, Citation2001f, Citation2001e, Citation2003b, Citation2009a, Citation2009b, Citation2010c, Citation2010d, Citation2010h, Citation2010k, Citation2010j, Citation2010b; Thompson et al. Citation2016).
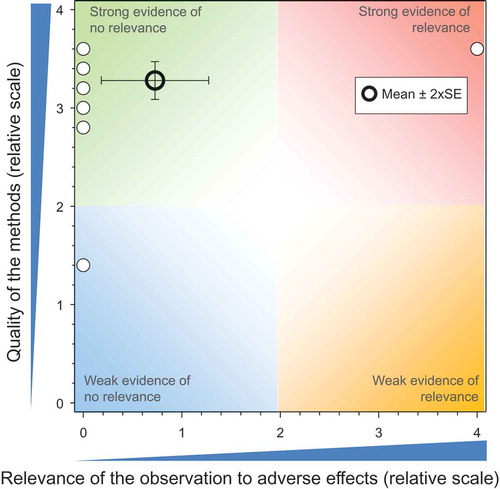
The data from studies in which honeybees were exposed to TMX in combination with other neonicotinoids were excluded to mitigate the potential for a Type-2 error. Most of the studies were field studies conducted under good laboratory practice guidelines whose quality was diminished by insufficient independent replication, failure to measure concentrations in field-collected, bee-relevant matrices, or failure to apply statistical procedures to the data, presumably because there were either no effects observed or because the experimental design was not conducive to comparison. The QoM of the studies on treated seeds was 1.46 ± SE of 0.03, and the values ranged from 0.8 to 3.2 ().
Figure 4. Quality and relevance of effects in honeybees exposed in controlled field studies via crops treated with TMX as seed treatments, n = 267. Symbols may obscure others, see SI for all responses. There were no data points obscured by the legend.
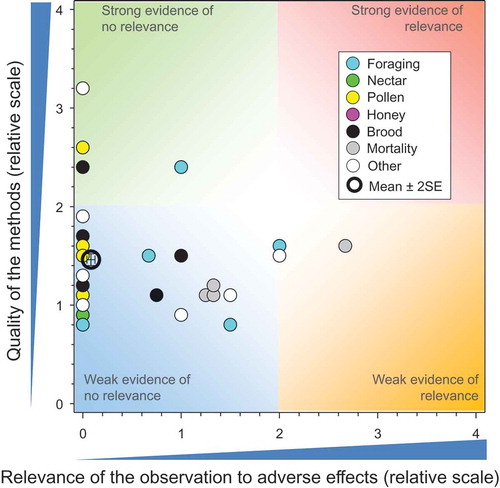
The relevance of responses were similar, regardless of whether the study was weak (e.g., mean relevance = 0.08 ± SE of 0.02) or moderately strong (values ranged from 0 to 2.67) because, essentially, most studies indicated that the exposure of honeybees to plants that had been grown from seed treated with TMX resulted in no adverse effects on a multitude of measurement endpoints that included mortality (larvae, pupae, adults, workers, drones and queen); strength of the colony (hive weights, number of workers, total number of adult bees, overwintering performance, food consumption rates, etc.); development of the colony (development of brood and queen, number of eggs, larvae, capped cells, and pupae, amount of honey, nectar, and pollen stores); colony health (Varroa mite infestation, viruses, or disease); foraging intensity and activity; flight dynamics (intensity and activity); behavior (trembling, agitation, immobilization, incoordination, hyper- or hypo-responsiveness etc.). Combinations of these metrics were generally measured over time (repeated measures) with multiple colony condition assessments (CCAs) conducted periodically (2–9 per study) for multiseasonal studies. For most studies, the assessment methods and procedures followed those recommended by EPPO (Citation1992) and SETAC (Citation1995).
Most of the earlier studies included in the QWoE were designed to assess exposure and, in these studies, honeybees were used to collect pollen and nectar for the analysis of exposures. The effects data associated with these earlier studies were generally qualitative observations collected over relatively short periods of time. As the observational metrics of the honeybees were incidental to the study rather than the direct focus, it is not surprising that these studies indicated that TMX did not present a risk to honeybees. The results of the more recent field and semi-field studies that have focused on the effects on honeybees indicate that the exposure concentrations in nectar and pollen were either insufficient to cause effects on apical endpoints or minimal exposure of honeybees was occurring. The residues measured in field-collected guttation fluid of TMX-treated seeds were sufficiently high to cause direct toxicity to honeybees; however, there was no apparent correlation with adverse effects to honeybees, presumably because the honeybees were rarely observed to ingest guttation fluid (see above). The absence of effects or preponderance of no-effects was attributed, in part, to resilience that has evolved in the social system of honeybees where the colony functions as an “organism” with highly conserved traits (Henry et al. Citation2015). The QWoE (see SI) clearly demonstrates that environmentally realistic concentrations of TMX result in no adverse effects to honeybees at the colony level of biological organization when used appropriately as a seed treatment. Evidence suggests that direct exposure of honeybees to TMX via dust from the operation of pneumatic seeders without deflectors could represent a risk to foraging honeybees (see below). However, recent advancements in the development of better adhesives and lubricants as well as modifications to pneumatic seeding machines have for the most part mitigated exposure of honeybees to neonicotinoid-contaminated exhaust dust.
