Abstract
To increase U.S. petroleum energy-independence, the University of Texas at Arlington (UT Arlington) has developed a coal liquefaction process that uses a hydrogenated solvent and a proprietary catalyst to convert lignite coal to crude oil. This paper reports on part of the environmental evaluation of the liquefaction process: the evaluation of the solid residual from liquefying the coal, called inertinite, as a potential adsorbent for air and water purification. Inertinite samples derived from Arkansas and Texas lignite coals were used as test samples.
In the activated carbon creation process, inertinite samples were heated in a tube furnace (Lindberg, Type 55035, Arlington, UT) at temperatures ranging between 300 and 850 °C for time spans of 60, 90, and 120 min, using steam and carbon dioxide as oxidizing gases. Activated inertinite samples were then characterized by ultra-high-purity nitrogen adsorption isotherms at 77 K using a high-speed surface area and pore size analyzer (Quantachrome, Nova 2200e, Kingsville, TX). Surface area and total pore volume were determined using the Brunauer, Emmet, and Teller method, for the inertinite samples, as well as for four commercially available activated carbons (gas-phase adsorbents Calgon Fluepac-B and BPL 4 × 6; liquid-phase adsorbents Filtrasorb 200 and Carbsorb 30). In addition, adsorption isotherms were developed for inertinite and the two commercially available gas-phase carbons, using methyl ethyl ketone (MEK) as an example compound. Adsorption capacity was measured gravimetrically with a symmetric vapor sorption analyzer (VTI, Inc., Model SGA-100, Kingsville, TX). Also, liquid-phase adsorption experiments were conducted using methyl orange as an example organic compound. The study showed that using inertinite from coal can be beneficially reused as an adsorbent for air or water pollution control, although its surface area and adsorption capacity are not as high as those for commercially available activated carbons.
The United States currently imports two-thirds of its crude oil, leaving its transportation system especially vulnerable to disruptions in international crude supplies. UT Arlington has developed a liquefaction process that converts coal, abundant in the United States, to crude oil. This work demonstrated that the undissolvable solid coal residual from the liquefaction process, called inertinite, can be converted to an activated carbon adsorbent. Although its surface area and adsorption capacity are not as high as those for commercially available carbons, the inertinite source material would be available at no cost, and its beneficial reuse would avoid the need for disposal.
Supplemental materials are available for this article. Go to the publisher's online edition of the Journal of the Air & Waste Management Association for properties of the commercial activated carbons tested in this study.
Introduction
The United States currently imports two-thirds of its crude oil, or 9.76 million barrels per day out of 14.72 million barrels per day (CitationWebber, 2009a). The transportation sector consumes 70% of this crude oil, which is refined into gasoline, diesel fuel, jet fuel, and other products (CitationWebber, 2009a). In fact, 96% of the U.S. transportation system energy needs come from crude oil, with natural gas supplying 2% and renewable energy 1% (CitationWebber, 2009b). Hence, the U.S. transportation system is highly vulnerable to potential disruption of non-domestic sources of crude oil.
To reduce this vulnerability, the U.S. Defense Advance Research Projects Agency (DARPA) has sponsored research on synthetic production of crude oil. In a DARPA-sponsored project, the University of Texas at Arlington (UT Arlington) developed a unique direct liquefaction technique, which enables lignite coal to be converted to a sweet crude oil (CitationBillo et al., 2010). The U.S. known coal reserves, the largest in the world, would last 236 years at current levels of use (CitationWebber, 2009a). Hence, converting coal to crude oil would increase U.S. energy independence and reduce the vulnerability of the transportation sector to disruptions in the international supply of crude.
The UT Arlington coal liquefaction process uses a hydrogenated solvent and a proprietary catalyst to extract all of the volatile carbon-based components from lignite as well as some of the fixed carbon; altogether, up to 60% of the lignite's carbon is converted to synthetic crude (CitationBillo et al., 2010). This is comparable to conversion rates reported for other coal-to-liquid processes in the literature. However, the UT Arlington process has several advantages over competitive processes, including (1) the use of lower-grade coal (lignite) as a feedstock, whereas many other processes require higher-grade feedstocks (sub-bituminous or bituminous coal) (CitationComolli et al., 1990, Citation1999; CitationLi et al., 2008; CitationRedlich et al., 1999; CitationSasaki et al., 2000; CitationValente and Cronauer, 2005), which are more expensive; (2) liquefaction process conditions that are milder than those reported for competitive processes (CitationBillo et al., 2010). Although a pilot plant is still in the planning stages, the cost of crude oil produced via the UT Arlington process is anticipated to be about half that of conventional West Texas crude, based on cost estimates from laboratory data. The synthetic oil produced can subsequently be refined to form gasoline, diesel, jet fuel, and other end products.
The DARPA research included evaluation of air, water, and solid waste impacts of the UT Arlington coal liquefaction process. Traditional air pollutant and greenhouse gas impacts of the process are reported elsewhere (CitationKositkanawuth et al., 2011). This paper focuses on one aspect of potential solid waste impacts. In the liquefaction process, a solid residue remains as a byproduct, known as inertinite. Inertinite, shown in , is the insoluble part of the coal that does not dissolve to form crude oil when treated with the digesting agent. Some of the options for the reuse of this inertinite are reuse as an adsorbent for air and water pollution control, reuse in asphalt and concrete pavements, or burning as fuel, since it is 50–70% carbon.
The objective of the work reported here was to evaluate the potential use of inertinite as an activated carbon (AC) for air and water purification. Adsorption captures a pollutant on the surface of a solid adsorbent, in a process that can be either physical or chemical. The greater the surface area of the solid, the greater generally is the adsorption capacity (mass of pollutant adsorbed per mass of adsorbent) (CitationCooper and Alley, 1994). Advantages of adsorption versus other pollution control methods include high removal efficiency, low space requirements, and ability to regenerate and reuse the adsorbent.
Activated carbon has long been recognized as one of the most versatile adsorbents due to its high porosity and resulting high surface area; it is the most common adsorbent for air and water pollution control applications. Activated carbon is manufactured by heating raw materials such as coal, wood, or coconut shell to 600–900 °C in an inert atmosphere, followed by heating to 600–1200 °C in an oxidizing atmosphere (CitationCooper and Alley, 1994). This process volatilizes a portion of the internal carbon, creating an internal network of pores with high surface area. Depending on the raw material, the resulting activated carbon has different physical/chemical characteristics, such as surface area, porosity, and surface functional groups.
