ABSTRACT
The purpose of this study was to determine the toxicological and pharmacokinetic properties of sucralose-6-acetate, a structural analog of the artificial sweetener sucralose. Sucralose-6-acetate is an intermediate and impurity in the manufacture of sucralose, and recent commercial sucralose samples were found to contain up to 0.67% sucralose-6-acetate. Studies in a rodent model found that sucralose-6-acetate is also present in fecal samples with levels up to 10% relative to sucralose which suggest that sucralose is also acetylated in the intestines. A MultiFlow® assay, a high-throughput genotoxicity screening tool, and a micronucleus (MN) test that detects cytogenetic damage both indicated that sucralose-6-acetate is genotoxic. The mechanism of action was classified as clastogenic (produces DNA strand breaks) using the MultiFlow® assay. The amount of sucralose-6-acetate in a single daily sucralose-sweetened drink might far exceed the threshold of toxicological concern for genotoxicity (TTCgenotox) of 0.15 µg/person/day. The RepliGut® System was employed to expose human intestinal epithelium to sucralose-6-acetate and sucralose, and an RNA-seq analysis was performed to determine gene expression induced by these exposures. Sucralose-6-acetate significantly increased the expression of genes associated with inflammation, oxidative stress, and cancer with greatest expression for the metallothionein 1 G gene (MT1G). Measurements of transepithelial electrical resistance (TEER) and permeability in human transverse colon epithelium indicated that sucralose-6-acetate and sucralose both impaired intestinal barrier integrity. Sucralose-6-acetate also inhibited two members of the cytochrome P450 family (CYP1A2 and CYP2C19). Overall, the toxicological and pharmacokinetic findings for sucralose-6-acetate raise significant health concerns regarding the safety and regulatory status of sucralose itself.
Introduction
Background: Discovery and synthesis
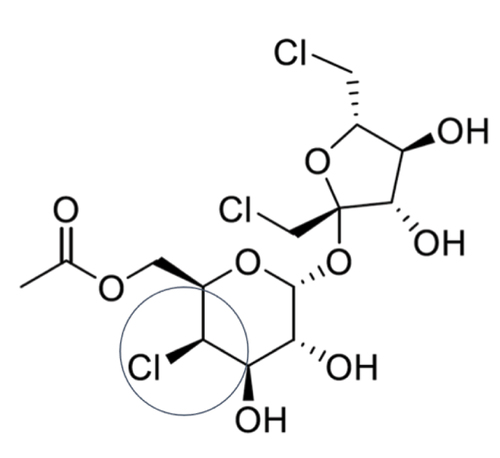
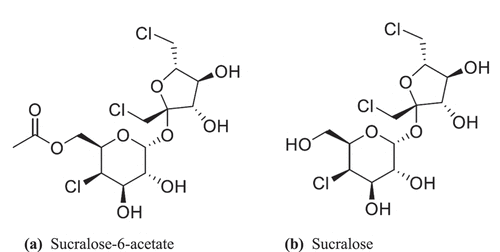
Sucralose is a chlorinated artificial sweeter that is used worldwide as a sugar substitute in thousands of food, beverage, and pharmaceutical products (Schiffman and Rother Citation2013). The sweet taste of sucralose was discovered at Queen Elizabeth College in London (Hough and Phadnis Citation1976) as part of a program to chemically modify sucrose (table sugar) for possible industrial applications. One structural modification was a chlorinated version of a novel disaccharide fructogalactose in which three hydroxyl groups were replaced by chlorine atoms with the chemical name 1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside. This compound was originally called trichlorogalactosucrose (TGS) but was subsequently given the name sucralose. The sweetness potency of sucralose is approximately 385 to 650-fold greater than sucrose by weight depending on the specific application (DuBois et al. Citation1991; Schiffman, Sattely-Miller, and Bishay Citation2008). The manufacturing process involves the synthesis of sucralose-6-acetate in multiple steps from sucrose with subsequent deacylation to produce sucralose (Hao Citation2011; Mufti and Khan Citation1983; Wang et al. Citation2011). The chemical structures of sucralose-6-acetate and sucralose are presented in . The sucralose-6-acetate intermediate generated during the manufacturing process is retained as an impurity in commercial sources of sucralose (Catani et al. Citation2006; OpAns Citation2021; United States Food and Drug Administration US FDA Citation2021; Werness Citation2021).
Figure 1. Chemical structures of sucralose-6-acetate (molecular weight 439.7, CAS number 105066-21-5) and sucralose (molecular weight 397.6, CAS number 56038-13-2).
Historical safety claims
Sucralose has regulatory approval in North America, Europe, and Asia along with the establishment of Acceptable Daily Intake (ADI) levels (Canada Gazette Citation1991; European Union EU Citation2004; Japanese Ministry of Health and Welfare JMHW Citation1999; Scientific Committee on Food SCF Citation2000; United States Food and Drug Administration US FDA Citation1998, Citation1999). Regulatory approval and establishment of ADIs were based upon historical studies performed during the 1980s and early 1990s in rats, mice, dogs, rabbits, and humans and ultimately published in part in the year 2000 (Baird et al. Citation2000; Goldsmith Citation2000; Grice and Goldsmith Citation2000; John, Wood, and Hawkins Citation2000a, Citation2000b; Kille et al. Citation2000a, Citation2000b; Roberts et al. Citation2000; Sims et al. Citation2000; Wood, John, and Hawkins Citation2000). These historical studies made the following 6 claims regarding sucralose and constituted the rationale for its regulatory approval:
1) Stability in vivo: Passes through the intestine unchanged.
2) Gut Microflora: No effect on gut microflora.
3) Intestinal Barrier: No effect on intestinal tissue.
4) Bioaccumulation: Does not bioaccumulate.
5) Metabolism: No effect on metabolism including blood glucose or insulin.
6) Biological/Toxicological Impact: Not genotoxic with no associated biologically significant consequences, and is heat stable.
Based on these historical claims, an ADI of 15 mg/kg body weight/day for humans was established by the Joint FAO/WHO Expert Committee on Food Additives JECFA (Citation1991) and by the Scientific Committee on Food SCF (Citation2000). A lower ADI of 5 mg/kg body weight/day was set by the United States Food and Drug Administration US FDA (Citation1998).
Independent research contrary to historical safety studies
Many scientific research investigations since regulatory approval, however, do not corroborate any of the 6 early historical claims regarding the biological fate or safety of sucralose.
Stability in vivo
Two acetylated sucralose biotransformation products were found in urine and feces of rats dosed with sucralose (Bornemann et al. Citation2018), and this finding is inconsistent with the historical claim that sucralose is stable and excreted unchanged (i.e., not metabolized) in the intestine. The more abundant acetylated metabolite was identified as sucralose-6-acetate (Werness and Schiffman Citation2020), and its relative contribution to the biological consequences of sucralose exposure has not yet been determined.
Gut microflora
Ingestion of sucralose by humans and/or animals within approved ADI levels was found to disrupt the microbiome in the gastrointestinal tract (GIT) (Abou-Donia et al. Citation2008; Bian et al. Citation2017; Méndez-García et al. Citation2022; Suez et al. Citation2022; Zhang et al. Citation2022). Maternal ingestion of sucralose in pregnant and lactating mice also impacted their progeny’s microbiome (Dai et al. Citation2020, Citation2021; Olivier-Van Stichelen, Rother, and Hanover Citation2019). Sucralose has also been detected in human breast milk samples from lactating women which indicates it is ingested by nursing infants (Sylvetsky et al. Citation2015).
Intestinal barrier
Sucralose also impacts the intestinal tissue. Sucralose ingestion induced histopathological changes including lymphocytic infiltrates into the intestinal epithelium, glandular disorganization, and epithelial scarring (Abou-Donia et al. Citation2008), increased bacterial infiltration into the ileal lamina propria in Crohn’s disease – like ileitis (Rodriguez-Palacios et al. Citation2018), elevated % CD3+T cells, CD19+B cells, and IgA+ plasma cells in Peyer’s patches (Rosales-Gómez et al. Citation2018), significantly increased levels of fecal chymotrypsin and trypsin and reduced fecal β-glucuronidase (Li et al. Citation2016), initiated lymphocyte aggregation in the ileum and colon (Zheng et al. Citation2022), and promoted inflammation and colitis-associated colorectal cancer risk (Guo et al. Citation2021; Li et al. Citation2020; Wang et al. Citation2019). Further, maternal ingestion of sucralose inhibited intestinal development, disrupted barrier function, and induced Paneth cell defects in offspring (Dai et al. Citation2020, Citation2021). In vitro studies demonstrated that sucralose enhanced biofilm formation along with bacterial invasion into gut epithelial cells (Shil and Chichger Citation2021) and disrupted tight junctions and barrier function in an intestinal epithelial model (Shil et al. Citation2020).
Bioaccumulation
Sucralose was found to bioaccumulate in adipose tissue of rats and was present two weeks after cessation of a 40-day feeding period even though it had disappeared from the urine and feces (Bornemann et al. Citation2018). An in vitro study reported that sucralose increased lipid accumulation and expression of adipocyte differentiation genes in cultured adipocytes (Azad et al. Citation2020).
Metabolism
Consumption of sucralose was noted to alter glucose and/or insulin concentrations in the plasma of some human subjects when delivered in liquids or capsules (Lertrit et al. Citation2018; Méndez-García et al. Citation2022; Pepino et al. Citation2013; Romo-Romo et al. Citation2018; Schiffman and Rother Citation2013; Suez et al. Citation2022) and when accompanied by carbohydrate (Dalenberg et al. Citation2020) or another non-caloric sweetener (Young et al. Citation2017). Maternal ingestion of sucralose during pregnancy impacted the progeny’s metabolism including downregulation of hepatic detoxification mechanisms and changes in bacterial metabolites (Olivier-Van Stichelen, Rother, and Hanover Citation2019). Additional studies reported that sucralose might affect incretin hormones including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) as well as the sodium-dependent glucose co‑transporter‑1 (SGLT‑1) (Kreuch et al. Citation2018; Lertrit et al. Citation2018; Margolskee et al. Citation2007; Sun et al. Citation2017; Young et al. Citation2017). Further, sucralose was demonstrated to blunt thyroid function (Pałkowska-Goździk, Bigos, and Rosołowska-Huszcz Citation2018). Chronic sucralose ingestion at levels that have regulatory approval in the United States and Europe also modify the fecal metabolome (Bian et al. Citation2017), liver proteome (Liu et al. Citation2019), and induce the expression of two intestinal proteins involved in first-pass metabolism, specifically P-glycoprotein (P-gp) and cytochrome P450 (CYP3A4) (Abou-Donia et al. Citation2008).
Biological/Toxicological impact
Additional independent research investigations since regulatory approval reported toxicological findings including genotoxicity and cancer risk following sucralose exposure. Sucralose, administered in feed beginning prenatally through the lifespan, induced hematopoietic neoplasias such as leukemias in male mice (Soffritti et al. Citation2016). Significant elevation in the number and size of colorectal tumors was detected in a murine model of colorectal cancer after sucralose treatment (Li et al. Citation2020). Four different studies utilizing a comet assay found that sucralose damaged DNA (Pasqualli et al. Citation2020; Raya et al. Citation2020; Sasaki et al. Citation2002; Van Eyk Citation2015). Sucralose also enhanced antimicrobial resistance and mutation frequency of E.coli (Qu et al. Citation2017). Further, heating sucralose with glycerol or lipids was found to generate chloropropanols, a potentially toxic class of compounds (Rahn and Yaylayan Citation2010), and this finding was supported by subsequent investigations that also reported thermal instability of sucralose accompanied by generation of hazardous polychlorinated compounds even in mild temperature conditions (De Oliveira, de Menezes, and Catharino Citation2015; Eisenreich, Gürtler, and Schäfer Citation2020).
Rationale for additional safety studies
It has not yet been established if adverse biological findings reported since regulatory approval are due to exposure to sucralose itself, to acetylated sucralose (e.g., sucralose-6-acetate), or both. Exposure to sucralose-6-acetate might occur during sucralose ingestion or result from metabolism of sucralose in the intestine. The amount of sucralose-6-acetate retained as an impurity in recent commercial sucralose samples varied with levels up to 0.67% (Werness Citation2021). McNeil Specialty Products Company acknowledged the presence of sucralose-6-acetate in batches of sucralose in their Food Additive Petition to the US FDA prior to approval (stated as 0.8% but <1.3%) (United States Food and Drug Administration US FDA Citation2021). However, the concentration of sucralose-6-acetate relative to sucralose in feces of rats dosed with sucralose was significantly greater than amounts in commercial sucralose samples, with fecal sucralose-6-acetate levels up to 10% (Bornemann et al. Citation2018; Werness and Schiffman Citation2020). This significant rise of the ratio of sucralose-6-acetate to sucralose in fecal samples may result from acetylation of sucralose by commensal bacteria in the GIT. Commensal bacteria in the intestine are known to acetylate xenobiotic compounds (Delomenie et al. Citation2001; Dull, Salata, and Goldman Citation1987). Previously Labare and Alexander (Citation1994) noted that microorganisms including bacteria were found to metabolize sucralose in sewage and soil samples. Further, Sun et al. (Citation2017) reported that a bacterium, Bacillus amyloliquefaciens, is capable of both acetylating and deacetylating sucralose. Overall, these findings indicate that biological exposure to sucralose-6-acetate may occur from ingestion of commercial impure sucralose as well as from acetylation of sucralose in the intestine.
The potential adverse health effects of exposure to sucralose-6-acetate are not yet known because this chemical has not been tested individually with a toxicology battery of tests to assess human risks. A comprehensive and systematic search of the scientific literature including government websites, chemical databases, patent literature, and scientific publications did not produce any apparent information on genotoxicity and cytotoxicity attributed to sucralose-6-acetate.
Additional studies undertaken
In this report, a series of 8 experiments were performed to screen for toxicological and pharmacokinetic properties of sucralose-6-acetate along with its structural parent sucralose (control). The following tests were utilized in these 8 experiments.
An in vitro MultiFlow® assay, a rapid high-throughput screening tool that predicts clastogenicity (induced DNA strand breakages) and aneugenicity (alterations in chromosome numbers), was used to determine genotoxic potential (Bryce et al. Citation2017; Citation2018).
A traditional in vitro Mammalian Cell Micronucleus Assay in thymidine kinase 6 (TK6) cells was performed to assess potential cytogenetic/chromosomal damage (Organi-sation for Economic Cooperation and Development OECD Citation2016).
The in silico Model Applier Leadscope® program was employed to detect structural chemical features with the potential to induce genetic mutations (Dearfield et al. Citation2017).
A traditional Bacterial Reverse Mutation Test (Ames test) was performed to assess mutagenic potential (Organisation for Economic Cooperation and Development OECD Citation2020).
Transepithelial Electrical Resistance (TEER) and permeability of human transverse colon were assessed using the RepliGut® system to investigate potential impairment and integrity of the intestinal barrier (Allbritton et al. Citation2021; Altis Biosystems, Durham, North Carolina, USA).
RNA-seq (RNA-sequencing) was utilized to investigate gene expression and identify differentially expressed genes in human intestinal epithelium (Marioni et al. Citation2008; Wang, Gerstein, and Snyder Citation2009).
Liver Microsome Stability Assays were employed to assess potential hepatic biotransformation (Houston Citation1994).
A Cytochrome P450 (CYP450) Inhibition Study was conducted to assess potential blunting of detoxification enzymes that might lead to drug-drug interactions (Obach et al. Citation2006).
These toxicological and pharmacokinetic tests were conducted in independent analytical labs that were selected based upon their expertise in specific in vitro techniques. This test battery of multiple assays was undertaken because no single test is able to conclusively identify the numerous potential toxicological and pharmacokinetic properties of a chemical compound.
Methods
Test articles
Two test articles, sucralose-6-acetate and sucralose, were utilized in 8 experiments to determine their toxicological and pharmacokinetic properties. Sucralose-6-acetate (4,1,6-trichloro sucralose-6-acetate) was synthesized by Jiangyin PharmaAdvance, Inc., 6 Dongsheng, West Road, Building D1, Jiangyin, Jiangsu Province, P. R. China 214431. The sucralose-6-acetate conformed to structure with a purity of 99.7% and was certified by 1H NMR spectrum, mass spectrum and high-performance liquid chromatography with an evaporative light scattering detector (HPLC-ELSD). Sucralose, used for control and comparison in several studies, was obtained from Sigma-Aldrich. It contained 0.5% sucralose-6-acetate as determined by HPLC-MS/MS (OpAns, Durham, North Carolina, USA) which is consistent with commercial food-grade sucralose.