The major limitations of the field studies conducted to date are the lack of statistical rigor and power in the experimental designs. Statistical procedures that can address complex issues that arise when dealing with multiple measures collected over time from multiple sites (independent) or reference plots are available, and these tools have been used in only a few studies. Currently, there are no standard methods for analyzing data generated from higher tiered studies such as those assessed in this section. Moreover, when applied appropriately, these methods would eliminate or reduce much of the uncertainty that arises when deciding if the effect is the result of the neonicotinoid or attributable to other noncontaminant variables.
Recent research examining the potential impacts of neonicotinoids, in particular TMX, on honeybees (Blacquière et al. Citation2012; Schneider et al. Citation2012) suggests that the effects on individual honeybee behavior (i.e., feeding and foraging) might be occurring but there is no quantifiable consequence to the function of the colony and most studies assessed herein demonstrated a low relevance associated with these endpoints. Henry et al. (Citation2015) observed that “despite an apparent link between neonicotinoid exposure and increased mortality of individual free-ranging foragers, colonies appeared to be able to compensate for the excess mortality so as to preserve unaltered performance in terms of population size and honey production” and that this was achieved by “delaying drone brood production in favour of increased worker brood production”. The weight of evidence indicates that bee-relevant matrices (i.e., nectar collected by honeybees or from plants, honey, pollen or bee bread) rarely had measured TMX residues above the chronic NOAED of 8.6 ng/bee/day for honeybees. Moreover, when exceedances occurred, there were no apparent adverse effects to apical endpoints. Recent research has demonstrated that neonicotinoids might function as an immunosuppressant with the implication that exposure of honeybees to these chemicals renders them more susceptible to pests and pathogens (Di Prisco et al. Citation2013). Neither of these negative adverse effects has been demonstrated from field studies; however, the molecular mechanisms have been proposed and demonstrated.
Effects of TMX on honeybees resulting from exposures to dust during seeding of seeds treated with TMX
Other forms of application potentially resulting in the exposure of honeybees to TMX included the application of the chemical as residues in dust formulated to mimic that which would arise during seeding of TMX-treated seed (Syngenta Citation2010a) or that resulted from actual seeding with TMX-treated seed (Syngenta Citation2012d, Citation2012a, Citation2012b, Citation2013a). Four field studies were designed to examine the potential impacts or effects on honeybees exposed via drift of dust containing TMX during and immediately following seeding of treated seed (). The objective of these studies was to evaluate the magnitude and extent of effects on honeybees of residues of dust on plant surfaces and in bee-relevant matrices as described in the previous section. Direct effects on honeybees either foraging or flying in adjacent fields with flowering plants (Syngenta Citation2013a, Syngenta Citation2012b, Citation2012a, Citation2012d) were assessed. For these field studies, the results indicated that the effects ranged from either no adverse effects on any endpoints (n = 9 of 10) to adverse effects on only one endpoint out of 13 for each of three studies (Syngenta Citation2012b, Citation2012a, Citation2012d). The study by Stanley et al. (Citation2015) was based on incorrect comparisons and was not included in the narrative. The details are provided in the associated SI. Mortality of honeybees associated with the treatment with TMX was greater than that in the control treatment at various times during three phases (pre-exposure, exposure, and post-exposure) of these studies. However, for the most part, these effects (mortality of honeybees) were neither consistent nor persistent over the entire period of observation. Nevertheless, there is an apparent risk for honeybees exposed to dust residues either directly or indirectly while foraging during the exposure period. The score for QoM for the six dust-related studies (n = 59 observations) was 1.65 ± SE of 0.03 and ranged from weak to moderate, but the relevance associated with the discernable adverse effects was consistently low 0.09 ± SE of 0.06 and variable. Because there were few persistent adverse impacts on apical endpoints, the relevance of the responses remained equally low (). For four of the six studies, mortality was the only endpoint adversely affected.
Conclusions
The overlay of the mean and 2 × SE values from the above four major groupings of studies () provides a graphical summary of the weights of evidence of all the studies included in this assessment. These results indicate that TMX, as currently used in good agricultural practices as a seed treatment, does not present a significant risk to honeybees at the level of the colony.
Figure 6. Overview of the conclusions of the QWoE for the effects of TMX on honeybees at the level of the colony. Total number of responses = 512. There were no data points obscured by the legend.
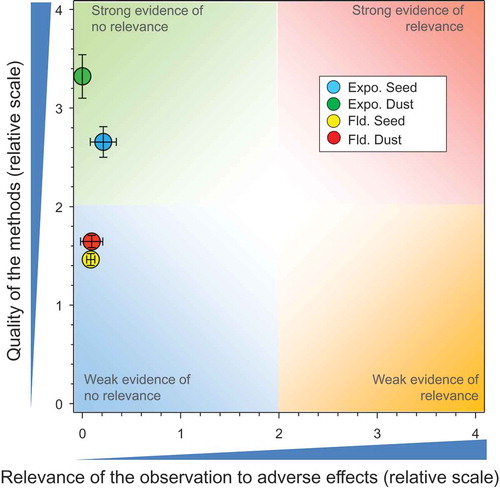
As indicated earlier, the effects estimated from the exposure of honeybees to TMX via nectar, guttation fluid, and pollen are based on a conservative assumption that honeybees are exposed exclusively to treated sources and obtain no food from untreated plants. The field studies where honeybees are exposed to TMX under more realistic conditions, whether from treated seed, or other routes of exposure, all show very little relevance for adverse effects.