Coal is the traditional raw material for producing activated carbon. In spite of its abundant uses, applications of activated carbon are sometimes restricted due to its higher cost (CitationBhatnagar and Sillanpää, 2010). Use of waste materials as adsorbents can reduce waste disposal costs, as well as reduce adsorbent costs (CitationBhatnagar and Sillanpää, 2010). Accordingly, a variety of waste materials have been explored as potential activated carbon adsorbents, including sewage sludge (CitationAksu and Yerner, 1998; CitationChen et al., 2005; CitationKojima et al., 2002; CitationRio et al., 2005; CitationThawornchaisit and Pakulanon, 2007), tires (CitationBhatnagar and Sillanpää, 2010), newspaper (CitationHamabe et al., 1998; CitationShimada et al., 1999), cork (CitationOlivella et al., 2011), ion-exchange resin (CitationLong et al., 2008), seaweed (CitationGhimire et al., 2007) and various agricultural wastes Morus plant (CitationGopalswami et al., 2010), jatropha husk (CitationNamasivayam et al., 2007), coir pith (CitationKadirvelu and Namasivayam, 2000; CitationNamasivayam and Kavitha, 2002; CitationNamasivayam and Sangeetha, 2006; CitationNamasivayam et al., 2001; CitationParab et al., 2006), maize waste (CitationEl-Geundi, 1991; CitationElizalde-González et al., 2006), rice husk (CitationAhmaruzzaman and Sharma, 2005; CitationAkhtar et al., 2006; CitationChandrasekhar and Pramada, 2006; CitationKannan and Sundaram, 2001; CitationKaur and Prasad, 2001; CitationKumar and Bandyopadhyay, 2006; CitationLakshmi et al., 2009; CitationMahvi et al., 2004; CitationMalik, 2003; CitationMcKay et al., 1999; CitationMohanty et al., 2006; CitationSrinivasan et al., 1988; CitationWang and Lin, 2008; CitationZafar et al., 2007), wheat bran (CitationBulut and Baysal, 2006; CitationFatma et al., 2007), tea and coffee waste (CitationBoudrahem et al., 2009; CitationErŏglu et al., 2009; CitationMinamisawa et al., 2005; CitationMurugesan et al., 2006; CitationUddin et al., 2009), coconut waste (Citationde Sousa et al., 2010; CitationHameed et al., 2008a, Citation2008b; CitationHo and Ofomaja, 2006; CitationSenthilkumaar et al., 2006; CitationTan et al., 2008; CitationVieira et al., 2009), peanut hull/husk (CitationHan et al., 2008; CitationOliveira et al., 2009; CitationZhu et al., 2009), banana peel (CitationAchak et al., 2009; CitationAnnadurai et al., 2002; CitationMemon et al., 2008, Citation2009), and orange peel (CitationAjmal et al., 2000; CitationArami et al., 2005; CitationLi et al., 2008; CitationNamasivayam et al., 1996; CitationSivaraj et al., 2001) ). Wastes from coal combustion, including coal cinder/ash (CitationMontagnaro and Santoro, 2009; CitationYang et al., 2009) and fly ash (CitationAkgerman and Zardkoohi, 1996; CitationAksu and Yener, 1999; Citation2001; CitationAlinnor, 2007; CitationBatabyal et al., 1995; CitationChen et al., 2010; CitationHaribabu et al., 1993; CitationJanos et al., 2003; CitationKao et al., 2000; CitationKhanna and Malhotra, 1977; CitationLi et al., 2009; CitationPanday et al., 1985; CitationSarkar et al., 2003; CitationSen and Arnab, 1987; CitationSingh and Rawat, 1994; CitationSingh et al., 1994; CitationViraraghavan and Ramakrishna, 1999; CitationWoolard et al., 2002), have been explored as adsorbents, made from species other than carbon. CitationZhang et al. (2011) and CitationZhou et al. (2008) prepared activated carbon from residue from a bituminous coal liquefaction process. This study examines activated carbon prepared from the residue of a lignite liquefaction process.
Experimental Procedure
Creation of activated carbon adsorbent
To create the activated carbon, inertinite samples derived from Arkansas lignite coal (samples ARK1–ARK6) were heated in a tube furnace (Lindberg, Type 55035, Arlington, UT) in an inert N2 atmosphere (2–4 L/min flow rate) at 300 or 500 °C for 60 min. Subsequently, the samples were heated in the tube furnace in an oxidizing atmosphere of carbon dioxide (2–4 L/min flow rate) at 300 or 500 °C for 60 min (Gangupomu et al., 2010). Sample composition before and after pretreatment is shown in Pretreatment of inertinite samples from Texas lignite low‐ash lignite (LAL) and NRG coal (samples LAL1–LAL8 and NRG1–NRG8) was conducted at temperatures ranging from 600 to 850 °C, as shown in Higher pretreatment temperatures were used for the LAL and NRG samples because the Arkansas sample heated to 500 °C for both N2 and CO2 had the highest surface area (268 and 256 g/m2 for samples ARK5 and ARK6, respectively). It was thus assumed that temperatures >500 °C would produce even greater sample surface area for the LAL and NRG samples.
Table 1. CHN Composition of adsorbents derived from inertinite
For the inertinite derived from LAL and NRG, pretreatment was conducted using steam, since it is cheaper and more common than N2 and CO2. Steam was not used for pretreatment of the inertinite samples derived from Arkansas coal; N2 and CO2 were used instead. Preliminary pretreatment testing of inertinite from Arkansas coal had shown that steam volatilized essentially all of the carbon from the samples, with only 1% remaining. Although a significant fraction of the carbon volatilized with steam pretreatment of the LAL and NRG samples, the problem was not as severe as that encountered with the Arkansas coal. Future studies could explore whether combining steam with CO2 provides better activation results. Pretreatment times of 60, 90, and 120 min were explored for the LAL and NRG samples. Composition of the samples before and after pretreatment is shown in
For most of the LAL samples, at least 75% of the original carbon remained after pretreatment. For the NRG samples, the highest percent carbon remaining after pretreatment was 38%. The original carbon content of the NRG samples was actually higher (67.0%, compared to 61.3% for LAL). Perhaps treatment of the LAL samples to remove ash had already removed carbon that would readily volatilize. At 850 °C, neither the NRG nor the LAL samples had any carbon remaining in the sample; this indicates that at higher temperatures, the sample would have no carbon left and would be of limited use as an adsorbent.