Experiment 1: In vitro MultiFlow® DNA damage assay in TK6 cells
A rapid high-throughput flow cytometric assay (in vitro MultiFlow® Assay) was performed to assess the genotoxic potential of sucralose-6-acetate and sucralose in human TK6 cells using a 96-well format. This screening tool for DNA damage predicts whether compounds are clastogens, aneugens, or non-genotoxic based upon increases in two clastogen-sensitive biomarkers (γH2A× and p53) and two aneugen-sensitive biomarkers (p-H3 and polyploidy) (Bernacki et al. Citation2016; Bryce et al. Citation2014, Citation2016, Citation2017, Citation2018). The phosphorylated histone γH2A× is an indicator of double-strand DNA breaks, and translocation of tumor protein p53 to the nucleus is a marker of DNA damage response. Phospho-histone 3 (p-H3) accumulates in cells exposed to aneugens, and polyploidization is a consequence of aneugenic activity. The methodology for the MultiFlow® assay that assessed these endpoints of DNA damage response pathways was described previously (Bryce et al. Citation2017; Hung et al. Citation2020). The assay was conducted by BioReliance (Rockville, MD) under their protocol entitled In Vitro Clastogenic, Aneugenic, or Non-Genotoxic (CAN) FlowScreen Assay in TK6 Cells (BioReliance Citation2020a, Citation2021). High-performing mathematical algorithms were used to predict the mode of action (MoA) based upon the signatures of biomarkers for clastogenicity and aneugenicity using established Global Evaluation Factors (GEFs) that provide cutoff values indicating significant fold increases for each biomarker (Bryce et al. Citation2017).
Materials
Materials for liberation of nuclei, staining of chromatin, and immunological labeling of specific nuclear epitopes (MultiFlow® DNA Damage Kit – p53, γH2A×, Phospho-Histone H3 kit) were purchased from Litron Laboratories, Rochester, NY. The components and reagents in the proprietary kit included: Nuclei Release Solution with Counting Beads (lyses cells and provides absolute bead count), DNA Stain (propidium iodide) that labels free nuclei for identification in flow cytometric analysis, RNase Solution that removes RNA, p53 Antibody FITC that detects nuclear translocation of the protein p53, γH2A× Antibody Alexa Fluor® 647 that detects double-strand breaks, and Phospho-Histone H3 Antibody PE that detects mitotic cells. Multiflow® analyses were performed at two time points (4 and 24 hr) after treatment initiation with test articles. Latex microsphere counting beads were utilized to calculate nuclei density and cytotoxicity metrics. The Multiflow® reagent solution was prepared from these components according to the instruction manual for the kit.
An exogenous metabolic activation system (MutazymeTM, a Phenobarbital/β-Naphthoflavone (PB/NF) induced liver S9 derived from male Sprague Dawley rats) was obtained from Moltox®, Boone, NC (www.moltox.com). Four genotoxic compounds with either a clastogenic or aneugenic MoA were employed as controls. Methyl methanesulfonate (MMS) and carbendazim (100, 50, 25, or 12.5 µM for both compounds) were utilized as positive controls for the treatment without S9 (−S9) activation. Cyclophosphamide (80, 40, 20 or 10 µM) and benzo(a)pyrene (100, 50, 25, or 12.5 µM) were used as positive controls for the treatment with S9 (+S9) activation. The positive controls were employed to ensure responsiveness of the test system and appropriate clastogenic and/or aneugenic MoA prediction but not to provide a standard for comparison with test articles. Dimethyl sulfoxide (DMSO) was used as the solvent for positive controls.
TK6 cell culture test system
TK6 cells, lymphoblastoid cells of human origin, were obtained from the American Type Culture Collection (repository number CRL-8015), Manassas, VA. The TK6 cell line is p53 proficient, sensitive to different mechanisms of genotoxicity with a doubling time of 12–14 hr. The spontaneous frequency of mutations and chromosome alterations in TK6 cells does not differ significantly from primary human cells (Schwartz et al. Citation2004). The TK6 cells were cultured in T-75 cm2 flasks in RPMI 1640 medium with L-glutamine (Sigma-Aldrich) supplemented with 10% heat-inactivated horse serum and penicillin-streptomycin complete culture medium (CCM). Cultures were incubated at 37°C, 5% CO2 and ≥85% humidity. After 22–26 hr incubation, cell density in the T-75 cm2 flasks was calculated after cell counting. Target cell stocks required for the Multiflow® tests in both absence and presence of S9 were calculated; an appropriate volume of cell suspension was transferred to 50 ml tubes and centrifuged at 150 × g for 6 min. The culture medium was aspirated, and cell densities adjusted with the CCM solution to 2 × 105 cells/ml in the absence of S9, and to 2.2 × 105 cells/ml in the presence of S9, immediately prior to use.
Treatments, and flow cytometric analysis
The TK6 cells were exposed to 20 concentrations of sucralose-6-acetate (maximum 4.5489 mM or 2000 μg/ml) or 20 concentrations of sucralose (maximum 10 mM or 3980 μg/ml) with a dose spacing of 1.4142 (square root of 2) in the presence (+S9) and absence (−S9) of metabolic activation along with vehicle control alone using 96-well plates. The test articles were prepared using DMSO with a final DMSO concentration in the cell suspension below 1%. In the +S9 condition, cells were exposed to S9 (MutazymeTM) for 4 hr after which S9 was washed out, centrifuged twice (5 min at 340 × g), and re-incubated in fresh culture media. Aliquots were taken from +S9 treatment wells at 4 hr (prior to the wash step) and at 24 hr incubation and subsequently transferred to new plates that were pre-loaded with 50 µl MultiFlow®-kit reagent solution. In the -S9 condition, aliquots were also taken at 4 and 24 hr exposure and transferred to new plates that were also pre-loaded with MultiFlow®-kit reagent solution. Cells were incubated in the reagent mix according to instructions in the kit in order to simultaneously digest the cytoplasmic membranes in the harvested cells, liberate the nuclei, stain the chromatin with the fluorescent nucleic acid dye, and label γH2A×, p-H3, and p53 with fluorescent antibodies. Fluorescent microspheres in reagent mix were used to obtain nuclei-to-bead ratios as a simple cytotoxicity index at 4 and 24 hr. Analysis was performed by flow cytometry utilizing a BD FACSCanto II Flow Cytometer with BD FACSDivaTM software (BD Biosciences), and fold-shifts in biomarkers determined.
Analysis of cytometric results
High-performing mathematical algorithms (Bernacki et al. Citation2016; Bryce et al. Citation2016, Citation2017) were used to predict MoA based upon multi-endpoints of biomarkers for clastogenicity and aneugenicity using Global Evaluation Factors (GEFs) that provided cutoff values representing significant fold elevation for each biomarker. Conditions for making MoA calls were established for 3 cases. First, a clastogenic call was made for S9 treatments (+S9) by fold-increases in two consecutive concentrations that met or exceeded GEFs for at least two out of 4 clastogen-sensitive biomarkers with γH2A× required for at least one response:
≥1.44-fold 4-hr γH2A×,
≥1.31-fold 24-hr γH2A×,
≥1.23-fold 4-hr nuclear p53,
≥1.12-fold 24-hr nuclear p53.
Second, a clastogenic call was made for 24-hr treatments (−S9) by fold elevation in two consecutive concentrations that met or exceeded cutoffs for at least two out of 4 clastogen-sensitive biomarkers with γH2A× required for at least one response:
≥1.51-fold 4-hr γH2A×,
≥2.11-fold 24-hr γH2A×,
≥1.40-fold 4-hr nuclear p53,
≥1.45-fold 24-hr nuclear p53.
Third, an aneugenic signature was demonstrated by fold increases in two consecutive concentrations that met or exceeded cutoffs for at least two of the following aneugenic responses:
≥1.71-fold 4-hr phospho-histone H3,
≥1.52-fold 24-hr phospho-histone H3,
≥5.86-fold 24-hr polyploidy,
≥1.45-fold 24-hr nuclear p53.
The call was nongenotoxic under the test conditions if less than two clastogen-sensitive or two aneugen-sensitive biomarkers did not meet or exceed the above GEFs. Cytotoxicity was based upon reduction of nuclei counts for individual cultures.
Experiment 2: In vitro mammalian cell micronucleus test in TK6 cells
An in vitro mammalian cell micronucleus (MN) test in TK6 cells was used to determine if MN are present in the cytoplasm of cells that were exposed to sucralose-6-acetate. Micronuclei are small extra-nuclear structures that are produced by DNA breakage (clastogens) or are induced by numerical chromosomal aberrations (aneugens) (OECD 487, Citation2016). A rise in MN frequency is a biomarker of cytogenetic/chromosomal damage. The MN assay was performed by BioReliance (Citation2020b) using TK6 cells according to standard protocol guidelines of the Organisation of Economic Cooperation and Development (OECD 487, Citation2016).
TK6 cells and treatment
The assay was conducted by treating TK6 cells with a range of concentrations of the test article (sucralose-6-acetate) as well as with positive and vehicle controls. The procedure for preparation of TK6 cells was described above for the Multiflow® test, and cell density was adjusted to 2.5 × 105 cells/ml in the absence and presence of exogenous metabolic activation (S9). DMSO was the vehicle for the sucralose-6-acetate and served as the vehicle control for each treatment type. The potential of sucralose-6-acetate and/or its metabolites to induce MN in TK6 cells was assessed in the presence (4-hr treatment) and absence (27-hr treatment) with S9. After the 4-hr incubation, cells were centrifuged to remove the treatment medium, resuspended in CCM, and incubated for an additional 23 hr. Sucralose-6-acetate was assessed at the following concentrations. For the 4-hr incubation, the concentrations of sucralose-6-acetate were 2000, 1500, 1000, 750, 700, 600, 500, 400, 350, 300, 200, and 100 μg/ml. For the 27-hr exposure, the levels of sucralose-6-acetate were 2000, 1500, 1250, 1000, 750, 500, 250, 125, 100, 80, 40, and 20 μg/ml. Cyclophosphamide (2.5, 3 or 4 μg/ml prepared in water) was employed as a positive control for the 4-hr treatment, and vinblastine (10 or 12 ng/ml prepared in water) served as the positive control in the 27-hr treatment.
Micronucleus scoring and statistical analysis
Micronucleus scoring was performed after exposure to sucralose-6-acetate for a minimum of 2000 mononuclear cells and a minimum of 200 binucleated/multinucleated cells at 4 concentrations in the 4-hr and at 5 concentrations for the 27-hr treatment. Scoring included the number and frequency of MN in binucleated and multinucleated cells as well as mononuclear cells as increases in binucleated cells were evident at higher concentrations of sucralose-6-acetate in this study. Scoring was performed for 300, 500, 700 and 1000 µg/ml for the 4-hr exposure and 100, 250, 500, 750 and 1000 µg/ml for 27-hr incubation.
Significance was assessed with a Fisher’s Exact Test (Fisher Citation1954) relative to solvent control. The Cochran-Armitage trend test was performed to determine if there was a trend in the number of micronucleated cells across increasing concentrations of sucralose-6-acetate (Agresti Citation2002; Armitage Citation1955; Cochran Citation1954). The criterion for significance was set at p < .05. Calculations were made in Excel (Microsoft Corporation). A MN test for sucralose itself was not undertaken in the current study as it was conducted and reported previously (United States Food and Drug Administration US FDA Citation1998).
Experiment 3: In silico assessment of mutagenic potential by Leadscope®
Leadscope®, a quantitative structure activity tool (Leadscope® Citation2019), was used to predict the genotoxic potential of sucralose-6-acetate and sucralose in silico based upon the chemical structure and performed by Aclairo Pharmaceutical Development Group (Aclairo Citation2019). The Leadscope® model utilizes a large mutagenic toxicology database called SAR Genetox based on International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH M7) (United States Food and Drug Administration US FDA Citation2018) with validated structures to generate computational structure-activity predictions (Hevener Citation2018). This tool also provides bacterial mutation alerts for the active/primary portion or molecular fragment(s) of these molecules. The alert knowledge base was constructed from a systematic analysis of available bacterial mutagenicity data and scientific literature. The chemical structures of sucralose-6-acetate and its parent sucralose (control) were both entered into the Leadscope® model applier as MOL files, a file format that includes attributes associated with entire chemical structure (CTFile Formats Citation2005).
Experiment 4: Bacterial reverse mutation test (Ames Test)
A Bacterial Reverse Mutation Test was performed by BioReliance (Citation2020c) according to OECD guidelines (Organization of Economic Cooperation and Development OECD Citation2020). This classic mutagenicity test was employed to evaluate the potential of the sucralose-6-acetate, sucralose, and/or their metabolites to induce reverse mutations at loci of TA98, TA100, TA1535, and TA1537 strains of Salmonella typhimurium and the WP2 uvrA strain of Escherichia coli in the presence and absence of an exogenous metabolic activation system (S9). The test article, sucralose-6-acetate was prepared in DMSO, and sucralose was prepared in sterile deionized water. Both test articles were evaluated via the plate incorporation method at 8 concentrations 1.5, 5, 15, 50, 150, 500, 1500, and 5000 µg/plate. The final DMSO concentration in the cell suspension was at or below 1% v/v. The positive controls in the Bacterial Reverse Mutation Test for each bacterial strain are presented in .
Table 1. Positive controls for each bacterial strain with and without metabolic activation.
Experiment 5: Assessment of transepithelial electrical resistance (TEER) and permeability in human transverse colon epithelium
A high throughput in vitro intestinal stem cell platform (RepliGut®, Altis Biosystems, Durham, NC USA) was utilized to screen the effects of sucralose-6-acetate and sucralose on human transverse colon. The assay was performed by Altis Biosystems (Citation2020, Citation2021). The RepliGut® system is comprised of polarized intestinal cells grown on transwells that mimic normal intestinal barrier function (Allbritton et al. Citation2021). The effect of sucralose-6-acetate and sucralose on TEER and permeability was assessed in two separate trials. TEER is a measure of monolayer resistance which is an indirect measure of barrier function and integrity of tight junctions (Elbrecht, Long, and Hickman Citation2016; Srinivasan et al. Citation2015). In Trial 1, TEER was determined after exposure of the RepliGut® system to a single effective concentration of test article (10 mM sucralose-6-acetate or 10 mM sucralose). In Trial 2, TEER as well as permeability were assessed at a range of effective concentrations of sucralose-6-acetate (0.3125 mM to 10 mM) and sucralose (5 mM to 160 mM). In both trials there was an additional no-treatment (control) condition.
Transverse colon cells were plated directly onto RepliGut® transwell plates coated with a thin hydrogel. Cultures were monitored for cell confluence by eye using a bright field microscope. Epithelial cells became confluent after 4 days. Once confluent, media was changed to a proprietary Altis Differentiation Media (ADM). Cells were then grown for 2 days in the ADM. To monitor cell confluence while cells were grown in ADM, TEER was measured using an EVOM2 Epithelial Volt/Ohm Meter with an STX2 Electrode (World Precision Instruments).
After 48 hr in ADM, TEER was measured on each transwell. Subsequently, the test compound was added to both apical and basal sides of each transwell. Transwells were incubated with the test compound for 24 hr, and TEER was measured in all transwells in Trials 1 and 2. In Trial 2, a permeability assay using 40 kDa fluorescein isothiocyanate labeled dextran (FITC) was also performed on each transwell to measure flux of the dextran over 4 hr. While TEER is an indicator of ionic conductance, 40 kDa FTIC is an indicator of paracellular permeability (Utami et al. Citation2018).
In Trial 1, apical and basal supernatants were collected from all transwells prior to cell collection and transferred to tubes and stored at −80°C. The apical and basal supernatants were submitted for chromatographic analysis to determine if there was any conversion to sucralose-6-acetate in sucralose-exposed transverse colon cells or deacetylation to sucralose in cells exposed to sucralose-6-acetate (OpAns, Durham, NC USA). After cell collection, 500 μl RNA Lysis Buffer was added to the apical side of each transwell. Lysates were pipetted up and down 10 times and complete lysis was confirmed by using a bright field microscope. Lysates were transferred to individual tubes and stored at −80°C for RNA extraction and RNA-seq analysis.
Experiment 6: RNA-seq and gene expression in transverse colon
RNA was isolated, quantified, and quality checked by Altis Biosystems using the transverse colon cells exposed to sucralose-6-acetate and sucralose (along with the no-treatment control) in the RepliGut® system in Trial 1 of Experiment 5. There were 12 samples, 4 each from sucralose, sucralose-6-acetate, and untreated controls. RNA was isolated using a RNAqueous-Micro Total RNA Isolation Kit (Invitrogen Cat#AM1931) and stored at −80°C. RNA concentration was determined using a Qubit RNA HS Assay Kit (Thermo Fisher Scientific, Cat#Q32852) and a Qubit 3.0 Fluorometer. RNA Integrity (RIN) values were determined utilizing an RNA 6000 Pico Kit (Agilent, Cat#5067–1513) on a Bioanalyzer 2100 machine. Subsequently, RNA concentrations and RIN values were determined.
