Abstract
Three pilot-scale, horizontal-flow biofilm reactors (HFBRs 1–3) were used to treat methane (CH4)-contaminated air to assess the potential of this technology to manage emissions from agricultural activities, waste and wastewater treatment facilities, and landfills. The study was conducted over two phases (Phase 1, lasting 90 days and Phase 2, lasting 45 days). The reactors were operated at 10 °C (typical of ambient air and wastewater temperatures in northern Europe), and were simultaneously dosed with CH4-contaminated air and a synthetic wastewater (SWW). The influent loading rates to the reactors were 8.6 g CH4/m3/hr (4.3 g CH4/m2 TPSA/hr; where TPSA is top plan surface area). Despite the low operating temperatures, an overall average removal of 4.63 g CH4/m3/day was observed during Phase 2. The maximum removal efficiency (RE) for the trial was 88%. Potential (maximum) rates of methane oxidation were measured and indicated that biofilm samples taken from various regions in the HFBRs had mostly equal CH4 removal potential. In situ activity rates were dependent on which part of the reactor samples were obtained. The results indicate the potential of the HFBR, a simple and robust technology, to biologically treat CH4 emissions.
The results of this study indicate that the HFBR technology could be effectively applied to the reduction of greenhouse gas emissions from wastewater treatment plants and agricultural facilities at lower temperatures common to northern Europe. This could reduce the carbon footprint of waste treatment and agricultural livestock facilities. Activity tests indicate that methanotrophic communities can be supported at these temperatures. Furthermore, these data can lead to improved reactor design and optimization by allowing conditions to be engineered to allow for improved removal rates, particularly at lower temperatures. The technology is simple to construct and operate, and with some optimization of the liquid phase to improve mass transfer, the HFBR represents a viable, cost-effective solution for these emissions.
Introduction
Greenhouse gases such as methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) are often emitted in agriculture, from landfills, and during waste, and wastewater, treatment (CitationAbdulla and Al-Ghazzawi, 2000; CitationMelse and Van Der Werf, 2005). An estimated 30% of anthropogenic methane emissions in Europe come from the waste treatment sector (CitationHaubrichs and Widmann, 2006). The European Union (EU) has committed to reducing greenhouse gas emissions under the legally binding Kyoto Protocol. The control of methane emissions from waste and wastewater treatment facilities is therefore important. Traditional methods for treating greenhouse gas emissions from landfills, waste and wastewater treatment facilities, and agricultural activities include onsite flaring, chemical scrubbing, or catalytic oxidation. These can be subject to high capital costs, generate secondary pollutants, and have high maintenance costs (CitationPhillips, 2008). Therefore, gas treatment using biological methods is being employed more frequently due to the inherent advantages, including (i) neutralization of the pollutant, (ii) low capital and maintenance costs, and (iii) good overall performance (CitationNikiema et al., 2007). The treatment of CH4 emissions in biofilm reactors has become an established technique in recent years.
The main challenge involved when designing CH4 treating biofilm reactors is the low solubility of CH4 in water, meaning relatively long retention times are required (CitationStreese and Stegmann, 2003). The required retention time will increase as temperature decreases. Previous studies found a relationship between the substrate utilization rate (k) at temperature (T) for methane bioelimination (CitationNikiema et al., 2009b). This can be written as ( Equationeq 1).
This model predicted CH4 oxidation at temperatures between 25 and 35 °C, which can be significantly higher than those found in many regions, for example, northern Europe. Recent work has focused on examining the optimization of operational parameters (CitationNikiema et al., 2009a; CitationNikiema and Heitz, 2009), nutrient feeds (CitationNikiema et al., 2009a), and solid and liquid phases (CitationNikiema et al., 2009a; CitationRocha-Rios et al., 2009). Thus the development and testing of a biofilm reactor capable of operating at lower temperatures in an efficient manner (i.e., without an excessive footprint) is desirable.
Full-scale compost biofilters have been most effectively applied to the treatment of CH4 emissions from animal store houses (CitationMelse and Van Der Werf, 2005). Recent reviews and experimental work on biological treatment of methane technology have shown that with optimization of liquid nutrient feeds, loading rates, and biofilter configuration, retention times can be significantly reduced and loading rates increased without compromising removal efficiency (CitationNikiema et al., 2009a; 2007). Mass transfer limitations traditionally associated with biological conversion of CH4 have been alleviated by adding an additional liquid phase (silicone oil), which helps improve the solubility of methane into the liquid phase (CitationRocha-Rios et al., 2009).