Determination of AC surface area and pore volume
A good activated carbon has various characteristics, including a large internal surface area and good accessibility to internal pores. Commercial activated carbon grades have an internal surface area of 500 up to 1500 m2/g and a large pore volume of more than 30 cm3/100 g (CitationPaul, 2005).
Activated inertinite samples were characterized by ultra-high-purity nitrogen (UHP-N2) adsorption isotherms at 77 K using a high-speed surface area and pore size analyzer (Quantachrome, Nova 2200e, Kingsville, TX). The cryogenic temperature (77 K) was achieved using liquid nitrogen (Matheson Tri-Gas). Each sample was prepared by a degassing process to a clean sample that was volatile organic compound– and water-free. Inertinite samples derived from Arkansas coal were degassed under vacuum for 2 hr at 200 °C; inertinite samples derived from LAL and NRG coal were degassed for 3 hr. The additional degassing time for the LAL and NRG samples was needed to ensure that they were fully free of impurities and water.
Surface area and total pore volume were determined from the N2 adsorption isotherms using the Brunauer, Emmet, and Teller (BET) method. The BET surface area requires points on the adsorption isotherm in the linear region of the BET plot, usually in the P/P 0 range of 0.05 to 0.35 for the determination of the surface area. The average pore size was calculated based on the total pore volume and surface area (CitationQuantachrome Instruments, 2007). Results are shown in
Table 2. Adsorbents derived from inertinite: Pretreatment time/temperature impacts on surface area and pore volume
As can be seen from , samples LAL1 and NRG1, which were treated in steam for 60 min at 600 °C, had higher surface areas than the other samples (295 and 269 m2/g, respectively) and thus are highlighted in bold. LAL1 had the third highest pore volume (17.5 cm3/100 g) among the LAL samples, and NRG1 had the highest pore volume (20.8 cm3/100 g) among the NRG samples. Because 500 °C had produced the highest surface area for the Arkansas coal samples, temperatures less than 600 °C were not tested for the LAL and NRG samples. In retrospect, however, pretreatment at lower temperatures such as 500 °C may have increased the surface area for inertinite derived from LAL and NRG coal.
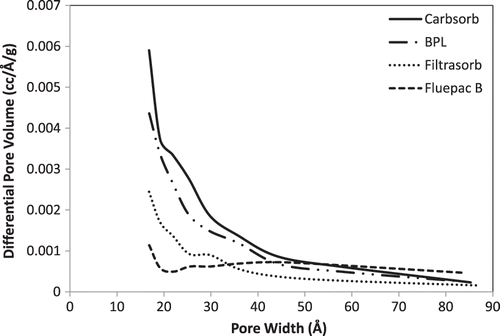
For comparison, surface area and pore volume of examples of commercially available carbons were measured. As examples of gas-phase adsorbents, Calgon Fluepac-B powdered and BPL 4×6 granular activated carbon were chosen for comparison, since the nature of the inertinite adsorbent is between a powder and a granule. As examples of liquid-phase adsorbents, Calgon Filtrasorb 200 and Carbsorb 30 were chosen. Properties of the Calgon carbons are provided in the supplemental information. presents measured values of surface area and pore volume for the commercial carbons, along with the LAL and NRG samples having the highest surface area.
Table 3. Surface area and pore volumes of commercial activated carbons versus inertinite
As shown in , the highest surface areas for the LAL and NRG samples (295 and 269 m2/g, respectively, for samples LAL1 and NRG1) were considerably lower than for the commercially available activated carbons (ranging from 522 to 1229 m2/g). The pore volumes for samples LAL1 and NRG1 (17.5 and 20.8 cm3/100 g, respectively) were also considerably lower than for the commercial samples (ranging from 23.9 to 59.2 cm3/100 g).
The lower surface areas and pore volumes of the inertinite-derived samples may be due to a variety of factors related to properties of the inertinite raw material and the activation process. Pretreatment may for some reason have not been very effective in opening pore volume on the inertinite samples. The bulk density of the LAL and NRG samples was much greater than that for the commercial samples, perhaps due to less volatilization of internal molecules to create pore space. Beneficial reuse of a waste material and low cost would be reasons to use activated carbons derived from inertinite, even though their surface areas are not as high as the presumably more expensive commercially available materials.
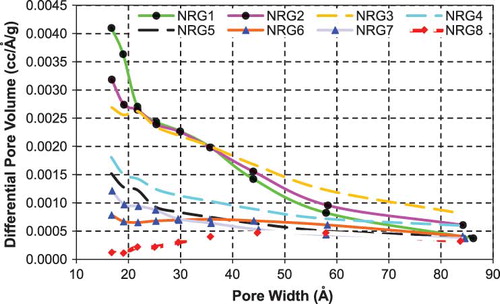
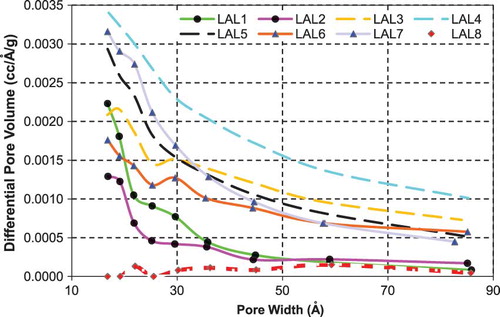
Mesopore size distributions for the commercially available activated carbons, inertinite-derived carbons from NRG coal, and inertinite-derived carbons from LAL coal are shown in , , and , respectively. Pore volumes for the commercial activated carbons are generally larger.
Although surface functional groups such as acidic or basic groups were not identified in this study, this would doing so would provide useful information in future studies.