RNA-seq (RNA-sequencing) was employed to analyze the transcriptome and determine changes in gene expression due to exposure to sucralose-6-acetate and sucralose, each relative to control (no treatment). RNAseq was conducted at the North Carolina State University (NCSU) Genomic Sciences Laboratory, and samples were run as 150bp paired-end reads on the NovaSeq 6000 (Illumina, Inc., San Diego, CA, USA). Raw reads were trimmed for adapter and quality using Trim Galore version 0.6.1 (Babraham Bioinformatics Citation2019) with the two-color flag set. Trim Galore calls cutadapt (v2.1) (Martin Citation2011) for adapter trimming. Quality cutoff was set to 20 and minimum sequence length for both reads was set to 20bp. All reads passed the initial quality and length filters. Bases kept post-trimming ranged from 95.7 to 98% of total bases analyzed. A second round of trimming to account for poly-G runs that may have been introduced during sequencing was performed with fastp (version 0.19.10) (Chen et al. Citation2018) with adapter trimming disabled and polyG tail trimming enforced with 98.77 to 99.36% reads retained post-trim.
Trimmed reads were aligned to the human genome as represented in the GRCh38 (Genome Reference Consortium Human Build 38, National Center for Biotechnology Information, USA) no alt analysis set using HISAT2 (v2.1.0) with the – dta-cufflinks flag set for downstream processing (Kim et al. Citation2019; Kim, Langmead, and Salzberg Citation2015; Pertea et al. Citation2016). Alignment rates ranged from 95.92 to 97.62%. The resulting SAM (Sequence Alignment Map) files were sorted and converted to BAM (Binary Alignment Map) binary files using samtools (Li et al. Citation2009). Stringtie2 (Pertea et al. Citation2015) was deployed for the individual BAM files for each replicate to assemble the alignments into potential transcripts and resulting replicate gene transfer format (GTF) files were merged into a single expressed transcriptome. Coverage tables were produced with the Stringtie -eB command to create a Ballgown object directory.
The Ballgown R package (Frazee et al. Citation2015) was deployed to access count data. Hierarchical clustering using Euclidean distances in gene expression between different replicates indicated that one of the 4 controls (the Control-2 replicate) was an outlier. It was therefore removed from further analysis. All remaining replicates passed quality control based upon PCA/MDS and hierarchical clustering analysis. Gene expression values were passed through a low-abundance filter such that any gene with a variance of less than 1 in expression across all samples was removed from further analysis. Four different comparisons were then made for the data: Sucralose-6-Acetate vs Control, Sucralose vs Control, Sucralose vs Sucralose-6-Acetate, and a three-way comparison for all samples. Significance was determined by a false-discovery-rate q-value of 0.05. Over-representation testing of significant genes was accomplished using gProfiler’s Gost service using default parameters, version: e101_eg48_p14_baf17f0 (Raudvere et al. Citation2019).
Experiment 7: Liver microsome stability assays for half-life (T1/2) determination
A microsomal stability assay was performed by BioDuro-Sundia (Shanghai, China) using a standardized protocol to determine the in vitro half-life T1/2 (min) of the two test articles, sucralose-6-acetate and sucralose, in the presence of liver microsomes that contain membrane-bound metabolizing enzymes including cytochrome P450 (CYP450). The goal of this assay was directed at determining Phase I metabolism in 5 different species (human, monkey, dog, rat, and mouse) using dihydronicotinamide-adenine dinucleotide phosphate/NADPH (ACROS Cat#328742500) as an enzyme co-factor. Microsome stability was assessed using a singlet incubation of 100 μM of each test article at 5 time points (0, 5, 15, 30, and 60 min). Samples were assessed using liquid chromatography/tandem mass spectrometry (LC/MS/MS) at peak area ratios to determine T1/2 along with an estimation of intrinsic clearance (Clint). The fraction of each test article removed by the liver (extraction ratio) was calculated for each of the 5 different species based upon physiological variables reported by Houston (Citation1994) and Davies and Morris (Citation1993). Subsequent liver stability assays were also conducted by Cyprotex (Watertown, MA USA) in human liver microsomes both with and without NADPH using standardized protocols (Cyprotex Citation2022).
Experiment 8: Inhibition of cytochrome P450 (CYP450) xenobiotic detoxification enzymes in human liver microsomes
A cytochrome P450 (CYP450) inhibition assay was conducted by BioDuro-Sundia (Shanghai, China) to determine if sucralose-6-acetate or sucralose are inhibitors of CYP enzymes that metabolize exogenous as well as endogenous compounds. CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5 were selected for assessment as recommended by the United States Food and Drug Administration (Citation2020). The probe substrates for each CYP isoform along with the inhibitors used as positive controls are provided in . The CYP isoform-specific substrates were incubated with human liver microsomes along with sucralose-6-acetate or sucralose according to standardized protocols of BioDuro-Sundia. In Trial 1, sucralose-6-acetate was incubated in duplicate at 0, 0.137, 0.412, 1.23, 3.7, 11.1, 33.3 and 100 µM, and sucralose was incubated in duplicate at 0, 4.12, 12.3, 37, 111, 333, and 1000 µM (BioDuro-Sundia Citation2021a). In Trial 2, sucralose-6-acetate was reassessed to compare the results with Trial 1 and incubated in duplicate at 0, 0.098, 0.39, 1.56, 6.3, 25, and 100 μM (BioDuro-Sundia Citation2022). At the end of each incubation, the amount of probe substrate remaining was monitored by LC/MS/MS, and IC50 (half-maximal inhibitory concentration) values for sucralose and sucralose-6-acetate were computed.
Table 2. CYP450 inhibition study.
Results
Experiment 1: In vitro MultiFlow® DNA damage assay in TK6 cells
The results of the MultiFlow® assay in TK6 cells for sucralose-6-acetate indicated that sucralose-6-acetate exhibited a prototypical clastogenic signature for both +S9 and -S9 conditions. The fold-increases in γH2A× and nuclear p53 relative to control for the +S9 and -S9 treatments are shown in respectively. A clastogenic call was made for the +S9 treatment in because fold-elevation in 3 consecutive concentrations of sucralose-6-acetate met or exceeded the GEF cutoffs for the 24-hr γH2A× (1.31) and the 24-hr nuclear p53 (1.12) biomarkers. The lowest observed concentration of genotoxicity with S9 for sucralose-6-acetate was 353 µg/ml (803 µM). A clastogenic call was detected for 24-hr treatment without S9 in as fold-increases in 2 consecutive concentrations exceeded the cutoffs for fold 4-hr nuclear p53 (1.40) and 24-hr nuclear p53 (1.45), and one concentration exceeded the cutoff for 24-hr γH2A× (2.11). The lowest observed concentration for genotoxicity of sucralose-6-acetate without S9 was 707 µg/ml (1607 µM or 1.607 mM). Thus, clastogenicity call for sucralose-6-acetate occurred at a lower concentration with S9 metabolic activation than it did without S9 activation. Sucralose-6-acetate did not display an aneugenic signature.
Table 3. MultiFlow® assay for the sucralose-6-acetate (+S9) condition.
Table 4. MultiFlow® assay for the sucralose-6-acetate (−S9) condition.
The MultiFlow® assay for sucralose with concentrations up to 3980 µg/ml yielded no marked predictions of genotoxicity, with or without S9, in TK6 cells. However, there were 4 successive increasing concentrations of sucralose beginning at 994 µg/ml (2.5 mM) in the non-activated treatment that resulted in a greater than 2-fold rise in γH2A×, a marker for DNA breaks.
Experiment 2: In vitro mammalian cell MN test in TK6 cells
The results of the in vitro mammalian cell micronucleus test in TK6 cells shown in indicated that sucralose-6-acetate was positive in the 27-hr treatment without S9 but not with S9. That is, sucralose-6-acetate (−S9) elevated the MN frequency which is a biomarker of cytogenetic/chromosomal damage. The occurrence of binucleated cells was also enhanced at higher concentrations. For this reason, the MN frequency was determined in combined mono-, bi-, and multi-nucleated cells. When all populations of cells were combined and assessed (mono-, bi-, and multi-nucleated), there was a significant rise at 1000 μg/ml in the 27 hr -S9 treatment. A Cochran Armitage Trend Test of the top 3 concentrations of 500, 750 and 1000 µg/ml (1137, 1705, and 2274 µM) showed a significant concentration-dependent elevation within that concentration range, and the MN frequency was outside of the historical vehicle control limit. Data indicate the sucralose-6-acetate is genotoxic which is consistent with the MultiFlow® assay.
Table 5. Micronucleus test: 27-hr treatment without S9.
Table 6. Micronucleus test: 4-hr treatment with S9.
A MN test for sucralose itself was not performed in the current study as a previous MN test submitted during regulatory assessment was inconclusive (United States Food and Drug Administration US FDA Citation1998) and the MultiFlow® assay was not positive.
Experiment 3: In silico assessment of mutagenic potential by Leadscope®
The consensus call by the Leadscope® quantitative structure activity tool predicted that sucralose-6-acetate may be mutagenic and induce permanent transmissible genetic variations. Leadscope® also provided a bacterial mutation alert for the secondary alkyl halide as the active/primary portion or molecular fragment of concern for sucralose-6-acetate (). The program recommended a follow up with a bacterial reverse mutation test using standard strains of Salmonella typhimurium and/or E. coli. These findings for sucralose were ambiguous.
Experiment 4: Bacterial reverse mutation test (Ames Test)
Data from the bacterial reverse mutation tests in indicated that sucralose-6-acetate and sucralose were both negative (non-mutagenic) under the conditions, and according to the criteria, of the study protocol. Sucralose-6-acetate and sucralose, and/or their metabolites, did not induce reverse mutations in 4 strains of Salmonella typhimurium (TA98, TA100, TA1535, and TA1537) or in the WP2 uvrA strain of Escherichia coli in the presence and absence of an exogenous metabolic activation system (S9). These results do not confirm the in silico prediction by Leadscope® and suggest that sucralose-6-acetate and sucralose are not mutagenic. These observations indicate that although sucralose-6-acetate was genotoxic in both the MultiFlow® and MN test, DNA damage initiated by sucralose-6-acetate may not lead to permanent alterations in further generations of cells because it is not mutagenic.
Table 7. Bacterial reverse mutation test for sucralose-6-acetate.
Table 8. Bacterial reverse mutation test for sucralose.
Experiment 5: Assessment of transepithelial electrical resistance (TEER) and permeability in human transverse colon epithelium
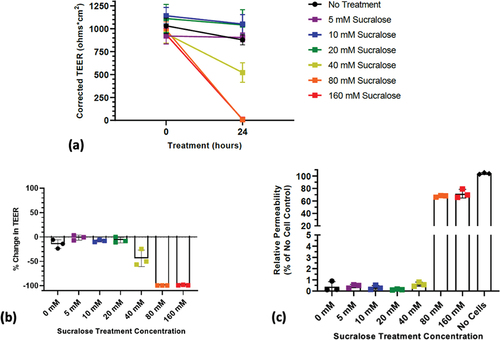
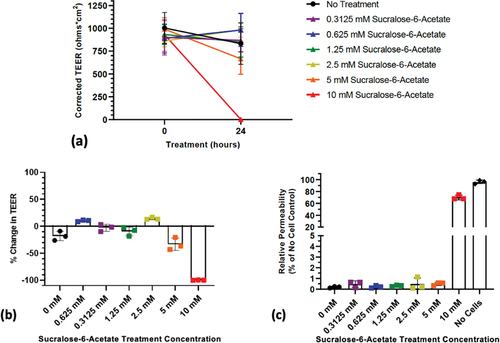
Both sucralose-6-acetate and sucralose altered TEER and permeability in human colonic epithelial monolayers at mM concentrations in the absence of bacteria. The results in below illustrate that a reduction in TEER from a single treatment for 24 hr with sucralose-6-acetate begins at 5 mM with a complete collapse at 10 mM. In , the relative permeability to 40 kDa FTIC-dextran significantly increased after exposure to 10 mM sucralose-6-acetate.
Figure 3. Response of human transverse colon epithelium to sucralose-6-acetate: (a) Corrected TEER of transverse colon monolayers showing that a reduction began to occur at 5 mM with a total loss at 10 mM; (b) Percent change in TEER of transverse colon computed from corrected TEER; (c) Change in permeability to 40 kDa FTIC-dextran transitions at 10 mM.
show that a reduction in TEER from a single treatment for 24 hr with sucralose begins at 40 mM with a complete collapse at 80 mM. illustrates that the relative permeability to 40 kDa FTIC-dextran was significantly elevated after incubation with 80 mM and 160 mM sucralose.
Experiment 6: RNA-seq and gene expression in transverse colon
Differential gene expression analysis was used to determine changes in gene expression in human transverse colon induced by sucralose-6-acetate and sucralose, each relative to control (no treatment). A total of 12,553 genes were analyzed after low-abundance filtering. Four comparisons using the R package Ballgown’s “stattest” function were: Sucralose-6-Acetate vs Control, Sucralose vs Control, Sucralose vs Sucralose-6-Acetate, and a three-way comparison for all samples (Sucralose vs Sucralose-6-Acetate vs Control).
Sucralose-6-acetate vs control
Thirty-four (34) genes were differentially expressed between sucralose-6-acetate and control samples, and 23 of these were identified. The expression of 16 of the identified genes significantly increased in the sucralose-6-acetate samples as compared to controls (), and the expression of 7 of the identified genes significantly decreased in the sucralose-6-acetate samples compared to controls (). In , three additional named but uncharacterized genes including LOC399900, LOC105371483, and LOC107986058 also exhibited significantly increased expression in sucralose-6-acetate than control. Twenty (20) of the 23 identified genes encode proteins while 2 of these are non-coding RNAs and 1 is a pseudogene. A brief description of each gene is presented in along with fold change and significance values. The fold changes for sucralose-6-acetate relative to control for three genes, metallothionein 1 G (MT1G), serine hydroxymethyltransferase (SHMT2), and activating transcription factor 3 (ATF3) were exceptionally large at 253.82, 81.23, and 54.49 respectively.
Table 9. Differential gene expression for sucralose-6-acetate with fold change >1.
Table 10. Differential gene expression for sucralose-6-acetate with fold change <1.
Sucralose vs control
Only two protein coding genes were differentially expressed between sucralose vs control samples. A brief description of these two genes is presented in along with fold change and significance values. For COX10, gene expression was higher in sucralose than control. For FAM166A, gene expression was lower in sucralose than control.
Table 11. Differential gene expression for sucralose.
Sucralose vs sucralose-6-acetate
There were 186 genes that were differentially expressed in the sucralose vs sucralose-6-acetate comparison. For 62 genes, expression in sucralose samples was higher than in sucralose-6-acetate samples with an average fold elevation of 4.63 ± 4.53. For 126 genes, expression was higher in sucralose-6-acetate than in sucralose but with a lower average fold change of only 0.28 ± 0.2. The Farnesyl-Diphosphate Farnesyltransferase gene (FDFT1) that encodes the first specific enzyme in cholesterol biosynthesis (Genecards Citation2023; The Human Protein Atlas Citation2023) displayed the largest change in expression in sucralose with a 30.93-fold rise relative to sucralose-6-acetate (P(q) value 3.6E–4 (0.02)). Other genes for which the expression was 3-fold higher or more in sucralose than in sucralose-6-acetate include: TFRC (cellular iron uptake), PFKP (glycolysis regulation), RHOT1 (mitochondrial trafficking), MRPL16 (protein synthesis within the mitochondrion), PRDX4 (protection against oxidative stress), PLCB3 (production of the secondary messengers diacylglycerol and inositol 1,4,5-triphosphate), ABO (production of ABO blood group proteins), FAM3D (insulin regulation), ACADVL (energy from fats), OGDH (biochemical conversions during the Krebs cycle), ACTR1A (microtubule-based vesicle motility), VPS13A (lipids transfer between membranes), and PTPRA (cell adhesion and proliferation)
Three-way comparison: Sucralose vs Sucralose-6-Acetate vs Control
There were 464 genes identified as differentially expressed. There was an over-representation in 7 Gene Ontology (Ashburner et al. Citation2000; The Gene Ontology Consortium Citation2019) categories listed under “Cellular Component.” In addition, 43 total regulatory motifs from TRANSFAC (Wingender Citation2008) were significantly over-represented as well as 33 terms from the Human Protein Atlas (The Human Protein Atlas Citation2023; Uhlén et al. Citation2015). The Cellular components were cytoplasm, cytosol, integral component of Golgi membrane, intracellular, intracellular membrane-bound organelle, intrinsic component of Golgi membrane, and membrane-bound organelle. The Human Protein Atlas indicated expression in 33 different tissue types originating in the small intestine, bronchus, colon, appendix, duodenum, salivary gland, pancreas, rectum, urinary bladder, stomach, lung, prostate, endometrium and kidneys. Transcription factor binding sites were associated with 266 of the genes. The 23 transcription factors identified were as follows: AP-2gamma: Elk-1, AP-2gamma, BEN, Churchill, E2F–1, E2F–2, E2F–3:HES-7, E2F–3, E2F–4, E2F–7, E2F, ETF, IRX-1, MAZ, MOVO-B, Sp1, TCF-1, TR4, WT1, ZF5, ZIC4, p300, pax-6.
