Abstract
In this paper, the toluene adsorption/desorption properties of modified 13X molecular sieves (M-13X) are discussed. M-13X molecular sieves were prepared by acidic and steam treatments of 13X molecular sieves. The structural parameters of M-13X were evaluated and compared with those of other molecular sieves (HY, HZSM-5, Cs7NaMOR, and a commercial 13X). The results show that the specific surface area, average pore diameter, and pore volume of M-13X were 414.17 m2/g, 2.98 nm, and 0.31 mL/g, respectively. The pore size distribution of M-13X was 1.8–3.0 nm. Because of its larger Si/Al ratio (Si/Al = 6.77), the hydrophobicity of M-13X is much higher than that of 13X (Si/Al = 1.28), indicating that it is particularly well suited to toluene control applications. The saturation adsorption capacity of M-13X was 0.045 g/g for simulated toluene at a temperature of 293 K and a relative humidity of 50%. The optimal regeneration temperature of M-13X was 473 K for 120 min with a hot air flow rate of 140 L/min.
The modified 13X molecular sieves (M-13X) are adsorbents with a high adsorption capacity and great hydrophobicity, suitable for the treatment of VOCs. The purpose of the present investigation is to provide a practical guide for their design.
Introduction
Volatile organic compounds (VOCs) are organic compounds such as aliphatic hydrocarbons, aromatic hydrocarbons, esters, ethers, and halohydrocarbons that have a high vapor pressure under normal room-temperature conditions (CitationHarper, 2000; CitationLi et al., 2009; CitationTseng et al., 2001). Sources of VOC emissions include fuel combustion, transport, industrial processes, solvent use, and storage and distribution of fuels (CitationChang et al., 2002; CitationLi, 1996). In China, manmade VOC emissions are expected to increase from 19.4 Tg in 2005 to 25.9 Tg in 2020, along with an increase in VOCs attributed to industrial production from 17% to 24% (CitationWei et al., 2011; CitationZhao, 2009). The development of an efficient and safe technology for the reuse of VOCs is therefore needed (CitationWei et al., 2010).
During the past decade, many efficient and practical technologies for VOC abatement have been developed (CitationGuieysse et al., 2008; CitationGupta et al., 2002; CitationMonneyron et al., 2008), including adsorption, photocatalytic destruction, biological processes, plasma decomposition, and catalytic combustion (CitationBorwankar et al., 2010; CitationChinte et al., 2009; CitationChristian et al., 2010; CitationCui et al., 2009; CitationKoziel et al., 2010; CitationToshiaki et al., 2010; CitationXue et al., 2011). Adsorption, combined with other techniques such as condensation and catalytic combustion, is considered to be a promising method for VOC removal or solvent recovery. In practical VOC removal processes, the primary requirements of the adsorbent include (1) a high adsorption capacity (large accessible pore volume), (2) uniform channels, (3) hydrophobic properties, and (4) ease of regeneration (CitationDouglas, 2011. CitationPolyakov et al., 1993). Activated carbon is an excellent adsorbent for nonpolar compounds; it has a large number of cavities and a large surface area (600–1500 m2/g). However, activated carbon also has some disadvantages; for example, it is a fire risk, and it is hygroscopic (CitationMakowski et al., 2007), which might have a negative effect on the adsorption of humid gas streams containing VOCs. Molecular sieves with crystalline aluminosilicate frameworks, another type of adsorbent, have recently attracted considerable attention (CitationChun et al., 2011; CitationKresge et al., 1992). Furthermore, as well as a porous structure, molecular sieves have other excellent properties, such as the potential for shape-selective catalysis and fire resistance, which might be of benefit in the treatment of VOCs in gas streams and enable them to overcome some of the disadvantages associated with activated carbon (CitationLi, 2009). CitationBao et al. (2011) and CitationHu et al. (2010) studied the adsorption performances of MCM-41and silicalite-1 molecular sieves for benzene under both dry and wet conditions, and found that water vapor reduced the benzene-adsorption capacity.
In the present study, a new modified 13X molecular sieve (M-13X) was investigated. The structural properties of M-13X, such as pore volume, average pore size, specific surface area, pore distribution, molecular composition, and Si/Al ratio, were measured. Water isotherms for M-13X and 13X were used to investigate the water adsorption behavior on sample adsorbents. Toluene was used as a probe molecule, and the adsorption/desorption properties of M-13X under different relative humidities and temperatures were studied.