Example testing of AC for air and water pollutant adsorption
Although surface area and pore volume tests are typically sufficient for comparing the effectiveness of adsorbents, additional testing was done with an example air pollutant and water pollutant. The adsorption capacities of the two inertinite-derived activated carbons with the highest surface area were compared with those of the commercially available activated carbons using methyl ethyl ketone (MEK) and methyl orange as example gas- and liquid-phase contaminants, respectively. MEK was chosen because it is one of the compounds compatible with the seals on the vapor sorption analyzer. It is a widely used industrial solvent. Although it was delisted in 2005 from the Environmental Protection Agency's Hazardous Air Pollutant list, it is still of concern because of its ozone-formation potential. Methyl orange, although not a common industrial pollutant, is frequently used in liquid-phase adsorption studies (CitationChen et al., 2010; CitationHosseini et al., 2011; CitationHuang, 2010; CitationLiu et al., 2009; CitationTian et al., 2009; CitationVisa et al., 2009; CitationYao et al., 2011).
Gas-phase adsorption tests
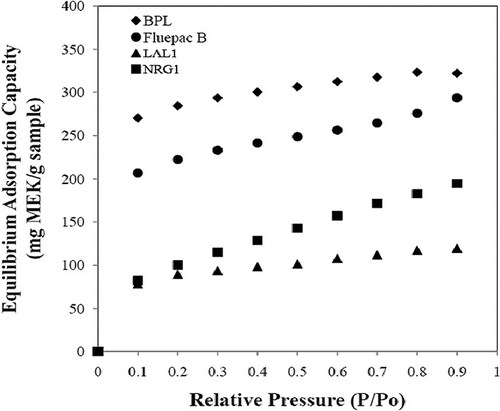
Equilibrium adsorption capacities of MEK at relative pressures ranging from 0.1 to 0.9 were measured gravimetrically with a symmetric vapor sorption analyzer (VTI, Inc., Model SGA-100, Kingsville, TX) at 25 °C. Results are shown in Adsorption capacities of the commercial activated carbons for MEK exceeded those of the inertinite-derived adsorbents for all partial pressures.
Liquid-phase adsorption tests
Inertinite from LAL and NRG coals was mixed by hand in an approximate ratio of 90%:10%, and then pretreated at 600 °C for 60 min in steam. One gram of the resulting carbon was placed in 150 mL of solution, with an initial methyl orange concentration of 150 g/L (CitationAhnert et al., 2003; CitationAmerican Society for Testing and Materials [ASTM]). Liquid-phase concentrations of methyl orange were measured as a function of time using a ultraviolet-visible (UV-VIS) spectrophotometer (UV-2550, Shimadzu, Arlington, UT) until the concentration remained constant with time (equilibrium was reached). The two commercially available liquid-phase adsorbents were tested in a similar manner. Tests were conducted at room temperature (25 °C).
For the inertinite sample, the equilibrium adsorption capacity was 17.1 mg methyl orange/g carbon. Both the Filtrasorb 200 and Carbsorb 30 adsorbed all the methyl orange in solution, indicating that their adsorption capacity is at least 22.5 mg/g, and probably higher. Thus, the maximum adsorption capacity of the commercially available carbons for methyl orange is at least 32% higher than for the inertinite-derived adsorbent (this test did not determine the absolute maximum adsorption capacity of the commercial carbons for methyl orange). The higher adsorption capacity of the commercial carbons is not surprising, given that the surface area and pore volumes for the Filtrasorb and Carbsorb samples were higher.
AC production potential from inertinite, and the global AC market
The UT Arlington coal-to-liquids process is anticipated to produce around 250 kg inertinite per ton of coal. During the activation process that produced the highest surface area, 44–60% of the original mass of the inertinite was volatilized. Assuming 50% mass lost to volatilization, 125 kg of activated adsorbent could be produced per ton of coal. Because 1 ton of coal produces three barrels of oil, 41.7 kg activated carbon could be produced per barrel of crude. If a plant produced 100,000 barrels per day of crude, 4.2 thousand metric tons of activated carbon would be produced per day, or 1521 thousand metric tons per year (assuming plant operation 365 days/year).
Activated carbon is used in drinking water treatment; industrial air purification, including solvent recovery; food and beverage processing, including sweetener processing; motor vehicle emission canisters; and pharmaceutical production. Global activated carbon demand is predicted to rise 5.2% yearly through 2012 (CitationFreedonia Group, 2008). summarizes U.S. activated carbon demand by industry sector for 2007, and forecasted demand for 2012 and 2017 (CitationBhatnagar and Sillanpää, 2010). The numbers reported in for air purification for 2012 and 2017 are likely underestimates, due to federal regulations requiring mercury control. According to one source, use of activated carbon for air purification is anticipated to increase to 160 thousand metric tons by 2013, making total U.S. demand around 380 thousand metric tons (CitationReisch, 2008).
Table 4. U.S. activated carbon demand by market
summarizes world activated carbon demand by region for 2007, and forecasted demand for 2012 and 2017. similarly underestimates air purification use of activated carbon in North America, due to mercury regulations. Accounting for this increase, global demand would be around 1300 thousand metric tons in 2013, and 1600 thousand metric tons per year in 2017.
Table 5. Global activated carbon demand by region
Thus, the anticipated supply of activated carbon from one plant producing 100,000 barrels per day (1521 thousand metric tons per year) would almost supply the annual global demand (1600 thousand metric tons per year in 2017). Hence, assuming that other coal-to-liquid conversion plants are built, additional uses would need to be found for the inertinite. Options include its reuse in asphalt and concrete pavements, or burning as fuel, since it is 50–70% C.
Summary
Inertinite from coal can be beneficially reused as an adsorbent for air or water pollution control. Beneficial reuse of the inertinite waste material, and low cost, would be reasons to use activated carbon derived from inertinite, even though its surface areas and adsorption capacities are not as high as the more expensive commercially available materials.
uawm_a_660269_sup_25623053.docx
Download MS Word (12.8 KB)Acknowledgments
This research was funded by the Defense Advanced Projects Research Agency (DARPA), Contract No. HR0011-09-C-0108. Any opinions, findings and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the DARPA. The authors would like to acknowledge Dr. Shreeyukta Singh and Maher D. Alrashdan for pretreatment of the samples and Avinash Athelli and Aditya Singh for conducting the surface area and pore size analysis experiments.
References
- Achak , M. , Hafidi , A. , Ouazzani , N. , Sayadi , S. and Mandi , L. 2009 . Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: Kinetic and equilibrium studies . J. Hazard. Mater. , 166 : 117 – 125 .