When CH4 gas concentrations are below approximately 5% volume CH4/volume air, energy recovery is not feasible (CitationEl-Fadel and Massoud, 2001). Examples include older landfills where methane emissions are commonly at lower concentrations (CitationHaubrichs and Widmann, 2006). In agricultural facilities, CH4 emissions can be associated with enteric fermentation in ruminants and fermentation of manure in animal houses. An average-sized animal house (100 dairy cattle) produces an airflow of about 60000 m3/hr at a CH4 concentration of 0.0003–0.015% (volume CH4/volume air) (CitationMelse and Van Der Werf, 2005). CH4 emissions, in wastewater treatment plants, can be generated due to due to anaerobic decay in bottom deposits (such as in treatment lagoons and holding tanks) and also during anaerobic treatment process used to stabilize wastewater sludge (CitationMetcalf and Eddy, 2003). Global CH4 emissions from municipal and industrial wastewater are estimated to be 1.3–2.4 Tg CH4/year, about 5% of global emissions. Frequently, the amount generated does not justify the installation of an energy recovery system and in such cases; alternative treatment methods such as flaring or biological oxidation are more appropriate (CitationEl-Fadel and Massoud, 2001).
In this study, a novel horizontal-flow biofilm reactor (HFBR) design was applied to the treatment of an air mixture containing CH4. The study was carried out at 10 °C, which can be typical of onsite temperature in Ireland, northern Europe, and other temperate climates. The HFBR has previously been shown to be cost-effective, sustainable, and efficient as a wastewater treatment technology (CitationClifford et al., 2010). The unique flow regime in the HFBR ensures good contact with the biofilm in the reactor and alleviates problems that can be associated with conventional biofilm reactors such as clogging, channeling, compaction, and pressure drop. The removal efficiency (RE) of each reactor was measured, flow and loading rates were recorded, and samples of the biofilm were removed from various regions in the system to analyze in situ and potential activity rates within the reactors.
Materials and Methods
Design and construction of HFBR
The pilot-scale HFBR units comprised a stack of 60 horizontal plastic sheets positioned one above the other. Each sheet measured 0.2 m × 0.2 m in plan with integrated frustums 8 mm high and 6 mm diameter at 15 mm centers. The stack of sheets was enclosed in an airtight reactor. Six sampling ports, located vertically along each reactor, allowed intermediate gas and synthetic wastewater (SWW) samples to be taken. This allowed the reactors to be divided into seven distinct sampling regions. Contaminant removal profiles could thus be established for each reactor ().
Figure 1. Schematic of the experimental setup; biofilm growth is supported on the surface and fustrums of the horizontal sheets.
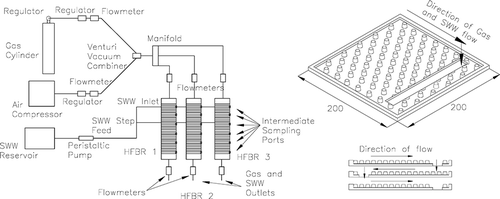
Each reactor was designed such that it was sealable but could be opened for visual inspection and biofilm sampling. The working volume of each reactor was 20 L and the top plan surface area (TPSA) of the plastic medium was 0.04 m2 (0.2 m × 0.2 m), providing a total medium plan area of 2.4 m2 (60 sheets × 0.04 m2/sheet). The air mixture was introduced at the top of each reactor, just above Sheet 1, and flowed horizontally across each sheet before moving to the sheet below and flowing along that sheet in the opposite direction. This pattern continued throughout the reactor. To provide nutrients to the biofilm, a synthetic wastewater (SWW 1) was dosed intermittently (for 10 min each hour) into the reactors in a step-feed manner—75% (6 L/day) were dosed onto Sheet 1 and 25% (2 L/day) onto Sheet 30—as dosing all of the SWW onto Sheet 1 likely would encourage thick biofilm development in this region with limited potential for biofilm development in lower regions. The step-feed allowed for better distribution of nutrients and more consistent biofilm growth throughout the HFBRs, while also likely promoting the development of various different biological zones (CitationClifford et al., 2010). SWW 1 was chosen because it has similar characteristics to influent wastewater at a municipal wastewater treatment plant, which may have provided a suitable, cost-effective, liquid nutrient feed on site. SWW 1 composition is presented in .
