Abstract
To increase U.S. petroleum energy independence, the University of Texas at Arlington (UT Arlington) has developed a direct coal liquefaction process which uses a hydrogenated solvent and a proprietary catalyst to convert lignite coal to crude oil. This sweet crude can be refined to form JP-8 military jet fuel, as well as other end products like gasoline and diesel. This paper presents an analysis of air pollutants resulting from using UT Arlington's liquefaction process to produce crude and then JP-8, compared with 2 alternative processes: conventional crude extraction and refining (CCER), and the Fischer-Tropsch process. For each of the 3 processes, air pollutant emissions through production of JP-8 fuel were considered, including emissions from upstream extraction/production, transportation, and conversion/refining. Air pollutants from the direct liquefaction process were measured using a LandTEC GEM2000 Plus, Draeger color detector tubes, OhioLumex RA-915 Light Hg Analyzer, and SRI 8610 gas chromatograph with thermal conductivity detector.
According to the screening analysis presented here, producing jet fuel from UT Arlington crude results in lower levels of pollutants compared to international conventional crude extraction/refining. Compared to US domestic CCER, the UTA process emits lower levels of CO2-e, NOx, and Hg, and higher levels of CO and SO2. Emissions from the UT Arlington process for producing JP-8 are estimated to be lower than for the Fischer-Tropsch process for all pollutants, with the exception of CO2-e, which were high for the UT Arlington process due to nitrous oxide emissions from crude refining. When comparing emissions from conventional lignite combustion to produce electricity, versus UT Arlington coal liquefaction to make JP-8 and subsequent JP-8 transport, emissions from the UT Arlington process are estimated to be lower for all air pollutants, per MJ of power delivered to the end user.
The United States currently imports two-thirds of its crude oil, leaving its transportation system especially vulnerable to disruptions in international crude supplies. At current use rates, U.S. coal reserves (262 billion short tons, including 23 billion short tons lignite) would last 236 years. Accordingly, the University of Texas at Arlington (UT Arlington) has developed a process that converts lignite to crude oil, at about half the cost of regular crude. According to the screening analysis presented here, producing jet fuel from UT Arlington crude generates lower levels of pollutants compared to international conventional crude extraction/refining (CCER).
Introduction
The United States currently imports two-thirds of its crude oil, or 9.76 million barrels per day out of 14.72 million barrels per day (CitationWebber, 2009). The transportation sector consumes 70% of this crude oil, which is refined into gasoline, diesel fuel, jet fuel, and other products (CitationWebber, 2009). In fact, 96% of the U.S. transportation system energy needs come from crude oil, with natural gas supplying 2% and renewable energy 1% (CitationWebber, 2007). Hence, the U.S. transportation system is highly vulnerable to potential disruption of nondomestic sources of crude oil.
To reduce this vulnerability, the U.S. Defense Advance Research Projects Agency (DARPA) has sponsored research on synthetic production of crude oil to supply JP-8. In a DARPA-sponsored project, the University of Texas at Arlington (UT Arlington) developed a unique direct liquefaction technique that enables lignite coal to be converted to a sweet crude oil (CitationBillo et al., 2010). The U.S. recoverable coal reserves, the largest in the world, are about 262 billion short tons, which would last 236 years at current levels of use (CitationEnergy Information Administration, 2010; CitationWebber, 2009). Excluding oil sands and shale, the United States has 21 billion barrels of conventional crude resources (CitationWebber, 2009). At current levels of use (5 million barrels per day of domestic crude), this is enough to last about 12 years (CitationWebber, 2009). Assuming average lignite energy content of 16.7 MJ/kg (HHV) (CitationDavis, 2000), the energy that could be extracted from U.S. recoverable lignite reserves (23.2 billion short tons; CitationEnergy Information Administration, 2010) would be 7.0 × 1014 MJ. Using the U.S. barrel of oil equivalent value (the approximate energy released by burning a barrel of crude oil) of 6118 MJ (HHV) (CitationInternal Revenue Service, 2011), the energy that could be extracted from U.S. conventional crude resources would be 1.3 × 1014 MJ, which is less than that from recoverable lignite. Hence, converting coal to crude oil, and lignite coal to crude oil in particular, would increase U.S. energy independence and reduce the vulnerability of the transportation sector to disruptions in the international supply of crude.
The UT Arlington coal liquefaction process uses a hydrogenated solvent and a proprietary catalyst to extract all of the volatile carbon-based components from lignite as well as some of the fixed carbon, such that up to 60% of the available carbon in the lignite is converted to synthetic crude (CitationBillo et al., 2010). This is comparable to conversion rates reported for other processes in the literature. However, the UT Arlington process has several advantages over competitive processes, including: (1) the use of lower grade coal (lignite) as a feedstock relative to the other processes that require higher grade, and more expensive, feedstocks (sub-bituminous and bituminous) (CitationComolli et al., 1999; 1999; CitationLi et al., 2008; CitationRedlich et al., 1999; CitationSasaki et al., 2000; CitationValente and Cronauer, 2005); and (2) liquefaction process conditions that are milder than the reported competitive processes (CitationBillo et al., 2010). Although a pilot plant is still in the planning stages, the cost of crude oil produced via the UT Arlington process is anticipated to be about half that of conventional West Texas crude, based on cost estimates from laboratory data. The synthetic oil produced can subsequently be refined to form gasoline, diesel, jet fuel, and other end products. Additional details regarding the UT Arlington process are available from the authors, but are not published here because a patent is pending.
