Abstract
In the disposal of electronic waste, cathode ray tube (CRT) funnel glass remains an urgent environmental problem because of its high lead content. This research developed mechanical activation as a pretreatment process, and it proved to be an effective method for extracting lead from CRT funnel glass. The effects of mechanical activation on the structural changes of CRT funnel glass were investigated using X-ray diffraction (XRD), particle size analysis, specific surface area (SSA), and a scanning electron microscope (SEM). Nitric acid leaching behaviors of the activated CRT funnel glass were studied by varying several parameters: leaching time, liquid-to-solid ratio, acid concentration, and heating temperature, as well as various conditions of activation. The lead recovery rate was observed to increase rapidly, particularly with increases in activation time and leaching temperature, but to vary relatively less under other experimental parameters. Under the optimal leaching conditions, the lead recovery rate for funnel glass activated for 2 hr at the rotational speed of 500 rpm (by ball mill) reached 92.5%, compared with 1.2% from the unactivated sample.
CRT funnel glass containing lead has become a serious environmental problem facing the whole world. In order to dispose of CRT funnel glass, some technologies have been developed. However, these technologies are associated with higher operation and maintenance costs. In this study, mechanical activation was introduced to change the physicochemical properties of CRT funnel glass, which can transform the glass into an easily dissolved one. Under atmospheric pressure leaching conditions, good recovery rate for lead can be achieved and the residue has wide uses. The process can be applied to treat other leaded glass or lead-containing wastes.
Supplemental Materials: s paper. Go to the publisher's online edition of the Journal of the Air and Waste Management System.
Introduction
The low cost of consumer electrical and electronic equipment (EEE)—especially computers and television sets—has led to its widespread acquisition and use all over the world. At the same time, frequent improvements in equipment features and functions have decreased the average life span of consumer electronic devices, thereby increasing the volume of e-waste generated from the obsolete devices (CitationLee et al., 2004; CitationWilliams et al., 2008).
End-of-life (EOL) cathode ray tubes (CTRs) make up a large proportion of waste electrical and electronic equipment (WEEE). Computer monitors and TV sets containing cathode ray tubes represent approximately 80% of the total electronic waste worldwide (CitationMéar et al., 2006b; CitationHerat, 2008). In China, TV sets are the dominant contributor and account for 47.7% by weight of the total e-waste (CitationYang et al., 2008). With the rapid development of display device technology, the CRT has gradually been replaced by the liquid crystal display (CitationPoon, 2008). Therefore a great number of EOL CRTs are discarded annually. With more and more televisions and computers reaching their end of life, the electronic industry is confronted with the urgent problem of dealing with the used CRTs.
The bulk of a CRT is glass—typically representing 42 wt% of the TV's or monitor's weight—and glass is responsible for most of the hazardous substances within a CRT. The hazardous characteristics of CRT glass have been determined by using a toxicity characteristic leashing procedure (TCLP) adopted by the U.S. Environmental Protection Agency (EPA) to determine the leaching toxicity of wastes (CitationJang and Townsend, 2003; CitationMusson et al., 2000; CitationSpalvins et al., 2008). CRT glass contains three main types of glass: panel glass, funnel glass, and neck glass. Panel glass has a high content of barium and strontium, and accounts for about two thirds of the weight of the entire CRT (CitationAndreola et al., 2005). Funnel glass is a type of lead silicate glass of 22–28 wt% lead, and accounts for about one third of the weight of the glass in the CRT (CitationIndustry Council for Electronics Equipment Recycling [ICEER], 2004; CitationWilliams et al., 2008). Neck glass is another type, containing 40 wt% lead. Between them, funnel and neck glass contribute an average of about 4 pounds of lead to a typical CRT, with some variation depending on the size, age, and manufacturer of the device (CitationAndreola et al., 2005a; CitationNnorom et al., 2011).
In the disposal of electronic waste, dealing with CRT glass has become an urgent environmental problem faced by the entire world. It is especially critical to find a way to process the waste funnel glass, which has a high lead content. The development of technologies for CRT glass detoxification and reutilization is therefore extremely urgent, especially in China, as a large portion of WEEE are generated there. Research by Yu and colleagues (CitationYu et al., 2010) showed that developing nations will have to deal with more old computers than developed countries by 2018 or sooner, because of the lax supervision and environmental regulations concerning e-waste in the developing world.