The HFBRs were housed in a purpose-built external laboratory with a controlled ambient temperature of 10 °C. Methane gas, supplied from a cylinder, was mixed with compressed air to form an air mixture with the desired concentrations (1% v CH4/v ambient air). Flowmeters and pressure regulators allowed flow rates and gas mix proportions to be controlled and varied (). Both influent and effluent flow rates were monitored to ensure that there was no leakage from the reactors. Flow and loading parameters are given in
Table 1. Flow and loading parameters for HFBR reactors
Table 2. Composition of SWW (CitationOdegaard and Rusten, 1980)
Various compositions were trialed in order to investigate the effects nutrient concentrations had on biological CH4 oxidation ().
Sampling and analysis
The study was divided into two phases (Phase 1 and Phase 2) comprising 90 and 45 days, respectively. During Phase 1 the reactors were operated in triplicate, with SWW 1 as the nutrient feed to each of the reactors. During Phase 2 the units were operated independently, with different nutrient feeds added to each reactor (SWW 2 to HFBR 1, SWW 3 to HFBR 2, and SWW 4 to HFBR 3).
Throughout the study, influent, effluent, and profile samples of the gas mixtures were taken from each reactor and analyzed. The profile samples were taken from the sampling ports located along the vertical profile of each reactor.
Gas samples were taken with a syringe and analyzed for CH4 concentrations using a Varian CP-3800 GC gas chromatograph (JVA Analytical; Dublin, Ireland). An Agilent 7890A GC (Agilent Technologies Ireland Ltd.; Cork, Ireland) was used to measure the CO2 concentration.
SWW samples from influent, effluent, and intermediate sampling ports were taken regularly. Suspended solids (SS), chemical oxygen demand (COD), and total nitrogen (TN) were measured in accordance with standard methods (American Public Health Association, American Water Works Association, and CitationWater Environment Federation [APHA-AWWA-WEF], 2005). Ammonium nitrogen (NH4-N), nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), and phosphate phosphorous (PO4-P) concentrations were determined using a Thermo Clinical Labsystems, Konelab 20 nutrient analyzer (Fisher Scientific, Waltham, MA, USA).
Preparation of seed cultures
An enrichment strategy was employed to cultivate a mixed microbial community capable of CH4 oxidation at low temperatures. The enrichment was carried out over 4 months by feeding CH4 to a mixture of landfill soil, compost, landfill leachate, and compost leachate, at 10 °C in batch cultures. GC analysis was used to monitor CH4 loss in the enrichment cultures, which were subcultured to new medium every 2–3 weeks. The biomass was suspended in an adapted Whittenbury medium (CitationWhittenbury et al., 1970). An aliquot of 2 L of the enriched biomass was then added to each of the HFBRs to bioaugment the existing biofilm and this was recirculated through the reactors for 7 days using the peristaltic pumps.
Determination of maximum potential methane-oxidation activity (potential activity test)
To develop and apply a potential activity assay for CH4 oxidation, samples of biofilm were removed from the reactors before the beginning of Phase 2 for analysis. Each reactor was divided into six regions (each region comprising six sheets) for the purpose of sampling, with a sample taken from each region on each sampling day. For each sample region (e.g., Sheets 1–6), a composite biofilm sample was prepared by combining an equal volume of biofilm from each sheet within that region. The concentration of volatile suspended solids (VSS) was determined as the combustible fraction (loss on ignition at 550 °C) of the total suspended solids. Activity tests were set up as batch cultures in 40 mL glass hypo-vials by adding 0.005 g VSS biofilm with 10 mL adapted Whittenbury medium (CitationWhittenbury et al., 1970). The vials were sealed with butyl rubber bungs and aluminum crimps. A volume of 3 mL of headspace was removed using a syringe and was replaced with 100% CH4, to give a final headspace CH4 concentration of 10%. The vials were incubated in the dark at 10 °C on a shaker at 80 rpm. The concentration of CH4 in the headspace was monitored over time using gas chromatography. All samples were set up in triplicate along with negative controls containing no biofilm. Activity was calculated based on the rate of cumulative methane loss over time and was expressed as mol CH4/g VSS/day at standard temperature and pressure. This allowed an indirect comparison with actual or in situ removal in the HFBR units.