The DARPA research included evaluation of air, water, and solid waste impacts of the UT Arlington coal liquefaction process (CitationBillo et al., 2010). Solid waste impacts are reported elsewhere (CitationGangupomu et al., 2012). Section 526 in the Energy Independence and Security Act (EISA) of 2007 states that synthetic fuels purchased by federal agencies must have life-cycle greenhouse gas profiles less than or equal to an equivalent petroleum-based fuel (CitationU.S. Government Printing Office, 2007). This paper thus presents an analysis of greenhouse gas emissions and conventional air pollutants resulting from UT Arlington's process, compared with two alternative processes for producing crude: conventional crude extraction and refining, and the Fischer–Tropsch process. The Fischer–Tropsch process is a series of chemical reactions that convert carbon monoxide and hydrogen into liquid hydrocarbons. Coal is first gasified to form carbon monoxide and hydrogen. Although the process was first developed in the 1920s, it has received recent attention as a source of liquid petroleum fuels. In addition, emissions from traditional use of lignite to produce coal-fired power are estimated for comparison with emissions from the UT Arlington process.
Scope of study
Comparison of Emissions From Three Alternative Processes for Producing Crude
The study design is summarized in . The study considered the following life-cycle stages of the aviation fuel life cycle (Air Force Research Lab, 2009):
| • | Life-cycle stage 1: Raw material acquisition. | ||||
| • | Life-cycle stage 2: Raw material transport. | ||||
| • | Life-cycle stage 3: Liquid fuels production. | ||||
Table 1. Three processes for producing crude life-cycle study design
shows specifically the life cycle stages that were evaluated for the three crude-production processes. Air pollutant emissions from raw material acquisition through production of JP-8 fuel were considered (JP-8 was of particular interest to DARPA in this project). Life cycle stages 4, product transport and refueling, 5, use/aircraft operation, and 6, end of life, were not considered. Refining of crude from the UT Arlington process would occur at existing refineries; thus, JP-8 transport emissions for stage 4 can be assumed to be the same regardless of the source of crude. At this time, data needed for comparing emissions from stage 5, use/aircraft operation, is not available; emissions from an actual jet engine combusting JP-8 produced from UT Arlington crude have not been measured. Combustion of JP-8 produced from UT Arlington crude would be anticipated to produce emissions similar to those of JP-8 from other crude sources for most pollutants, given that its chromatographic profile indicates a similar composition. For sulfur dioxide (SO2) in particular, JP-8 from UT Arlington crude would be anticipated to produce lower emissions compared to JP-8 from heavy crudes, since the UT Arlington crude is sweet, with a sulfur content <0.5% (CitationWebber, 2009). However, measurements are needed to confirm this.
Figure 1. (a) Life-cycle stages for conventional crude extraction and refining. (b) Life-cycle stages for the Fischer–Tropsch process. (c) Life-cycle stages for the coal liquefaction process.
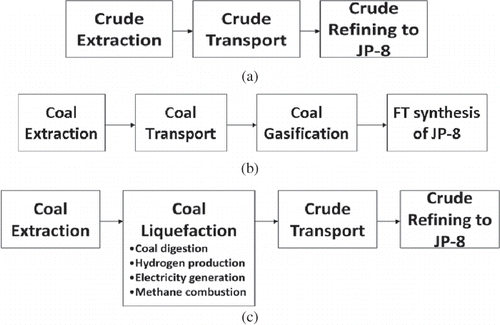
The temporal representation after the study was 2005. The CitationAir Force Research Laboratory (2009) guidance concerning estimating greenhouse gas footprints of aviation fuels recommends using baseline numbers from a CitationNational Energy Technology Lab (NETL) 2008 report for comparison of greenhouse gas emissions from synthetic and alternate fuels. This NETL report provides emission estimates for 2005.
The life cycle of JP-8 was evaluated, since this fuel was of particular interest to DARPA. The reporting metric was air pollutant emissions in kilograms per produced barrel of jet fuel. Two of the crude production processes start with coal: The UT Arlington process produces 3 barrels of fuel per ton of coal, and Fischer–Tropsch process produces 2 barrels per ton of fuel. This difference in yields was included in the analysis, since emissions were compared per barrel of jet fuel produced.
Life-cycle impacts analyzed included air pollutant emissions of sulfur dioxide (SO2), nitrogen oxides (NOx), mercury (Hg), and carbon monoxide (CO). Emissions of greenhouse gases carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) were also compared. CO2-equivalent emissions were calculated by multiplying methane emissions by 25 and nitrous oxide emissions by 298, to account for their greater global-warming potential on a 100-year time horizon, according to the CitationIntergovernmental Panel on Climate Change (2007), since their heat-trapping capacities differ.
Emission estimates in this study assume controls on SO2, NOx, and CO that are required to meet federal regulations, such as new source performance standards. For Hg and CO2, which are subject to new federal regulations, emission estimates are provided with and without controls for the five largest sources included in the analysis: crude refinery, electric power plant, hydrogen production plant, Fischer–Tropsch plant, and UT Arlington coal liquefaction plant. For these five large sources, Hg is assumed to be reduced by 95% using sulfur-impregnated activated carbon injection (CitationStiegel, 2009), and CO2 is assumed to be reduced by 90% via capture and sequestration (after CitationMarano and Ciferno, 2001). Although Hg and CO2 controls could also be applied to smaller emission sources like railroads and barges, such controls are less likely at this time and are therefore not included.