- Ahmaruzzaman , M. and Sharma , D.K. 2005 . Adsorption of phenols from wastewater . J. Colloid Interface Sci. , 287 : 14 – 24 .
- Ahnert , F. , Arafat , H.A. and Pinto , N. G. 2003 . A Study of the influence of hydrophobicity of activated carbon on the adsorption equilibrium of aromatics in non-aqueous media . Adsorption , 9 : 311 – 319 .
- Ajmal , M. , Rao , R.A.K. , Ahmad , R. and Ahmad , J. 2000 . Adsorption studies on citrus reticulate (fruit peel of orange): Removal and recovery of Ni(II) from electroplating wastewater . J. Hazard. Mater. , 79 : 117 – 131 .
- Akgerman , A. and Zardkoohi , M. 1996 . Adsorption of phenolic compounds on flyash . J. Chem. Eng. Data , 41 : 185 – 187 .
- Akhtar , M. , Bhanger , M.I. , Iqbal , S. and Hasany , S.M. 2006 . Sorption potential of rice husk for the removal of 2,4-dichlorophenol from aqueous solutions: Kinetic and thermodynamic investigations . J. Hazard. Mater. , 128 : 44 – 52 .
- Aksu , Z. and Yener , J. 1999 . The usage of dried activated sludge and fly ash wastes in phenol biosorption/adsorption: Comparison with granular activated carbon . J. Environ. Sci. Health Part A , 34 : 1777 – 1796 .
- Aksu , Z. and Yerner , J. 1998 . Investigation of the biosorption of phenol and monochlorinated phenols on the dried activated sludge . Process Biochem. , 33 : 649 – 655 .
- Aksu , Z. and Yener , J. 2001 . A comparative adsorption/biosorption study of monochlorinated phenols onto various sorbents . Waste Manage. , 21 : 695 – 702 .
- Alinnor , I.J. 2007 . Adsorption of heavy metal ions from aqueous solution by fly ash . Fuel , 86 : 853 – 857 .
- American Society for Testing and Materials (ASTM) . 2008 . ASTM Method D 3860-98. Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm Technique , West Conshohocken , PA : ASTM .
- Annadurai , G. , Juang , R.-S. and Lee , D.-J. 2002 . Use of cellulose-based wastes for adsorption of dyes from aqueous solutions . J. Hazard. Mater. , 92 : 262 – 274 .
- Arami , M. , Limaee , N.Y. , Mahmoodi , N.M. and Tabrizi , N.S. 2005 . Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies . J. Colloid Interface Sci. , 288 : 371 – 376 .
- Batabyal , D. , Sahu , A. and Chaudhuri , S.K. 1995 . Kinetics and mechanism of removal of 2,4-dimethylphenol from aqueous solutions with coal fly ash . Sep. Technol. , 5 : 179 – 186 .
- Bhatnagar , A. and Sillanpää , M. 2010 . Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review . Chem. Eng. J. , 157 : 277 – 296 .
- Billo , R.E , Choi , H. , Dennis , B.H. , MacDonnell , F. , Priest , J.W. and Sattler , M. 2010 . A Carbon Extraction Process for Converting Southwestern Lignite to JP-8 , Arlington , TX : UT Arlington . Final Report, DARPA Project HR0011-09-C-0108. December 2010
- Boudrahem , F. , Aissani-Benissad , F. and Aït-Amar , H. 2009 . Batch sorption dynamics and equilibrium for the removal of lead ions from aqueous phase using activated carbon developed from coffee residue activated with zinc chloride . J. Environ. Manage. , 90 : 3031 – 3039 .
- Bulut , Y. and Baysal , Z. 2006 . Removal of Pb(II) from wastewater using wheat bran . J. Environ. Manage. , 78 : 107 – 113 .
- Chandrasekhar , S. and Pramada , P.N. 2006 . Rice husk ash as an adsorbent for methylene blue-effect of ashing temperature . Adsorption , 12 : 27 – 43 .
- Chen , J. , Ouyang , T. , Lai , L. and Cao , W. Utilization of coal fly ash for remediation of metal contaminated soil in mining sites . Presented at the 4th International Conference on Bioinformatics and Biomedical Engineering . June 18–20 2010 , Chengdu , China . Piscataway , NJ : IEEE Computer Society .
- Chen , C.Y. , Wang , P. and Zhuang , Y.Y. 2005 . Dye removal from wastewater using the adsorbent developed from sewage sludge . J. Environ. Sci. (China) , 17 : 1018 – 1021 .
- Chen , S. , Zhang , J. , Zhang , C. , Yue , Q. , Li , Y. and Li , C. 2010 . Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis . Desalination , 252 : 149 – 156 .
- Comolli , A.G. , Johanson , E.S. , Panvelker , S. and Popper , G. Catalytic two-stage liquefaction (CTSL) process Wyoming sub-bituminous coal . Paper presented at the Proc. Int. Conf. Coal Slurry Technol . April 23–26 1990 , Clearwater , FL . Washington , DC : Coal & Slurry Technology Association .
- Comolli , A.G.,. , Lee , T.L.K. , Popper , G.A. and Zhou , P. 1999 . The Shenhua coal direct liquefaction plant . Fuel Process. Technol. , 59 : 207 – 215 .
- Cooper , C.V. and Alley , F. C. 1994 . Air Pollution Control: A Design Approach. Long Grave , IL : Waveland Press .
- de Sousa , D.A. , de Oliveira , E. , Da , M. , Nogueira , C. and Espósito , B.P. 2010 . Development of a heavy metal sorption system through the P S functionalization, of coconut (Cocos nucifera) fibers . Bioresour. Technol. , 101 : 138 – 143 .
- El-Geundi , M.S. 1991 . Colour removal from textile effluents by adsorption techniques . Water Res. , 25 : 271 – 273 .
- Elizalde-González , M. P. , Geyer , W. , María , P. , Guevara-Villa , M.R.G. , Mattusch , J. , Peláez-Cid , A.A. and Wennrich , R. 2006 . Characterization of an adsorbent prepared from maize waste and adsorption of three classes of textile dyes . Colloids Surf. A Physicochem. Eng. Asp. , 278 : 89 – 97 .