Many studies have been carried out on the recycling of scrap CRT glass to manufacture other products, such as foam glass (CitationMéar et al., 2006a, Citation2006c; CitationBernardo and Albertini, 2006), ceramic glaze (CitationAndreola et al., 2005b, Citation2007; CitationBernardo, 2007), flux (CitationAndreola et al., 2008), fine aggregate in cement mortar (CitationLing and Poon, 2011), crystalline silicotitanate (CitationChen et al., 2010), and so on. These solutions would be sufficient if there were not potential environmental risks from the lead remaining in the products made from waste CRT glass. It is necessary to remove the lead from CRT funnel glass in order to detoxify it, before reusing it in other products. Some technologies have been developed for this purpose, such as the self-propagating process (CitationChen et al., 2009), the reduction process (Yot and CitationMéar, 2009) by reaction with silicon carbide and titanium nitride, ultrasonically enhanced leaching (CitationSaterlay et al., 2001), and subcritical water (CitationMiyoshi et al., 2004) and mechanical activation (CitationYuan et al., 2012) to enhance leaching. However, the lead is so tightly entangled in the network structure of the glass that it is very difficult to extract. Thus, a pretreatment process that could dissolve CRT funnel glass into its constituent components would greatly enhance its reuse capabilities.
The influence of mechanical activation, particularly by dry milling, on the dissolution of finely milled minerals has recently attracted much attention (CitationHeineke, 1984; CitationBaláž, 2003, Citation2008; CitationGuo et al., 2010), but little information is available on its application to glass materials. In this study, a mechanical activation method was introduced as a pretreatment process for activating scrap CRT funnel glass, and the activated samples then underwent acid leaching. The basic information obtained from these processes is reported here.
Experimental
Materials
EOL funnel glass (with inner and outer coatings removed) was provided by Henan Ancai Gaoke Corporation. The funnel glass block was broken into small pieces, pulverized with a ball mill, and then sized in order to obtain powders with a dimension range of 124∼178 μm. The resulting powder was dried at 105 °C for 24 hr. The average chemical composition of the specimens investigated, determined by using X-ray fluorescence spectroscopy (SXF-1200; Shimadzu, Tokyo, Japan), is shown in . Analytical-reagent-grade nitric acid was used as the chelating reagent in this research work.
Table 1. Chemical composition of the end-of-life CRT funnel glass analyzed by X-ray fluorescence (XRF)
Methods
Mechanical activation
The funnel glass powder was mechanically activated by grinding with a planetary ball mill (P-7; Fritsch, Idar-Oberstein, Germany). For ball milling, seven zirconia balls of 15 mm diameter and a 45-mL bowl were used. In each batch, 4 g of funnel glass powder was milled in an ambient atmosphere, at 100, 300, 500, and 700 rpm with duration times of 30, 60, 120, 180 and 240 min. The mechanical activation process was undertaken at the set rotation speed. All the samples were subjected to leaching after the mechanical activation, with no further treatment.
Characterization
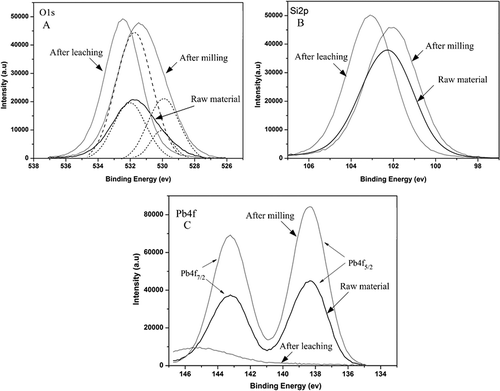
The particle sizes of funnel glass samples before and after mechanical activation treatment were measured by a Microtrac particle size analyzer (MT3300; Tokyo, Japan). The median particle size, D 50, was used in this paper. The specific surface areas (SSAs) of the activated samples were measured with a nitrogen gas adsorption instrument (ASAP2010; Micromeritics, Georgia, USA) based on the Brunauer-Emmett-Teller (BET) method. The samples were investigated by X-ray diffraction (XRD; Rigaku, Tokyo, Japan), using Cu Kα radiation with a step size of 0.02° and a recorded range for 2θ from 5° to 60° (λ = 1.5604 Å). The microstructures of the samples were investigated by scanning electron microscopy (SEM; Hatachi 6600; Tokyo, Japan). X-ray photoelectron spectroscopy (XPS; PHI 5600 ESCA system; Ulvac-Phi Inc., Kanagawa, Japan) was performed to obtain information on the chemical binding energy of the samples before and after activation. The Pb4f XPS lines were recorded under ultra-high-vacuum conditions with a pass energy of 187.9 eV. The C1s signal was chosen to determine the degree of contamination in order to correct the energy scale for charging effects because of the insulating property of the glass surface. Scans were recorded for C1s, Si2p, O1s, and Pb4f signals by steps of 0.125 eV.
Leaching in nitric acid solution
Leaching experiments on the glass samples were carried out in a closed 100-mL three-necked flask in a water batch, which was placed on a thermostatically controlled magnetic stirrer. The leaching process was with the following parameters: nitric acid concentrations of 0.3, 0.5, 1.0, 3.0, 5.0, and 7.0 mol/L; leaching times of 20, 40, 60, 120, and 240 min; leaching temperatures of 30, 65, 80, and 95 °C; and liquid-to-solid ratios (L/S) of 150:1, 100:1, 75:1, 50:1, and 25:1. After leaching, solid-liquid separation was performed by vacuum filtration; a nylon filter membrane was used with a pore size of 0.45 μm. The amount of metal in the aqueous solution after separation was quantitatively analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES; Optima 3300SYS; Tokyo, Japan). The residue was dried at 105 °C for 24 hr and then characterized by XRD and XPS to analyze its structural morphology and structural differences in the surface region.