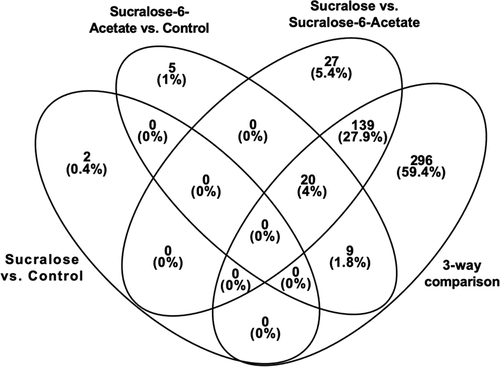
Genes in common for the four comparisons
The Venn diagram in illustrates the overlap of the loci (both named and unnamed genes) that were found in common for the 4 comparisons: Sucralose-6-Acetate vs Control, Sucralose vs Control, Sucralose vs Sucralose-6-Acetate, and the three-way comparison for all samples (Sucralose vs Sucralose-6-Acetate vs Control). The two genes in the Sucralose vs Control comparison were not found in any of the three other comparisons. Twenty loci were in common between comparisons that contain sucralose-6-acetate. Of those 20, the 16 named genes (ABO, ATE1-AS1, CASKIN1, CHST3, ELP5, EWSR1, KCNQ1DN, MCM2, MT1G, MTMR9, TNFSF14, UVRAG-DT, ZSCAN10) along with LOC399900, LOC105371483, and LOC107986058 are included in .
Figure 5. Venn diagram that shows the overlap of loci (including both named and unnamed genes) that were found in common for the four comparisons: Sucralose-6-Acetate vs Control, Sucralose vs Control, Sucralose vs Sucralose-6-Acetate, and the three-way comparison for all samples (Sucralose vs Sucralose-6-Acetate vs Control).
Experiment 7: Liver microsome stability assays for half-life (T1/2) determination
The results of the liver microsome stability assays in human, monkey, dog, rat, and mouse microsomes with NADPH are shown in for both sucralose-6-acetate and sucralose. The fraction of sucralose-6-acetate removed by the liver (extraction ratio) was calculated for sucralose-6-acetate using data from Houston (Citation1994) and Davies and Morris (Citation1993). A subsequent study in human liver microsomes obtained a value of T1/2 of 37.6 min (with and without NADPH) for sucralose-6-acetate and >180 for sucralose (with and without NADPH) (Cyprotex Citation2022). Data indicate that NADPH is not necessary for extraction of sucralose-6-acetate.
Table 12. T1/2 (min), intrinsic clearance (CLint), hepatic clearance (CLhep), and extraction ratios determined in microsome stability assays with NADPH (BioDuro-Sundia Citation2021b).
Experiment 8: Inhibition of cytochrome P450 (CYP450) xenobiotic detoxification enzymes in human liver microsomes
Sucralose-6-acetate was found to be an inhibitor of CYP1A2 and CYP2C19 in human liver microsomes while no significant inhibition was detected for CYP2C9, CYP2D6, or CYP3A4/5. No marked inhibitory effect on any CYP450 enzymes was noted for sucralose. The inhibition study was initially performed in duplicate for both sucralose-6-acetate and sucralose and repeated in duplicate 8 months later for sucralose-6-acetate to confirm the results. The mean IC50 (µM) values for the initial and replication investigations for sucralose-6-acetate are given in . In the initial study, the mean IC50 values for CYP1A2 and CYP2C19 were 42.9 µM and 89.3 µM. In the repetition study, the mean IC50 values for CYP1A2 and CYP2C19 were 65.1 µM and 46.3 µM. report the patterns of response of CYP1A2 and CYP2C19 from sucralose-6-acetate that were used to determine IC50 (µM). indicates that no marked CYP inhibition occurred for sucralose.
Figure 6(a). CYP450 inhibition – initial study: IC50 (µM) curves for sucralose-6-acetate for CYP1A2 and CYP2C19. Results from BioDuro-Sundia (Citation2021a).
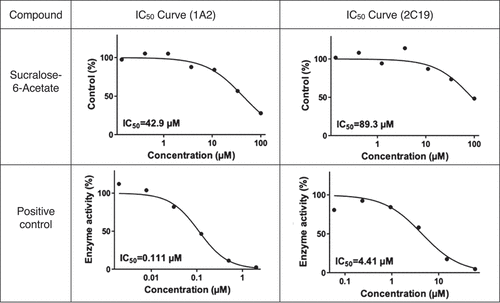
Figure 6(b). CYP450 inhibition – repeated study: IC50 (µM) curves for sucralose-6-acetate for CYP1A2 and CYP2C19. Results from BioDuro-Sundia (Citation2022).
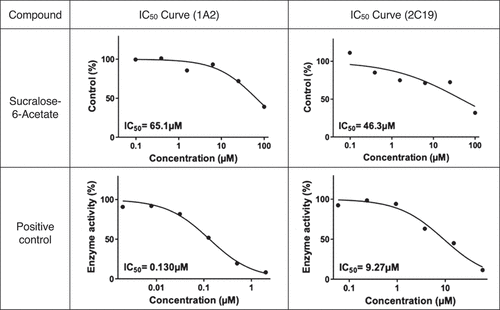
Table 13. CYP450 inhibition initial study: IC50 (µM) for sucralose-6-acetate (BioDuro-Sundia Citation2021a).
Table 14. CYP450 inhibition replication study: IC50 (µM) for sucralose-6-acetate (BioDuro-Sundia Citation2022).
Table 15. CYP450 inhibition study: IC50 (µM) for sucralose study (BioDuro-Sundia Citation2021a).
Discussion
The experiments conducted in this study found that sucralose-6-acetate was genotoxic, and the MoA was classified as clastogenic (induced DNA strand breaks). Further, exposure of human intestinal epithelium to sucralose-6-acetate as well as sucralose itself damaged tight junctions and impaired intestinal barrier function at mM concentrations. The transcriptome from human intestinal tissue determined using RNA-seq noted that sucralose-6-acetate significantly increased expression of genes associated with inflammation, oxidative stress, and cancer. Sucralose-6-acetate also inhibited two members of the CYP450 family at low µM concentrations that might potentially interfere with metabolism of endogenous and exogenous chemicals including medications.
Genotoxicity
The sucralose impurity and metabolite, sucralose-6-acetate, was found to be genotoxic in human lymphoblastoid cells in both the MultiFlow® high throughput assay as well as the standard MN test. In the MultiFlow® test, the lowest observed concentration for genotoxicity for sucralose-6-acetate without metabolic activation was 1.607 mM while the lowest observed level for genotoxicity with metabolic activation was 803 µM. The finding that metabolic activation resulted in a lower observed concentration for genotoxicity suggests that sucralose-6-acetate may be converted into additional DNA reactive metabolites. The MoA was concluded to be clastogenic (induction of DNA strand breaks) utilizing the MultiFlow® assay. These observations for sucralose-6-acetate genotoxicity by the Multiflow® assay were corroborated by cytogenetic/chromosomal damage in the Mammalian Cell Micronucleus Assay.
The potential adverse health effects attributed to genotoxicity of sucralose-6-acetate have not yet been addressed in archival scientific publications. Further, there has been no apparent systematic post-marketing surveillance of health effects from sucralose exposure by the manufacturer since this sweetener entered the food supply. provides three examples of the level of exposure to sucralose-6-acetate that might occur following ingestion of a single beverage sweetened with sucralose. The exposure is based upon the presence of sucralose-6-acetate in current commercial sucralose samples of levels up to 0.67% (Werness Citation2021).
Table 16. Three examples of estimated sucralose-6-acetate content in common beverages.
While some regulatory agencies hold that there is no acceptable level of genotoxic exposure, other institutions evaluated the genotoxic risk associated with potential exposure and MoA. A threshold of toxicological concern for genotoxicity (TTCgenotox) of 0.15 µg/person/day (0.0025 µg/kg bw/day for a 60 kg person) was suggested for chemicals at low levels in the diet (European Food Safety Authority EFSA Citation2016; Gooderham et al. Citation2020; Kroes et al. Citation2004; Serafimova, Coja, and Kass Citation2021). Data in indicate that single servings of sucralose-containing drinks may contain levels of sucralose-6-acetate that exceed a TTCgenotox of 0.15 µg/person/day by 4 orders of magnitude or more.
The actual exposure to sucralose-6-acetate, however, likely far exceeds levels in due to repeated dosing, biotransformation of sucralose to sucralose-6-acetate in the intestine, and potential of bioaccumulation. Repeated daily dosing enhances exposure to sucralose-6-acetate because this impurity was reported to persist in the body for at least 11 days after cessation of intake of sucralose (Bornemann et al. Citation2018). The systemic persistence of sucralose-6-acetate occurred even though this compound was shown in the present study to undergo partial extraction in the liver. Biotransformation of sucralose to sucralose-6-acetate was demonstrated to occur in the intestinal tract, elevating the % beyond that in commercial sucralose samples by a factor of approximately 20 (Bornemann et al. Citation2018; Werness Citation2021). Further, sucralose-6-acetate is more lipophilic than sucralose itself which may favor bioaccumulation through enhanced intestinal absorption and transport across cellular barriers.
Previous questions regarding sucralose genotoxicity were raised but the contribution of sucralose-6-acetate to these effects was not apparently investigated. Early tests of potential sucralose genotoxicity in a mouse MN test and a chromosomal aberration test in cultured human lymphocytes were inconclusive (United States Food and Drug Administration US FDA Citation1998). Four different comet assays (two in vivo and two in vitro) subsequently found that sucralose- initiated DNA damage in blood cells (Pasqualli et al. Citation2020), colon cell lines (Van Eyk Citation2015), gastrointestinal organs (Sasaki et al. Citation2002), and brain, kidneys, and liver tissues (Raya et al. Citation2020). The in vitro comet assays employed by Pasqualli et al. (Citation2020) and Van Eyk (Citation2015) detected DNA damage in the µM and mM range respectively. Our current finding of the fact that sucralose-6-acetate is genotoxic may partially explain these prior observations regarding sucralose in comet tests. Dietary exposure to sucralose from the 12th day of fetal life through the lifespan was found to initiate a significant dose-related elevated incidence of malignant tumors and a significant dose-related increased incidence of hematopoietic neoplasias in male mice (Soffritti et al. Citation2016). The mechanism by which sucralose exposure beginning prenatally produces hematopoietic neoplasias is not known but compounds such as sucralose-6-acetate (with a molecular weight <500 Daltons and lipophilic properties) readily diffuse across lipid membranes of the placenta (Griffiths and Campbell Citation2015) and mammary tissue in lactating mothers (Sylvetsky et al. Citation2015) and might bioaccumulate over time.
Recent epidemiological studies found that early onset of colorectal and other cancers of the digestive system are rapidly increasing in many middle- and high-income nations, and this elevated incidence of human cancer was associated with dietary choices and dysbiosis (Ugai et al. Citation2022). The global rise in colorectal cancers parallels the burden of inflammatory bowel disease (IBD) that is also rising globally (Alatab et al. Citation2020). Sucralose consumption was noted as a causative factor in IBD as well as a risk factor for colorectal cancer in animal models (Guo et al. Citation2021; Li et al. Citation2020; Rosales-Gómez et al. Citation2018; Wang et al. Citation2019) but the potential contribution of sucralose-6-acetate to this finding is not yet known. Questions have been raised in the scientific literature whether sucralose consumption may also contribute to IBD in humans (Qin Citation2011).
Intestinal barrier function effects
The assessment of transepithelial electrical resistance (TEER) and permeability in human transverse colon epithelium in the current in vitro study found that sucralose-6-acetate and sucralose both disrupt gastrointestinal epithelial tight junctions and mucosal barrier function at mM concentrations in the absence of bacteria. A significant collapse of TEER occurred after a single 24-hr exposure to 40 mM sucralose which is only 6.7-fold greater than the concentration of sucralose currently approved by the European Union (Citation2004) for use in a single syrup-type food supplement at 2400 mg/kg (6 mM). Integrity of the intestinal epithelial barrier is dependent upon tight junctions, the specialized complexes which connect adjacent cells and provide a physical and functional barrier that limits or regulates passive diffusion of ions, solutes, macromolecules, and cells from the lumen through the paracellular space. Sucralose-6-acetate and sucralose reduced the transepithelial resistance and enabled ions and macromolecules to pass from the apical (luminal) to the basolateral side of intestinal epithelium through the paracellular pathways. Enhanced intestinal permeability (leaky gut) that enables passage of microorganisms and metabolites into the body plays a major role in IBD (Lee Citation2015; Welcker et al. Citation2004), chronic liver disease (Mohandas and Vairappan Citation2017), as well as pathogenesis of colorectal cancer (Sánchez-Alcoholado et al. Citation2020). Further, elevated intestinal permeability in conjunction with repeated ingestion and retention of colonic contents over days may increase intraluminal concentration, absorption, and systemic exposure to sucralose and sucralose-6-acetate resulting long-term in bioaccumulation and toxicity.
Previous studies showed that factors, in addition to the direct interaction of sucralose and sucralose-6-acetate with tight junctions, also play a role in disruption of the intestinal barrier from exposure to sucralose (Schiffman and Rother Citation2013). These factors include dysbiosis of microbial gut flora as well as inflammation associated with oxidative stress and elevated presence of proinflammatory cytokines. Abou-Donia et al. (Citation2008) first reported that strain-specific decrements in commensal anaerobic bacteria were associated with histopathological changes in the colon including lymphocytic infiltrates into the epithelium, epithelial scarring, atrophy/disorganization/architectural disruption, inflammation of glands, submucosal (and/or lamina propria) lymphoid aggregates, lymphoid follicles, mild depletion of goblet cells, and loss of superficial mucin. These effects occurred after 90 days of consumption of sucralose by rats at levels approved by regulatory agencies. Subsequent studies also demonstrated alterations in gut bacteria from sucralose exposure (Bian et al. Citation2017; Méndez-García et al. Citation2022; Suez et al. Citation2014, Citation2022; Zhang et al. Citation2022). These investigations do not support the historical claim in the Food Additive Petition for regulatory approval by the US FDA that “sucralose does not inhibit either aerobic or anaerobic microorganisms” (United States Food and Drug Administration US FDA Citation2021). Maternal ingestion of sucralose in mice during pregnancy also impacted their progeny’s microbiome (Dai et al. Citation2020, Citation2021; Olivier-Van Stichelen, Rother, and Hanover Citation2019). Gut microbes and their metabolites were shown to modulate the expression of tight junction proteins in both in vivo and in vitro models (Anderson et al. Citation2010; Bansal et al. Citation2010; Ewaschuk et al. Citation2008; Ukena et al. Citation2007). Inflammation is also associated with dysbiosis along with disruption of gut barrier integrity (Al Bander et al. Citation2020), and numerous studies reported that inflammatory biomarkers are induced by sucralose ingestion (Bian et al. Citation2017; Farid et al. Citation2020; Li et al. Citation2020; Rosales-Gómez et al. Citation2018; Shil and Chichger Citation2021). Intestinal inflammation might also induce genotoxicity in extraintestinal tissues (Westbrook et al. Citation2011).
Another conclusion that may be drawn from the current study of human transverse colon in the absence of bacteria is that bioconversion of sucralose to sucralose-6-acetate observed by Bornemann et al. (Citation2018) was mediated by bacteria and not host metabolism. Chromatographic analysis of the apical and basal supernatants illustrated no apparent conversion of sucralose to sucralose-6-acetate in the RepliGut® System (OpAns Citation2021). Thus, enrichment of the sucralose-6-acetate to sucralose ratio in fecal samples reported by Bornemann et al. (Citation2018) most likely results from bacterial metabolism rather than metabolism by acetyl coenzyme A (acetyl CoA) in host intestinal epithelium. Acetylation of sucralose in the gut may serve as a detoxification mechanism for bacteria (Koppel, Rekdal, and Balskus Citation2018) because it facilitates excretion of sucralose from microbial cells by diminishing its polarity. Sucralose-6-acetate may also contribute to the blunted growth of anaerobic bacteria in the gut that was reported after ingestion of sucralose by Abou-Donia et al. (Citation2008). Acetylation of pharmaceutical compounds such as 5-aminosalicylate drugs previously were found to inhibit growth of anaerobes (Delomenie et al. Citation2001).