Experimental Section
Experimental adsorbents and reagents
Adsorbents
Granular commercial 13X molecular sieves were provided by the Shanghai Zeolite Molecular Sieve Co., Ltd, China. Granular M-13X molecular sieves were prepared by acid and steam treatments of 13X molecular sieves according to the methods described by CitationGola et al. (2000). The 13X molecular sieves were steamed using inlet water vapor (0.6 g/g molecular sieve) at 923 K for 4 hr. Acid treatment was carried out using nitric acid (0.02 mol/L) at 373 K for 2 hr. The suspension was then filtered, washed with distilled water, and dried at 393 K. The M-13X and 13X molecular sieves were first outgassed under a vacuum at 378 K (a temperature suitable for pore water removal) until no weight loss was observed using an analytical balance.
Reagents
Analytically pure toluene (Quhua Reagent Co., Ltd., Hangzhou, China) was used as the probe molecule. Analytically pure sodium citrate (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) was used to absorb toluene remaining in the off-gas (CitationLan et al., 2008).
Experimental analyzers
Chemical–physical adsorption measurements
The specific surface area, average pore diameter, and pore volume were measured using a chemical–physical adsorption instrument (ASAP2010, Micromeritics, Norcross, GA). Surface areas were calculated using the Brunauer–Emmett–Teller (BET) model. The pore size distributions were obtained using the Barrett–Joyner–Halenda (BJH) model. Total pore volumes were measured at a relative pressure of 0.998.
Scanning electron microscopy and x-ray diffraction
The surface morphology of the adsorbents was determined using scanning electron microscopy (SEM; XL-30-ESEM, Philips, Eindhoven, The Netherlands) and by x-ray diffraction (XRD; D/MAX-2500PC. XRD, Rigaku, Tokyo, Japan) using Cu Kα radiation at 40 kV and 40 mA, with scanning from 10° to 60°.
X-Ray fluorescence
Molecular compositions were determined using x-ray fluorescence (XRF; ARL ADVANT'X IntelliPowerTM 4200, Thermo Scientific, Waltham, MA). XRF was performed with Rh Kα radiation at 60 kV and 140 mA, and UniQuant software was used to analyze the samples quantitatively.
Gas chromatography
Toluene concentrations were determined using a gas chromatograph (GC; Agilent 6980, Agilent, Santa Clara, CA) with flame ionization detectors and a capillary column of dimensions 30.0 m × 320 μm × 0.5 μm. The temperatures of the vaporization chamber, detector, and capillary column were 483 K, 473 K, and 363 K, respectively. The column flow rate was 1 mL/min, and the injection volume was 800 μL. The flow rate of nitrogen, which was used as the carrier gas, was 33.4 mL/min, and the split ratio was 30:1. The flow rates of hydrogen and air were controlled at 30 mL/min and 400 mL/min, respectively.
Experimental apparatus
The experimental fixed-bed reactor is shown in . Adsorption columns with Φ 30 mm × 600 mm were connected using stainless-steel pipes (Φ 15 mm). A stream of toluene diluted with dry air was obtained by passing air through a gas generator containing toluene. The diluted toluene gas entered an adsorption column packed with adsorbent (100 g). The small amount of gaseous toluene remaining in the off-gas from the adsorption column was absorbed using 5% sodium citrate. The experiment was stopped when the outlet toluene concentration reached the same level as that of the inlet stream.
Figure 1. Experimental flow in adsorptive fixed-bed reactor: (1) air compressor; (2) gas generator; (3) humidifier; (4) gas mixture buffer; (5) adsorption column; (6) heater; (7), (8), (9), (10), (11), and (12) sampling ports; (13) absorber; and (14) dehumidifier.
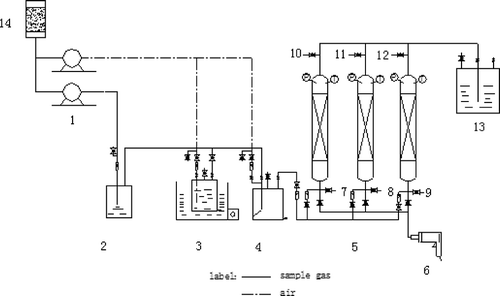
This investigation simulated practical conditions as follows. The concentration of toluene gas was controlled at 1000 ± 50 mg/m3, the gas flow rate was 17 L/min, and the temperature was kept constant at 293 K. The flow rate of hot air for regeneration was about 140 L/min, periodically; the toluene concentrations from the inlet and outlet were measured quantitatively using GC.