- Erŏglu , H. , Yapici , S. , Nuhŏglu , C. and Varŏglu , E. 2009 . An environmentally friendly process; Adsorption of radionuclide Tl-201 on fibrous waste tea . J. Hazard. Mater. , 163 : 607 – 617 .
- Fatma , C. , Dursun , O. , Ahmet , O. and Ayla , O. 2007 . Low cost removal of reactive dyes using wheat bran . J. Hazard. Mater. , 146 : 408 – 416 .
- Freedonia Group. 2008. World Activated Carbon to 2012. May 2008 www.freedoniagroup.com/World-Activated-Carbon.html (http://www.freedoniagroup.com/World-Activated-Carbon.html) (Accessed: 20 September 2010 ).
- Gangupomu , R.H. , Kositkanawuth , K. , Sattler , M.L. , Ramirez , D. , Dennis , B. , MacDonnell , F. , Billo , R. and Priest , J. June 2011 . “ Analysis and comparison of inertinite-derived adsorbent with conventional adsorbents ” . In Proceedings of the Air & Waste Management Association 104th Annual Conference June , 21 – 24 . Orlando , FL
- Ghimire , K.N. , Inoue , K. , Ohto , K. and Hayashida , T. 2007 . Adsorptive separation of metallic pollutants onto waste seaweeds, Porphyra yezoensis and Ulva japonica . Sep. Sci. Technol. , 42 : 2003 – 2018 .
- Gopalswami , P.M. , Ponnusamy , S. , Sivakumar , N. and Ilamparithi , A. 2010 . Methylene blue adsorption onto low cost powdered activated carbon from agricultural waste—Morus plant . Nature Environ. Pollut. Technol. , 9 : 317 – 322 .
- Hamabe , H. , Okayama , T. , Simada , M. , Iida , T. and Kawarda , K. Adsorption characteristics of activated carbon from waste newspaper . Proceedings of the Pulp and Paper Research Conference . June 15–16 1998 , Tokyo , Japan. pp. 14 – 17 . Tokyo , , Japan : Japan Techinical Association of the Pulp and Paper Industry .
- Hameed , B.H. , Mahmoud , D.K. and Ahmad , A.L. 2008a . Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste . J. Hazard. Mater. , 158 : 65 – 72 .
- Hameed , B.H. , Tan , I.A.W. and Ahmad , A.L. 2008b . Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon . Chem. Eng. J. , 144 : 235 – 244 .
- Han , R. , Han , P. , Cai , Z. , Zhao , Z. and Tang , M. 2008 . Kinetics and isotherms of neutral red adsorption on peanut husk . J. Environ. Sci. , 20 : 1035 – 1041 .
- Haribabu , E. , Upadhya , Y.D. and Upadhyay , S.N. 1993 . Removal of phenols from effluents by fly ash . Int. J. Environ. Stud. , 43 : 169 – 176 .
- Ho , Y.-S. and Ofomaja , A.E. 2006 . Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent . Biochem. Eng. J. , 30 : 117 – 123 .
- Hosseini , S. , Khan , M.A. , Malekbala , M.R. , Cheah , W. and Choong , T.S.Y. 2011 . Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and desorption studies . Chem. Eng. J. , 171 : 1124 – 1131 .
- Huang , J. 2010 . Adsorption thermodynamics of methyl orange from aqueous solution onto a hyper-cross-linked polystyrene resin modified with phenolic hydroxy groups . Adsorpt. Sci. Technol. , 28 : 397 – 405 .
- Janos , P. , Buchtova , H. and Ryznarova , M. 2003 . Sorption of dyes from aqueous solutions onto fly ash . Water Res. , 37 : 4938 – 4944 .
- Kadirvelu , K. and Namasivayam , C. 2000 . Agricultural by-product as metal adsorbent: Sorption of lead(II) from aqueous solution onto coirpith carbon . Environ. Technol. , 21 : 1091 – 1097 .
- Kannan , N. and Sundaram , M.M. 2001 . Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study . Dyes Pigments , 51 : 25 – 40 .
- Kao , P.C. , Tzeng , J.H. and Huang , T.L. 2000 . Removal of chlorophenols from aqueous solution by fly ash . J. Hazard. Mater. , 76 : 237 – 249 .
- Kaur , S. and Prasad , N. 2001 . Adsorption of dyes on rice husk ash . Indian J. Chem. A , 40 : 388 – 391 .
- Khanna , P. and Malhotra , S.K. 1977 . Kinetics and mechanism of phenol adsorption of flyash . Indian J. Environ. Health. , 19 : 224 – 237 .
- Kojima , N. , Mitomo , A. , Itaya , Y. , Mori , S. and Yoshida , S. 2002 . Adsorption removal of pollutants by active cokes produced from sludge in the energy recycle process of wastes . Waste Manage. , 22 : 399 – 404 .
- Kositkanawuth , K. , Gangupomu , R. H. , Sattler , M. L. , Dennis , B. , MacDonnell , F. , Billo , R. and Priest , J. Evaluation of emissions from three alternatives for producing crude . Proceedings of the 104th Annual Conference of the Air & Waste Management Association . Orlando , Florida . Pittsburgh , PA : Air & Waste Management Association .
- Kumar , U. and Bandyopadhyay , M. 2006 . Sorption of cadmium from aqueous solution using pretreated rice husk . Bioresour. Technol. , 97 : 104 – 109 .
- Lakshmi , U.R. , Srivastava , V.C. , Mall , I.D. and Lataye , D.H. 2009 . Rice husk ash as an effective adsorbent: Evaluation of adsorptive characteristics for indigo carmine dye . J. Environ. Manage. , 90 : 710 – 720 .
- Li , X. , Hu , H. , Jin , L. , Hu , S. and Wu , B.O. 2008 . Approach for promoting liquid yield in direct liquefaction of Shenhua coal . Fuel Process. Technol. , 89 : 1090 – 1095 .
- Li , X. , Tang , Y. , Cao , X. , Lu , D. , Luo , F. and Shao , W. 2008 . Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel . Colloids Surf. A Physicochem. Eng. Aspects , 317 : 512 – 521 .
- Li , Y. , Zhang , F.-S. and Xiu , F.-R. 2009 . Arsenic(V) removal from aqueous system using adsorbent developed from a high iron-containing fly ash . Sci. Total Environ. , 407 : 5780 – 5786 .