Gene expression
The RNA-seq and gene expression analysis in the current study indicates that sucralose-6-acetate upregulated expression of genes in transverse colon that are associated with biological responses to harmful chemicals and conditions. The metallothionein 1 G (MT1G) displayed the largest change in expression in sucralose-6-acetate with a 253.82-fold rise relative to untreated control. Metallothionein (MT) genes encode proteins that are biomarkers of inflammation, oxidative stress, and cancer as well as cellular toxicity from insecticides, herbicides, metals, and other xenobiotic compounds (Bauman et al. Citation1991; Dai et al. Citation2021; Migliaccio et al. Citation2020; Ostrakhovitch et al. Citation2006; Rodrigo et al. Citation2020; Ruttkay-Nedecky et al. Citation2013; Si and Lang Citation2018; Tong et al. Citation2020). MT1G upregulation accelerates the G1/S transition in the growth phase of acute promyelocytic leukemia cells (Hirako and Takahashi Citation2021). Enhanced expression of MTs was also reported in IBD (Brüwer et al. Citation2001; Dooley et al. Citation2004). As noted previously, hematopoietic neoplasias (Soffritti et al. Citation2016) and IBD (Li et al. Citation2016; Citation2020; Wang et al. Citation2019; Guo et al. Citation2021; Rodriguez-Palacios et al. Citation2018) occur in sucralose-fed rodent models. However, the potential contribution of sucralose-6-acetate to the genesis of hematopoietic neoplasias or IBD has not apparently been investigated.
Three additional genes, SHMT2, ATF3 and carbohydrate sulfotransferase 3 (CHST3), were also markedly expressed by sucralose-6-acetate with 81.23, 54.49, and 9.26-fold elevation relative to untreated control. SHMT2 encodes a key mitochondrial enzyme, serine hydroxymethyltransferase-2, that catalyzes the reaction of serine to glycine that is found in high concentrations in intestinal epithelial cells. SHMT2 initiates lymphoma development through epigenetic tumor suppressor silencing (Parsa et al. Citation2020), drives the progression of colorectal cancer (Cui et al. Citation2022; Liu et al. Citation2021), potentiates the aggressive process of oral squamous cell carcinoma (Zheng et al. Citation2022) and promotes tumorigenesis in rhabdomyosarcoma (Nguyen et al. Citation2021). ATF3 encodes a member of the mammalian activation transcription factor/cAMP responsive element-binding (CREB) protein family of transcription factors. ATF3 is a marker of oxidative stress (Ketola et al. Citation2012) and plays a role in modulation of metabolism, immunity, and oncogenesis (Yin et al. Citation2008; Ku and Cheng Citation2020). The carbohydrate sulfotransferase 3 (CHST3) gene encodes an enzyme (chondroitin 6-O-sulfotransferase 1 or C6ST–1) that plays a role in the formation of chondroitin 6-sulfate (MedlinePlus Citation2023). Chondroitin 6-sulfate is involved in development and maintenance of the skeleton as well as naïve T lymphocytes (Uchimura et al. Citation2002). Chondroitin 6-sulfate expression is upregulated in human glioma cells, and this upregulation is correlated with glioma malignancy (Pan et al. Citation2020).
Other genes for which expression was greater for sucralose-6-acetate than control were also implicated in cancer in some tissues. Minichromosome maintenance complex component 2 (MCM2) is a prognostic marker of poor prognosis in squamous cell/adenosquamous carcinoma and adenocarcinoma of the gallbladder (Liu et al. Citation2016) and hepatocellular carcinoma (Tang et al. Citation2022). Elevated expression of zinc finger and SCAN domain containing 10 (ZSCAN10) in glioma tissues was associated with poor prognosis in glioma (Jiang et al., Citation2019). EWS RNA Binding Protein 1 (EWSR1) is a prognostic marker of unfavorable outcomes liver cancer (Jiang et al. Citation2021). Tumor necrosis factor superfamily member 14 (TNFSF14) is upregulated and a prognostic marker of poor outcome in renal cell cancer (Xu et al. Citation2020) and SET nuclear proto-oncogene is an unfavorable prognostic marker in liver cancer (Van Nguyen et al. Citation2021). Elongator acetyltransferase complex subunit 5 (ELP5) plays a role in tumorigenicity of melanoma cells (Close et al. Citation2012).
Only two genes were differentially expressed between sucralose and control samples. COX10 displayed higher numerical expression in the sucralose incubation as compared to control. The COX10 gene encodes a component of the mitochondrial electron transport chain and is required for natural killer (NK) cell expansion (Mah-Som et al. Citation2021). FAM166A which plays a role in spermatogenesis (The Human Protein Atlas, Citation2023) exhibited a higher numerical expression in control than sucralose. This finding of lower expression of FAM166A in the sucralose exposure relative to control did not occur with sucralose-6-acetate such that the reduction in expression of this spermatogenesis-associated gene appears to be initiated by sucralose itself. The effects of diminished expression of the spermatogenesis gene FAM166A by sucralose are not known. A historical 28-day gavage study on the glycolytic activity of sucralose in the rat spermatozoa showed no marked effect (Kille et al. Citation2000b). However, the 28-day test period may be insufficient to assess the entire duration of spermatogenesis in rats which is 52 days (Clouthier et al. Citation1996). A four-week feeding study found that a commercial artificial sweetener containing sucralose as well as acesulfame-K altered spermatogenesis in mice (Al-Qudsi and Al-Dosssary, Citation2020). Human feeding studies of the influence of sucralose on spermatogenesis were not undertaken but exposure to organochlorine compounds is associated with altered semen quality, DNA fragmentation, and chromosome aneuploidy in human males (Giulioni et al. Citation2022).
Within the current study, results of the sucralose vs sucralose-6-acetate comparison of gene expression are consistent with the sucralose-6-acetate vs control comparison that indicate sucralose-6-acetate impaired normal cellular functioning. The expression of genes for essential and fundamental cellular functions were lower in sucralose-6-acetate relative to sucralose. The Farnesyl-diphosphate farnesyltransferase (FDFT1) gene that encodes the first specific enzyme in cholesterol biosynthesis was markedly expressed by sucralose relative to sucralose-6-acetate with a fold change of 30.93. Cholesterol is not only essential for stability of cell membranes but also for tight junction formation (Shigetomi et al. Citation2023). Taken together data obtained from the sucralose vs sucralose-6-acetate comparison along with increased expression of MT1G, SHMT2, ATF3, and CHST3 in the sucralose-6-acetate vs control comparison indicated that exposure of human intestinal epithelium to sucralose-6-acetate disrupts essential cellular processes.
Previous studies reported changes in gene expression related to inflammation after exposure to sucralose. Increased gene expression of hepatic inflammatory markers (MMP-2 and iNOS) was detected in sucralose-treated mice (Bian et al. Citation2017) although it is not known if this was due to direct stimulation of liver cells by sucralose or sucralose-6-acetate, or to functional/metabolic alterations subsequent to sucralose exposure. Sucralose also enhanced adipogenesis and antioxidant gene expression in an in vitro study of human adipose tissue (Kundu et al. Citation2020). The upregulation of the antioxidant gene GPX3 was interpreted as a compensatory response to elevated intracellular accumulation of reactive oxygen species (ROS). Another in vitro investigation in adipose tissue found that sucralose upregulated PPARγ, a suppressor of NF-κB-mediated pro-inflammatory responses (Azad et al. Citation2020). Consumption of diet soda sweetened with sucralose as well as acesulfame-K (another artificial sweetener) altered inflammatory transcriptome pathways including NF-κB signaling in subcutaneous adipose tissue (Sylvetsky et al. Citation2020).
Pharmacokinetics
The study of the half-life (T1/2) in liver microsomes indicates that sucralose-6-acetate is extracted to a certain extent by the liver with a greater effect in humans than rats, but absorption and metabolism of sucralose-6-acetate have not yet been fully characterized. The extraction does not appear to involve Phase 1 metabolism in human liver microsomes since it is independent of NADPH. Although the extraction rate is higher for sucralose-6-acetate than for sucralose, sucralose-6-acetate was detected in urine for 5 days longer than sucralose after discontinuation of sucralose intake. This might be attributed to greater lipophilicity and hence elevated bioaccumulation potential of sucralose-6-acetate.
The investigation of xenobiotic detoxification enzymes in human liver microsomes noted that sucralose-6-acetate is an inhibitor of two members of the cytochrome P450 (CYP450) family (CYP1A2 and CYP2C19). Inhibition of these enzymes may potentially affect bioavailability of drugs and levels of endogenous substrates. No significant inhibitory effect on CYP450 enzymes was found for sucralose. Inhibition of CYP1A2 and CYP2C19 might reduce metabolism of endogenous and exogenous chemicals and precipitate potentially adverse metabolic effects.
CYP1A2 metabolizes many endogenous compounds such as retinols, melatonin, steroids (including estradiol), estrogens, uroporphyrinogen and arachidonic acids. Inhibition of CYP1A2 may potentially increase estradiol levels, and there is an association between estradiol and breast cancer (Cummings et al. Citation2002; DrugBank Citation2022; PubChem Citation2022). Inhibition of CYP1A2 may also elevate plasma levels of caffeine (a substrate of CYP1A2), potentially exacerbating anxiety, sleep problems, and even high blood pressure.
Inhibition of CYP1A2 by sucralose-6-acetate may also potentially increase plasma concentrations of pharmaceuticals that are CYP1A2 substrates. Representative CYP1A2 substrates (along with their biological functions/indications) include alosetron (irritable bowel syndrome), axitinib (renal cell carcinoma), caffeine (CNS stimulant), clozapine (antipsychotic), flutamide (prostate cancer), frovatriptan (migraine), melatonin (sleep-wake cycle), mexiletine (heart arrhythmias), mirtazapine (antidepressant), olanzapine (antipsychotic), rasagiline (Parkinson’s disease), tacrine (Alzheimer’s disease), theophylline (bronchodilator), tizanidine (muscle relaxer), and triamterene (diuretic). Inhibition of CYP2C19 by sucralose-6-acetate might potentially elevate plasma concentrations of CYP2C19 substrates. Representative CYP2C19 substrates that are known to be significantly affected by CYP2C19 inhibitors (along with their biological functions/indications) include abrocitinib (atopic dermatitis), cannabidiol (seizures), carisoprodol (muscle relaxant), cilostazol (claudication), citalopram (antidepressant), clobazam (sedative), clopidogrel (blood thinner), diazepam (anxiety), esomeprazole (gastroesophageal reflux), methadone (narcotic addiction), omeprazole (gastroesophageal reflux), phenytoin (seizures), and tofacitinib (rheumatoid arthritis) (DrugBank Citation2022; PubChem Citation2022).
The inhibition of CYP1A2 and CYP2C19 by sucralose-6-acetate reported here was observed in vitro in human liver microsomes in the absence of bacteria. However, additional pharmacokinetic effects, including inhibition or induction, attributed to sucralose-6-acetate may occur in vivo because intestinal flora and inflammation are well-known to alter the expression and activity of CYP450 metabolizing enzymes as well as transporters (Claus et al. Citation2011; Kuno et al. Citation2016; Toda et al. Citation2009; Selwyn et al. Citation2016; Togao et al. Citation2020; Collins and Patterson Citation2020; Hu et al. Citation2021; Lenoir et al. Citation2021). Further, oxidative stress was noted to induce cytochrome P450 3A4 (CYP3A4) (Nagai et al. Citation2004; Strolin-Benedetti et al. Citation1999) as well as the transporter P-glycoprotein/P-gp (Abu-Qare, Elmasry, and Abou-Donia Citation2003; Callaghan et al. Citation2008; Feng et al. Citation2019; Shchulkin et al. Citation2021), and induction of both CYP3A4 and P-gp were reported in vivo after 12 weeks of sucralose ingestion (Abou-Donia et al. Citation2008).
Conclusions
The 8 projects performed in this study add to the large and growing scientific literature that report adverse biological impacts attributed to exposure to sucralose. In the current investigation sucralose-6-acetate, a sucralose impurity and metabolite, was found to be genotoxic with a clastogenic MoA associated with induction of breaks in DNA. Exposure of intestinal epithelium in vitro to mM concentrations of both sucralose-6-acetate and sucralose in the absence of intestinal bacteria impaired the integrity of intestinal barrier function. Sucralose-6-acetate induced expression of genes in intestinal epithelium associated with inflammation, oxidative stress, and cancer including MT1G and SHMT2. Sucralose-6-acetate also blocked two members of the cytochrome P450 family (CYP1A2 and CYP2C19) that metabolize both endogenous and xenobiotic compounds that might consequently lead to adverse toxicological exposures. These findings raise health and safety concerns regarding the continued presence of sucralose in the food supply and indicate that a regulatory status review needs to be undertaken.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data that support the findings of this study are available from the corresponding author, SSS, upon reasonable request.
Additional information
Funding
References
- Abou-Donia, M. B., E. M. El-Masry, A. A. Abdel-Rahman, R. E. McLendon, and S. S. Schiffman. 2008. Splenda alters gut microflora and increases intestinal P-glycoprotein and cytochrome P-450 in male rats. J. Toxicol. Environ. Health Part A 71 (21):1415–29. doi:10.1080/15287390802328630.
- Abu-Qare, A., E. Elmasry, and M. Abou-Donia. 2003. A role for P-glycoprotein in environmental toxicology. J. Toxicol. Environ. Health Part B 6 (3):279–88. doi:10.1080/10937400306466.
- Aclairo. 2019. Leadscope® analysis of sucralose-6-acetate and sucralose. Vienna, VA, USA: Aclairo® pharmaceutical development group.
- Agresti, A. 2002. Categorical Data Analysis. 2nd ed. Hoboken, NJ, US: John Wiley & Sons, Inc. https://onlinelibrary.wiley.com/doi/book/10.1002/0471249688?utm_sq=gxwftwi3be.
- Alatab, S., S. G. Sepanlou, K. Ikuta, H. Vahedi, C. Bisignano, S. Safiri, A. Sadeghi, M. R. Nixon, A. Abdoli, H. Abolhassani, et al. 2020. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 5 (1):17–30. doi:10.1016/S2468-1253(19)30333-4.
- Al Bander, Z., M. D. Nitert, A. Mousa, and N. Naderpoor. 2020. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health 17 (20):7618. doi:10.3390/ijerph17207618.
- Allbritton, N., Y. Wang, C. Sims, S. Magness, and S. Bultman. 2021. Methods to generate gastrointestinal epithelial tissue constructs. United States Patent US 11,193,110 B2, issued December 7, 2021. https://patentimages.storage.googleapis.com/69/fc/f6/07af3119a3ed6b/US11193110.pdf
- Al-Qudsi, F., and A. Al-Dosssary. 2020. Commercial artificial sweeteners affect spermatogenesis in mice. Int. J. Life Sci. Pharma Res. 10:L6–15. doi:10.22376/ijpbs/lpr.2020.10.4.L6-15.
- Altis Biosystems. 2020. Investigation of the effects of sucralose and sucralose-6-acetate on human colonic epithelial monolayers. Study director Bailey Zwarycz PhD. Altis Biosystems, 6 Davis Drive, Durham, NC 27709, USA.
- Altis Biosystems. 2021. Further investigating the effects of sucralose and sucralose-6-acetate on human colonic epithelial monolayers. Study director Bailey Zwarycz, PhD. Altis Biosystems, 6 Davis Drive, Durham, NC 27709, USA.
- Anderson, R. C., A. L. Cookson, W. C. McNabb, Z. Park, M. J. McCann, W. J. Kelly, and N. C. Roy. 2010. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 10:316. doi:10.1186/1471-2180-10-316.
- Armitage, P. 1955. Tests for linear trends in proportions and frequencies. Biometrics 11 (3):375–86. doi:10.2307/3001775.
- Ashburner, M., C. A. Ball, J. A. Blake, D. Botstein, H. Butler, J. M. Cherry, A. P. Davis, K. Dolinski, S. S. Dwight, J. T. Eppig, et al. 2000. Gene ontology: Tool for the unification of biology. Nat. Genetics 25 (1):25–29. doi:10.1038/75556.
- Azad, M. B., A. Archibald, M. M. Tomczyk, A. Head, K. G. Cheung, R. J. de Souza, A. B. Becker, P. J. Mandhane, S. E. Turvey, T. J. Moraes, et al. 2020. Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: Evidence from humans, mice, and cells. Int. J. Obes. 44 (10):2137–48. doi:10.1038/s41366-020-0575-x.
- Babraham Bioinformatics. 2019. Trim galore Version 0.6.1. https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/.
- Baird, I. M., N. W. Shephard, R. J. Merritt, and G. Hildick-Smith. 2000. Repeated dose study of sucralose tolerance in human subjects. Food Chem. Toxicol. 38:S123–29. doi:10.1016/s0278-6915(00)00035-1.
- Bansal, T., R. C. Alaniz, T. K. Wood, and A. Jayaraman. 2010. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. National Acad. Sci. USA 107 (1):228–33. doi:10.1073/pnas.0906112107.
- Bauman, J. W., J. Liu, Y. P. Liu, and C. D. Klaassen. 1991. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol. Appl. Pharmacol. 110 (2):347–54. doi:10.1016/S0041-008X(05)80017-1.