The UT Arlington process would have different land and water quality impacts compared with conventional crude extraction/refining and Fischer–Tropsch. Some of the land impacts could potentially impact greenhouse gas emissions. For example, land changes associated with increased lignite mining could result in emissions from land clearing, changes in biomass carbon stocks, lost forest sequestration, and/or changes in soil carbon stocks (CitationAir Force Research Laboratory, 2009). Evaluation of these impacts was beyond the scope of this study. Potential water quality and solid waste impacts of the UT Arlington process itself were discussed in the project report (CitationBillo et al., 2010).
According to the CitationAir Force Research Laboratory (2009) guidance concerning estimating greenhouse gas footprints of aviation fuels, this study would be considered a preliminary Level III Screening analysis. At the time that a pilot plant is constructed using the UT Arlington process, additional measurements of pollutant emissions should be conducted, and a Level II Standard or Level 1 Comprehensive analysis completed.
Comparison of Emissions From Two Alternate Uses for Lignite
Emissions from two alternate uses for lignite were also compared: conventional combustion in a power plant that produces electricity, and conversion into crude via the UT Arlington liquefaction process. Air pollutant emissions were estimated from lignite extraction through electric power delivered to an end user in one case, and from lignite extraction through transport of JP-8 to an end user in the other. The functional unit for this part of the analysis was thus megajoules of energy delivered for use (liquid fuel or electricity). Air pollutants evaluated were the same as for the three crude production alternatives. Land and water impacts were beyond the scope of the comparison.
Emissions Estimates
Conventional crude extraction and refining
and provide emission estimates for crude extraction, transport, and refining, provided by the National Energy Technology Laboratory (NETL) for the year 2005 (CitationNETL, 2008). The CitationAir Force Research Laboratory (2009) guidance concerning estimating greenhouse gas footprints of aviation fuels recommends use of this NETL report as a baseline for comparisons of greenhouse gas emissions from synthetic and alternate fuels. provides emissions for U.S. domestic crude only; provides national average emissions for crude used in the United States, from both domestic and international sources. Emissions estimates in and were taken directly from the NETL report, except that the conversion of 1 m3 fuel = 6.29 bbl fuel was made. The NETL study assumes that one barrel of crude is converted to one barrel of jet fuel in the refining process, although in actuality the refining process produces a distribution of fuels of various densities. The study includes refining of crude to kerosene-type jet fuel, which is similar to JP-8 (and therefore refining emissions are assumed to be the same).
Table 2. Emissions from conventional crude extraction and refining – U.S. domestic crude only
Table 3. Emissions from conventional crude extraction and refining—U.S. national average (including imported crude)
The domestic refining emission estimates from NETL would include pollution controls typical of refineries as of 2005. These would include controls on NOx, SO2, and CO when needed, but not Hg or CO2. Thus, a column has been added to that assumes 95% and 90% reductions in mercury and CO2, respectively.
Fischer–Tropsch process
Emission estimates for coal extraction, transport, gasification, and the Fischer–Tropsch (F-T) synthesis are given in , and are described in more detail in the following. If the Fisher–Tropsch plant were located at the coal mine, in a mine-mouth scenario, the transport step would be removed, and emissions would be correspondingly lower.
Table 4. Emissions from the Fischer–Tropsch process
Emissions from coal extraction
and provide estimates for emissions from surface coal mining due to fuel use, since lignite is surface mined; emission estimates specific to lignite extraction could not be found. References for emission factors and activity levels are given in the notes to the table. Emission factors for natural gas combustion are uncontrolled. Mercury emissions from electricity generation assume 95% and 90% control of Hg and CO2, respectively. Coal mine methane emissions specific to lignite could not be found; hence, coal mine methane emissions were taken to be the same as those from surface mining of Wyoming Powder River Basin Coal (0.142 kg methane/ton of coal) (CitationMarano and Ciferno, 2001). Since the F-T process produces 2 barrels of JP-8 per ton of coal, emissions of coal mine methane are taken to be 0.071 kg methane/bbl JP-8.
Table 5. Emissions of greenhouse gases from surface coal mining fuel use
Table 6. Emissions of traditional air pollutants from surface coal mining fuel use
Emissions from coal transport
provides estimates for emissions from coal transport, with data sources indicated in the notes to the table. Since the location of F-T plants to be built in the future is not known, emissions estimates from CitationJaramillo et al. (2007) for coal transport from mines to electric generating units were used to represent emissions from coal transport from mines to Fischer–Tropsch plants. For estimating mercury emissions, rail, barges, and trucks were all assumed to use diesel fuel. For each transport mode, the mercury content parts per billion value was multiplied by energy intensity, average transport distance, and diesel density (135 kg/bbl) and divided by diesel heat content (5.51 MMBtu LHV/bbl) to obtain kilograms of mercury emitted per ton of coal transported (CitationNETL, 2008).
Table 7. Emissions from coal transport
Emissions from coal gasification and Fischer–Tropsch synthesis
provides emission estimates for coal gasification and Fischer–Tropsch synthesis to JP-8. Emission estimates are from CitationMarano and Ciferno (2001), with the exception of mercury, which is discussed in the following. These estimates are assumed to apply to 2005, the year of the crude extraction and refining estimates. Average NETL estimates for conversion of bituminous and sub-bituminous coal are included (designated Options 1 and 4 in the report). Emission estimates for a Fischer–Tropsch conversion process involving lignite are not available, to our knowledge.