In situ methane removal test
To monitor the CH4 removal rates in the different regions of the reactors, gas samples were taken from the intermediate sampling ports of each of the reactors and analyzed using gas chromatography. This process was repeated 14 times for each reactor during Phase 1. Methane oxidation activity in each region was then calculated as mol CH4 removed/m2 TPSA-day. This allowed an indirect comparison with the potential activity tests carried out under idealized conditions in the laboratory.
Results
Phase 1—Initial reactor performance (days 1–90)
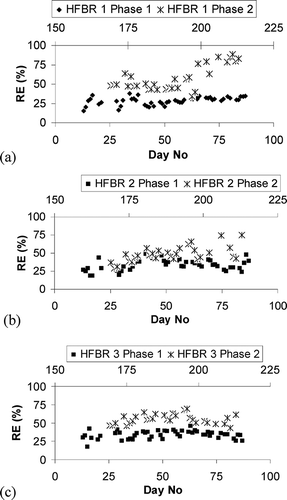
After inoculation with the seed material, three identical reactors were continuously fed with SWW 1 () for 90 days. The flow and loading parameters outlined in were applied to each unit (HFBRs 1, 2, and 3). After a 14-day start-up phase, steady-state removals were observed; thereafter effluent methane concentrations remained reasonably constant. Removal data for each of the CH4 HFBR units during the 76-day steady-state period is summarized in
Table 3. Initial removal efficiency data for the CH4 HFBR units
Profile analyses indicated that methane oxidation rates were reasonably constant throughout the reactor, with the exception of the uppermost region (Sheets 1–4). The majority of COD removal from the SWW occurred in this uppermost region (Sheets 1–4). This indicated that the presence of external organic carbon in the SWW had an inhibitory effect on CH4 removal. Average removal efficiencies for all three HFBRs for specific regions are shown in The discreet removal refers to the percentage of the influent methane removed in a particular region.
Table 4. Removal efficiency data for each sampling region in CH4 HFBR units
Phase 2 (days 168–213)
Effect of varying feed composition
After the initial phase, SWWs 2, 3, and 4 () were applied to HFBRs 1, 2, and 3, respectively, for 45 days. The flow and loading parameters outlined in were applied to each unit as before (HFBRs 1, 2, and 3). The aim of this phase was to examine the effect of varying the nutrient feed. For onsite applications, it may be desirable to use wastewater on site as a source of nutrients for the reactor. However, this may be at the cost of lower oxidation rates and thus specific nutrient feeds, though potentially costing more, may lead to more economic reactor performance. The positive effect of adding high concentrations of nitrogen to the nutrient feed in a CH4 bioreactor has been previously demonstrated (CitationNikiema and Heitz, 2009). According to some research (CitationNikiema et al., 2009a), this nitrogen should be added in the form of nitrate (NO3) rather than as ammonium (NH4) because methanotrophic activity can be diverted to nitrification of the NH4 in the feed. There are some conflicting reports, however, as to which species of nitrogen should be added, with other work suggesting that nitrogen added in the form of ammonium can improve CH4 oxidation (CitationBodelier et al., 2000). It was observed in Phase 1 that the region with the most extensive carbon removal also had the lowest CH4 removal. The effect of eliminating carbon from the nutrient supply was therefore examined by removing glucose and dried milk from the SWW influent to HFBRs 1 and 3. SWW 2 had external organic carbon removed and was applied to HFBR 1. SWW 3 comprised additional nitrogen (the additional nitrogen was in the form of nitrate with ammonium also being present as in SWW 2) and was applied to HFBR 2. SWW 4, applied to HFBR 3, had external organic carbon and external ammonium removed and had increased nitrogen concentrations ().