The quality of the studies in the QWoE assessments was variable, but the results of the studies are consistent and point to the same conclusion. The overall weight of evidence based on many studies thus does not falsify the main null hypothesis being tested, that TMX has no negative impacts on viability and survival of the honeybee colony.
Declaration of interest
No potential conflicts of interest were reported by the authors.
Paper-4-TMX-WoE-SI-2017-07-29.pdf
Download PDF (7.4 MB)Acknowledgments
The authors wish to thank Ms. Jennifer Miller (Miller Environmental Science Inc.) for QA services and Mr. Spencer West for assistance with literature searches and obtaining the references and associated SI. The authors wish to thank Syngenta, LLC for providing access to the unpublished reports and for funding the study. The decision to publish this assessment was the authors’, and they were solely responsible for the content and the opinions herein. We are indebted to Dr EJ Marshall and Prof Keith Walters for editing these papers and to the reviewers for the helpful and constructive comments.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Notes
1 The NOAEC and LOAEC were derived from the characterization of the data in the report of the study (Syngenta Citation2015) in terms of the scoring guide for effects (SI ). Our NOAEC and LOAEC were less than those derived by the authors of the report (measured concentrations of 39.7 and 73.8 µg TMX/kg, respectively) and provide a measure of conservatism in this assessment. However, the difference in NOAEC had almost no influence on the outcome of the assessment of risk.
References
- [NWQMC] National Water Quality Monitoring Council. 2016. Water quality data. NWQMC. Accessed January, 2016. http://waterqualitydata.us/portal/.
- [OMECC] Ontario Ministry of Environment and Climate Change. 2017. Stream neonicotinoid monitoring study OMECC. Accessed January 25, 2017. https://www.ontario.ca/search/data-catalogue?sort=asc&query=Neonicotinoid.
- [PubChem] PubChem. 2016. Clothianidin. PubChem. Accessed April, 2016. http://pubchem.ncbi.nlm.nih.gov/compound/Thiamethoxam#section=Top.
- Anderson, T. A., C. J. Salice, R. A. Erickson, S. T. McMurry, S. B. Cox, and L. M. Smith. 2013. Effects of landuse and precipitation on pesticides and water quality in playa lakes of the southern high plains. Chemosphere 92:84–90. doi:10.1016/j.chemosphere.2013.02.054.
- Bargańska, Ż., M. Ślebioda, and J. Namieśnik. 2013. Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 31:196–201. doi:10.1016/j.foodcont.2012.09.049.
- BCPC. 2015. The Pesticide Manual (12th ed.), ed. Tomlin, C. D. S., Alton, UK: British Crop Protection Council.
- Biocca, M., R. Fanigliulo, P. Gallo, P. Pulcini, and D. Pochi. 2015. The assessment of dust drift from pneumatic drills using static tests and in-field validation. Crop Protect 71:109–15. doi:10.1016/j.cropro.2015.02.006.
- Blacquière, T., G. Smagghe, C. A. M. Van Gestel, and V. Mommaerts. 2012. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 21:973–92. doi:10.1007/s10646-012-0863-x.
- Botias, C., A. David, J. Horwood, A. Abdul-Sada, E. Nicholls, E. Hill, and D. Goulson. 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environmental Science & Technology 49:12731–40. doi:10.1021/acs.est.5b03459.
- Codling, G., Y. Al Naggar, J. P. Giesy, and A. J. Robertson. 2016. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 144:2321–28. doi:http://dx.doi.org/10.1016/j.chemosphere.2015.10.13.
- David, A., C. Botías, A. Abdul-Sada, E. Nicholls, E. L. Rotheray, E. M. Hill, and D. Goulson. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environment International 88:169–78. doi:10.1016/j.envint.2015.12.011.
- Di Prisco, G., V. Cavaliere, D. Annoscia, P. Varricchio, E. Caprio, F. Nazzi, G. Gargiulo, and F. Pennacchio. 2013. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences USA 110:18466–71. doi:10.1073/pnas.1314923110.
- Dively, G. P., and A. Kamel. 2012. Insecticide residues in pollen and nectar of a cucurbit crop and their potential exposure to pollinators. Journal of Agricultural and Food Chemistry 60:4449–56. doi:10.1021/jf205393x.
- EFSA. 2014. Conclusion on the peer review of the pesticide risk assessment for bees for the active substance thiamethoxam. EFSA Journal 11:3067. doi:10.2903/j.efsa.2013.3067.
- EPPO. 1992. Guideline on test methods for evaluating the side-effects of plant protection products on honey bees. EPPO Bulletin 22:203–08.