The NETL emission estimates were based on F-T plant designs developed for DOE by Nexant in the 1990s, with coal-to-liquid conversion facilitated by an iron catalyst. Estimates from the maximum distillate option were chosen for this study, as this would be the option that would produce the most jet fuel. The design met all applicable federal statutes at the time of design for airborne emissions of SO2, NOx, CO, and PM, including the U.S. Environmental Protection Agency (EPA) New Source Performance Standards (NSPS). The basic NETL report assumes no carbon capture and sequestration, although a case study at the end does include various capture and sequestration options. Accordingly, a column with 90% reduction in CO2 has been added to .
In gasification, coal is combined with oxygen and steam to produce a combustible gas, waste gases, char, and ash. A portion of mercury would be left behind in the char and ash; however, this portion is small, due to the high temperatures associated with gasification (CitationStiegel, 2009). As a worst case, it was assumed here that all mercury contained in the coal volatilizes and becomes part of the combustible gas or waste gas stream. According to the CitationU.S. EPA (1997), mercury concentrations in 3331 coal samples from the 50 coal beds having the highest production in the United States ranged from 0.003 to 3.8 ppm by weight, as analyzed by USGS. The U.S. EPA averaged concentrations for each bed; the average of the U.S. EPA averages was 0.22 ppm. All of this mercury was assumed to volatilize in the columns in without Hg control; 95% removal of mercury was assumed in the “with control” columns.
UT Arlington's Coal Liquefaction Process
Emissions from all phases of UT Arlington's coal liquefaction process, from coal extraction through crude refining to JP-8, are given in . A coal transport step is not included, because mine-mouth operation is assumed, with lignite being converted to jet fuel in a plant located near the mine. Emissions from each of the life-cycle phases is discussed in more detail in the following.
Table 8. Emissions from the UT Arlington coal liquefaction process (all phases from coal extraction to crude refining to JP-8)
Emissions from coal extraction
Emissions estimates for coal extraction were taken from , except that the coal liquefaction process was assumed to yield 3 barrels jet fuel/1 ton coal. Laboratory tests have produced 3 barrels crude oil per ton of coal, and 1 barrel of jet fuel was assumed per barrel of crude, to remain consistent with the NETL assumption used for conventional crude extraction and refining. With potential improvements in yield of the coal liquefaction process, emissions per barrel of jet fuel would decrease.
Coal mine methane emissions specific to lignite could not be found; hence, coal mine methane emissions were taken to be the same as those from surface mining of Wyoming Powder River Basin Coal (0.142 kg methane/ton of coal) (CitationMarano and Ciferno, 2001). Since the UT Arlington process produces 3 barrels of JP-8 per ton of coal, emissions of coal mine methane are taken to be 0.047 kg methane/bbl JP-8.
Emissions from coal liquefaction
Emissions from coal liquefaction at a pilot-scale or full-scale plant would include emissions from the coal digestion process itself, as well as from burning methane for energy on-site, use of electricity to power the plant, and emissions from production of hydrogen (emissions from trucking hydrogen to the plant are not included, as the process is likely at some point be done on-site). Emissions from the digestion process itself are discussed first. Two cases were considered: the digestion process with no pollution controls, and then the digestion process with pollution controls.
Pollutants generated from the coal digestion process were collected in Tedlar bags and measured using the instruments shown in . Widely varied digestion process conditions (around 50) were tested, with variations of catalyst, temperature, and pressure. Some of these conditions did not appreciably digest the coal, and thus would not be used in a pilot plant. Measurement results are shown in for runs with process conditions that effectively digested the coal and thus would be likely to be used in a scaled-up process. These runs had yields greater than 40%, meaning that 40% of the available carbon in the coal was converted to crude. “NRG” and “Luminant” in the first column refer to two types of lignite tested.
Table 9. Instruments used to measure air pollutants from the coal digestion step
Table 10. Air pollutants measured from the UT Arlington coal digestion step
For most digestion runs, not enough gas was generated to enable every pollutant to be measured for that run; the LandTEC and Hg Analyzer in particular required large gas volumes for testing. If a pollutant was not measured, its cell is left blank. In cases when a concentration was below the minimum on the instrument scale, measured values are shown with a less than sign. For the NRG4 and NRG6 runs, measurements of carbon dioxide and methane were made with a gas chromatograph instead of the LandTEC, as a check. Only one of the runs with conditions likely to be used in a scaled-up process contained a mercury reading (198 ng/m3); this reading was considerably below the mercury readings for most of the other runs, with process conditions not likely to be scaled up. Thus, the mercury reading of 198 ng/m3 may underestimate actual emissions from coal digestion, depending on the mercury content of the coal. For CO, no measurements were taken on runs ultimately considered to have process conditions that might be used in a pilot plant; the three measurements taken on other runs were thus averaged as an estimate of CO emissions from digestion. Measurements of CO in particular, as well as the other pollutants, should be conducted at a pilot-scale facility to provide improved emissions estimates. The uncertainty in the CO emissions, however, becomes less of an issue if CO is assumed to be used on-site to produce H2 needed for the process, as discussed next.
shows emissions from coal digestion, as well as other emissions that would occur from a liquefaction plant, including emissions from burning methane for energy on-site, use of electricity to power the plant (such emissions would occur off-site), and emissions from production of hydrogen. The methane generated from the digestion process itself is assumed to be burned for energy on-site or sold for use as fuel, so methane emissions are set to zero. Pollutant emission factors for methane combustion were taken to be the same as those for natural gas combustion, since natural gas is predominantly methane (CitationDavis, 2000). The emission factor for NOx is controlled, assuming low NOx burners. The others are uncontrolled values. Mercury emissions from electricity generation assume 95% and 90% control of Hg and CO2, respectively. For hydrogen, emission factors were taken from CitationSpath and Mann (2000) of the National Renewable Energy Laboratory, assuming the hydrogen is generated from natural gas steam reforming. The emission factors assume that the reformer furnace is equipped with a low NOx burner.