Results and Discussion
Effect of mechanical activation on CRT funnel glass
The morphologies of the initial and mechanically activated glass samples observed by SEM are shown in Milling operation at the low speed of 100 rpm did not cause evident changes in either morphology. However, when the rotational speed was increased to 300 rpm or above, obvious changes were observed in morphology. In order to clearly show the surface morphology of the activated sample, the SEM images of smaller particles (rotational speed above 300 rpm) were chosen. From , the funnel glass particles were observed to be composed of two parts: agglomerates consisting of primary particles in nano order, and some larger powders, not fully broken. This implies that running the milling operation at a rotational speed of 300 rpm is not strong enough to break all of the particles into nano order. When the rotational speed reached 500 rpm, though, all the particles were very fine, with a cotton-like surface. They were in a state of agglomeration, which is a typical phenomenon observed with mechanically activated samples. The typical changes after mechanical activation were observed with the increase of SSA and the decrease of median particle size. When the rotational speed was above 300 rpm (2.71 ± 0.02 m2/g), the median particle size changed slightly, but the SSA still rapidly increased to 5.37 ± 0.05 m2/g (500 rpm). Further extension of rotational speed to 700 rpm (5.36 ± 0.01 m2/g) did not cause evident changes in these two values.
Figure 1. SEM images of CRT funnel glass after mechanical activation for 2 hr at different rotational speeds under an ambient atmosphere: (A) raw material; (B) 100 rpm; (C) 300 rpm; (D) 500 rpm; (E) 700 rpm.
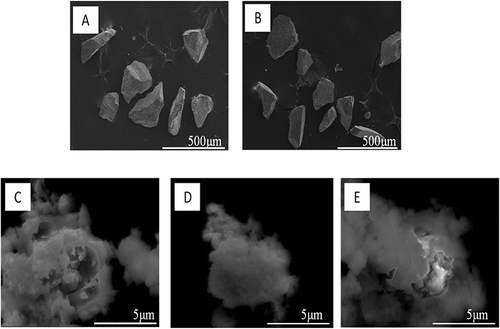
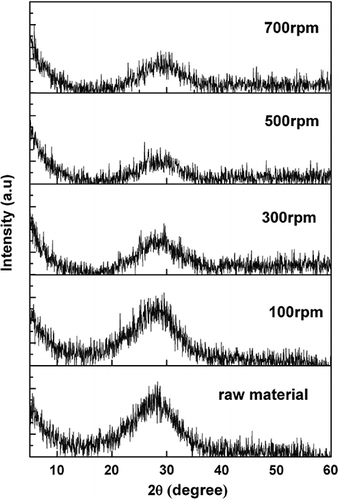
Figure 2 shows the XRD patterns of the samples milled for 120 min at different rotational speeds. Glass is one of the amorphous materials in which there occurs short-range order and long-range disorder (CitationShanxi University of Science and Technology, 2006). According to , it is clear that all of the initial glass samples, as well as the activated samples, exhibited a typical glass phase pattern with a glass hump around 29°. The CRT funnel glass was still amorphous, and no crystalline formed after the mechanical activation process.
Figure 2. X-ray diffraction patterns of raw material and 120-min-activated CRT funnel glass at different rotational speeds.
In this experiment, the main purpose was to increase lead recovery by general acid leaching with the aid of mechanical activation. shows the changes in recovery rates of lead, aluminum, and Silica leached from the activated samples with variations of the rotational speed of the milling operation. Lead recovery was the key point in this research, and the chemical reaction ( Equationeq 1) of funnel glass in acid solution can be used to represent the lead dissolution (CitationBonnet et al., 2003).
Figure 3. Dependence of recovery rate of major elements on milling rotational speed, under the following conditions: leaching temperature of 95 °C, HNO3 concentration 3 mol/L, and leaching time of 1 hr.
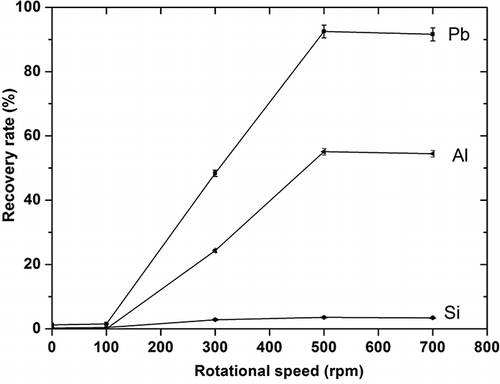
It is understood that silica composition is not soluble in an acid solution. The results shown in confirm this reaction pathway, where the silica recovery rate remained low with all the samples, irrespective of the milling operation. However, recovery rates of cation compositions of both lead and aluminum were found to change tremendously after activation and reach as high as 92.5% and 55.0%, respectively, from the sample milled at 500 rpm.