- Henry, M., N. Cerrutti, P. Aupinel, A. Decourtye, M. Gayrard, J.-F. Odoux, A. Pissard, C. Rüger, and V. Bretagnolle. 2015. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proceedings of the National Academy of Sciences 282:2015–110. doi:10.1098/rspb.2015.2110.
- Hilton, M. J., T. D. Jarvis, and D. C. Ricketts. 2016. The degradation rate of thiamethoxam in European field studies. Pest Management Science 72:388–97. doi:10.1002/ps.4024.
- Hladik, M. L., and D. W. Kolpin. 2016. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environmental Chemistry 13:12–20. doi:http://dx.doi.org/10.1071/EN15061.
- Hladik, M. L., M. Vandever, and K. L. Smalling. 2016. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Science of the Total Environment 542 (Part A):469–77. doi:10.1016/j.scitotenv.2015.10.077.
- Jeschke, P., R. Nauen, M. Schindler, and A. Elbert. 2011. Overview of the status and global strategy for neonicotinoids. Journal of Agricultural and Food Chemistry 59:2897–908.doi:10.1021/jf101303g.
- Jones, A., and G. Turnbull. 2016. Neonicotinoid concentrations in UK honey from 2013. Pest Management Science 72:1897–900. doi:10.1002/ps.4227.
- Kasiotis, K. M., C. Anagnostopoulos, P. Anastasiadou, and K. Machera. 2014. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Science of the Total Environment 485-486:633–42. doi:10.1016/j.scitotenv.2014.03.042.
- Krupke, C. H., G. J. Hunt, B. D. Eitzer, G. Andino, and K. Given. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7:e29268. doi:10.1371/journal.pone.0029268.
- Lawrence, T. J., E. M. Culbert, A. S. Felsot, V. R. Hebert, and W. S. Sheppard. 2016. Survey and risk assessment of Apis mellifera (Hymenoptera: Apidae) exposure to neonicotinoid pesticides in urban, rural, and agricultural settings. Journal of Economic Entomology 109:520–28. doi:10.1093/jee/tov397.
- Main, A. R., J. Fehr, K. Liber, J. V. Headley, K. M. Peru, and C. A. Morrissey. 2017. Reduction of neonicotinoid insecticide residues in Prairie wetlands by common wetland plants. Science of the Total Environment 579:1193–202. doi:10.1016/j.scitotenv.2016.11.102.
- Main, A. R., J. V. Headley, K. M. Peru, N. L. Michel, A. J. Cessna, and C. A. Morrissey. 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE 9:e92821. doi:10.1371/journal.pone.0092821.
- Main, A. R., N. L. Michel, M. C. Cavallaro, J. V. Headley, K. M. Peru, and C. A. Morrissey. 2016. Snowmelt transport of neonicotinoid insecticides to Canadian Prairie wetlands. Agriculture, Ecosystems & Environment 215:76–84. doi:10.1016/j.agee.2015.09.011.
- Mullin, C. A., M. Frazier, J. L. Frazier, S. Ashcraft, R. Simonds, D. Vanengelsdorp, and J. S. Pettis. 2010. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 5:e9754. doi:10.1371/journal.pone.0009754.
- Pacífico Da Silva, I., F. A. S. Oliveira, H. P. Pedroza, I. C. N. Gadelha, M. M. Melo, and B. Soto-Blanco. 2015. Pesticide exposure of honeybees (Apis mellifera) pollinating melon crops. Apidologie 46:703–15. doi:10.1007/s13592-015-0360-3.
- Pilling, E., P. Campbell, M. Coulson, N. Ruddle, and I. Tornier. 2013. A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS ONE 8:e77193. doi:10.1371/journal.pone.0077193.
- Pohorecka, K., P. Skubida, A. Miszczak, P. Semkiw, P. Sikorski, K. Zagibajło, D. Teper, Z. Kołtowski, M. Skubida, D. Zdańska, and A. Bober. 2012. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. Journal of Apicultural Science 56. doi:10.2478/v10289-012-0029-3.
- Sanchez-Hernandez, L., D. Hernandez-Dominguez, M. T. Martin, M. J. Nozal, M. Higes, and J. L. Bernal Yague. 2016. Residues of neonicotinoids and their metabolites in honey and pollen from sunflower and maize seed dressing crops. Journal of Chromatography 1428:220–7. doi:10.1016/j.chroma.2015.10.066.
- Schaafsma, A., V. Limay-Rios, T. Baute, J. Smith, and Y. Xue. 2015. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in Southwestern Ontario. PLoS ONE 10:e0118139. doi:10.1371/journal.pone.0118139.
- Schneider, C. W., J. Tautz, B. Grunewald, and S. Fuchs. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7:e30023. doi:10.1371/journal.pone.0030023.
- SETAC, editor. 1995. Procedures for Assessing the Environmental Fate and Ecotoxicity of Pesticides. Pensacola, Florida: SETAC.
- Solomon, K. R., and G. L. Stephenson. 2017a. Quantitative weight of evidence assessment of higher tier studies on the toxicity and risks of neonicotinoid insecticides in honeybees 1: Methods. Journal Toxicogical Environment Health B. This issue.