- Bernacki, D. T., S. M. Bryce, J. C. Bemis, D. Kirkland, and S. D. Dertinger. 2016. γH2AX and p53 responses in TK6 cells discriminate promutagens and nongenotoxicants in the presence of rat liver S9. Environ. Mol. Mutagen. 57 (7):546–58. doi:10.1002/em.22028.
- Bian, X., L. Chi, B. Gao, P. Tu, H. Ru, and K. Lu. 2017. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front. Physiol. 8:487. doi:10.3389/fphys.2017.00487.
- BioDuro-Sundia. 2021a. CYP inhibition determination, Trial 1. Shanghai, China: Study director: Jaing Pu.
- BioDuro-Sundia. 2021b. Microsomal stability assay of sucralose-6-acetate and sucralose. Shanghai, China: BioDuro-Sundia. Study director: Jaing Pu. https://bioduro-sundia.com/adme-microsomal-stability-assay/.
- BioDuro-Sundia. 2022. CYP inhibition determination, Trial 2, repetition of sucralose-6-acetate. Shanghai, China: Study director: Jaing Pu.
- BioReliance. 2020a. CAN MultiFLOW High-throughput 96-well assay using human TK6 cells to screen for Clastogens, Aneugens: Sucralose-6-Acetate. Study AG05LV.365.BTL. Study director: Shambhu Roy, PhD, DABT, ERT, 9630 Medical Center Drive, Rockville, MD.
- BioReliance. 2020b. In Vitro Mammalian Cell Micronucleus Screening Assay in TK6 Cells. 2 Arm Treatment (Treatments of 4 hours +S9 and 27 hours -S9). Study AG05LV.366ICH.BTL. Study directors: Pavan Gollapudi, PhD and Shambhu Roy, PhD, Rockville, MD.
- BioReliance. 2020c. Bacterial Reverse Mutation Assay: Sucralose and Sucralose-6-acetate. Study AG05LU-LV.501028.BTL. Study director: Shannon Bruce, MFS, 9630 Medical Center Drive, Rockville, MD.
- BioReliance. 2021. CAN MultiFLOW High-throughput 96-well assay using human TK6 cells to screen for Clastogens, Aneugens: Sucralose. Study, AG05LU.365.BTL. Study director: Pavan Gollapudi, PhD, 9630 Medical Center Drive, Rockville, MD.
- Bornemann, V., S. C. Werness, L. Buslinger, and S. S. Schiffman. 2018. Intestinal metabolism and bioaccumulation of sucralose in adipose tissue in the rat. J. Toxicol. Environ. Health Part A 81 (18):913–23. doi:10.1080/15287394.2018.1502560.
- Brüwer, M., K. W. Schmid, K. A. Metz, C. F. Krieglstein, N. Senninger, and G. Schürmann. 2001. Increased expression of metallothionein in inflammatory bowel disease. Inflamm. Res. 50 (6):289–93. doi:10.1007/PL00000246.
- Bryce, S. M., J. C. Bemis, J. A. Mereness, R. A. Spellman, J. Moss, D. Dickinson, M. J. Schuler, and S. D. Dertinger. 2014. Interpreting in vitro micronucleus positive results: Simple biomarker matrix discriminates clastogens, aneugens, and misleading positive agents. Environ. Mol. Mutagen. 55 (7):542–55. doi:10.1002/em.21868.
- Bryce, S. M., D. T. Bernacki, J. C. Bemis, and S. D. Dertinger. 2016. Genotoxic mode of action predictions from a multiplexed flow cytometric assay and a machine learning approach. Environ. Mol. Mutagen. 57 (3):171–89. doi:10.1002/em.21996.
- Bryce, S. M., D. T. Bernacki, J. C. Bemis, R. A. Spellman, M. E. Engel, M. Schuler, E. Lorge, P. T. Heikkinen, U. Hemmann, V. Thybaud, et al. 2017. Interlaboratory evaluation of a multiplexed high information content in vitro genotoxicity assay. Environ. Mol. Mutagen. 58 (3):146–61. doi:10.1002/em.22083.
- Bryce, S. M., D. T. Bernacki, S. L. Smith-Roe, K. L. Witt, J. C. Bemis, and S. D. Dertinger. 2018. Investigating the generalizability of the MultiFlow® DNA damage assay and several companion machine learning models with a set of 103 diverse test chemicals. Toxicol. Sci. 162 (1):146–66. doi:10.1093/toxsci/kfx235.
- Callaghan, R., E. Crowley, I. D. Potter, S. Kerr, and I. D. Kerr. 2008. P‐glycoprotein: So many ways to turn it on. J. Clin. Pharmacol. 48 (3):365–78. doi:10.1177/0091270007311568.
- Canada Gazette. 1991. Food and drug regulations, amendment [Sucralose] (SOR/91-527). Canada Gazette II 125 (20):3125–30.
- Catani, S. J., J. E. Wiley, N. M. Vernon, C. M. Merkel, and E. Micinski. 2006. Process for improving sucralose purity and yield. United States Patent 6,998,480 B2, February 14, 2006. https://patentimages.storage.googleapis.com/52/51/f8/fa99e898ec5443/US6998480.pdf.
- Chen, S., Y. Zhou, Y. Chen, and J. Gu. 2018. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34 (17):i884–90. doi:10.1093/bioinformatics/bty560.
- Claus, S. P., S. L. Ellero, B. Berger, L. Krause, A. Bruttin, J. Molina, A. Paris, E. J. Want, I. de Waziers, O. Cloarec, et al. 2011. Colonization-induced host-gut microbial metabolic interaction. mBio 2 (2):10.1128/mBio.00271-10. e00271-10.
- Close, P., M. Gillard, A. Ladang, Z. Jiang, J. Papuga, N. Hawkes, L. Nguyen, J. -P. Chapelle, F. Bouillenne, J. Svejstrup, et al. 2012. DERP6 (ELP5) and C3ORF75 (ELP6) regulate tumorigenicity and migration of melanoma cells as subunits of Elongator. J. Biol. Chem. 287 (39):32535–45. doi:10.1074/jbc.m112.402727.
- Clouthier, D. E., M. R. Avarbock, S. D. Maika, R. E. Hammer, and R. L. Brinster. 1996. Rat spermatogenesis in mouse testis. Nature 381 (6581):418–21. doi:10.1038/381418a0.
- Cochran, W. G. 1954. Some methods for strengthening the common χ2 tests. Biometrics 10 (4):417–51. doi:10.2307/3001616.
- Collins, S. L., and A. D. Patterson. 2020. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 10 (1):19–32. doi:10.1016/j.apsb.2019.12.001.
- CTFile Formats. 2005. https://web.archive.org/web/20070630061308/http://www.mdl.com/downloads/public/ctfile/ctfile.pdf.
- Cui, X., Y. Cui, T. Du, X. Jiang, C. Song, S. Zhang, C. Ma, Y. Liu, Q. Ni, Y. Gao, et al. 2022. SHMT2 drives the progression of colorectal cancer by regulating UHRF1 expression. Can. J. Gastroenterol. Hepatol. 2022:3758697. doi:10.1155/2022/3758697.
- Cummings, S. R., T. Duong, E. Kenyon, J. A. Cauley, M. Whitehead, and K. A. Krueger. 2002. Serum estradiol level and risk of breast cancer during treatment with raloxifene. J. Am. Med. Assoc. 287 (2):216–20. doi:10.1001/jama.287.2.216.
- Cyprotex. 2022. Microsomal stability assay of sucralose-6-acetate and sucralose with and without NADPH. Cyprotex, 313 Pleasant St., Watertown, MA 02472, USA.
- Dai, X., Z. Guo, D. Chen, L. Li, X. Song, T. Liu, G. Jin, Y. Li, Y. Liu, A. Ajiguli, et al. 2020. Maternal sucralose intake alters gut microbiota of offspring and exacerbates hepatic steatosis in adulthood. Gut Microbes. 11 (4):1043–63. doi:10.1080/19490976.2020.1738187.
- Dai, X., C. Wang, Z. Guo, Y. Li, T. Liu, G. Jin, S. Wang, B. Wang, K. Jiang, and H. Cao. 2021. Maternal sucralose exposure induces Paneth cell defects and exacerbates gut dysbiosis of progeny mice. Food Funct. 12 (24):12634–46. doi:10.1039/d1fo02921e.
- Dai, H., L. Wang, L. Li, Z. Huang, and L. Ye. 2021. Metallothionein 1: A new spotlight on inflammatory diseases. Front. Immunol. 12:739918. doi:10.3389/fimmu.2021.739918.
- Dalenberg, J. R., B. P. Patel, R. Denis, M. G. Veldhuizen, Y. Nakamura, P. C. Vinke, S. Luquet, and D. M. Small. 2020. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 31 (3):493–502. doi:10.1016/j.cmet.2020.01.014.
- Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10 (7):1093–95. doi:10.1023/a:1018943613122.
- Dearfield, K. L., B. B. Gollapudi, J. C. Bemis, R. D. Benz, G. R. Douglas, R. K. Elespuru, G. E. Johnson, D. J. Kirkland, M. J. LeBaron, A. P. Li, et al. 2017. Next generation testing strategy for assessment of genomic damage: A conceptual framework and considerations. Environ. Mol. Mutagen. 58 (5):264–83. doi:10.1002/em.22045.
- Delomenie, C., S. Fouix, S. Longuemaux, N. Brahimi, C. Bizet, B. Picard, E. Denamur, and J. -M. Dupret. 2001. Identification and functional characterization of arylamine N-acetyltransferases in eubacteria: Evidence for highly selective acetylation of 5-aminosalicylic acid. J. Bacteriol. 183 (11):3417–27. doi:10.1128/JB.183.11.3417-3427.2001.
- De Oliveira, D. N., M. de Menezes, and R. R. Catharino. 2015. Thermal degradation of sucralose: A combination of analytical methods to determine stability and chlorinated byproducts. Sci. Rep. 5 (1):9598. doi:10.1038/srep09598.
- Dooley, T. P., E. V. Curto, S. P. Reddy, R. L. Davis, G. W. Lambert, T. W. Wilborn, and C. O. Elson. 2004. Regulation of gene expression in inflammatory bowel disease and correlation with IBD drugs: Screening by DNA microarrays. Inflamm. Bowel Dis. 10 (1):1–14. doi:10.1097/00054725-200401000-00001.
- DrugBank. 2022. https://go.drugbank.com
- DuBois, G. E., D. E. Walters, S. S. Schiffman, Z. S. Warwick, B. J. Booth, S. D. Pecore, K. Gibes, B. T. Carr, and L. M. Brands. 1991. Concentration-response relationships of sweeteners: A systematic study. In Sweeteners. Discovery, Molecular Design, and Chemoreception. ACS Symposium Series 450, ed. D. E. Walters, F. T. Orthoefer, and G. E. DuBois, pp. 261–76. Washington, D.C: American Chemical Society.
- Dull, B. J., K. Salata, and P. Goldman. 1987. Role of the intestinal flora in the acetylation of sulfasalazine metabolites. Biochem. Pharmacol. 36 (21):3772–74. doi:10.1016/0006-2952(87)90034-7.
- Eisenreich, A., R. Gürtler, and B. Schäfer. 2020. Heating of food containing sucralose might result in the generation of potentially toxic chlorinated compounds. Food Chem. 321:126700. doi:10.1016/j.foodchem.2020.126700.
- Elbrecht, D. H., C. J. Long, and J. J. Hickman. 2016. Transepithelial/Endothelial Electrical Resistance (TEER) theory and applications for microfluidic body-on-a-chip devices. J. Rare Dis. Res. Treat. 1 (3):46–52. doi:10.29245/2572-9411/2016/3.1026.
- European Food Safety Authority (EFSA). 2016. Review of the threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Supporting Publ. 13 (3):1–50. doi:10.2903/sp.efsa.2016.EN-1006.
- European Union (EU). 2004. Directive 2003/115/EC of the European Parliament and of the Council of 22 December 2003 amending Directive 94/35/EC on sweeteners for use in foodstuffs. Off. J. Eur. Union 47 (L24):65–71. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:024:0065:0071:EN:PDF.
- Ewaschuk, J. B., H. Diaz, L. Meddings, B. Diederichs, A. Dmytrash, J. Backer, M. Looijer-van Langen, and K. L. Madsen. 2008. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol-Gastr. L. 295 (5):G1025–34. doi:10.1152/ajpgi.90227.2008.
- Farid, A., M. Hesham, M. El-Dewak, and A. Amin. 2020. The hidden hazardous effects of stevia and sucralose consumption in male and female albino mice in comparison to sucrose. Saudi Pharm. J. 28 (10):1290–300. doi:10.1016/j.jsps.2020.08.019.
- Feng, Q., W. Yang, Z. Gao, X. Ruan, and Y. Zhang. 2019. Up-regulation of P-gp via NF-κB activation confers protection against oxidative damage in the retinal pigment epithelium cells. Exp. Eye Res. 181:367–73. doi:10.1016/j.exer.2018.11.024.
- Fisher, R. A. 1954. Statistical methods for research workers. Edinburg: Oliver and Boyd. ISBN 0-05-002170-2.
- Frazee, A. C., G. Pertea, A. E. Jaffe, B. Langmead, S. L. Salzberg, and J. T. Leek. 2015. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 33 (3):243–46. doi:10.1038/nbt.3172.
- GeneCards. 2023. https://www.genecards.org/
- The Gene Ontology Consortium. 2019. The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 47 (D1):D330–38. doi:10.1093/nar/gky1055.
- Giulioni, C., V. Maurizi, D. Castellani, S. Scarcella, E. Skrami, G. Balercia, and A. B. Galosi. 2022. The environmental and occupational influence of pesticides on male fertility: A systematic review of human studies. Andrology 10 (7):1250–71. doi:10.1111/andr.13228.
- Goldsmith, L. A. 2000. Acute and subchronic toxicity of sucralose. Food Chem. Toxicol. 38:S53–69. doi:10.1016/s0278-6915(00)00028-4.
- Gooderham, N. J., S. M. Cohen, G. Eisenbrand, S. Fukushima, F. P. Guengerich, S. S. Hecht, I. M. C. M. Rietjens, T. J. Rosol, M. Bastaki, M. J. Linman, et al. 2020. The safety evaluation of food flavoring substances: The role of genotoxicity studies. Crit. Rev. Toxicol. 50 (1):1–27. doi:10.1080/10408444.2020.1712589.
- Grice, H. C., and L. A. Goldsmith. 2000. Sucralose—an overview of the toxicity data. Food Chem. Toxicol. 38:S1–6. doi:10.1016/s0278-6915(00)00023-5.
- Griffiths, S. K., and J. P. Campbell. 2015. Placental structure, function and drug transfer. Continuing Edu. Anaesth. Crit. Care Pain 15 (2):84–89. doi:10.1093/bjaceaccp/mku013.
- Guo, M., X. Liu, Y. Tan, F. Kang, X. Zhu, X. Fan, C. Wang, R. Wang, Y. Liu, X. Qin, et al. 2021. Sucralose enhances the susceptibility to dextran sulfate sodium (DSS) induced colitis in mice with changes in gut microbiota. Food Funct. 12 (19):9380–90. doi:10.1039/d1fo01351c.
- Hao, X. 2011. Process for the preparation of sucralose. United States Patent US7,932,380 B2. Apr. 26, 2011. https://patentimages.storage.googleapis.com/7c/7e/01/cafc2dd77e7bbf/US7932380.pdf.
- Hevener, K. E. 2018. Computational toxicology methods in chemical library design and high-throughput screening hit validation. Meth. Mol. Biol. 1800:275–85. doi:10.1007/978-1-4939-7899-1_13.
- Hirako, N., and S. Takahashi. 2021. Upregulation of metallothionein-1G accelerates G1/S transition in the growth phase of acute promyelocytic leukemia NB4 cells. Ann. Clin. Lab. Sci. 51:38–43. PMID:33653779.
- Hough, L., and S. P. Phadnis. 1976. Enhancement in the sweetness of sucrose. Nature 263 (5580):800. doi:10.1038/263800a0.
- Houston, J. B. 1994. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem. Pharmacol. 47 (9):1469–79. doi:10.1016/0006-2952(94)90520-7.
- Hu, N., X. Liu, Q. Mu, M. Yu, H. Wang, Y. Jiang, R. Chen, and L. Wang. 2021. The gut microbiota contributes to the modulation of intestinal CYP3A1 and P-gp in streptozotocin-induced type 1 diabetic rats. Eur. J. Pharm. Sci. 162:105833. doi:10.1016/j.ejps.2021.105833.
- The Human Protein Atlas. 2023. https://www.proteinatlas.org/.
- Hung, P.-H., M. Savidge, M. De, J. Kang, S. M. Healy, and L. G. Valerio Jr. 2020. In vitro and in silico genetic toxicity screening of flavor compounds and other ingredients in tobacco products with emphasis on ENDS. J. Appl. Toxicol. 40 (11):1566–87. doi:10.1002/jat.4020.