In all cases, significant improvements in reactor performance were observed ( and ). Although the addition of NaNO3 resulted in improved performance in HFBR 2, the most notable increase was observed in HFBR 1, demonstrating the positive effect of omitting external carbon from the SWW. Improvements in average removals were also observed in HFBR 3, which had no organic carbon and had NO3 as the main nitrogen source.
Table 5. Removal efficiency data following modifications to feed
Carbon dioxide (CO2) production
Aerobic biodegradation of CH4 can be given as follows (CitationNikiema et al., 2005):
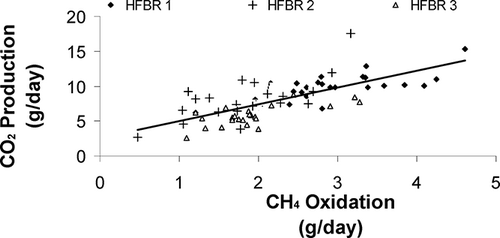
Thus CO2 production rates can also give valuable information on biomass yields due to methane oxidation. summarizes CO2 production in the HFBR units. shows a linear trend between methane CH4 oxidation and CO2 production for the three units. For each reactor, a good correlation between CH4 oxidation and CO2 production was found (correlation coefficients were 0.72, 0.69, and 0.71 for HFBRs 1, 2, and 3, respectively). From effluent analyses about 0.255 moles of CO2 exited HFBR 1, about 0.199 moles of CO2 exited HFBR 2, and about 0.145 moles of CO2 exited HFBR 3 every day. This comprised CO2 entering the reactor in the air stream (about 0.0455 mol/day), the aerobic oxidation of organic carbon, and methane oxidation. A carbon mass balance for each reactor during Phase 2 is presented in below.
Table 6. Carbon dioxide production for each HFBR unit
Table 7. Carbon mass balance for each reactor
Filtered COD (CODf) in the effluent was consistently low and previous studies on the HFBR (at similar loading rates and using a similar SWW) have shown that this mostly comprised nonbiodegradable COD (CitationClifford et al., 2010). This is further indicated by the observation that nitrification was prominent in the reactor. Filtered effluent concentrations from each reactor (40 samples for each reactor) averaged (i) 36.6, 40.5, and 34.3 mg COD/L for HFBRs 1, 2, and 3, respectively; (ii) 1.5, 1.7, and 1.2 mg NH4/L for HFBRs 1, 2, and 3, respectively; and (iii) 17.4, 14.8, and 15.5 mg NO3/L for HFBRs 1, 2, and 3, respectively.
From and assuming new cells to be represented as C5H7NO2 (CitationMetcalf and Eddy, 2003), it can be estimated that the biomass growth is approximately 0.30, 1.96, and 0.19 g new cells/day in HFBRs 1, 2, and 3, respectively, due to methane oxidation and nutrient uptake from the SWW. Carbon in the influent SWW was calculated based on its composition ().
Biomass production in HFBR 2 was significantly higher than in HFBRs 1 and 3. The most probable reason for this is the higher concentration of heterotrophic microorganisms present in HFBR 2 due to the organic carbon in SWW 3 (). These heterotrophs were observed to produce a thicker biofilm/biomass, particularly on the uppermost sheets.
Microbial activity analysis
Samples of the biomass, taken immediately after the modifications to SWW were made, were used for potential methane oxidation activity assays. In situ activity, which was determined from the profile CH4 removal data (expressed in terms of TPSA), was compared with maximum potential activity ().
Table 8. Potential and in situ activity for each reactor
Maximum methane oxidation assays indicated that the oxidation potential of the biofilm varied between zones. In general, the zones furthest away from the SWW inlets had lower potential for methane oxidation. This was most obvious in HFBRs 1 and 3 and although the difference in oxidation potential between zones in HFBR 2 was less, it followed the same trend. The difference is most likely due to nutrient depletion in the SWW stream as it flows through the HFBRs. The potential methane oxidation rates also appeared consistently higher than in situ rates.
Discussion
The performance of the novel HFBR technology was investigated for its efficacy in treating nuisance gases. Following the addition of methanotrophic seed material, good CH4 removal was achieved despite the low operating temperatures. In a prior experiment, municipal activated sludge was investigated as a possible seed material; however, after a number of weeks of operation limited CH4 removal was observed. When prepared methanotrophic seed material was added in a bioaugmentation of the existing biofilm, steady-state removals of 2.8 g CH4/m3/hr were observed after 2 weeks under the same operating conditions. The preparation of a methanotrophic seed material is therefore an important step in the construction of a CH4-oxidizing biofilm reactor.