Table 11. Emissions from UT Arlington coal liquefaction—Liquefaction phase only
Digestion emissions are given in without any emission controls, as a worst-case scenario, as well as with emission controls. For the control scenario, it is assumed that a 98% efficient wet limestone scrubber is used to remove SO2 produced during digestion (CitationCooper and Alley, 2011). NOx controls on the digestion process itself are not considered because emissions are zero; 95% and 90% control of Hg and CO2 are assumed, as discussed earlier. CO is all used on-site to produce H2, which is needed for the process, so emissions from the digestion process, as well as from methane combustion, are taken as zero.
Emissions from production of the hydrogenated solvent are not included in , since the solvent is able to be reused, so these emissions represent a one-time event and would be anticipated to be small compared with emissions from the ongoing crude production process. Similarly, emissions associated with production of the catalyst are not included, since the catalyst is able to be reused.
The emissions in are for the coal liquefaction plant itself; they do not include emissions from coal extraction or crude transport to a refinery and subsequent refining. Emissions for these other steps of the complete process are given in .
Emissions from crude transport and refining
Estimates in for domestic crude transport and refining are from , discussed earlier. The distance that crude from lignite would have to be transported to a refinery would likely be similar to the distance for conventional crude. The highest concentration of refineries in the United States is along the coasts of Texas and Louisiana (CitationWebber, 2009). Texas leads the United States in conventional crude production (CitationWebber, 2009). Since production of crude from lignite would also likely occur in Texas, transport distances were assumed to be similar.
It was assumed that emissions from refining our crude would be similar to those from refining conventional crude. The crude produced from the UT Arlington process is a sweet crude, with sulfur content <0.5%; sweet crude produces fewer SO2 emissions than sour crude (CitationWebber, 2009). Assuming the same emissions from JP-8 from the different sources of crude is thus a conservative assumption for SO2.
Emissions from lignite combustion to produce electricity
Emission estimates for lignite extraction and combustion are given in . Coal-fired power plants are typically located near mine sites, so that transport of the coal from the mine to the power plant does not need to be included as a life-cycle stage. Emissions from coal extraction were estimated in the Fischer–Tropsch section earlier in this paper, and were converted to grams per megajoule of fuel, using the heating value of lignite given later in . The higher heating value of lignite was used; using the lower heating value would increase emissions from coal extraction. Emission factors for coal combustion were taken from the U.S. EPA AP-42 Section 1.7, “Lignite Combustion” (U.S. EPA, 1998b). A firing configuration of pulverized coal in a dry bottom wall-fired boiler was assumed. Controlled emission factors were chosen for NOx and CO (over-fire air and low NOx burners), as well as for SO2 (wet scrubber with 87.5% efficiency). Emissions with no control of Hg or CO2 are presented, as well as with 95 and 90% control, respectively. The power plant boiler for generating steam was assumed to be 90–98% efficient, the steam turbines were assumed to be 40–45% efficient, and the electricity generators were assumed to be 98–99% efficient, for an overall power plant efficiency of 35–44% (CitationWebber, 2009). A mid-range value of 40% was selected. Transmission lines were assumed to be 90% efficient (CitationWebber, 2009), giving an overall efficiency for electricity generation and transmission of 36%.
Table 12. Emissions from conventional lignite combustion
Emissions from JP-8 transport
As stated previously, an objective of this work was to compare emissions from two end uses of lignite. Emission estimates for JP-8 transport to an end user were needed. Estimates of emissions from JP-8 transport are provided in , and were added to the emissions from coal extraction through crude refining to JP-8, which were already estimated in a previous section. Emissions from the UT Arlington process with controls were used. Emissions in kilograms per barrel were converted to grams per megajoule of fuel, using a heating value (LHV) for JP-8 of 5.23 MMBtu/bbl (CitationNETL, 2008).
Table 13. Emissions from UT Arlington's coal liquefaction process, including JP-8 transport
Comparison of emissions from three crude-making processes
compares total emissions from the three crude-making processes, through the step of refining to jet fuel. For Fischer–Tropsch and the UT Arlington liquefaction process, the best-case scenarios including Hg and CO2 controls are compared in . Emissions of pollutants from the three processes are also compared in .
Table 14. Comparison of total emissions from three processes for making crude
Table 15. Carbon content and heating value of various coal types
Figure 2. Comparison of emissions from three processes for making JP-8: conventional crude extraction and refining (CCER), Fischer–Tropsch synthesis (FT), and the UT Arlington coal liquefaction process (UTA).