When the change in lead recovery rate shown in is compared with that of specific surface area, it can be seen that lead recovery rates for all the samples increased with the increase in specific surface area, and at a larger specific surface area lead recovery rate is thought to be approximately proportional to the specific surface area. Compared with raw material, the activated sample achieved a higher lead recovery rate under the same leaching conditions. From , it can easily be seen that element recovery rates increased with the increase in rotational speed in the range of 100∼500 rpm. At a rotational speed below 100 rpm, the element recovery rates for activated samples were found to be only slightly different from those for raw material, because insufficient energy could be applied to break the glass structure. At the rotational speed of 500 rpm, there was enough energy to fully break the glass, but more energy (up to 700 rpm) did not lead to any further increase in the element recovery rates. This result indicates that the rotational speed of the ball mill is an important factor in the lead recovery rate from the glass samples. Similar phenomena have been reported for materials dissolution by Tromans and colleagues (CitationTromans and Meech, 2001).
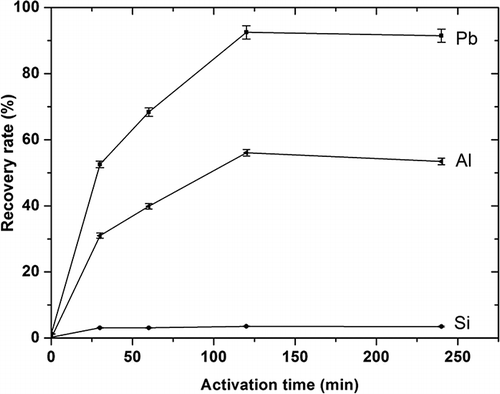
Besides rotational speed, the effect of milling time on dissolution behavior was also investigated; the results at 500 rpm are shown in Clearly, with an increase in milling time, the recovery rates of lead and aluminum increased rapidly and reached high levels at 120 min, with no further increase at longer time periods, whereas the rate for silicon remained low without evident dissolution for any time period. The results were similar for different rotational speeds. Clearly, there exist best conditions of activation treatment for lead recovery from glass samples. Prolonging treatment or increasing speed to over 700 rpm actually results in a slight decrease in lead dissolution.
Effect of leaching conditions
Leaching conditions as well as activation conditions have been found to affect lead recovery from CRT funnel glass. These effects are discussed in this section, using the samples mechanically activated for 120 min at the rotational speed of 500 rpm. In this study, a single-factor experiment was carried out. The variable leaching conditions were leaching temperature, acid concentration, liquid-to-solid ratio, and leaching time. The interactions among factors will be analyzed through orthogonal experiments in future work.
Influence of leaching temperature
Leaching temperature was examined first and the results are shown in , with constant conditions of nitric acid concentration 3.0 mol/L, L/S 150:1, and leaching time for 1 hr, at temperatures of 30, 65, 80, and 95 °C. With the increase in leaching temperature, lead and aluminum recovery rates were clearly observed to increase from 26.4% and 12.3% at 30 °C to 92.5% and 55.0% at 95 °C. To some extent, increase in the leaching temperature also tended to enhance the silica leaching rate, but the increase was much less remarkable. Silica forms the network structure of the glass and is very difficult to extract (CitationScholze, 1982; CitationBonnet et al., 2003). The recovery rate for silica was essentially 0% at 30 °C and rose to approximately 3.5% at 95 °C.
Figure 5. Dependence of recovery rate of major elements on the leaching temperature, under the following conditions: leaching time of 1 hr and HNO3 concentration 3 mol/L.
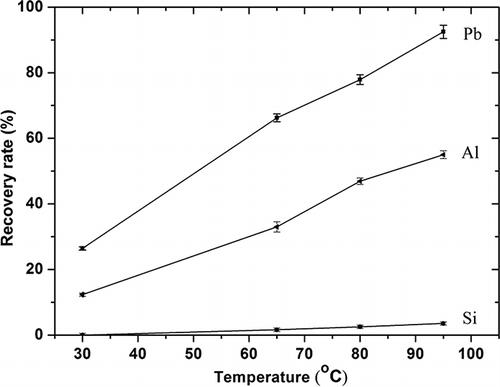
It appears that the higher the leaching temperature, the higher the recovery rate of lead. For experiments varying other parameters, a leaching temperature of 95 °C, just below the boiling point of water, was used.