The maximum observed elimination capacity (EC) was 7.6 g CH4/m3/hr. Although this compares favorably with previous studies that observed ECs in the range 0.5–9.0 g CH4/m3/hr (CitationMelse and Van Der Werf, 2005), it is conservative when compared with other recent studies, which observed EC values as high as 60 g CH4/m3/hr, albeit at more favorable temperatures than this study (CitationNikiema et al., 2009a; CitationNikiema and Heitz, 2009). This indicates that with further optimization, the HFBR technology could be effectively applied to the reduction of greenhouse gas emissions in a simple, cost-effective manner, allaying public concerns and reducing the carbon footprint of waste treatment facilities. As mass transfer is the limiting factor, current studies are examining methods of optimizing the liquid phase for methane removal (such as using additional or alternative liquid phases) in order to reduce Empty Bad Retention Time (EBRT) and increase EC.
As described, potential methane oxidation rates appeared consistently higher than in situ rates. This may be due to methanotrophic bacteria in the reactors being inhibited by more competitive microbes, including, for example, heterotrophic (carbon-oxidizing) or nitrifying bacteria, which would not have been the case during the potential activity tests.
Other factors that may have contributed to the difference in activity include differences in the influent CH4 concentration, which was lower in the reactors than it was in the vials, as was the contact time between the biofilm and the CH4. There were also differences in the nutrient solutions: SWW 1 was used in the reactors and the adapted Whittenbury medium was used during the potential activity tests.
Further studies are necessary to determine the exact reasons for the difference in potential and in situ oxidation; however, the results of the activity tests do indicate that the methanotrophic bacteria can be applied successfully to the biological treatment of CH4-containing emissions at lower temperatures and that further optimization of the technology and in situ environmental conditions could improve performance significantly.
The improvements that were observed following the omission of organic carbon and addition of nitrate nitrogen suggest that secondary treated wastewater could be a practical, cost-effective nutrient feed at full scale. However, it should be noted that the best performance (HFBR 1, Phase 2) was observed where organic carbon in the liquid feed was omitted, and ammonium nitrogen was used as the nitrogen source. This could indicate that wastewater, which has a low organic carbon concentration and has not been nitrified, may also provide a cost-effective liquid feed.
It is intended also to apply the HFBR to odor removal. Experiments examining the treatment of hydrogen sulfide and ammonia gases in the HFBR are currently underway.
Conclusions
The main conclusions of this study are the following:
| • | Three laboratory-scale reactors were commissioned and loaded with an average of 8.6 g CH4/m3/hr. | ||||
| • | The reactors reached a steady-state CH4 removal rate of 32% when municipal-strength, synthetic wastewater (SWW) was applied. | ||||
| • | Various combinations of carbon and nitrogen in the SWW were then examined (Phase 2), resulting in significant increases in the REs. Average RE increased up to 79.5% with maximum efficiencies of up to 88% observed. | ||||
| • | CO2 production was also measured, giving a more complete insight into methanotrophic activity in the CH4 reactors. It was found that about 0.255 moles of CO2 exited HFBR 1, about 0.199 moles of CO2 exited HFBR 2, and about 0.145 moles of CO2 exited HFBR 3 every day. | ||||
| • | Good correlation between CO2 production and CH4 oxidation was also demonstrated (correlation coefficients were 0.72 for HFBR 1, 0.69 for HFBR 2, and 0.71 for HFBR 3). | ||||
| • | Samples of the biofilm taken from the HFBRs showed the excellent methane oxidation potential of the biomass in the reactors. | ||||
| • | Further work is necessary on the role of nitrogen forms in biological reactors treating methane under various conditions. | ||||
Acknowledgments
The authors would like to gratefully acknowledge financial support from Science Foundation Ireland (SFI) and Enterprise Ireland.
