Abstract
The emission of particulate matter (PM10 and PM2.5) and ammonia (NH3) by aeration processes at wastewater treatment plants (WWTPs) with and without odor control units was examined. Local concentrations of PM2.5, PM10, and NH3 at the aeration basins were within urban ranges. Emission fluxes of NH3 and PM2.5 for a medium-sized WWTP were determined to be 136 g day−1 and 43 g day−1, respectively, which are not substantial emission fluxes for urban environments. Odor control treatment using a granulated activated carbon bed reduced aerosol and NH3 emissions substantially. Detection of sterols, in particular the fecal sterol campesterol, in the PM clearly demonstrates aerosolization of wastewater components in the aeration process. The presence of campesterol in PM2.5 at a remote fenceline location in a WWTP facility illustrates that wastewater components are aerosolized in the fine PM fraction and transported beyond the facilities.
Wastewater treatment plants are potential emission sources of particulate matter and gases. This study characterized particulate matter emissions from aeration basins and quantified emissions fluxes of particulate matter and NH3. While fine and coarse particles as well as NH3 are being emitted, the overall emissions are small compared to other urban sources. However, fecal steroid presence in particles at the fence of a treatment plant demonstrates that wastewater material is getting aerosolized and transported beyond the facilities.
Introduction
Atmospheric particulate matter (PM) can be derived from a variety of primary and secondary sources. Anthropogenic sources, including primary emissions of particles from industrial sources, can substantially impact local environments. Industrial sources can include power plants as well as refining, smelting, and metal processing operations. Wastewater treatment plants (WWTPs) can also be industrial emissions sources (e.g., CitationRadke and Herrmann, 2003; CitationRadke, 2005; CitationUpadhyay et al., 2011a). WWTPs are common in urban environments; more than 16,000 WWTPs are located in the United States alone (CitationU.S. EPA, 1996). Many of these are in close proximity to the populated areas they serve, and hence any atmospheric emissions will directly impact neighborhood and regional air quality.
Within a WWTP, a likely origin of air emissions and wastewater material transferred to the atmosphere is the frequently used aeration basins. In the common activated sludge processes, outdoor air is compressed and diffused into deep aeration basins to allow diffusion of oxygen into water that serves as an electron acceptor for biological degradation of organic matter in sewage as well as biological oxidation of organic nitrogen to ammonia or nitrate. The diffused aeration process results in bursting bubbles at the liquid surface through the same mechanisms that generate sea salt particles (e.g., CitationTyree et al., 2007). Recent studies have suggested that activated sludge aeration basins produce a substantial flux of organic aerosols and bioaerosols (e.g., CitationRadke et al., 2005).
Several studies have examined the health effects of WWTP emissions (CitationGray, 1989; CitationBauer et al., 2002; CitationGlassmeyer et al., 2005; CitationCarducci et al., 2008). Most studies have focused on the exposure of plant workers (CitationGrisoli et al., 2009), especially in terms of bioaerosol exposure (CitationLeclerc et al., 2001; CitationBauer et al., 2002; CitationCarducci et al., 2008). Several authors have reported a particular illness that strikes plant workers, called “sewage worker's syndrome” (CitationFannin et al., 1985; CitationClark, 1987), which is characterized by general malaise, weakness, acute rhinitis, and fever. Some researchers also found a significant association between exposure to WWTP emissions and the incidence of respiratory and enteric illness, as well as seropositivity in plant workers and residents of neighboring areas to several viral strains contained in sewage (e.g., CitationHeng, 1994). All of these studies showed the potential for adverse effects from WWTP PM emissions (e.g., viruses, bacteria, pathogens) on human populations. Hence, a determination of the WWTP contribution to the PM in the ambient atmosphere and an evaluation of the potential health effects on neighboring populations seem warranted.