Results and Discussion
Characterization of M-13X
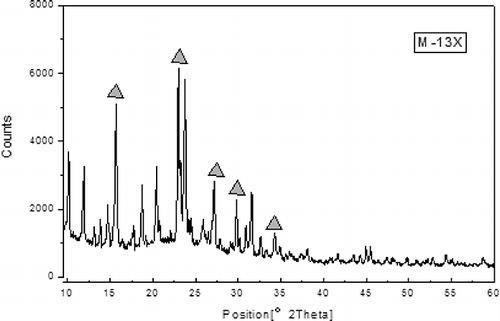
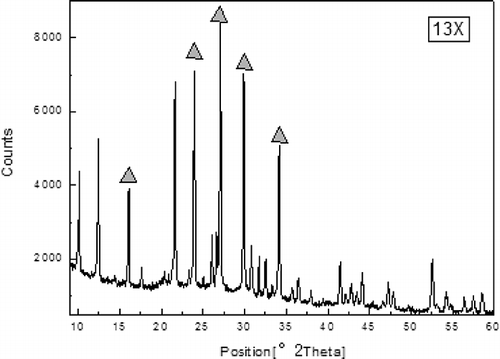
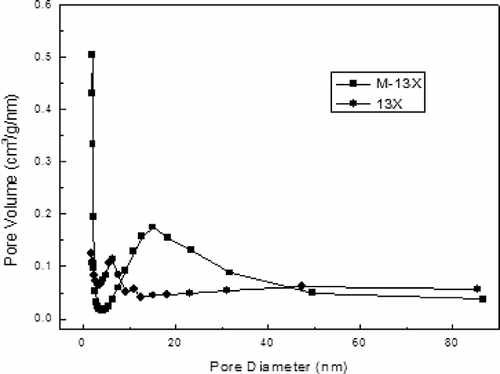
and show that M-13X and 13X have similar x-ray diffraction patterns, indicating that crystalline frameworks were not destroyed during the modified process (CitationCheng et al., 2008). The sharp peaks labeled in the , revealing the presence of a highly organized crystalline structure in 13X (CitationAlonso-Vicario et al., 2010), were also observed in M-13X. However, some new diffraction peaks appeared in M-13X, indicating that different crystalline phases were formed. In addition, the lower strengths of related peaks also indicated that the sizes of crystalline grains turned smaller during the modified process. Crystallite details of M-13X and 13X were observed further by SEM, as illustrated in . The crystals of 13X and M-13X were grainy, but the crystal size in M-13X was less regular than in 13X. In M-13X the intercrystalline pores were blocked by small crystals, which would reduce the adsorption capacity (CitationYao et al., 2010). An increase in the Si/Al ratio resulted in shrinkage of the unit cell, leading to a decrease in the particle size, because the Al–O bond length is 0.171 nm, whereas the Si–O bond length is only 0.164 nm (CitationLloyd, 2011). The pore size distributions of M-13X and 13X, shown in , demonstrate that the pore sizes of M-13X were mainly in the range 1.8–3.0 nm, and they were a little more uniform than those of 13X. Furthermore, the average micropore widths of both M-13X and 13X were observed at around 1.9 nm, so they could be easily accessed by pollutant macromolecules.
The specific surface area, pore volume, and average pore diameter of M-13X, which were measured using chemical–physical adsorption, were 414.17 m2/g, 0.31 mL/g, and 2.98 nm, respectively. The specific surface area of M-13X, listed in , was similar to those of HZSM-5 and Cs7NaMOR, but the pore volume was about twice those of HZSM-5 and Cs7NaMOR. A comparison of 13X and M-13X shows that their porous structural parameters were very similar. The XRF data for M-13X and 13X are shown in ; they indicate that the Si/Al ratio of M-13X was much improved. The Si/Al ratio of M-13X was 6.77, which was almost 5.3 times that of 13X (1.28). The reason was that cations (Na+, Ca2+, Mg2+, etc.) in 13X were exchanged by H+ from solution, forming hydroxyl bridges; the bridging hydroxyls were then converted to Si–OH as a result of the reaction of H+ and framework Al, so Al was extracted, which improved the Si/Al ratio (CitationXu et al., 2004; CitationYang et al., 2004,). A high Si/Al ratio would decrease the polarity of the molecular sieve and greatly improve the hydrophobicity.