Influence of nitric acid concentration
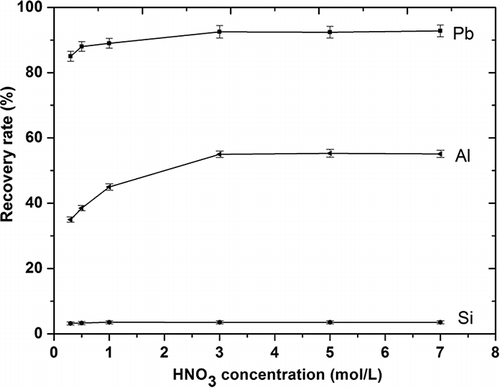
The influence of nitric acid concentration on the recovery rates for lead, aluminum, and silica from funnel glass mechanically activated for 2 hr at the rotational speed of 500 rpm was investigated as shown in The leaching conditions were leaching temperature 95 °C, leaching time 1 hr, and L/S 150. The recovery rate of lead from activated CRT funnel glass increased slightly from 85.0% to 92.5% with the nitric acid concentration of 0.3∼3.0 mol/L, whereas there was no remarkable change when the nitric acid concentration exceeded 3.0 mol/L; the rate remained at approximately 92.0%. The leaching processes of silica and aluminum, as network formers, were more difficult than for network modifier lead. The recovery of silica was very small, approximately 3.0%, and did not change appreciably with the increase in the acid concentration. The aluminum recovery rate did increase with the increase of nitric acid concentration. It reached its maximum value of 55.0% when the acid concentration reached 3.0 mol/L, although further extension of the acid concentration did not obtain a significantly higher recovery rate for aluminum. As previously stated, it could be concluded that acid concentration has a slight positive effect on the leaching behavior of a glass modifier from CRT funnel glass after mechanical activation.
Influence of liquid-to-solid ratio
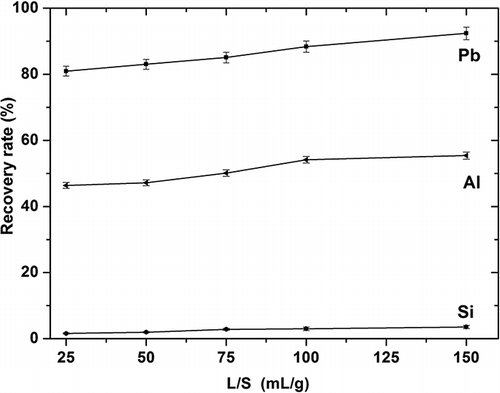
The effect of the liquid-to-solid ratio on the recovery rates for lead, aluminum, and silica from funnel glass was studied using nitric acid 3.0 mol/L, leaching temperature of 95 °C and leaching duration of 1 hr. The recovery rates under these conditions are plotted in From these data, it can be seen that the L/S ratio significantly affected the leaching recovery rates for lead and aluminum, but only slightly affected the rate for silica. Recovery rates for lead and aluminum increased from 80.0% and 46.0% at L/S 25 to 92.5% and 55.0% at L/S 150, respectively. At the same ratios, the silica recovery rate did not significantly change, rising only from slightly above 0.0% to 2.0%. Many studies have shown that high silica dissolution has a negative effect on the filtration rate (CitationMa, 2007). Determining a reasonable L/S ratio for both reasonable lead recovery and positive silica influence on the filtration rate will require further testing before any practical application can be developed.
Influence of leaching time
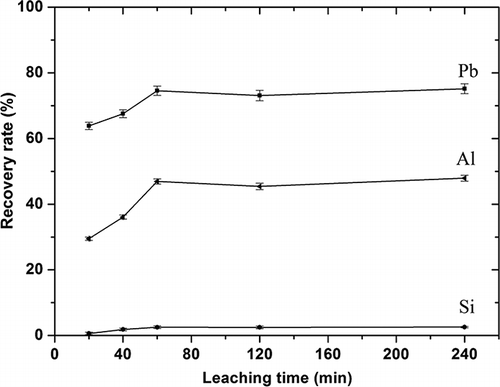
The recovery of major elements from activated CRT funnel glass using various leaching durations was also investigated in this research, as shown in The leaching conditions were as follows: nitric acid concentration 3.0 mol/L, leaching temperature of 80 °C, L/S 150, and leaching durations of 60–250 min. During the first 60 min, lead and aluminum recovery rates increased rapidly as leaching progressed. The recovery rates of lead and aluminum were 75.0% and 47.0%, respectively, within 60 min. However, no significant increase in lead and aluminum extraction was observed with extended leaching times. The leaching behavior of silica was similar to that of lead and aluminum. Thus, leaching time should be kept to 1 hr for lower energy consumption and higher productivity.
Characterization of samples after acid leaching
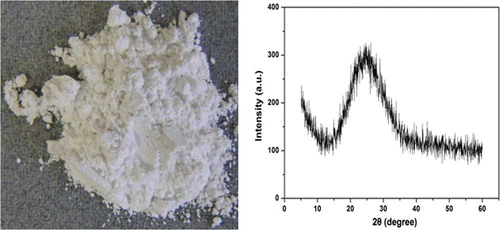
One research study reported on the leaching behavior of CRT funnel glass pieces of a given size and protective layer formed during the leaching process (CitationYamashita et al., 2010). However, to the best of our knowledge, the structural changes in activated CRT funnel powder after leaching has not been reported. In this study, the sample after leaching (residue) in 3.0 mol/L HNO3 solution at 95 °C for 1 hr was characterized by XRD and its photo image is shown in The residue after leaching is a superfine silica powder in amorphous form, which has a number of practical applications. For detecting the changes in the chemical environments of elements in glass structures, XPS has proved to be a powerful analytical tool. In this study, the chemical bonding environments of O1s, Si2p, and Pb4f in unactivated and activated CRT funnel glass, and leaching residue were analyzed by XPS, and the results are shown in
Figure 9. Photo and XRD pattern of residue after leaching in 3 mol/L HNO3 at 95 °C for 1 hr (L/S = 150). (Color figure available online).