Limited data are available on the chemical composition of particles emitted by aeration basins. Radke and coworkers in Germany showed that trace species, including sterols and surfactants, can be readily aerosolized and are present in substantial concentrations in PM inside and in the vicinity of WWTPs (CitationRadke and Herrmann, 2003; CitationRadke, 2005; CitationBeck and Radke, 2006). Other investigators (CitationThorn et al., 2002; CitationPräzmo et al., 2003) investigated the presence of endotoxins in the air at sewage treatment plants. One study investigated WWTPs as a source of synthetic musk species in the urban atmosphere (CitationUpadhyay et al., 2011a).
The off-gases from aeration basins carry gaseous and particulate emissions. Volatile species, including methane, ammonia, odorous sulfide, and organic compounds, partition into the aeration gas. Odorous gases can be controlled by enclosing aeration basins and treating off-gases with activated carbon or gas scrubbers. No published studies have investigated the ability of these odor control technologies to remove PM from the exhaust stream. In addition, although many studies have focused on NH3 emissions from agricultural sewage (e.g., manure lagoons), little is known about emission fluxes from urban WWTPs.
This work aims to investigate and assess the importance of PM emissions from aeration basins, as both total PM and organic species of particular concern for human health or the environment. Furthermore, emissions of NH3 by aeration basins are evaluated. Finally, the effect of activated carbon odor control technology for removal of PM and ammonia in exhaust gases is evaluated, and emission rates are quantified.
Experimental
Field sampling
Gas and PM samples were collected at two different publicly owned WWTPs in the Phoenix (AZ) metropolitan area (). From October 28, 2008 to November 14, 2008 and from January 10, 2009 to January 23, 2009 samples were collected at a state-of-the-art reclamation facility that has a wastewater treatment capacity of 18 million gallons per day (MGD) (0.79 m3 s−1) and includes a covered aeration basin (CAB) and odor control facilities. Samples were collected from the atmosphere inside the enclosed aeration basin as well as from the odor control system exhaust.
Table 1. Overview of sampling campaigns and facilities
At the WWTP facility with the CAB, blowers are used to draw the aeration and ventilation air from one end of the basin. The exhaust airflow is greater than the aeration flow to minimize the escape of odorous vapors through the ventilation openings. The exhaust flow is then forced through a granular activated carbon (GAC) bed to remove odorous vapors. The aeration and exhaust flows are forced by blowers that are operated at constant rates.
PM samples were collected in the basin headspace near the exhaust duct and at the exhaust stack of the carbon adsorber. These samples were analyzed to determine the concentrations of PM components. The exhaust flow rate was calculated by measuring the diameter and velocity of gas flow from the carbon adsorber stack. The total exhaust flow rate was 5.5 m3 s−1 at each stack; thus, four stacks exhausted 22 m3 s−1.
A second series of measurements was made at a WWTP that included several uncovered aeration basins. This facility, referred to as OAB (“open aeration basins”; no odor control), had a treatment capacity of 200 MGD or 8.76 m3 s−1 of wastewater; here samples were collected from May 20, 2009 to June 1, 2009 at the edge of the aeration basins as well as at a fenceline location approximately 215 m away from the aeration basins.
PM2.5 (particles with aerodynamic diameter <2.5 μm) and PM10 (particles with aerodynamic diameter <10 μm) were collected using a sampling system consisting of two sampling trains, each with a size-selective cyclone inlet, two annular denuders, and a three-stage filter pack. The cyclone inlets had 50% cut-point diameters of 2.5 μm (2000-30EH, URG, Chapel Hill, NC) and 10 μm (2000-30ENB, URG) for PM2.5 and PM10 collection, respectively. The designed flow rate (critical orifice) through each sampling train was 16.7 L min−1 to achieve the cyclone cut-point diameter. Typical sampling times ranged from 8 to 24 hr. PM samples for mass and ion analysis were collected on Teflon filters (46.2 mm diameter, Whatman) contained in filter packs (2000-30F, URG). Gas-phase ammonia was collected on annular denuders (2000-30x242-3CSS, URG) that were coated with citric acid prior to use. A second denuder and filter sampler unit was used to collect samples on quartz fiber filters (47 mm, Pall Life Sciences, prefired at 600 °C for 12 hr) for organic and elemental carbon analysis.