Table 1. Structural parameters and adsorption properties of adsorbents
Table 2. Molecular composition of adsorbents (%)
Breakthrough curves
Breakthrough curves, a common technique for evaluating the adsorption times of adsorbents, were used in this study. A ratio of outlet toluene concentration to inlet concentration of 5% meant that the adsorption bed had been broken through. When the outlet toluene concentration reached the same level as that of the inlet stream, this meant that the adsorbents were saturated.
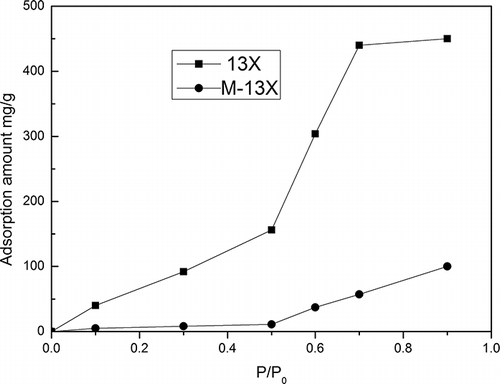
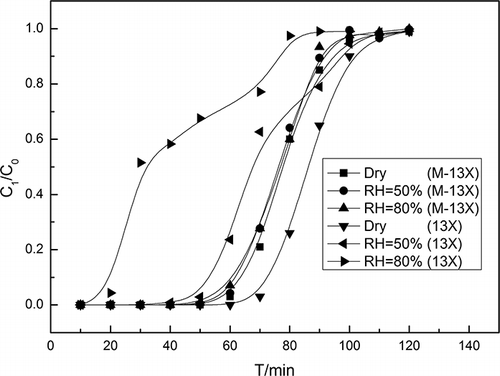
However, in practical situations, the VOC stream usually contains a large amount of water vapor, about 80% relative humidity (CitationZhao et al., 1998). It is therefore necessary to observe the effects of humidity on the adsorption process. The adsorption isotherms of water vapor on 13X and M-13X are shown in . As expected, M-13X, which had a low water loading, was much more hydrophobic than 13X. In addition, the adsorption isotherms showed that capillary condensation occurred at 0.5–0.7 for 13X, showing that 13X was unsuitable as an adsorbent for relative humidity above 50%. The breakthrough curves of M-13X and 13X under different relative humidities are shown in . For 13X, the breakthrough time decreased from 50 min to 20 min, and the saturation time was reduced from 110 min to 80 min, as the relative humidity increased from 50% to 80%; this indicated that the moisture resistance of 13X was poor. In contrast, the breakthrough curve at 50% relative humidity on M-13X almost coincided with that at 80% relative humidity, which demonstrated that M-13X had excellent hydrophobicity, in accordance with its high Si/Al ratio.
Comparison of saturation capacity on various adsorbents
One of the most important indicators of adsorption properties is the adsorption saturation capacity, which is calculated using the following equation (CitationChun et al., 2009):
The adsorption performances of M-13X and 13X were investigated at a temperature of 293 K and a gas flow rate of 17 L/min for the toluene concentration range 900–1100 mg/m3. Based on the data in , the saturation adsorption capacities of M-13X and 13X, calculated using Equationeq 1, are shown in . Obviously, the saturation adsorption capacity of 13X is slightly higher than that of M-13X under dry conditions. However, the saturation adsorption capacity of 13X dramatically decreased from 0.055 g/g to 0.037g/g when the relative humidity was increased to 50%. The saturation adsorption capacity of M-13X was higher than that of 13X; for 13X, competitive adsorption of water decreased the volume available for toluene adsorption. The adsorption properties of other molecular sieves are also listed in , and it can be seen that the saturation capacities of M-13X and HZSM-5 are quite similar to each other. The data also show that the saturation capacities of HY and Cs7NaMOR are less than twice that of M-13X.
Desorption properties
The desorption performance has a profound effect on the adsorbent service life. Usually, adsorptive reactions are exothermic (CitationWilliam et al., 1997), and therefore adsorbents can be regenerated using hot air or hot water vapor.