- Solomon, K. R., and G. L. Stephenson. 2017b. Quantitative weight of evidence assessment of higher tier studies on the toxicity and risks of neonicotinoid insecticides in honeybees 3: Clothianidin. Journal Toxicogical Environment Health B. This issue.
- Stanley, J., K. Sah, S. K. Jain, J. C. Bhatt, and S. N. Sushil. 2015. Evaluation of pesticide toxicity at their field recommended doses to honeybees, Apis cerana and A. mellifera through laboratory, semi-field and field studies. Chemosphere 119:668–74. doi:10.1016/j.chemosphere.2014.07.039.
- Stephenson, G. L., and K. R. Solomon. 2017. Quantitative weight of evidence assessment of higher tier studies on the toxicity and risks of neonicotinoid insecticides in honeybees 2: Imidacloprid. Journal Toxicogical Environment Health B. This issue.
- Stewart, S. D., G. M. Lorenz, A. L. Catchot, J. Gore, D. Cook, J. Skinner, T. C. Mueller, D. R. Johnson, J. Zawislak, and J. Barber. 2014. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the Mid-Southern United States. Environmental Science & Technology 48:9762–69. doi:10.1021/es501657w.
- Stoner, K. A., and B. D. Eitzer. 2012. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 7:e39114. doi:10.1371/journal.pone.0039114.
- Struger, J., J. Grabuski, S. Cagampan, E. Sverko, D. McGoldrick, and C. H. Marvin. 2017. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 169:516–23. doi:10.1016/j.chemosphere.2016.11.036.
- Syngenta. 1998a. Tunnel test: Effects of sunflowers grown from seeds dressed with A-9567B on Honey Bees (Apis mellifera). (Unpublished Report). Report CGA293343_0957 - A9567B (S98NCR3769V074), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 1998b. Semi-field test: Effects of oil-seed winter-rape grown from seeds dressed with A9700 B on the Honey Bee (Apis mellifera L.). (Unpublished Report). Report CGA293343_0822 - A9700B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 1999. BeeSCAN monitoring in summer rape grown from seed dressed with Cruiser A 9567 B. (Unpublished Report). Report CGA293343_1351 - A9567B, Syngenta Ltd, Munchwilen, Switzerland.
- Syngenta. 2000a. Report on analytical study 100/99 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in honey and sun flower heads collected in study 983769. (Unpublished Report). Report CGA293343_1363 - A9567B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2000b. Two field trials to determine the effects of HELIXTM seed treatment on honeybees foraging on canola flowers. (Unpublished Report). Report CGA293343_1384 - A11642A (CER03214/99), Syngenta Ltd, Guelph, Canada.
- Syngenta. 2000c. Report on analytical study 107/00 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in sun flower (Heads and flowers), honey, nectar, and pollen collected in study 31062/00. (Unpublished Report). Report CGA293343_1365 - A9567B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2000d. Report on analytical study 107/00 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in sun flower (Heads), honey, and pollen collected in study S99NCB1556V046 (Unpublished Report). Report CGA293343_1366 - A9567B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2000e. Report on analytical study 106/00 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in sun flower (Heads and flowers), honey, nectar, and pollen collected in study 31061/00. (Unpublished Report). Report CGA293343_1364 - A9567B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2000f. Report on analytical study 104/00 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in sun flower (Heads and leafs), honey, pollen, and bee (Honey stomach content) collected in study 99332/S1-BFEU. (Unpublished Report). Report CGA293343_1367 - A9567B, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2000g. Tunnel test- effects of sunflowers grown from seeds dressed with CGA 293343 70 WS (A-9567 B) on Honey Bees (Apis mellifera). (Unpublished Report). Report CGA293343_1408 - A9567B (S99NCB1556VO46), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001a. Field test: effects of oil-seed spring-rape grown from seeds dressed with CGA 293343 WS 70 (A 9567 B) on the Honey Bee (Apis mellifera L.) (Conducted in Southern Germany near Pforzheim). (Unpublished Report). Report CGA293343_1376 - A9567B (99125/01-BFEU), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001b. Report on analytical study 103/01 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in sunflowers (Flowers, leaves), honey, honey stomach content, and pollen, collected in study 20001072/I1-BFEU. (Unpublished Report). Report CGA293343_1411- A9567B, Syngenta Ltd., Basel, Switzerland.
- Syngenta. 2001c. Report on analytical study 102/01 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in winter rape (Leaves, blossoms), honey, honey stomach content, and pollen, collected in study 99393/01-BFEU. (Unpublished Report). Report CGA293343_1830, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001d. Report on analytical study 112/01 determination of analytes thiamethoxam (CGA 293343) and CGA 322704 in oil seed rape (Flowers), honey, honey stomach content, and pollen, collected in study 00 10 48 016. (Unpublished Report). Report CGA293343_1832, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001e. Semi-field test: Side effects of oil-seed spring-rape (Brassica napus) dressed with different rates of CGA 293343 on the Honey Bee (Apis mellifera L.). (Unpublished Report). Report CGA293343_1833 - A9807C, A9567C (20001077/01-BZEU). Syngenta Ltd., Basel, Switzerland.