- Liu , Z. , Zhou , A. , Wang , G. and Zhao , X. 2009 . Adsorption behavior of methyl orange onto modified ultrafine coal powder . Chin. J. Chem. Eng. , 17 : 942 – 948 .
- Long , C. , Lu , J. , Li , A. , Hu , D. , Liu , F. and Zhang , Q. 2008 . Adsorption of naphthalene onto the carbon adsorbent from waste ion exchange resin: Equilibrium and kinetic characteristics . J. Hazard. Mater. , 150 : 656 – 661 .
- Mahvi , A.H. , Maleki , A. and Eslami , A. 2004 . Potential of rice husk and rice husk ash for phenol removal in aqueous systems . Am. J. Appl. Sci. , 1 : 321 – 326 .
- Malik , P.K. 2003 . Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of acid yellow 36 . Dyes Pigments , 56 : 239 – 249 .
- McKay , G. , Porter , J.F. and Prasad , G.R. 1999 . The removal of dye colours from aqueous solutions by adsorption on low-cost materials . Water Air Soil Pollut. , 114 : 423 – 438 .
- Memon , J.R. , Memon , S.Q. , Bhanger , M.I. , El-Turki , A. , Hallam , K.R. and Allen , G.C. 2009 . Banana peel: A green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater . Colloids Surf. B Biointerfaces , 70 : 232 – 237 .
- Memon , J.R. , Memon , S.Q. , Bhanger , M.I. , ZuhraMemon , G. , El-Turki , A. and Allen , G.C. 2008 . Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal . Colloids Surf. B Biointerfaces , 66 : 260 – 265 .
- Minamisawa , M. , Nakajima , S. , Minamisawa , H. , Yoshida , S. and Takai , N. 2005 . “ Removal of Copper (II) and Cadmium(II) from Water using Roasted Coffee Beans ” . In Environmental Chemistry Green Chemistry and Pollutants in Ecosystems , Edited by: Lichtfouse , E. , Schwarzbauer , J. and Robert , D. 259 – 265 . Berlin, Heidelberg : Springer .
- Mohanty , K. , Naidu , J.T. , Meikap , B.C. and Biswas , M.N. 2006 . Removal of crystal violet from wastewater by activated carbons prepared from rice husk . Ind. Eng. Chem. Res. , 45 : 5165 – 5171 .
- Montagnaro , F. and Santoro , L. 2009 . Reuse of coal combustion ashes as dyes and heavy metal adsorbents: Effect of sieving and demineralization on waste properties and adsorption capacity . Chem. Eng. J. , 150 : 174 – 180 .
- Murugesan , G.S. , Sathishkumar , M. and Swaminathan , K. 2006 . Arsenic removal from groundwater by pretreated waste tea fungal biomass . Bioresour. Technol. , 97 : 483 – 487 .
- Namasivayam , C. and Kavitha , D. 2002 . Removal of Congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste . Dyes Pigments , 54 : 47 – 58 .
- Namasivayam , C. , Muniasamy , N. , Gayatri , K. , Rani , M. and Ranganathan , K. 1996 . Removal of dyes from aqueous solutions by cellulosic waste orange peel . Bioresour. Technol. , 57 : 37 – 43 .
- Namasivayam , C. , Radhika , R. and Suba , S. 2001 . Uptake of dyes by a promising locally available agricultural solid waste: Coir pith . Waste Manage. , 21 : 381 – 387 .
- Namasivayam , C. and Sangeetha , D. 2006 . Recycling of agricultural solid waste, coir pith: Removal of anions, heavy metals, organics and dyes from water by adsorption onto ZnCl2 activated coir pith carbon . J. Hazard. Mater. , 135 : 449 – 452 .
- Namasivayam , C. , Sangeetha , D. and Gunasekaran , R. 2007 . Removal of anions, heavy metals, organics and dyes from water by adsorption onto a new activated carbon from Jatropha husk, an agro-industrial solid waste . Process Saf. Environ. Protect. , 85 : 181 – 184 .
- Oliveira , F.D. , Paula , J.H. , Freitas , O.M. and Figueiredo , S.A. 2009 . Copper and lead removal by peanut hulls: Equilibrium and kinetic studies . Desalination , 248 : 931 – 940 .
- Olivella , M.À. , Jove , P. and Oliveras , A. 2011 . The use of cork waste as a biosorbent for persistent organic pollutants—Study of adsorption/desorption of polycyclic aromatic hydrocarbons . J. Environ. Sci. Health A Toxicol. Hazard. Subst. Environ. Eng. , 46 : 824 – 832 .
- Panday , K.K. , Prasad , G. and Singh , V.N. 1985 . Copper(II) removal from aqueous solutions by fly ash . Water Res. , 19 : 869 – 873 .
- Parab , H. , Joshi , S. , Shenoy , N. , Lali , A. , Sarma , U.S. and Sudersanan , M. 2006 . Determination of kinetic and equilibrium parameters of the batch adsorption of Co(II), Cr(III) and Ni(II) onto coir pith . Process Biochem. , 41 : 609 – 615 .
- Paul, P.J. 2005. Value Added Products from Gasification-Activated Carbon. The Combustion, Gasification and Propulsion Laboratory (CGPL) at the Indian Institute of Science (IISc). http://cgpl.iisc.ernet.in/site/Default.aspx (http://cgpl.iisc.ernet.in/site/Default.aspx) (Accessed: 20 March 2011 ).
- Quantachrome Instruments . 2007 . NOVA Operation Manual; High Speed Gas Sorption Analyzer Versions 9.0 and Above , Boynton Beach , FL : Quantachrome Instruments .
- Redlich , P.J. , Hulston , C.K.J. , Jackson , W.R. , Larkins , F.P. and Marshall , M. 1999 . Hydrogenation of sub-bituminous and bituminous coals pre-treated with water-soluble nickel-molybdenum or cobalt-molybdenum catalysts . Fuel , 78 : 83 – 88 .
- Reisch , M. 2008 . Getting rid of mercury . Chem. Eng. News , 86 November 24 : 22 – 23 .