- Japanese Ministry of Health and Welfare (JMHW). 1999. Approval of new high-intensity Sweetener: Sucralose: Revision of the enforcement regulations under the food sanitation law and of the standards and specifications for food and food additives, etc.(published in Official Gazette, No. 2678, July 30, 1999). Japanese Ministry of Health and Welfare (JMHW), Ministry of Health and Welfare Ordinance No.75 (Ministerial Ordinance to Revise Part of the Enforcement regulations under the Food Sanitation Law) and Ministry of Health and Welfare Announcement No. 167.
- Jiang, Y., H. Huang, X. Zhu, M. Wu, M. Ye, B. Xiao, C. Yu, H. Fang, F. Liu, and S. Lv. 2019. ZSCAN10 promotes cell proliferation, upregulates OCT4 expression, and activates Wnt/β-catenin signaling in glioma. Int. J. Clin. Exp. Pathol. 12 (3):700–10.
- Jiang, W., T. Wu, X. Shi, and J. Xu. 2021. Overexpression of EWSR1 (Ewing sarcoma breakpoint region 1/EWS RNA binding protein 1) predicts poor survival in patients with hepatocellular carcinoma. Bioengineered 12 (1):7941–49. doi:10.1080/21655979.2021.1982844.
- John, B. A., S. G. Wood, and D. R. Hawkins. 2000a. The pharmacokinetics and metabolism of sucralose in the mouse. Food Chem. Toxicol. 38:S107–10. doi:10.1016/s0278-6915(00)00032-6.
- John, B. A., S. G. Wood, and D. R. Hawkins. 2000b. The pharmacokinetics and metabolism of sucralose in the rabbit. Food Chem. Toxicol. 38:S111–13. doi:10.1016/s0278-6915(00)00033-8.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). 1991. Trichlorogalactosucrose, in: Toxicological evaluation of certain food additives and contaminants. 37th JECFA Report, June 5–14, 1990, World Health Organization (WHO), Geneva, Switzerland , WHO Food Additives Series, No. 28, pp. 219–28. http://www.inchem.org/documents/jecfa/jecmono/v28je14.htm.
- Ketola, K., M. Hilvo, T. Hyötyläinen, A. Vuoristo, A. -L. Ruskeepää, M. Orešič, O. Kallioniemi, and K. Iljin. 2012. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br. J. Cancer 106 (1):99–106. doi:10.1038/bjc.2011.530.
- Kille, J. W., W. C. Ford, P. McAnulty, J. M. Tesh, F. W. Ross, and C. R. Willoughby. 2000b. Sucralose: Lack of effects on sperm glycolysis and reproduction in the rat. Food Chem. Toxicol. 38:S19–29. doi:10.1016/s0278-6915(00)00025-9.
- Kille, J. W., J. M. Tesh, P. A. McAnulty, F. W. Ross, C. R. Willoughby, G. P. Bailey, O. K. Wilby, and S. A. Tesh. 2000a. Sucralose: Assessment of teratogenic potential in the rat and the rabbit. Food Chem. Toxicol. 38:S43–52. doi:10.1016/s0278-6915(00)00027-2.
- Kim, D., B. Langmead, and S. L. Salzberg. 2015. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12 (4):357–60. doi:10.1038/nmeth.3317.
- Kim, D., J. M. Paggi, C. Park, C. Bennett, and S. L. Salzberg. 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37 (8):907–15. doi:10.1038/s41587-019-0201-4.
- Koppel, N., V. M. Rekdal, and E. P. Balskus. 2018. Chemical transformation of xenobiotics by the human gut microbiota. Science 356 (6344):eaag2770. doi:10.1126/science.aag2770.
- Kreuch, D., D. J. Keating, T. Wu, M. Horowitz, C. K. Rayner, and R. L. Young. 2018. Gut mechanisms linking intestinal sweet sensing to glycemic control. Front. Endocrinol. 9:741. doi:10.3389/fendo.2018.00741.
- Kroes, R., A. G. Renwick, M. Cheeseman, J. Kleiner, I. Mangelsdorf, A. Piersma, B. Schilter, J. Schlatter, F. van Schothorst, J. G. Vos, et al. 2004. Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. 42 (1):65–83. doi:10.1016/j.fct.2003.08.006.
- Ku, H. -C., and C. -F. Cheng. 2020. Master regulator activating transcription factor 3 (ATF3) in metabolic homeostasis and cancer. Front. Endocrinol. 11:556. doi:10.3389/fendo.2020.00556.
- Kundu, N., C. C. Domingues, J. Patel, M. Aljishi, N. Ahmadi, M. Fakhri, A. C. Sylvetsky, and S. Sen. 2020. Sucralose promotes accumulation of reactive oxygen species (ROS) and adipogenesis in mesenchymal stromal cells. Stem Cell Res. Therapy 11 (1):1–7. doi:10.1186/s13287-020-01753-0.
- Kuno, T., M. Hirayama-Kurogi, S. Ito, and S. Ohtsuki. 2016. Effect of intestinal flora on protein expression of drug-metabolizing enzymes and transporters in the liver and kidney of germ-free and antibiotics-treated mice. Mol. Pharm. 13 (8):2691–701. doi:10.1021/acs.molpharmaceut.6b00259.
- Labare, M. P., and M. Alexander. 1994. Microbial cometabolism of sucralose, a chlorinated disaccharide, in environmental samples. Appl. Microbiol. Biotechnol. 42 (1):173–78. doi:10.1007/bf00170242.
- Leadscope®. 2019. www.leadscope.com
- Lee, S. H. 2015. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intestinal Res. 13 (1):11. doi:10.5217/ir.2015.13.1.11.
- Lenoir, C., V. Rollason, J. A. Desmeules, and C. F. Samer. 2021. Influence of inflammation on cytochromes P450 activity in adults: A systematic review of the literature. Front. Pharmacol. 12:733935. doi:10.3389/fphar.2021.733935.
- Lertrit, A., S. Srimachai, S. Saetung, S. Chanprasertyothin, L. -O. Chailurkit, C. Areevut, P. Katekao, B. Ongphiphadhanakul, and C. Sriphrapradang. 2018. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutrition 55-56:125–30. doi:https://doi.org/10.1016/j.nut.2018.04.001.
- Li, H., B. Handsaker, A. Wysoker, T. Fennell, J. Ruan, N. Homer, G. Marth, G. Abecasis, and R. Durbin. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25 (16):2078–79. doi:10.1093/bioinformatics/btp352.
- Li, X., Y. Liu, Y. Wang, X. Li, X. Liu, M. Guo, Y. Tan, X. Qin, X. Wang, and M. Jiang. 2020. Sucralose promotes colitis-associated colorectal cancer risk in a murine model along with changes in microbiota. Front. Oncol. 10:710. doi:10.3389/fonc.2020.00710.
- Liu, C. -W., L. Chi, P. Tu, J. Xue, H. Ru, and K. Lu. 2019. Quantitative proteomics reveals systematic dysregulations of liver protein metabolism in sucralose-treated mice. J. Proteomics 196:1–10. doi:10.1016/j.jprot.2019.01.011.
- Liu, C., L. Wang, X. Liu, Y. Tan, L. Tao, Y. Xiao, P. Deng, H. Wang, Q. Deng, Y. Lin, et al. 2021. Cytoplasmic SHMT2 drives the progression and metastasis of colorectal cancer by inhibiting β-catenin degradation. Theranostics 11 (6):2966–86. doi:10.7150/thno.48699.
- Liu, Z., Z. Yang, S. Jiang, Q. Zou, Y. Yuan, J. Li, D. Li, L. Liang, M. Chen, and S. Chen. 2016. MCM2 and TIP30 are prognostic markers in squamous cell/adenosquamous carcinoma and adenocarcinoma of the gallbladder. Mol. Med. Rep. 14 (5):4581–92. doi:10.3892/mmr.2016.5851.
- Li, R., J. Zheng, M. Jiang, Y. Liu, X. Qin, and X. Wang. 2016. Increased digestive proteases and decreased β-glucuronidase in feces of rats treated with sucralose and saccharin—Another critical evidence that these dietary chemicals may be important causative factors for inflammatory bowel disease. Inflamm. Bowel Dis. 22 (8):E29–30. doi:10.1097/mib.0000000000000859.
- Mah-Som, A. Y., M. P. Keppel, J. M. Tobin, A. Kolicheski, N. Saucier, V. Sexl, A. R. French, J. A. Wagner, T. A. Fehniger, and M. A. Cooper. 2021. Reliance on Cox10 and oxidative metabolism for antigen-specific NK cell expansion. Cell Rep. 35 (9):109209. doi:10.1016/j.celrep.2021.109209.
- Margolskee, R. F., J. Dyer, Z. Kokrashvili, K. S. H. Salmon, E. Ilegems, K. Daly, E. L. Maillet, Y. Ninomiya, B. Mosinger, and S. P. Shirazi-Beechey. 2007. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. National Acad. Sci. USA 104 (38):15075–80. doi:10.1073/pnas.0706678104.
- Marioni, J. C., C. E. Mason, S. M. Mane, M. Stephens, and Y. Gilad. 2008. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 18 (9):1509–17. doi:10.1101/gr.079558.108.
- Martin, M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMB Net J. 17 (1):10–12. doi:10.14806/ej.17.1.200.
- MedlinePlus. 2023. CHST3 gene. https://medlineplus.gov/genetics/gene/chst3/#:~:text=The%20CHST3%20gene%20provides%20instructions,and%20maintenance%20of%20the%20skeleton
- Méndez-García, L. A., N. Bueno-Hernández, M. A. Cid-Soto, K. L. De León, V. M. Mendoza-Martínez, A. J. Espinosa-Flores, M. Carrero-Aguirre, M. Esquivel-Velázquez, M. León-Hernández, R. Viurcos-Sanabria, et al. 2022. Ten-week sucralose consumption induces gut dysbiosis and altered glucose and insulin levels in healthy young adults. Microorganisms 10 (2):434. doi:10.3390/microorganisms10020434.
- Migliaccio, V., L. Lionetti, R. Putti, and R. Scudiero. 2020. Exposure to dichlorodiphenyldichloroethylene (DDE) and metallothionein levels in rats fed with normocaloric or high-fat diet: A review. Int. J. Mol. Sci. 21 (5):1903. doi:10.3390/ijms21051903.
- Mohandas, S., and B. Vairappan. 2017. Role of pregnane X-receptor in regulating bacterial translocation in chronic liver diseases. World J. Hepatol. 9 (32):1210. doi:10.4254/wjh.v9.i32.1210.
- Mufti, K. S., and R. A. Khan. 1983. Process for the preparation of 4,1’,6’-trichloro-4,1’,6’-trideoxygalactosucrose (TGS). United States Patent 4,380,476, Apr. 19, 1983. https://patentimages.storage.googleapis.com/65/97/df/34eeba00c9c026/US4380476.pdf
- Nagai, F., E. Kato, and H. -O. Tamura. 2004. Oxidative stress induces GSTP1 and CYP3A4 expression in the human erythroleukemia cell line, K562. Biol. Pharm. Bull. 27 (4):492–95. doi:10.1248/bpb.27.492.
- Nguyen, T. H., P. L. Vemu, G. E. Hoy, S. Boudjadi, B. Chatterjee, J. F. Shern, J. Khan, W. Sun, and F. G. Barr. 2021. Serine hydroxymethyltransferase 2 expression promotes tumorigenesis in rhabdomyosarcoma with 12q13-q14 amplification. J. Clin. Invest. 131 (15):e138022. doi:10.1172/JCI138022.
- Obach, R. S., R. L. Walsky, K. Venkatakrishnan, E. A. Gaman, J. B. Houston, and L. M. Tremaine. 2006. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J. Pharmacol. Exp. Ther. 316 (1):336–48. doi:10.1124/jpet.105.093229.
- Olivier-Van Stichelen, S., K. I. Rother, and J. A. Hanover. 2019. Maternal exposure to non-nutritive sweeteners impacts progeny’s metabolism and microbiome. Front. Microbiol. 10:1360. doi:10.3389/fmicb.2019.01360.
- OpAns. 2021. HPLC-MS/MS quantification of sucralose and sucralose-6-acetate from transwell apical and basal reservoir supernatants as well as samples of sucralose from Sigma-Aldrich, Study directors Jose Mendoza PhD and Tony Leesnitzer. OpAns, LLC. 4134 S. Alston Ave., Durham, NC 27713, USA.
- Organization of Economic Cooperation and Development (OECD). 2016. Test No. 487: In Vitro Mammalian cell micronucleus test, OECD guideline for the testing of chemicals, Section 4. Paris: OECD Publishing. doi:10.1787/9789264264861-en.
- Organization of Economic Cooperation and Development (OECD). 2020. Test No. 471: Bacterial reverse mutation test. OECD guideline for the testing of chemicals, Section 4. Paris: OECD Publishing. doi:10.1787/9789264071247-en.
- Ostrakhovitch, E. A., P. -E. Olsson, S. Jiang, and M. G. Cherian. 2006. Interaction of metallothionein with tumor suppressor p53 protein. FEBS Lett. 580 (5):1235–38. doi:10.1016/j.febslet.2006.01.036.
- Pałkowska-Goździk, E., A. Bigos, and D. Rosołowska-Huszcz. 2018. Type of sweet flavour carrier affects thyroid axis activity in male rats. Eur. J. Nutr. 57 (2):773–82. doi:https://doi.org/10.1007/s00394-016-1367-x.
- Pan, H., W. Xue, W. Zhao, and M. Schachner. 2020. Expression and function of chondroitin 4‐sulfate and chondroitin 6‐sulfate in human glioma. The FASEB J. 34 (2):2853–68. doi:10.1096/fj.201901621RRR.
- Parsa, S., A. Ortega-Molina, H. -Y. Ying, M. Jiang, M. Teater, J. Wang, C. Zhao, E. Reznik, J. P. Pasion, D. Kuo, et al. 2020. The serine hydroxymethyltransferase-2 (SHMT2) initiates lymphoma development through epigenetic tumor suppressor silencing. Nat. Cancer 1 (6):653–64. doi:10.1038/s43018-020-0080-0.
- Pasqualli, T., P. E. E. Chaves, L. da Veiga Pereira, É. Adílio Serpa, L. F. S. de Oliveira, and M. M. Machado. 2020. Sucralose causes non‐selective CD4 and CD8 lymphotoxicity via probable regulation of the MAPK8/APTX/EID1 genes: An in vitro/in silico study. Clin. Exp. Pharmacol. Physiol. 47:1751–57. doi:10.1111/1440-1681.13362.
- Pepino, M. Y., C. D. Tiemann, B. W. Patterson, B. M. Wice, and S. Klein. 2013. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 36 (9):2530–35. doi:https://doi.org/10.2337/dc12-2221.
- Pertea, M., D. Kim, G. M. Pertea, J. T. Leek, and S. L. Salzberg. 2016. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11 (9):1650–67. doi:10.1038/nprot.2016.095.
- Pertea, M., G. M. Pertea, C. M. Antonescu, T. -C. Chang, J. T. Mendell, and S. L. Salzberg. 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33 (3):290–95. doi:10.1038/nbt.3122.
- PubChem. 2022. https://pubchem.ncbi.nlm.nih.gov/
- Qin, X. 2011. What caused the recent worldwide increase of inflammatory bowel disease: Should sucralose be added as a suspect? Inflamm. Bowel Dis. 17 (10):E139. doi:10.1002/ibd.21823.
- Qu, Y., R. Li, M. Jiang, and X. Wang. 2017. Sucralose increases antimicrobial resistance and stimulates recovery of Escherichia coli mutants. Curr. Microbiol. 74 (7):885–88. doi:10.1007/s00284-017-1255-5.
- Rahn, A., and V. A. Yaylayan. 2010. Thermal degradation of sucralose and its potential in generating chloropropanols in the presence of glycerol. Food Chem. 118 (1):56–61. doi:10.1016/j.foodchem.2009.04.133.
- Raudvere, U. L., I. Kolberg, T. Kuzmin, P. Arak, H. P. Adler, J. Vilo, and J. Vilo. 2019. G: Profiler: A web server for functional enrichment analysis and conversions of gene lists. Nucleic Acids Res. 47 (W1):W191–98. https://biit.cs.ut.ee/gprofiler/gost.
- Raya, S. A., A. M. Aboul-Enein, M. M. El-Nikeety, R. S. Mohamed, and W. M. Abdelwahid. 2020. In vivo comet assay of food additives’ combinations and their effects on biochemical parameters in albino rats. Biointerface Res. Appl. Chem. 11 (2):9170–83. doi:10.33263/briac112.91709183.
- Roberts, A., A. G. Renwick, J. Sims, and D. J. Snodin. 2000. Sucralose metabolism and pharmacokinetics in man. Food Chem. Toxicol. 38:S31–41. doi:10.1016/s0278-6915(00)00026-0.