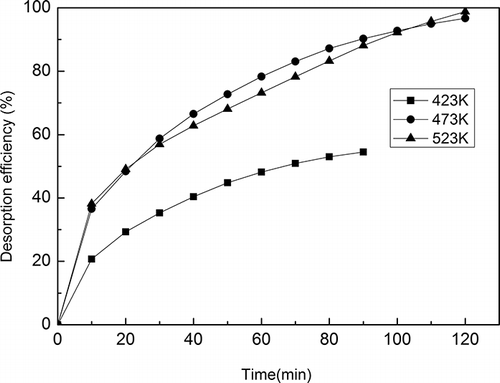
In these experiments, a hot air stream was used for the regeneration of M-13X, and the typical sectional flow rate of the hot air stream was 140 L/min. The desorption properties of M-13X at different temperatures were investigated. Desorption efficiency was defined as the ratio of the amount desorbed by heating to the difference between the amount initially adsorbed and the amount that is reversibly adsorbed (CitationKim and Ahn, 2012). As shown in , all the curves increased dramatically in the first 20 min, which indicated that the externally adsorbed toluene was desorbed quickly. After 50 min, the curves rose slowly, showing that toluene which had diffused into the M-13X channels was released slowly. After 90 min, the desorption efficiencies of M-13X were 55%, 90%, and 88% at regenerating temperatures of 423 K, 473 K, and 523 K, respectively. No more toluene was desorbed at 423 K after 90 min. However, a maximum desorption efficiency of 98% was observed after 120 min at 473 K and 523 K. There was no obvious improvement in the desorption efficiency at 523 K compared with that at 473 K. The regeneration temperature of molecular sieves depends on the properties of adsorbates and the strength of interaction between adsorbates and adsorbent. According to the previous study (CitationShen et al., 2009), 423 K was not enough for the regeneration since the optimal regeneration temperature of 13X was 473–573 K. Considering the energy utilization, 473 K is a reasonable regeneration temperature of M-13X in the present study.
Conclusion
In conclusion, M-13X is a potential adsorbent for the treatment of VOCs in high-humidity streams. XRD, XRF, chemical–physical adsorption measurements, and BET and BJH models were used to characterize the physical performances of M-13X and 13X. The results revealed that the Si/Al ratio of M-13X was much higher than that of 13X. Other properties, such as specific area, pore volume, and average pore diameter, were unchanged, but the crystal size and array changed, and this needs to be addressed in future research. In addition, the pore size distribution of M-13X was more uniform than that of 13X; this is one of the primary requirements for an excellent adsorbent. Breakthrough curve experiments using toluene indicated that the saturation capacity of M-13X were slightly higher than that of 13X under the tested conditions. This may be because of the high hydrophobicity of M-13X as a result of its high Si/Al ratio. The hydrophobicity was confirmed by investigating the water isotherms and toluene adsorption under different relative humidities; the results indicated that M-13X has super-hydrophobic properties. The desorption efficiency curves demonstrated that the optimal regeneration temperature for toluene adsorption was 473 K, taking account of energy saving.
References
- Alonso-Vicario , A. , Ochoa-Gómez , J. , Gil-Río , S. , ómez-Jiménez-Aberasturi , P. G , Ramírez-López , C. , Torrecilla-Soria , J. and Domínguez , A. 2010 . Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites . Micropor. Mesopor. Mater , 134 : 100 – 107 . doi: 10.1016/j.atmosenv.2005.11.074
- Borwankar , D. , Fowler , M. and Anderson , W. 2010 . Incorporating energy generation into volatile organic compound (VOC) emission treatment using a solid oxide fuel cell: A model-based approach . Energy Fuels , 24 : 4693 – 4702 . doi: 10.1021/ef901249g
- Bao , J. , Hu , Q. , Li , Q. and Hao , Z. 2011 . Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry . J. Hazard. Mater , 186 : 1615 – 1624 . doi: 10.1016/j.matlet.2010.09.058
- Baek , S. , Kim , J. and Ihm , S. 2004 . Design of dual functional adsorbent/catalyst system for the control of VOC's by using metal-loaded hydrophobic Y-zeolites . Catal. Today , 93–95 : 575 – 581 . doi: 10.1016/j.cattod.2004.06.107
- Chang , C. , Lee , C. and Wu , Y. 2002 . Assessment of the strategies for reducing volatile organic compound emissions in the automotive industry in Taiwan . Resources Conserv. Recycling , 34 : 117 – 128 . doi: 10.1016/S0921-3449(01)00096-9
- Cheng , X. , Wang , J. , Guo , J. and Long , Y. 2008 . Binder-free ZSM-5 zeolite caralysts modified with framework de-alumination . Acta Chim. Sin , 66 : 2099 – 2106 . doi: 10.3724/SP.J.1077.2008.00592
- Chinte , H. , Bai , H. and Karthik , M. 2009 . Ordered mesoporous silica particles and Si-MCM-41 for the adsorption of acetone: A comparative study . Separ. Purif. Technol , 64 : 265 – 272 .