References
- Abdulla , F.A. and Al-Ghazzawi , Z.D. 2000 . Methane emissions from domestic waste management facilities in Jordan—Applicability of IPCC methodology . J. Air Waste Manage. Assoc. , 50 : 234 – 239 . doi: 10.1080/10473289.2000. 10464011
- American Public Health Association , American Water Works Association and Water Environment Federation . 2005 . Standard Methods for Examination of Water and Wastewater , 21st , Washington , DC : American Public Health Association .
- Bodelier , P.L. , Roslev , P. , Henckel , T. and Frenzel1 , P. 2000 . Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots . Nature , 403 : 421 – 424 .
- Clifford , E. , Nielsen , M. , ørensen , K. S and Rodgers , M. 2010 . Nitrogen dynamics and removal in a horizontal flow biofilm reactor for wastewater treatment . Water Res. , 44 : 3819 – 3828 . doi: 10.1016/j.watres.2010.04.042
- El-Fadel , M. and Massoud , M. 2001 . Methane emissions from wastewater management . Environ. Pollut. , 114 : 177 – 185 . doi: 10.1016/S0269-74910000222-0
- Haubrichs , R. and Widmann , R. 2006 . Evaluation of aerated biofilter systems for microbial methane oxidation of poor landfill gas . Waste Manage. , 26 : 408 – 416 . doi: 10.1016/j.wasman.2005.11.008
- Melse , R.W. and Van Der Werf , A.W. 2005 . Biofiltration for mitigation of methane emission from animal husbandry . Environ. Sci. Technol. , 39 : 5460 – 5468 . doi: 10.1021/es048048q
- Metcalf and Eddy . 2003 . Wastewater Engineering Treatment and Reuse , 4th , New York : McGraw Hill .
- Nikiema , J. , Bibeau , L. , Lavoie , J. , Brzezinski , R. , Vigneux , J. and Heitz , M. 2005 . Biofiltration of methane: An experimental study . Chem. Eng. J. , 113 : 111 – 117 . doi: 10.1016/j.cej.2005.04.005
- Nikiema , J. , Brzezinski , R. and Heitz , M. 2007 . Elimination of methane generated from landfills by biofiltration: A review . Rev. Environ. Sci. Biotechnol. , 6 : 261 – 284 . doi: 10.1007/s11157-006-9114-z
- Nikiema , J. , Girard , M. , Brzezinski , R. and Heitz , M. 2009a . Biofiltration of methane using an inorganic filter bed: Influence of Inlet load and nitrogen concentration . Can. J. Civ. Eng. , 36 : 1903 – 1910 . doi: 10.1139/L09-144
- Nikiema , J. and Heitz , M. 2009 . The influence of the gas flow rate during methane biofiltration on an inorganic packing material . Can. J. Civ. Eng. , 87 : 136 – 142 .
- Nikiema , J. , Payre , G. and Heitz , M. 2009b . A mathematical steady state model for methane bioelimination in a closed biofilter . Chem. Eng. J. , 150 : 418 – 425 . doi: 10.1016/j.cej.2009.01.032
- Odegaard , H. and Rusten , B. 1980 . “ Nitrogen removal in rotating biological contactors without the use of external carbon source ” . In Proceedings of the First National Symposium/Workshop On Rotating Biological Contactor Technology, Champion, PA, February 4–6, 1980 , Vol.2 , 1301 – 1317 . US Army Construction Engineering Research Laboratory .
- Phillips , J.P. 2008 . “ Development of innovative solutions for biological treatment of odour and VOC emissions from municipal wastewater treatment applications ” . In Proceedings of the Water Environment Federation, WEFTEC 2008, Sessions 61 – 70 . 5317 – 5326 .
- Rocha-Rios , J. , Bordel , S. and Hernandez , S. 2009 . Methane degradation in two-phase partition bioreactors . Chem. Eng. J. , 152 : 289 – 292 . doi: 10.1016/j.cej.2009.04.028
- Streese , J. and Stegmann , R. 2003 . Microbial oxidation of methane from old landfills in biofilters . Waste Manage. , 23 : 573 – 580 . doi: 10.1016/S0956-053X0300097-7
- Whittenbury , R. , Phillips , K.C. and Wilkinson , J.F. 1970 . Enrichment, isolation and some properties of methane-utilizing bacteria . J. Gen. Microbiol. , 61 : 205 – 218 . doi: 10.1099/00221287-61-2-205