The profiles of the O1s spectra changed for the three samples, as shown in Two peaks were observed in the O1s spectra—for raw material and for the activated sample, which were attributed to the bridging oxygen in Si–O–Si (532.2 eV) and the nonbridging oxygen in Si–O–X (530 eV, where X = Na, Pb, Al, etc.). Only one peak was observed after leaching, and its spectral profile was almost the same as that of the higher-energy component present before leaching (CitationMizuno et al., 2005). The major contribution to the O1s signal is from the bridging oxygen as the fraction of Si–O–Si bond in the sample increases. Due to the leaching of lead and other metals, the nonbridging oxygen signal is attenuated to the limit of detection (CitationMnzenberg et al., 1998).
The Si2p peaks of unactivated and activated samples were characterized by similar binding energies around 102.2 eV, as illustrated in It should be noted that such signals in the samples after leaching have been observed to shift toward a higher binding energy of 103.1 eV. A similar result was achieved by Bertoncello and colleagues (CitationBertoncello et al., 2004). As shown in (the Pb4f binding energies line), Pb4f5/2 from the unactivated samples (CRT funnel glass) appeared at 138.4 eV, which was close to the value for PbSiO3 (138.7 eV) (CitationMéar et al., 2005). Thus, it could be concluded that lead ions in funnel glass are mainly (+α), which is consistent with the research of Méar and colleagues (CitationMéar et al., 2007; Yot and CitationMéar, 2009). The binding energies of Pb4f in unactivated and activated samples did not change, and Pb4f disappeared from the leached samples because lead was removed after leaching.
According to the O1s, Si2p, and Pb4f signals for unactivated and activated CRT funnel glass illustrated in , the small shifts in the binding energy indicate that the chemical state of these elements did not change significantly upon mechanical activation. The observed increases in the binding energy of the Si2p and O1s peaks in the residue are therefore indicative of an increase in the number of Si–O bonds per silicon atom and may be leading to enhanced polymerization of the silica present in glass.
According to the XRD pattern of the residue after leaching shown in , the residue remained in an amorphous form. It consisted primarily of superfine bright silica powder. The residue has many uses, such as in the production of rubber, paint, or pesticide, and could have significant economic benefits and wide application. Characterization of the residue after leaching as porous silica, such as the Toxicity Characteristic Leaching Procedure (TCLP; EPA), SSA, and water absorption, is under investigation, and subsequent reports are expected to be submitted concerning the related data and demonstrating the potential production of high-value silica from waste glass.
Conclusions
Mechanical activation was applied to improve lead recovery from CRT funnel glass, using traditional acid solution leaching, in this study. It was an effective method to promote the leaching process of funnel glass. The lead recovery rate was found to depend on both milling and leaching operations. Enhanced lead recovery was attributed to changes induced by the milling operation—on particle size, SSA, and morphology, as well as on the local structure of the glass sample. The maximum lead recovery rate reached 92.5% for funnel glass mechanically activated for 2 hr at the rotational speed of 500 rpm under the leaching conditions of nitric acid solution 3 mol/L, heating temperature 95 °C, leaching time 1 hr, and liquid-to-solid ratio of 150 mL/g. Lead recovery from unactivated CRT funnel glass, on the other hand, was only 1.2%, under the above-mentioned leaching conditions. The method developed in this study could also be used to process other types of leaded glass.
Supplementary Materials
Download MS Word (47 KB)Acknowledgments
This study was financially supported by the National Nature Science Foundation of China (21177069) and the Short-time Study Abroad Program for Ph.D. Candidates from Tsinghua University. The authors are also grateful to the Henan Aicai Gaoke Company for providing CRT funnel glass.
References
- Andreola , F. , Barbiere , L. , Corradi , A. and Lancellotti , I. 2005a . Cathode ray tube glass recycling: An example of clean technology . Waste. Manage. Res. , 23 : 314 – 321 . doi: 10.1177/0734242X05054422
- Andreola , F. , Barbieri , L. , Corrade , A. and Lancellotti , I. 2007 . CRT glass state of the art, a case study: Recycling in ceramic glazes . J. Eur. Ceram. Soc. , 27 : 1623 – 1629 . doi: 10.1016/j.jeurceramsoc.2006.05.009
- Andreola , F. , Barbieri , L. , Corradi , A. , Lancellotti , I. , Falcone , R. and Hreglich , S. 2005b . Glass-ceramics obtained by the recycling of end of life cathode ray tubes glasses . Waste Manage. , 25 : 183 – 189 . doi: 10.1016/j.wasman.2004.12.007
- Andreola , F. , Barbieri , L. , Karamanova , E. , Lancellotti , I. and Pelino , M. 2008 . Recycling of CRT panel glass as fluxing agent in the porcelain stoneware tile production . Ceram. Int. , 34 : 1289 – 1295 . doi: 10.1016/j.ceramint.2007.03.013
- Baláž , P. 2003 . Mechanical activation in hydrometallurgy . Int. J. Miner. Process. , 72 : 341 – 354 .