- Chun , H. , Lu , H. , Zeng , L. and Huang , H. 2009 . Relative performance of zeolites and activated carbon in gaseous phase adsorption and desorption of toluene . Environ. Pollut. Control , 31 ( 4 ) : 38 – 42 .
- Christian , K. and Veiga , M. 2010 . Technologies for the abatement of odors and volatile organic and inorganic compounds . Chem. Eng. Trans , 23 : 1 – 6 .
- Chun , M. , Feng , Y. and Ma , L. 2011 . Characterization and hydroisomerization performance of SAPO-11 molecular sieves synthesized by dry gel conversion . Micropor. Mesopor. Mater , 147 : 205 – 211 .
- Cui , S. , Chen , M. , Ma , C. and Zheng , X. 2009 . Pd-Mn/stainless steel wire mesh catalyst for catalytic oxidation of toluene, acetone and ethyl acetate . Chin. J. Chem , 27 : 1903 – 1906 .
- Douglas , M. 2011 . Molecular sieve separations . Chem. Ing. Tech , 2 ( 83 ) : 44 – 52 .
- Gola , A. , Rebours , B. , Milazzo , E. , Lynch , J. , Benazzi , E. , Lacombo , S. , Delevove , L. and Fernandez , C. 2000 . Effect of leaching agent in the dealumination of stabilized Y zeolites . Micropor. Mesopor. Mater , 40 : 73 – 83 . doi: 10.1016/S1387-1811(00)00243-2
- Guieysse , B. , Hort , C. , Platel , V. , Munoz , R. , Ondarts , M. and Revah , S. 2008 . Biological treatment of indoor air for VOC removal: Potential and challenges . Biotechnol. Adv , 26 ( 5 ) : 398 – 410 . doi: 10.1016/j.biotechadv.2008.03.005
- Gupta , V. and Verma , N. 2002 . Removal of volatile organic compounds by cryogenic condensation followed by adsorption . Chem. Eng. Sci , 57 ( 14 ) : 2679 – 2696 . doi: 10.1016/S0009-2509(02)00158-6
- Harper , M. 2000 . Sorbent trapping of volatile organic compounds from air . J. Chromatogr. A , 885 : 129 – 151 . doi: 10.1016/S0021-9673(00)00363-0
- Hu , Q. , Dou , B. , Tian , H. , Li , J. , Li , P. and Hao , Z. 2010 . Mesoporous silicalite-1 nanospheres and their properties of adsorption and hydrophobicity . Micropor. Mesopor. Mater , 129 : 30 – 36 . doi: 10.1016/j.micromeso.2009.08.029
- Koziel , J. A. , Yang , X. , van Leewen , H. , Jenks , W. and Laor , Y. 2010 . Treatment of odorous VOCs with ultraviolet light . Chem. Eng. Trans , 23 : 363 – 368 .
- Kresge , C. , Leonowicz , M. , Roth , W. , Vartuli , J. and Beck , J. 1992 . Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism . Nature , 359 : 710 – 712 . doi: 10.1038/359710a0
- Kim , K. and Ahn , H. 2012 . The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating . Micropor. Mesopor. Mater , 152 : 78 – 83 . doi: 10.1016/j.micromeso.2011.11.051
- Lloyd , L. 2011 . Handbook of Industrial Catalysts , Berlin : Springer .
- Li , M. 1996 . Organic pollution, purification and analysis overview . Shanxi Machinery , 1 : 18 – 20 .
- Li , N. 2009 . Synthesis and Characterization of Hierarchical-Structured Mordenite , Dissertation, Dalian University of Technology .
- Lan , R. , Chen , C. and Yan , Y. 2008 . The research on treating gaseous toluene by using sodium citrate . Resources Environ. Eng , 2 ( 22 ) : 250 – 253 .