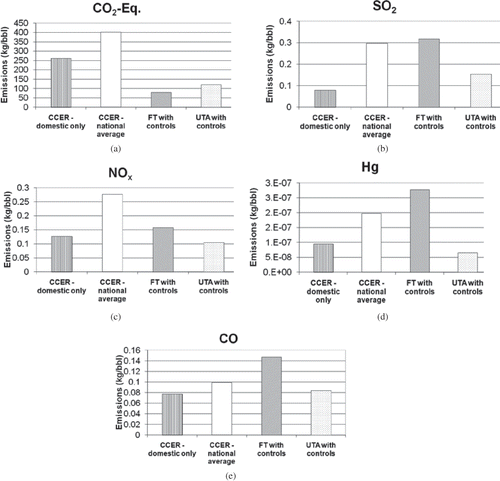
For all pollutants, emissions from conventional crude extraction and refining (CCER) are higher for the national average case, compared with the domestic only case. This is to be expected, since the national average case includes extraction and refining in countries with air quality regulations less stringent than those in the United States, and in which flaring is common practice.
CO2-equivalent emissions from the UT Arlington process are lower than those from conventional crude extraction and refining: 54% lower compared to the domestic-only case, and 70% lower compared to the national average case. CO2-equivalent emissions from the UT Arlington process are lower due to emissions from coal extraction (5.26 kg CO2-eq/bbl) being significantly lower than for crude extraction (156.3 and 235.8 kg CO2-eq/bbl for the domestic and national average cases, respectively). The lower emissions from coal extraction are due in part to the fact that methane emissions are lower: Coal mining releases less methane from underground geologic formations than conventional crude extraction.
Emissions of NOx and Hg are lower for the UT Arlington process with controls compared to both cases of conventional crude extraction and refining (domestic-only and national average). For SO2 and CO, emissions from the UT Arlington process are higher than CCER for the domestic-only case, but lower than CCER for the national average case. CO emissions from the UT Arlington process with controls are due primarily to coal extraction. CO emissions from coal extraction are high primarily due to burning of distillate fuel oil to power mining equipment. SO2 emissions from the UT Arlington process are due largely to generation of electricity needed to power the digestion process. As tighter federal controls on SO2 from power plants are implemented, SO2 emissions from the UT Arlington process will decrease.
Emissions from the UT Arlington process with controls are lower than for Fischer–Tropsch process with controls for all pollutants, with the exception of greenhouse gas CO2-e emissions. It was expected that emissions from the Fischer–Tropsch process would generally be higher, because the Fischer–Tropsch process is energy-intensive; a large amount of energy is required to gasify the coal. Direct liquefaction processes, which do not require gasifying the coal, generally release lower levels of air pollutants. In the case of greenhouse gas emissions, 62% (75.8 kg/bbl of 121.3 kg/bbl) of the CO2-e emissions from the UT Arlington process are associated with N2O released during crude refining. The N2O emission factor came from the Petroleum Baseline Model, with adjustments for changes in refinery operations based on crude oil density and sulfur content (CitationSkone, 2009). Assuming that the Petroleum Baseline Model large emission factors for N2O are accurate, methods for reducing those emissions should be investigated.
Uncertainties
The emission estimates compared in include various sources of uncertainty, including those listed next, which should be kept in mind in use of the estimates.
Uncertainties in CCER emission estimates
| • | Emissions for refining crude to kerosene-type jet fuel were assumed to be the same as for refining crude to JP-8. | ||||
| • | One barrel of crude was assumed to be converted to one barrel of jet fuel in the refining process, although in actuality the refining process produces a distribution of fuels of various densities. | ||||
| • | Emission estimates for crude refining are national averages, which may be higher or lower than emissions from a specific refinery. | ||||
Uncertainties in F-T emission estimates
| • | Fischer–Tropsch plant emission estimates were for conversion of bituminous and sub-bituminous coal, since estimates for conversion of lignite are not available, to our knowledge. Carbon contents and heating values of various coals are shown in . Assuming that coals with lower heating values require more energy input to gasify, emissions from lignite conversion would be higher than those for bituminous and sub-bituminous coal. | ||||
| • | F-T plant emission estimates were based on plant designs developed in the 1990s. Newer plant designs may produce different emissions. | ||||
| • | All mercury was assumed to volatilize during F-T conversion; this is a conservative estimate. In addition, mercury emissions would vary as coal mercury content varies. | ||||
| • | Emissions for coal extraction were based on average fuel usage for surface mining and “generic” emission factors for combustion of various fuel types (distillate fuel oil, gas, gasoline, etc.). | ||||
| • | Coal mine methane emissions specific to lignite could not be found; hence, coal mine methane emissions were taken to be the same as those from surface mining of Wyoming Powder River Basin sub-bituminous coal. | ||||
| • | The locations of F-T plants to be built in the future are unknown; hence, coal transport distances and modes from mines to electric generating units were used to represent coal transport from mines to Fischer–Tropsch plants. | ||||
Uncertainties in UT Arlington liquefaction process emission estimates
| • | Lab-scale emission measurements for the digestion process, which varied among runs, were assumed to be representative of emissions from a full-scale facility. | ||||
| • | Emissions from electricity generation were from the GREET model, and may be higher or lower than emissions from a specific power plant. | ||||
| • | Emission factors for methane combustion were from CitationDavis (2000) and AP-42 (U.S. EPA, 1998a). These factors represent averages over various boiler ages and models. | ||||
| • | Coal-mine methane emissions for lignite were assumed to be the same as for surface-mined sub-bituminous Wyoming Powder River Basin coal. | ||||
| • | Emissions from refining UT Arlington coal-derived crude were assumed to be similar to those from refining conventional crude. | ||||
Of the uncertainties just listed, those with the largest magnitude are most likely the use of Fischer–Tropsch plant emission estimates conversion of bituminous and sub-bituminous coal to represent those for lignite conversion, and the fact that UT Arlington process emission estimates were obtained from lab experiments run at different process conditions, rather than from a pilot-scale or full-scale plant. In the future, it is recommended that emission measurements be taken from a UT Arlington process pilot plant to confirm the lab-scale measurements.