Aerosol samples for organic trace analysis were collected using a ChemVol impactor (model 2400, Thermo Fisher Scientific, Waltham, MA) (CitationDemokritou et al., 2002) in which size-segregated particles were collected on different stages according to their aerodynamic diameter (Da): Da > 10 μm, 2.5 μm < Da < 10 μm, 1.0 μm < Da < 2.5 μm, 0.5 μm < Da < 1.0 μm, 0.1 μm < Da < 0.5 μm, and an after-filter for the particles with Da < 0.1 μm. The ChemVol sampler was operated at the designed flow rate of 760 L min−1. Polyurethane foam (PUF) substrates were used as the impaction media, and polypropylene foam (PPF) was used as the after filter media. All PUF and PPF substrates were precleaned and stored at −20°C before sample collection (CitationSun et al., 2009).
Chemical analysis
The mass concentrations of ambient PM were determined gravimetrically by weighing the Teflon filter samples pre and post sampling under controlled temperature (25°C) and humidity (relative humidity [RH] 45 ± 5%). Quartz fiber filter fractions were analyzed for organic carbon (OC) and elemental carbon (EC) by thermal-optical transmission using a well-established method (CitationBirch and Cary, 1996) on a Sunset Laboratories EC/OC analyzer.
Teflon filters were extracted in 10 mL of deionized (DI) water under sonication. The extracts were filtered through glass-fiber filters and the filtrates were refrigerated until analysis. Major anions were analyzed by ion chromatography (Dionex DX-600) using a Dionex AS11 column and suppressed conductivity detection. An on-line electrolytic eluent generator produced high-purity hydroxide eluent. Cations were determined on a Dionex DX-120 ion chromatography system using a Dionex cation-exchange CS12A column and suppressed conductivity detection.
The denuders were extracted using nanopure DI water. The resulting aqueous extracts were analyzed for NH4 + using ion chromatography as outlined earlier for the major ions.
Organic molecular compounds were extracted from PUF and PPF following a protocol of two extractions with isopropanol (Burdick & Jackson, high purity 99.9%) followed by three extractions with dichloromethane (Burdick & Jackson, high purity 99.9%). The extraction method is discussed thoroughly elsewhere (CitationSun et al., 2009). The extract was silylated to generate trimethylsilyl esters of sterols and other organic species containing hydroxyl groups. In a typical silylation procedure, 50 μL of concentrated sample extract was combined with 50 μL of bis(trimethylsilyl)trifluoroacetamide (BSTFA) and 10 μL of trimethylchlorosilane (TMCS), and the mixture was allowed to react for 2 hr at 70°C. Filter extracts were analyzed on an Agilent 6890 gas chromatograph (GC) coupled to an Agilent 5973 inert mass-selective detector (MSD). Separation was accomplished using an HP 5MS capillary column (30 m × 250 μm × 0.25 μm; 5% phenylmethylsiloxane film). Injections of 1-μL aliquots were performed in splitless mode, and helium (ultra-high purity) was used as the carrier gas. The MSD was operated in ion scan mode, and ions were produced by electron impact (EI) ionization. The GC temperature profile included an initial hold time of 10 min at 65 °C, followed by a temperature gradient of 10°C min−1 to a final temperature of 300°C that was held constant for 20 min. Authentic standards were used for identification and to obtain response factors for the majority of the quantified organic compounds. In the current work we focused on the sterols campesterol, cholesterol, β-sitosterol, coprostan-3-ol, stigmastanol, and stigmasterol.
Results and Discussion
PM2.5 and PM10 mass and OC concentrations
presents the PM mass and OC concentrations for PM2.5 (a) and PM10 (b) observed at the different locations during the study as well as urban PM concentrations measured in the Phoenix area (CitationUpadhyay et al., 2011b) as a reference. Overall mass and OC concentrations of both PM2.5 and PM10 are in the range of an urban area in general and Phoenix in particular. PM2.5 at the CAB is slightly higher but not atypical for closed, poorly vented environments. In the enclosed process, there is a substantial difference in off-gas concentrations between up- and downstream of the odor treatment unit. About 80% of both PM10 (23 to 6.7 μg/m3) and PM2.5 (24 to 5 μg/m3) was removed by the odor treatment unit.