- Baláž , P. 2008 . Mechanochemistry in Nanoscience and Minerals Engineering , Berlin : Verlag Berlin Heidelberg .
- Bernardo , E. and Albertini , F. 2006 . Glass foams from dismantled cathode ray tubes . Ceram. Int. , 32 : 603 – 608 . doi: 10.1016/j.ceramint.2005.04.019
- Bernardo , E. , Castellan , R. and Hreglich , S. 2007 . Sintered glass–ceramics from mixtures of wastes . Ceram. Int. , 33 : 27 – 33 . doi: 10.1016/j.ceramint.2005.07.012
- Bertoncello , R. , Milanese , L. , Bouquillon , A. , Dran , J.C. , Mille , B. and Salomon , J. 2004 . Leaching of lead silicate glasses in acid environment: compositional and structural changes . Appl. Phys. A , 79 : 193 – 198 . doi: 10.1007/s00339-004-2651-9
- Bonnet , C. , Bouquillon , A. , Turrell , S. , Deram , V. , Mille , B. , Salomon , J. , Thomassin , J.H. and Fiaud , C. 2003 . Alteration of lead silicate glasses due to leaching in heated acid solutions . J. Non-Cryst. , 323 : 214 – 220 . doi: 10.1016/S0022-3093(03)00279-5
- Chen , M.J. , Zhang , F.S. and Zhu , J.X. 2009 . Lead recovery and the feasibility of foam glass production from funnel glass of dismantled cathode ray tube through pyrovacuum process . J. Hazard. Mater. , 61 : 1109 – 1113 . doi: 10.1016/j.jhazmat.2008.04.084
- Chen , M.J. , Zhang , F.S. and Zhu , J.X. 2010 . Effective utilization of waste cathode ray tube glass—Crystalline silicotitanate synthesis . J. Hazard. Mater. , 182 : 45 – 49 . doi: 10.1016/j.jhazmat.2010.05.135
- Environmental Protection Agency . 1986 . Toxicity Characteristic Leaching Procedure . Federal Register. USA ,
- Guo , X. Y , Duan , X. D, G.H. and Mou , P. 2010 . A review of mechanochemistry applications in waste management . Waste Manage. , 30 : 4 – 10 . doi: 10.1016/j.wasman.2009.08.017
- Heineke , G. 1984 . Tribochemistry , Berlin : Carl Hanser Publishing .
- Herat , S. 2008 . Recycling of cathode ray tubes (CRTs) in electronic waste . Clean , 36 : 19 – 24 . doi: 10.1002/clen.200700082
- Industry Council for Electronics Equipment Recycling (ICEER) . 2004 . Material Recovery from Waste Cathode Ray Tubes (CRTs), Waste and Resources Action Programme. Report 70 , Banbury , UK : Waste and Resources Action Programme .
- Jang , Y.C. and Townsend , T.G. 2003 . Leaching of lead from computer printed wire boards and cathode ray tubes by municipal solid waste landfill leachates . Environ. Sci. Technol. , 37 : 4778 – 4784 . doi: 10.1021/es034155t
- Lee , C.H. , Chang , C.T. , Fan , K.S. and Chang , T.C. 2004 . An overview of recycling and treatment of scrap computers . J. Hazard. Mater. , 114 : 93 – 100 . doi: 10.1016/j.jhazmat.2004.07.013
- Ling , T.C. and Poon , C.S. 2011 . Utilization of recycled glass derived from cathode ray tube glass as fine aggregate in cement mortar . J. Hazard. Mater. , 192 : 451 – 456 . doi: 10.1016/j.jhazmat.2011.05.019
- Méar , F. , Yot , P. , Cambon , M. , Caplain , R. and Ribes , M. 2006a . Characterization of porous glasses prepared from Cathode Ray Tube (CRT) . Powder Technol. , 162 : 59 – 63 .