- Rodrigo, M. A. M., A. M. J. Jimemez, Y. Haddad, K. Bodoor, P. Adam, S. Krizkova, Z. Heger, and V. Adam. 2020. Metallothionein isoforms as double agents–their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Update. 52:100691. doi:10.1016/j.drup.2020.100691.
- Rodriguez-Palacios, A., A. Harding, P. Menghini, C. Himmelman, M. Retuerto, K. P. Nickerson, M. Lam, C. M. Croniger, M. H. McLean, S. K. Durum, et al. 2018. The artificial sweetener Splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease–like ileitis. Inflamm. Bowel Dis. 24 (5):1005–20. doi:10.1093/ibd/izy060.
- Romo-Romo, A., C. A. Aguilar-Salinas, G. X. Brito-Córdova, R. A. Gómez-Díaz, and P. Almeda-Valdes. 2018. Sucralose decreases insulin sensitivity in healthy subjects: A randomized controlled trial. Am. J. Clin. Nutr. 108 (3):485–91. doi:https://doi.org/10.1093/ajcn/nqy152.
- Rosales-Gómez, C. A., B. E. Martínez-Carrillo, A. A. Reséndiz-Albor, N. Ramírez-Durán, R. Valdés-Ramos, T. Mondragón-Velásquez, and J. A. Escoto-Herrera. 2018. Chronic consumption of sweeteners and its effect on glycaemia, cytokines, hormones, and lymphocytes of GALT in CD1 mice. Biomed. Res. Int. 2018:1345282. doi:10.1155/2018/1345282.
- Ruttkay-Nedecky, B., L. Nejdl, J. Gumulec, O. Zitka, M. Masarik, T. Eckschlager, M. Stiborova, V. Adam, and R. Kizek. 2013. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 14 (3):6044–66. doi:10.3390/ijms14036044.
- Sánchez-Alcoholado, L., R. Ordóñez, A. Otero, I. Plaza-Andrade, A. Laborda-Illanes, J. A. Medina, B. Ramos-Molina, J. Gómez-Millán, and M. I. Queipo-Ortuño. 2020. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int. J. Mol. Sci. 21 (18):6782. doi:10.3390/ijms21186782.
- Sasaki, Y. F., S. Kawaguchi, A. Kamaya, M. Ohshita, K. Kabasawa, K. Iwama, K. Taniguchi, and S. Tsuda. 2002. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 519 (1–2):103–19. doi:10.1016/s1383-5718(02)00128-6.
- Schiffman, S. S., and K. I. Rother. 2013. Sucralose, a synthetic organochlorine sweetener: Overview of biological issues J. Toxicol. Environ. Health Part B. 16 (7):399–451. doi:10.1080/10937404.2013.842523.
- Schiffman, S. S., E. A. Sattely-Miller, and I. E. Bishay. 2008. Sensory properties of neotame: Comparison with other sweeteners. Sweetness and Sweeteners: Biology, Chemistry and Psychophysics. ACS Symposium Series 979, ed. D. K. Weerasinghe, and G. E. DuBois, pp. 511–29. New York, NY: Oxford University Press.
- Schwartz, J. L., R. Jordan, H. H. Evans, M. Lenarczyk, and H. L. Liber. 2004. Baseline levels of chromosome instability in the human lymphoblastoid cell TK6. Mutagenesis 19 (6):477–82. doi:10.1093/mutage/geh060.
- Scientific Committee on Food (SCF). 2000. Opinion of the scientific committee on food on sucralose (Adopted by the SCF on 7 September 2000). European Commission Health & Consumer Protection Directorate-General Directorate C – Scientific Opinions C3 – Management of scientific committees II; scientific co-operation and networks. Scientific Committee on Food [SCF/CS/ADDS/EDUL/190 Final 12/9/2000]. https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out68_en.pdf
- Selwyn, F. P., S. L. Cheng, C. D. Klaassen, and J. Y. Cui. 2016. Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab. Dispos. 44 (2):262–74. doi:10.1124/dmd.115.067504.
- Serafimova, R., T. Coja, and G. E. Kass. 2021. Application of the threshold of toxicological concern (TTC) in food safety: Challenges and opportunities. Front. Toxicol. 3:655951. doi:10.3389/ftox.2021.655951.
- Shchulkin, A. V., Y. V. Abalenikhina, P. D. Erokhina, I. V. Chernykh, and E. N. Yakusheva. 2021. The role of P-glycoprotein in decreasing cell membranes permeability during oxidative stress. Biochem. 86 (2):197–206. doi:10.1134/S0006297921020085.
- Shigetomi, K., Y. Ono, K. Matsuzawa, and J. Ikenouchi. 2023. Cholesterol-rich domain formation mediated by ZO proteins is essential for tight junction formation. Proc. National Acad. Sci. USA 120 (8):e2217561120. doi:10.1073/pnas.2217561120.
- Shil, A., and H. Chichger. 2021. Artificial sweeteners negatively regulate pathogenic characteristics of two model gut bacteria, E. coli and E. faecalis. Int. J. Mol. Sci. 22 (10):5228. doi:10.3390/ijms22105228.
- Shil, A., O. Olusanya, Z. Ghufoor, B. Forson, J. Marks, and H. Chichger. 2020. Artificial sweeteners disrupt tight junctions and barrier function in the intestinal epithelium through activation of the sweet taste receptor, T1R3. Nutrients 12 (6):1862. doi:10.3390/nu12061862.
- Si, M., and J. Lang. 2018. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 11 (1):107. doi:10.1186/s13045-018-0645-x.
- Sims, J., A. Roberts, J. W. Daniel, and A. G. Renwick. 2000. The metabolic fate of sucralose in rats. Food Chem. Toxicol. 38:S115–21. doi:10.1016/S0278-6915(00)00034-X.
- Soffritti, M., M. Padovani, E. Tibaldi, L. Falcioni, F. Manservisi, M. Lauriola, L. Bua, M. Manservigi, and F. Belpoggi. 2016. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male Swiss mice. Int. J. Occup. Environ. Health 22 (1):7–17. doi:10.1080/10773525.2015.1106075.
- Srinivasan, B., A. R. Kolli, M. B. Esch, H. E. Abaci, M. L. Shuler, and J. J. Hickman. 2015. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20 (2):107–26. doi:10.1177/2211068214561025.
- Strolin-Benedetti, M., G. Brogin, M. Bani, F. Oesch, and J. G. Hengstler. 1999. Association of cytochrome P450 induction with oxidative stress in vivo as evidenced by 3-hydroxylation of salicylate. Xenobiotica 29 (11):1171–80. doi:10.1080/004982599238038.
- Suez, J., Y. Cohen, R. Valdés-Mas, U. Mor, M. Dori-Bachash, S. Federici, N. Zmora, A. Leshem, M. Heinemann, R. Linevsky, et al. 2022. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 185 (18):1–22. doi:10.1016/j.cell.2022.07.016.
- Suez, J., T. Korem, D. Zeevi, G. Zilberman-Schapira, C. A. Thaiss, O. Maza, D. Israeli, N. Zmora, S. Gilad, A. Weinberger, et al. 2014. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514 (7521):181–86. doi:10.1038/nature13793.
- Sun, J., L. Chen, B. Lou, Y. Bai, X. Yu, M. Zhao, and Z. Wang. 2017. Acetylation and deacetylation for sucralose preparation by a newly isolated Bacillus amyloliquefaciens WZS01. J. Biosci. Bioeng. 123 (5):576–80. doi:10.1016/j.jbiosc.2016.12.013.
- Sun, E. W., D. De Fontgalland, P. Rabbitt, P. Hollington, L. Sposato, S. L. Due, D. A. Wattchow, C. K. Rayner, A. M. Deane, R. L. Young, et al. 2017. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes 66 (8):2144–49. doi:10.2337/db17-0058.
- Sylvetsky, A. C., A. L. Gardner, V. Bauman, J. E. Blau, H. M. Garraffo, P. J. Walter, and K. I. Rother. 2015. Nonnutritive sweeteners in breast milk. J. Toxicol. Environ. Health Part A 78 (16):1029–32. doi:10.1080/15287394.2015.1053646.
- Sylvetsky, A. C., S. Sen, P. Merkel, F. Dore, D. B. Stern, C. J. Henry, H. Cai, P. J. Walter, K. A. Crandall, K. I. Rother, et al. 2020. Consumption of diet soda sweetened with sucralose and acesulfame‐potassium alters inflammatory transcriptome pathways in females with overweight and obesity. Mol. Nutr. Food Res. 64 (11):1901166. doi:10.1002/mnfr.201901166.
- Tang, Z., Y. Yang, W. Chen, E. Li, and T. Liang. 2022. Demethylation at enhancer upregulates MCM2 and NUP37 expression predicting poor survival in hepatocellular carcinoma patients. J. Transl. Med. 20 (1):49. doi:10.1186/s12967-022-03249-2.
- Toda, T., N. Saito, N. Ikarashi, K. Ito, M. Yamamoto, A. Ishige, K. Watanabe, and K. Sugiyama. 2009. Intestinal flora induces the expression of CYP3a in the mouse liver. Xenobiotica 39 (4):323–34. doi:10.1080/00498250802651984.
- Togao, M., K. Kawakami, J. Otsuka, G. Wagai, Y. Ohta‐takada, and S. Kado. 2020. Effects of gut microbiota on in vivo metabolism and tissue accumulation of cytochrome P450 3A metabolized drug: Midazolam. Biopharm. Drug Dispos. 41 (7):275–82. doi:10.1002/bdd.2244.
- Tong, Z. -B., J. Braisted, P. -H. Chu, and D. Gerhold. 2020. The MT1G gene in LUHMES neurons is a sensitive biomarker of neurotoxicity. Neurotox. Res. 38 (4):967–78. doi:10.1007/s12640-020-00272-3.
- Uchimura, K., K. Kadomatsu, H. Nishimura, H. Muramatsu, E. Nakamura, N. Kurosawa, O. Habuchi, F. M. El-Fasakhany, Y. Yoshikai, and T. Muramatsu. 2002. Functional analysis of the chondroitin 6-sulfotransferase gene in relation to lymphocyte subpopulations, brain development, and oversulfated chondroitin sulfates. J. Biol. Chem. 277 (2):1443–50. doi:10.1074/jbc.M104719200.
- Ugai, T., N. Sasamoto, H. -Y. Lee, M. Ando, M. Song, R. M. Tamimi, I. Kawachi, P. T. Campbell, E. L. Giovannucci, E. Weiderpass, et al. 2022. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 19 (10):656–73. doi:10.1038/s41571-022-00672-8.
- Uhlén, M., L. Fagerberg, B. M. Hallström, C. Lindskog, P. Oksvold, A. Mardinoglu, Å. Sivertsson, C. Kampf, E. Sjöstedt, A. Asplund, et al. 2015. Tissue-based map of the human proteome. Science 347 (6220):1260419. doi:10.1126/science.1260419.
- Ukena, S. N., A. Singh, U. Dringenberg, R. Engelhardt, U. Seidler, W. Hansen, A. D. Bruder, G. Franzke, A. Rogler, S. Suerbaum, et al. 2007. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLos One 2 (12):e1308. doi:10.1371/journal.pone.0001308.
- United States Food and Drug Administration (US FDA). 1998. Food additives permitted for direct addition to food for human consumption; sucralose. 21CFR Part 172 [Docket No. 87F-0086]. Fed. Regist. 63 (64):16417–33. http://www.gpo.gov/fdsys/pkg/FR-1998-04-03/pdf/98-8750.pdf.
- United States Food and Drug Administration (US FDA). 1999. Food additives permitted for direct addition to food for human consumption: Sucralose [21CFR Part 172; Docket No. 99F–0001]. Fed. Regist. 64 (155):43908–09. https://www.govinfo.gov/content/pkg/FR-1999-08-12/pdf/99-20888.pdf
- United States Food and Drug Administration (US FDA). 2018. M7(R1) assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m7r1-assessment-and-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential
- United States Food and Drug Administration (US FDA). 2020. In Vitro Drug Interaction Studies —Cytochrome P450 Enzyme- and Transporter Mediated Drug Interactions. Guidance for Industry. Center for Drug Evaluation and Research (CDER). https://www.fda.gov/media/134582/download
- United States Food and Drug Administration (US FDA). 2021. Data accessed from the sucralose food additive petition (FAP) filed by McNeil Specialty Products Co. Exhibit E: Safety, Page 002211. Obtained from the US FDA Office of Food Additive Safety on February 8, 2021.
- Utami, R. A., A. Hakiki, S. Asyarie, and D. S. Retnoningrum. 2018. Gliadin peptide facilitates FITC dextran transport across the non-everted gut sac of rat small intestine. Sci. Pharm. 86 (2):13. doi:10.3390/scipharm86020013.
- Van Eyk, A. D. 2015. The effect of five artificial sweeteners on Caco-2, HT-29 and HEK-293 cells. Drug. Chem. Toxicol. 38 (3):318–27. doi:10.3109/01480545.2014.966381.
- Van Nguyen, G., M. C. Tran, L. Van Nguyen, H. T. Quynh, and M. N. Nguyen. 2021. Up-regulation of SET nuclear proto-oncogene is associated with early recurrence and poorer prognosis of hepatocellular carcinoma. VNUHCM J. Health Sci. 2:110–21.
- Wang, Z., M. Gerstein, and M. Snyder. 2009. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 10 (1):57–63. doi:10.1038/nrg2484.
- Wang, X., J. Guo, Y. Liu, H. Yu, and X. Qin. 2019. Sucralose increased susceptibility to colitis in rats. Inflamm. Bowel Dis. 25 (2):e3–4. doi:10.1093/ibd/izy196.
- Wang, F., H. He, X. Yang, Y. Yu, and Z. Fan. 2011. Method of sucralose synthesis yield. United States Patent US 7,884,203 B2. Feb. 8, 2011. https://patentimages.storage.googleapis.com/6e/f0/41/27b2650c7022ac/US7884203.pdf
- Welcker, K., A. Martin, P. Kolle, M. Siebeck, and M. Gross. 2004. Increased intestinal permeability in patients with inflammatory bowel disease. Eur. J. Med. Res. 9 (10):456–60.
- Werness, S. 2021. Chemical analysis of 18 food-grade commercial samples of sucralose extracted with ethyl acetate to determine the presence of impurities including sucralose-6-acetate. Report to Department of Biomedical Engineering at North Carolina State University. Study director: Stephen Werness, Director of Mass Spectrometry Laboratory, Avazyme, Inc., 2202 Ellis Rd #A, Durham, NC 27703.
- Werness, S., and S. S. Schiffman. 2020. Comparison of chromatographic results from sucralose-6-acetate (synthesized by Jiangyin PharmaAdvance, Inc., P. R. China) with chromatographic and mass spectrometry data from Bornemann et al. 2018.
- Westbrook, A. M., B. Wei, J. Braun, and R. H. Schiestl. 2011. Intestinal inflammation induces genotoxicity to extraintestinal tissues and cell types in mice. Int. J. Cancer 129 (8):1815–25. doi:10.1002/ijc.26146.
- Wingender, E. 2008. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief. Bioinformatics 9 (4):326–32. doi:10.1093/bib/bbn016.
- Wood, S. G., B. A. John, and D. R. Hawkins. 2000. The pharmacokinetics and metabolism of sucralose in the dog. Food Chem. Toxicol. 38:S99–106. doi:10.1016/s0278-6915(00)00031-4.
- Xu, F., Y. Guan, P. Zhang, L. Xue, X. Yang, K. Gao, and T. Chong. 2020. The impact of TNFSF14 on prognosis and immune microenvironment in clear cell renal cell carcinoma. Genes Genomics 42 (9):1055–66. doi:10.1007/s13258-020-00974-0.
- Yin, X., J. W. Dewille, and T. Hai. 2008. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene 27 (15):2118–27. doi:10.1038/sj.onc.1210861.
- Young, R. L., N. J. Isaacs, G. Schober, T. Wu, N. Cvijanovic, N. Pezos, M. Bound, D. J. Keating, C. K. Rayner, and M. Horowitz. 2017. Impact of artificial sweeteners on glycaemic control in healthy humans (193). OP 33 Gastro-entero-pancreatic interactions. Diabetologia 60 (Suppl 1):S91. doi:10.1007/s00125-017-4350-z.
- Zhang, H., Y. Che, B. Xuan, X. Wu, and H. Li. 2022. Serine hydroxymethyltransferase 2 (SHMT2) potentiates the aggressive process of oral squamous cell carcinoma by binding to interleukin enhancer-binding factor 2 (ILF2). Bioengineered 13 (4):8785–97. doi:10.1080/21655979.2022.2051886.
- Zheng, Z., Y. Xiao, L. Ma, W. Lyu, H. Peng, X. Wang, Y. Ren, and J. Li. 2022. Low dose of sucralose alter gut microbiome in mice. Front. Nutr. 9:848392. doi:10.3389/fnut.2022.848392.