Comparison of Air Pollutants From Two Uses of Lignite
Emissions from two alternate uses for lignite were also compared: conventional combustion in a power plant that produces electricity, and conversion into crude via the UT Arlington liquefaction process. Air pollutant emissions were estimated per megajoule of power delivered for use: from lignite extraction through electric power delivered to an end user in one case, and from lignite extraction through transport of JP-8 to an end user in the other. compares total emissions from the two uses of lignite. The UT Arlington liquefaction process with emission controls was used. Emissions from the UT Arlington process are lower for all pollutants.
Table 16. Comparison of total emissions from two uses of lignite coal
(middle column) shows that the heating value of lignite per kilogram is lower than for other types of coal; however, the heating value per kilogram of carbon is highest for lignite (right-hand column). This means that lignite combustion will produce less carbon dioxide per megajoule of heat generated, compared with other forms of coal. If significant amounts of lignite were to be converted to crude oil, lignite consumption for power generation might decrease due to increased demand and prices for lignite. If lignite for power consumption were replaced by other forms of coal, this would increase carbon dioxide emissions from power generation.
Conclusions
| • | Emissions of NOx, Hg, and CO2-e from the UT Arlington process for producing crude and then JP-8 are estimated to be lower than those from conventional crude extraction and refining (CCER), for both domestically produced crude and national average CCER numbers, which include crude imports. Emissions of SO2 and CO for the UT Arlington process are estimated to be higher than for domestic CCER, but lower than for national average CCER. As tighter federal controls on SO2 from power plants are implemented, SO2 emissions from the UT Arlington process will decrease. | ||||
| • | Emissions from the UT Arlington process for producing JP-8 are estimated to be lower than for the Fischer–Tropsch process for all pollutants, with the exception of those of CO2-e, which were high for the UT Arlington process due to N2O emissions from crude refining. | ||||
| • | When comparing emissions from conventional lignite combustion to produce electricity, versus UT Arlington coal liquefaction to make JP-8 and subsequent JP-8 transport, emissions from the UT Arlington process are estimated to be lower for all air pollutants, per megajoule of power delivered to the end user. | ||||
| • | Emission measurements should be taken from a UT Arlington process pilot plant to confirm the lab-scale measurements. | ||||
Acknowledgments
This research was funded by the Defense Advanced Projects Research Agency (DARPA), contract HR0011-09-C-0108. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the DARPA.
References
- Air Force Research Laboratory. 2009. Framework and Guidance for Estimating Greenhouse Gas Footprints of Aviation Fuels. Report AFRL-RZ-WP-TR-2009–2206 http://www.netl.doe.gov/energy-analyses/pubs/EstGHGFtprnts AvFuels2009.pdf (http://www.netl.doe.gov/energy-analyses/pubs/EstGHGFtprnts AvFuels2009.pdf) (Accessed: 15 January 2012 ).
- Argonne National Laboratory. 2010. The Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) Model, version 1.8 c http://greet.es.anl.gov (http://greet.es.anl.gov) (Accessed: 15 January 2012 ).
- Billo , R.E. , Choi , H. , Dennis , B.H. , MacDonnell , F.M. , Priest , J.W. and Sattler , M.L. 2010 . A Carbon Extraction Process for Converting Southwestern Lignite to JP-8 Final Report, DARPA Project HR0011-09-C–0108
- Carnegie Mellon Green Design Institute. 1997. Economic Input-Output Life Cycle Assessment (EIO LCA) software www.eiolca.net (http://www.eiolca.net) (Accessed: 15 December 2009 ).
- Comolli , A.G. , Johanson , E.S. , Panvelker , S. and Popper , G. 1990 . “ Catalytic Two-Stage Liquefaction (CTSL) Process, Wyoming Sub-Bituminous Coal ” . In Paper presented at the International Conference on Coal and Slurry Technologies
- Comolli , A.G. , Lee , T.L.K. , Popper , G.A. and Zhou , P. 1999 . The Shenhua coal direct liquefaction plant . Fuel Process. Technol. , 59 : 207 – 215 . doi: 10.1016/S0378-38209900016-8
- Cooper , C.D. and Alley , F.C. 2011 . Air Pollution Control: A Design Approach , Long Grove , IL : Waveland Press .
- Davis , W.T. , ed. 2000 . Air Pollution Engineering Manual , New York , NY : John Wiley & Sons .
- Energy Information Administration. 2010. Annual Energy Review 2010 http://www.eia.gov/coal/annual/ (http://www.eia.gov/coal/annual/) (Accessed: 13 April 2012 ).
- Gangupomu , R.H. , Kositkanawuth , K. , Sattler , M.L. , Dennis , B. , MacDonnell , F.M. , Billo , R. and Priest , J. 2012 . Analysis and comparison of inertinite-derived adsorbent with conventional adsorbents . J. Air Waste Manage. Assoc. , 62 ( 5 ) : 489 – 499 . doi: 10.1080/10962247.2012.660269
- Intergovernmental Panel on Climate Change (IPCC). 2007. Fourth Assessment Report: Climate Change 2007 http://www.ipcc.ch/publications_and_data/publications_and_data_reports.shtml#1 (http://www.ipcc.ch/publications_and_data/publications_and_data_reports.shtml#1) (Accessed: 15 February 2011 ).