- Méar , F. , Yot , P. , Cambon , M. and Ribes , M. 2005 . The changes in lead silicate glasses induced by the addition of a reducing agent (TiN or SiC) . J. Non-Cryst. , 351 : 3314 – 3319 . doi: 10.1016/j.jnoncrysol.2005.08.019
- Méar , F. , Yot , P. , Cambon , M. and Ribes , M. 2006b . The characterization of waste cathode ray tube glass . Waste Manage. , 26 : 1468 – 1476 . doi: 10.1016/j.wasman.2005.11.017
- Méar , F. , Yot , P. , Kolobov , A.V. , Ribes , M. , Guimon , M.F. and Gonbeau , D. 2007 . Local structure around lead, barium and strontium in waste cathode ray tube glasses . J. Non-Cryst. , 353 : 4640 – 4646 . doi: 10.1016/j.jnoncrysol.2007.07.009
- Méar , F. , Yot , P. and Ribes , M. 2006c . Effects of temperature, reaction time and reducing agent content on the synthesis of macroporous foam glasses from waste funnel glasses . Mater. Lett. , 60 : 929 – 934 . doi: 10.1016/j.matlet.2005.10.046
- Ma , R.J. 2007 . Principle on Hydrometallurgy , Beijing : Metallurgical Industry Press .
- Miyoshi , H. , Chen , D.P. and Akai , T. 2004 . A novel process utilizing subcritical water to remove lead from wasted lead silicate glass . Chem. Lett. , 33 : 956 – 957 . doi: 10.1246/cl.2004.956
- Mizuno , M. , Takahashi , M. , Takaishi , T. and Yoko , T. 2005 . Leaching of lead and connectivity of plumbate networks in lead silicate glasses . J. Am. Ceram. Soc. , 88 : 2908 – 2912 . doi: 10.1111/j.1551-2916.2005.00508.x
- Mnzenberg , C.S. , Meisel , W. and Gutlich , P. 1998 . Changes of lead silicate glasses induced by leaching . J. Non-Cryst. , 238 : 83 – 90 .
- Musson , S.E. , Jang , Y.C. , Townsend , T.G. and Chung , I.H. 2000 . Characterization of lead leachability from cathode ray tubes using the toxicity characteristic leaching procedure . Environ. Sci. Technol. , 34 : 4376 – 4381 . doi: 10.1021/es0009020
- Nnorom , I.C. , Osibanjo , O. and Ogwuegbu , M.O.C. 2011 . Global disposal strategies for waste cathode ray tubes . Resour. Conserv. Recy. , 55 : 275 – 290 . doi: 10.1016/j.resconrec.2010.10.007
- Poon , C.S. 2008 . Management of CRT glass from discarded computer monitors and TV sets . Waste Manage. , 28 : 1499 doi: 10.1016/j.wasman.2008.06.001
- Saterlay , A.J. , Wilkins , S.J. and Compton , R.G. 2001 . Towards greener disposal of waste cathode ray tubes via ultrasonically enhanced lead leaching . Green Chem. , 3 : 149 – 155 . doi: 10.1039/b102671m
- Scholze , H. 1982 . Chemical durability of glasses . J. Non-Cryst. , 52 : 91 – 103 . doi: 10.1016/0022-3093(82)90283-6
- Shanxi University of Science and Technology . 2006 . Glass Technology , Beijing : China Light Industry Press .
- Spalvins , E. , Dubey , B. and Timothy , T.G. 2008 . Impact of electronic waste disposal on lead concentrations in landfill leachate . Environ. Sci. Technol. , 42 : 7452 – 7458 . doi: 10.1021/es8009277
- Tromans , D. and Meech , J.A. 2001 . Enhanced dissolution of minerals: stored energy, amorphism and mechanical activation . Miner Eng. , 14 : 1359 – 1377 . doi: 10.1016/S0892-6875(01)00151-0
- Williams , E. , Kahhat , R. , Allenby , B. , Kavazanjian , E. , Kim , J. and Xu , M. 2008 . Environmental, social, and economic implications of global reuse and recycling of personal computers . Environ. Sci. Technol. , 42 : 6446 – 6454 . doi: 10.1021/es702255z
- Yamashita , M. , Wannagon , A. , Matsumoto , S. , Akai , T. , Sugita , H. , Imoto , Y. , Komai , T. and Sakanakura , H. 2010 . Leaching behavior of CRT funnel glass . J. Hazard. Mater. , 184 : 58 – 64 . doi: 10.1016/j.jhazmat.2010.08.002
- Yang , J. , Lu , B. and Xu , C. 2008 . WEEE flow and mitigating measures in China . Waste Manage. , 28 : 1589 – 1597 . doi: 10.1016/j.wasman.2007.08.019
- Yot , P.G. and Méar , F. 2009 . Lead extraction from waste funnel cathode-ray tubes glasses by reaction with silicon carbide and titanium nitride . J. Hazard. Mater. , 172 : 117 – 123 . doi: 10.1016/j.jhazmat.2009.06.137
- Yu , J. , Williams , E. , Ju , M. and Yang , Y. 2010 . Forecasting global generation of obsolete personal computers . Environ. Sci. Technol. , 44 : 3232 – 3237 . doi: 10.1021/es903350q
- Yuan , W.Y. , Li , J.H. , Zhang , Q.W. and Saito , F. 2012 . Innovated application of mechanical activation to separate lead from scrap cathode ray tube funnel glass . Environ. Sci. Technol. , 46 : 4109 – 4114 . doi: 10.1021/es204387a