- Syngenta. 2001f. Field test: Side effects of sunflower grown from seeds dressed with A-9567 B on the Honey Bee (Apis mellifera L.) in Italy. (Unpublished Report). Report CGA293343_1391 - A9567B (20001072/I1-BFEU). Syngenta Ltd., Basel, Switzerland.
- Syngenta. 2001g. Assessment of side effects of CGA 293343 + CGA 329351 + CGA 173506 FS 321.3 (A 9807 C) applied as seed dressing of Brassica napus on the Honeybee Apis mellifera L. (Unpublished Report). Report CGA173506_5395 - A9807C (2003626), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001h. Field test: Side effects of oil-seed winter-rape grown from seeds dressed with Cruiser OSR (A9807 C) on the Honey Bee (Apis mellifera L.). (Unpublished Report). Report CGA173506_5423 - A9807C (99393/01-BFEU), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001i. Field test (Non-GLP): Side effects of oil-seed winter-rape grown from seeds dressed with A9807 C on the Honey Bee (Apis mellifera L.). (Unpublished Report). Report CGA173506_5452 - A9807C (991579), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001j. Field test: effects of oil-seed spring-rape grown from seeds dressed with CGA 293343 WS 70 (A 9567 B) on the Honey Bee (Apis mellifera L.) (Conducted in Northern Germany near Celle). (Unpublished Report). Report CGA293343_1360 - A9567B (99125/02-BFEU), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001k. Field test: Side effects of sunflowers grown from seeds dressed with CGA 293343 WS 70 (A 9567 B) on the Honey Bee (Apis mellifera L.) in Spain. (Unpublished Report). Report CGA293343_1361 - A9567B (99332/S1-BFEU), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001l. Field test: Side effects of sunflowers grown from seeds dressed with CGA 293343 350 FS (A-9700 B) on the Honeybee (Apis mellifera carnica). (Unpublished Report). Report CGA293343_1369 - A9700B (31061/00), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001m. Field test: Side effects of sunflowers grown from seeds dressed with CGA 293343 350 FS (A-9700 B) on the Honeybee (Apis mellifera carnica). (Unpublished Report). Report CGA293343_1370 - A9700B (31062/00), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2001n. Semi-field test (Tunnel): Side effects of sunflower grown from seeds dressed with A-9567 B on the Honey Bee (Apis mellifera) in Spain. (Unpublished Report). Report CGA293343_1375 - A9567B (20001072/S1-BZEU), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2002. Determination of residues of thiamethoxam (CGA 293343) and CGA 322704 in Maize plants and Maize pollen after seed dressing with A9567C. (Unpublished Report). Report CGA293343_1643, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2003a. Determination of analytes thiamethoxam (CGA 293343) and Its Metabolite CGA 322704 in or on Pollen, Nectar and Honey from sunflower collected in study 991567. (Unpublished Report). Report CGA293343_1704, Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2003b. Field test: Side effects of sunflower grown from seeds dressed with A-9700 B on Honey Bee (Apis mellifera L.) in Argentina. (Unpublished Report). Report CGA293343_1697 - A9700B (991567), Syngenta Ltd, Basel, Switzerland.
- Syngenta. 2007a. Thiamethoxam (CGA293343) and its Metabolite (CGA322704): A residue study with A10590C treated maize seed, investigating residues in crop, soil and honeybee products in Alsace, France. (Unpublished Report). Report CGA173506_7323 - A10590C (20051149/F1-BZEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007b. Thiamethoxam (CGA293343) and its Metabolite (CGA322704): A residue study with A9700B treated spring barley seed followed by A9807C treated winter oil-seed rape seed, investigating residues in crop and honeybee products in Northern France. (Unpublished Report). Report A9700B_10673 A9807C, Bracknell Berkshire, United Kingdom, Syngenta Ltd.