- Rio , S. , Faur-Brasquet , C. , Courcoux , P. , Le Coq , L. and Le Cloirec , P. 2005 . Experimental design methodology for the preparation of carbonaceous sorbents from sewage sludge by chemical activation-application to air and water treatments . Chemosphere , 58 : 423 – 437 .
- Sarkar , M. , Acharya , P.K. and Bhattacharya , B. 2003 . Modeling the adsorption kinetics of some priority organic pollutants in water from diffusion and activation energy parameters . J. Colloid Interface Sci. , 266 : 28 – 32 .
- Sasaki , M. , Kotanigawa , T. and Yoshida , T. 2000 . Liquefaction reactivity of methylated Illinois No. 6 coal . Energy Fuels , 14 : 76 – 82 .
- Sen , A.K. and Arnab , K.D. 1987 . Adsorption of mercury on coal fly-ash . Water Res. , 21 : 885 – 887 .
- Senthilkumaar , S. , Kalaamani , P. and Subburaam , C.V. 2006 . Liquid phase adsorption of crystal violet onto activated carbons derived from male flowers of coconut tree . J. Hazard. Mater. , B136 : 800 – 808 .
- Shimada , M. , Hamabe , H. , Iida , T. , Kawarada , K. and Okayama , T. 1999 . The properties of activated carbon made from waste newsprint paper . J. Porous Mater. , 6 : 191 – 196 .
- Singh , B.K. , Misra , N.M. and Rawat , N.S. 1994 . Sorption characteristics of phenols on flyash and impregnated flyash . Ind. J. Environ. Health , 36 : 1 – 7 .
- Singh , B.K. and Rawat , N.S. 1994 . Comparative sorption equilibrium studies of toxic phenols on fly ash and impregnated fly ash . J. Chem. Technol. Biotechnol. , 61 : 307 – 317 .
- Sivaraj , R. , Namasivayam , C. and Kadirvelu , K. 2001 . Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions . Waste Manage. , 21 : 105 – 110 .
- Srinivasan , K. , Balasubaramanian , N. and Ramakhrisna , T.V. 1988 . Studies on chromium removal by rice husk carbon . Indian J. Environ. Health. , 30 : 376 – 387 .
- Tan , I.A.W. , Ahmad , A.L. and Hameed , B.H. 2008 . Adsorption of basic dye on high-surfacearea activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies . J. Hazard. Mater. , 154 : 337 – 346 .
- Thawornchaisit , U. and Pakulanon , K. 2007 . Application of dried sewage sludge as phenol biosorbent . Bioresour. Technol. , 98 : 140 – 144 .
- Tian , K.-Q. , Li , F.-F. and Xing , J.-M. 2009 . Adsorption methyl orange using carbon materials from waste tobacco . Gongneng Cailiao J. Funct. Mater. , 40 : 1490 – 1492 .
- Uddin , M.T. , Islam , M.A. , Mahmud , S. and Rukanuzzaman , M. 2009 . Adsorptive removal of methylene blue by tea waste . J. Hazard. Mater. , 164 : 53 – 60 .
- Valente , A.M. and Cronauer , D.C. 2005 . Analyses of Illinois no. 6 coal liquefaction results generated in the Wilsonville, Alabama unit . Energy Fuels , 19 : 489 – 498 .
- Vieira , A.P. , Santana , S.A.A. , Bezerra , C.W.B. , Silva , H.A.S. , Chaves , J.A.P. , de Melo , J.C.P. , Da , E.C. , Filho , S. and Airoldi , C. 2009 . Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp . J. Hazard. Mater. , 166 : 272 – 1278 .
- Viraraghavan , T. and Ramakrishna , K.R. 1999 . Flyash for colour removal from synthetic dye solutions . Water Qual. Res. J. Can. , 34 : 505 – 517 .
- Visa , M. , Enesca , A. and Duta , A. 2009 . Simultaneous adsorption of methyl orange and heavy metals from solution using fly ash aria . Adv. Mater. Res. , 79–82 : 247 – 250 .
- Wang , L.H. and Lin , C.I. 2008 . Adsorption of lead(II) ion from aqueous solution using rice hull ash . Ind. Eng. Chem. Res. , 47 : 4891 – 4897 .
- Webber, M. E. 2009a. Fossil fuel [PowerPoint presentation]. August 2009 http://www.webberenergygroup.com/courseswww.webberenergygroup.com/courses (http://www.webberenergygroup.com/courseswww.webberenergygroup.com/courses) (Accessed: 21–24Jun2011 ).
- Webber, M. E. 2009b. Introduction: Energy uses, basics and fundamentals [PowerPoint presentation]. August 2009 http://www.webberenergygroup.com/courseswww.webberenergygroup.com/courses (http://www.webberenergygroup.com/courseswww.webberenergygroup.com/courses) (Accessed: 20 March 2011 ).
- Woolard , C.D. , Strong , J. and Erasmus , C.R. 2002 . Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams . Appl. Geochem. , 17 : 1159 – 1164 .
- Yang , J. , Wang , S. , Lu , Z. , Yang , J. and Lou , S. 2009 . Converter slag-coal cinder columns for the removal of phosphorous and other pollutants . J. Hazard. Mater. , 168 : 331 – 337 .
- Yao , Y. , Bing , H. , Feifei , X. and Xiaofeng , C. 2011 . Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes . Chem. Eng. J. , 170 : 82 – 89 .
- Zafar , M.N. , Nadeem , R. and Hanif , M.A. 2007 . Biosorption of nickel from protonated rice bran . J. Hazard. Mater. , 143 : 478 – 485 .
- Zhang , J. , Jin , L. , He , X. , Liu , S. and Hu , H. 2011 . Decomposition over activated carbons prepared from direct coal liquefaction residue by KOH activation with addition of SiO2 or SBA-15 . Int. J. Hydrogen Energy , 36 : 8978 – 8984 .
- Zhou , Y. , Xiao , N. , Qiu , J. , Sun , Y. , Sun , T. , Zhao , Z. , Zhang , Y. and Tsubaki , N. 2008 . Preparation of carbon microfibers from coal liquefaction residue . Fuel , 87 : 3474 – 3476 .
- Zhu , C.-S. , Wang , L.-P. and Chen , W.-B. 2009 . Removal of Cu(II) from aqueous solution by agricultural by-product: Peanut hull . J. Hazard. Mater. , 168 : 739 – 746 .