- Internal Revenue Service. 2011. Nonconventional Source Fuel Credit, Section 45K Inflation Adjustment Factor, and Section 45K Reference Price. Internal Revenue Bulletin: 2011-17 http://www.irs.gov/irb/2011-17_IRB/ar10.html (http://www.irs.gov/irb/2011-17_IRB/ar10.html) (Accessed: 13 April 2012 ).
- Jaramillo , P. , Griffin , W.M. and Matthews , H.S. 2007 . Comparative life-cycle air emissions of coal, domestic natural gas, LNG, and SNG for electricity generation . Environ. Sci. Technol. , 41 ( 17 ) : 6290 – 6296 . doi: 10.1021/es063031o
- Li , X. , Hu , H. , Jin , L. , Hu , S. and Wu , B. 2008 . Approach for promoting liquid yield in direct liquefaction of Shenhua coal . Fuel Process. Technol. , 89 : 1090 – 1095 . doi: 10.1016/j.fuproc.2008.05.003
- Marano, J.J., and J.P. Ciferno. 2001. Life-Cycle Greenhouse-Gas Emissions Inventory for Fischer–Tropsch Fuels. Prepared for U.S. Department of Energy National Energy Technology Laboratory. http://www.netl.doe.gov/technologies/coalpower/gasification/pubs/pdf/GHGfinalADOBE.pdf (http://www.netl.doe.gov/technologies/coalpower/gasification/pubs/pdf/GHGfinalADOBE.pdf)
- National Energy Technology Laboratory. 2008. Development of Baseline Data and Analysis of Life Cycle Greenhouse Gas Emissions of Petroleum-Based Fuels. DOE/NETL–2009/1346. http://www.netl.doe.gov/energy-analyses/pubs/NETL%20LCA%20Petroleum-Based%20Fuels%20Nov%202008.pdf (http://www.netl.doe.gov/energy-analyses/pubs/NETL%20LCA%20Petroleum-Based%20Fuels%20Nov%202008.pdf)
- Oak Ridge National Laboratory (ORNL). 2007. Transportation Energy Data Book: Edition 26. ORNL-6978 http://cta.ornl.gov/data/download26.shtml (http://cta.ornl.gov/data/download26.shtml) (Accessed: 10 June 2008 ).
- Redlich , P.J. , Hulston , C.K.J. , Jackson , W.R. , Larkins , F.P. and Marshall , M. 1999 . Hydrogenation of sub-bituminous and bituminous coals pre-treated with water-soluble nickel-molybdenum or cobalt-molybdenum catalysts . Fuel , 78 : 83 – 88 . doi: 10.1016/S0016-23619800122-7
- Sasaki , M. , Kotanigawa , T. and Yoshida , T. 2000 . Liquefaction reactivity of methylated Illinois no. 6 coal . Energy Fuels , 14 : 76 – 82 . doi: 10.1021/ef9800314
- Skone , T. 2009 . Draft petroleum life cycle analysis research conducted for National Energy Technology Laboratory
- Spath , P. and Mann , M. 2000 . “ Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming ” . In National Renewable Energy Laboratory, U.S. Department of Energy Laboratory, contract CE-AC36–99-GO10337
- Stiegel , G. 2009 . National Energy Technology Lab , Private communication .
- U.S. Environmental Protection Agency. 1997. Mercury Study Report to Congress. EPA-452/R–97–003. http://www.epa.gov/mercury/report.htm (http://www.epa.gov/mercury/report.htm)
- U.S. Environmental Protection Agency. 1998a. Emissions Factors & AP 42, Compilation of Air Pollutant Emission Factors,Section 1.4, “Natural Gas Combustion” http://www.epa.gov/ttn/chief/ap42/, (http://www.epa.gov/ttn/chief/ap42/,) (Accessed: 15 January 2012 ).
- U.S. Environmental Protection Agency. 1998b. Emissions Factors & AP 42, Compilation of Air Pollutant Emission Factors,Section 1.7, “Lignite Combustion” http://www.epa.gov/ttn/chief/ap42/, (http://www.epa.gov/ttn/chief/ap42/,) (Accessed: 15 October 2010 ).
- U.S. Government Printing Office. 2007. Energy Independence and Security Act (EISA), HR6 http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname= 110_cong_bills&docid=f:h6enr.txt.pdf (http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname= 110_cong_bills&docid=f:h6enr.txt.pdf) (Accessed: 19 January 2012 ).
- Valente , A.M. and Cronauer , D.C. 2005 . Analyses of Illinois no. 6 coal liquefaction results generated in the Wilsonville, Alabama Unit . Energy Fuels , 19 : 489 – 498 . doi: 10.1021/ef0400565
- Webber, M.E. 2007. Introduction: Energy Uses, Basics and Fundamentals www.webberenergygroup.com/courses (http://www.webberenergygroup.com/courses) (Accessed: 15 August 2009 ).
- Webber, M.E. 2009. Fossil Fuel www.webberenergygroup.com/courses (http://www.webberenergygroup.com/courses) (Accessed: 15 August 2009 ).
- Wilhelm , S.M. 2001 . Estimate of mercury emissions to the atmosphere from petroleum . Environ. Sci. Technol. , 35 ( 24 ) : 4704 – 4710 . doi: 10.1021/es001804h