- Syngenta. 2007c. Thiamethoxam (CGA293343) and its metabolite (CGA322704): A residue study with A9807C treated winter oil-seed rape seed, investigating residues in crop and honeybee products in Alsace (France). (Unpublished Report). Report CGA173506_7186 A9807C, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007d. Thiamethoxam (CGA293343) and its metabolite (CGA322704): A residue study with A9807C treated winter oil-seed rape seed, investigating residues in crop and honeybee products in Southern France. (Unpublished Report). Report CGA173506_7188 A9807C, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007e. Thiamethoxam: Deposition of dust from pneumatic drilling of A10590C treated maize seed in France during 2006. (Unpublished Report). Report CGA293343_3445, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007f. Thiamethoxam (CGA293343) and its Metabolite (CGA322704): A residue study with A10590C treated maize seed, investigating residues in crop, soil and honeybee products in Southern France. (Unpublished Report). Report CGA173506_7324 - A10590C (20051149/F2-BZEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007g. Thiamethoxam (CGA293343) and its Metabolite (CGA322704): A residue study with A10590C treated maize seed, investigating residues in crop, soil and honeybee products in northern France. (Unpublished Report). Report CGA173506_7333 - A10590C (2032750), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007h. Thiamethoxam (CGA293343) and its metabolite (CGA322704): A residue study with A9700B treated spring barley seed followed by A9807C treated winter oil-seed rape seed, investigating residues in crop, soil and honeybee products in Southern France. (Unpublished Report). Report CGA293343_ 3436 A9700B, A9807C (20051040/F2-BZEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007i. Thiamethoxam (CGA 293343) and its Metabolite (CGA322704): A residue study with A9700B treated spring barley seed followed by A9807C treated winter oilseed rape seed, investigating residues in crop, soil and honeybee products in Northern France. (Unpublished Report). Report CGA293343-3456 A9700B, A9807C (2032738), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2007j. Thiamethoxam (CGA 293343) and its Metabolite (CGA322704): A residue study with A9807C treated winter oilseed rape seed, investigating residues in crop and honeybee products in Northern France. (Unpublished Report). Report CGA1730506 _7187 A9807C (20051041/F2-BZEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2008a. Thiamethoxam; deposition of dust from Pneumatic DRILLING of A9700B treated maize seed in France during 2005. (Unpublished Report). Report A9700B_10842, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2008b. Thiamethoxam: Deposition of dust from Pneumatic drilling of A9700B+A9638A treated maize seed in France during 2008. (Unpublished Report). Report A9700B, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2009a. Thiamethoxam (CGA293343): A field study with A9807C treated winter oilseed rape seed, investigating effects on Honeybees (Apis mellifera L.) over Four Years in Alsace (France). (Unpublished Report). Report A9807C_10957 (20051041/F1-BFEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2009b. Thiamethoxam (CGA293343): A field study with A9807C treated winter oilseed rape seed, investigating effects on Honeybees (Apis mellifera L.) over Four years in Northern France. (Unpublished Report). Report A9807C_10958 - A9807C (20051041/F2-BFEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010a. Thiamethoxam (A9700B, A9584C) - A semi-field study with dust from A9700B treated Maize seeds and A9584C to evaluate effects on the Honeybee Apis mellifera L. (Hymenoptera, Apidae) in Phacelia tanacetifolia. (Unpublished Report). Report A9700B_10908 - A9700B, A9584C, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010b. Thiamethoxam (CGA293343) - A semi-field study with A9700B + A9638A treated Maize seed, followed by untreated flowering crop(s), investigating residues in crop(s), soil and honeybee products in burgundy (France), in 2009. (Unpublished Report). Report A9700B_10916 - A9700B (S08-01285), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010c. Thiamethoxam (CGA293343) - A field study with A9700B + A9638A treated Maize seed, investigating effects on Honeybees (Apis mellifera L.) over Four years in Alsace (France). (Unpublished Report). Report A9700B_10911 - A9700B (20061138/F1-BFEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010d. Thiamethoxam (CGA293343) - A field study with A9700B + A9638A treated maize seed, investigating effects on Honeybees (Apis mellifera L.) over Four Years in Lorraine (France). (Unpublished Report). Report A9700B_10912 - A9700B (20061138/F2-BFEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010e. Thiamethoxam – Investigating the deposition of dust from Pneumatic drilling of A9700B treated maize seeds in Italy during 2009. (Unpublished Report). Report CGA293343_11508-A9700BS, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010f. Thiamethoxam - Investigating the deposition of dust from Pneumatic drilling of A9700B treated Maize seeds in France during 2009. (Unpublished Report). Report A9700B_10917, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010g. Evaluation of deposition of seed treatment particles abraded from “PRODUCT” dressed maize seeds emitted during sowing, in adjacent oilseed rape and bare soil areas Final Report Amendment No. 1. (Unpublished Report). Report A9700B_10906, Syngenta Ltd, Frankfurt am Main, Germany.
- Syngenta. 2010h. Thiamethoxam (CGA293343) - A field study with A9700B + A9638A treated maize seed, investigating effects on Honeybees (Apis mellifera L.) over Four years in Southern France. (Unpublished Report). Report A9700B_10913 - A9700B (20061138/F3-BFEU), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010i. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during seeding and residues in guttation liquid, on Honeybee Colonies in Alsace (France) in 2009. (Unpublished Report). Report A9700B_10921 - A9700B (S09-01639), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010j. Thiamethoxam (CGA293343) - A semi-field study with A9700B + A9638A treated maize seed, followed by untreated flowering crop(s), investigating residues in crop(s), Soil and Honeybee products in Alsace (France), in 2009. (Unpublished Report). Report A9700B_10915 - A9700B (S08-01279), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010k. Thiamethoxam (CGA293343) - A semi-field study with A9700B + A9638A treated maize seed, followed by untreated flowering crop(s), investigating residues in crop(s), Soil and Honeybee products in Picardie (France), in 2009. (Unpublished Report). Report A9700B_10914 - A9700B (S08-01284), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2010l. Thiamethoxam - A semi-field study with maize seeds treated with A9700B and A14304E, Investigating residues in guttation liquid in 2009. (Unpublished Report). Report A9700B_10918 - A9700B, A14304E (S09-02828), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2011a. Investigating the dust deposition during sowing of seed-treated oilseed rape seeds with air assisted (Pneumatic) sowing machinery. (Unpublished Report). Report A9807C_10978 - CWFG - A9807C, Syngenta Ltd, Frankfurt am Main, Germany.