- Li , W. , Wang , J. and Gong , H. 2009 . Catalytic combustion of VOCs on non-noble metal catalyst . Catal. Today , 148 : 81 – 87 . doi: 10.1016/j.cattod.2009.03.007
- Monneyron , P. , Manero , M. and Mathé , S. 2008 . Combined selective adsorption and ozonation process for VOCs removal from air . Can. J. Chem. Eng , 85 ( 3 ) : 326 – 332 .
- Makowski , W. and Kustrowski , P. 2007 . Probing pore structure of microporous and mesoporous molecular sieves by quasi-equilibrated temperature programmed desorption and adsorption of N-nonane . Micropor. Mesopor. Mater , 102 : 283 – 289 . doi: 10.1016/j.micromeso.2007.01.009
- Polyakov , N. , Dubinin , M. , Kataeva , L. and Petuhova , G. 1993 . Porous structure and adsorption of active carbon . Pure Appl. Chem , 65 ( 10 ) : 2189 – 2192 . doi: 10.1351/pac199365102189
- Serra , R. M. , Miró , E. E. , Bolcatto , P. G. and Boix , A. V. 2012 . Experimental and theoretical studies about the adsorption of toluene on ZSM-5 and mordenite zeolites modified with Cs . Micropor. Mesopor. Mater , 147 : 17 – 29 . doi: 10.1016/j.micromeso.2011.05.016
- Serra , R. M. , Miró , E. E. , Sapag , M. K. and Boix , A. V. 2011 . Adsorption and diffusion of toluene on Na and Cs mordenites for hydrocarbon traps . Micropor. Mesopor. Mater , 138 ( 1–3 ) : 102 – 109 . doi: 10.1016/j.micromeso.2010.09.024
- Shen , H. , Su , S. and Zhong , Q. 2009 . Principles and Processes of Air Pollution Control Engineering , Beijing : Tsinghua University Press .
- Tseng , T. and Chu , H. 2001 . The catalytic incineration of styrene over an Mn2O3/Fe2O3 catalyst . Sci. Total Environ , 275 : 83 – 93 . doi: 10.1016/S0048-9697(00)00856-1
- Toshiaki , Y. , Asada , S. , Iida , T. and Ehara , Y. Nobel NOX and VOC treatment using concentration and plasma decomposition . Paper presented at annual meeting for Industry Applications Society on IEEE . Houston , TX . October 3–7 .
- William , L. 1997 . Industrial Air Pollution Control Systems , New York : McGraw-Hill Professional .
- Wei , W. , Wang , S. , Hao , J. and Cheng , S. 2011 . Projection of anthropogenic volatile organic compounds (VOCs) emissions in China for the period 2010–2020 . Atmos. Environ , 1 ( 13 ) : 1 – 9 . doi: 10.1016/j.atmosenv.2011.01.013
- Wei , Z. and Sun , J. 2010 . Removal of gaseous toluene by the combination of photocatalytic oxidation under complex light irradiation of UV and visible light and biological process . J. Hazard. Mater , 177 : 814 – 821 . doi: 10.1016/j.jhazmat.2009.12.106
- Xue , N. , Wang , Q. , Wu , C. , Zhao , P. and Xie , W. 2011 . Elimination of NH3 and odor from composting by biotrickling filter and preliminary exploration on molecular biology . Water Sci. Technol , 63 : 747 – 753 . doi: 10.2166/wst.2011.302
- Xu , R. and Pang , W. 2004 . Chemistry of Molecular Sieve and Material , Beijing : Science Publishing Company . doi: 10.1021/cm035066i
- Yang , S. and Meng , C. 2004 . Study on morderites treated by hydrochloric and oxalic acid and adsorption capacity . Liaoning Chem. Ind , 33 ( 3 ) : 125 – 126 .
- Yao , X. , Du , X. , Liu , Z. , Cui , Q. , Wang , H. and Yao , H. 2010 . Adsorption performance for n-hexane and characterization of 5A molecular sieves . Petrochem. Technol , 39 ( 7 ) : 757 – 761 .
- Zhao , X. , Ma , Q. and Lu , G. 1998 . VOC removal: Comparison of MCM-41 with hydrophobic zeolites and activated carbon . Energy Fuels , 12 : 1051 – 1054 .
- Zhao , Y. 2009 . The Theory Study of Atmospheric Reaction Mechanism of VOCs , Dissertation, Shan Dong University .