- Syngenta. 2011b. Determination of residues of thiamethoxam and its metabolites in sweet corn and sorghum pollen after drilling of seeds treated with A9700B, France 2010. (Unpublished Report). Report A9700B_10940 - A9700B (S10-01907), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2011c. Investigating the dust deposition during sowing of seed-treated oilseed rape seeds with air assisted (Pneumatic) sowing machinery. (Unpublished Report). Report A9807C_10977 - CWFG - A9807C, Syngenta Ltd, Frankfurt am Main, Germany.
- Syngenta. 2012a. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Alsace, France in 2010. Final Report Amendment 1. (Unpublished Report). Report A9700B S10-01857, Syngenta Ltd, Bracknell, United Kingdom.
- Syngenta. 2012b. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Lorraine (France) in 2010. Final Report Amendment 2. (Unpublished Report). Report A9700B S10-01859, Syngenta Ltd, Bracknell, United Kingdom.
- Syngenta. 2012c. Thiamethoxam - Investigating the deposition of dust from Pneumatic drilling of A9700B treated sunflower seeds in Northern Germany during 2011. (Unpublished Report). Report A9700B_10950 - A9700B, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2012d. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Stade, Germany in 2010. Final Report Amendment 1. (Unpublished Report). Report A9700B-S10-01860, Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2012e. Thiamethoxam and CGA322704 - A9807C - Monitoring of the deposition of thiamethoxam and CGA322704 dust emitted during sowing of oil-seed rape dressed with Cruiser OSR (Germany, 2008). (Unpublished Report). Report A9807C_10980 - A9807C, Syngenta Ltd, Maintal, Germany.
- Syngenta. 2012f. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Stade, Germany in 2010. (Unpublished Report). Report A9700B_10951, Syngenta Ltd, Niefern-Öschelbronn, Germany.
- Syngenta. 2012g. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Alsace, France in 2010. (Unpublished Report). Report A9700B_10952, Syngenta Ltd, Niefern-Öschelbronn, Germany.
- Syngenta. 2012h. Thiamethoxam FS (A9700B) - A field study with treated maize seeds, investigating the effects of residues from dust during drilling, residues in guttation liquid and flowering maize, on the Honeybee (Apis mellifera L.) in Lorraine (France) in 2010. (Unpublished Report). Report A9700B_10953, Syngenta Ltd, Niefern-Öschelbronn, Germany.
- Syngenta. 2013a. Thiamethoxam - monitoring of potential effects of the drilling of thiamethoxam FS treated maize seeds on honeybees, guttation monitoring of maize seedlings under agronomic use conditions and assessment of the relevance of guttation for Honeybees in Alsace (France). (Unpublished Report). Report CGA293343_11583 - A9807F (S10-01275), Syngenta Ltd.
- Syngenta. 2013b. Thiamethoxam – A field study to evaluate the magnitude of residues of thiamethoxam and its metabolite CGA322704 in Melon following a granular application of Actara® 5GR (A12180A) at transplanting in Spain 2012 Final report. (Unpublished Report). Report A12180A_10000 - A12180A (S12-02311), Syngenta Ltd, Bracknell, Berkshire, United Kingdom.
- Syngenta. 2014. Thiamethoxam/Difenoconazole/Metalaxyl-M/Fludioxonil FS (A11642A) – Residue Levels in or on Canola (Flowers, Pollen and Nectar) from Trials Conducted in Canada during 2012 and 2013 Final report. (Unpublished Report). Report A11642A (TK0116694), Syngenta Ltd, Guelph, Canada.
- Syngenta. 2015. Thiamethoxam technical – Honey Bee brood and colony level effects following Thiamethoxam Intake via Treated Sucrose Solution in a Field Study in North Carolina. (Unpublished Report). Report S14-02633, Syngenta Ltd, Greensboro, United States.
- Thompson, H., M. Coulson, N. Ruddle, S. Wilkins, and S. Harkin. 2016. Thiamethoxam: Assessing flight activity of honeybees foraging on treated oilseed rape using radio frequency identification technology. Environmental Toxicology and Chemistry 35:385–93. doi:10.1002/etc.3183.
- USEPA. 2014. Guidance for Assessing Pesticide Risks to Bees. Washington, DC: Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs. United States Environmental Protection Agency. https://www.epa.gov/sites/production/files/2014-06/documents/pollinator_risk_assessment_guidance_06_19_14.pdf
- Yamamoto, A., T. Terao, H. Hisatomi, H. Kawasaki, and R. Arakawa. 2012. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionisation. Journal of Environmental Monitoring 14:2189–94. doi:10.1039/c2em30296a.
- Zhang, B., X. Pan, L. Venne, S. Dunnum, S. T. McMurry, G. P. Cobb, and T. A. Anderson. 2008. Development of a method for the determination of 9 currently used cotton pesticides by gas chromatography with electron capture detection. Talanta 75:1055–60. doi:10.1016/j.talanta.2008.01.032.