Figure 1. PM2.5 (a) and PM10 (b) mass and organic carbon (OC) concentrations observed during the study and the average values for Phoenix (CitationUpadhyay et al., 2011b). Bars represent the range (min–max) of observations.
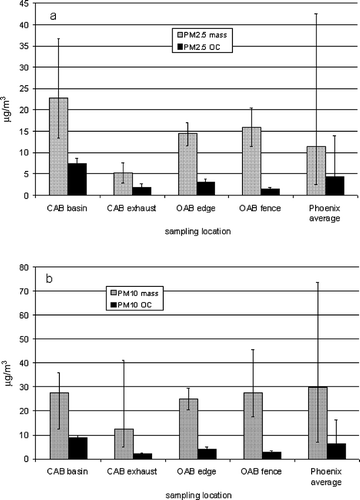
Although the overall mass and OC concentrations are not very high, they can still contain substantial amounts of aerosolized wastewater, including potential harmful species. First we evaluated the importance of the aeration basin as a PM emission source.
PM emissions from wastewater treatment operations
The fluxes of PM components from the surface of the CAB were calculated from the concentration and outflow measurements so that the present results may be generalized for other WWTP aeration basins and other sources of aerosols from bubble bursting. The aerosol flux of PM component i, Fi, was calculated as
This mass flux corresponds to a total daily emission rate of 43 g day−1. This is a very low number, considering that recent work (CitationWatson et al., 2011) estimated gasoline vehicle emissions of PM2.5 at <0.03 g km−1 for new and 0.06 g km−1 for older vehicles. Assuming a new vehicle emission factor of 0.03 g km−1, the daily emission rate of an aeration basin would correspond to about 1400 vehicle kilometers.
The aerosol flux from the WWTP aeration basin may be compared with laboratory measurements designed to study aerosol formation at the ocean surface (CitationTyree et al., 2007; CitationTyree and Allen, 2008). In the prior research, salt concentration, superficial bubbling velocity, and bubble size significantly affected aerosol flux from the bubbling surface. Superficial bubbling velocity, vb, was calculated as
These comparisons suggest that to understand the role of operating and design parameters on PM mass flux from aeration basins, laboratory experiments specifically designed to approximate these conditions are required. Calculations as well as the observational data suggest that WWTPs are not important sources of PM mass. Still, selected components of PM emitted by aeration basins could have substantial health effects, and select species are discussed in the following.
Inorganic PM composition
Out of concern for potential human health effects, we investigated the chemical composition of the emitted PM. shows the average relative ionic composition of PM2.5 and PM10 (by mass) in all sampling locations.
Figure 2. PM2.5 (a) and PM10 (b) average relative composition of major ions observed during the study.
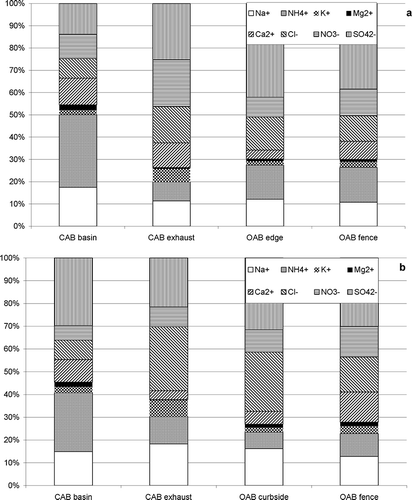
The major components and their relative abundance are very similar to the reported PM2.5 composition for the Phoenix area (e.g., CitationSoroosian et al., 2011). There is little variation in concentrations between the fence site and curbside. In the CAB some compositional change occurs; in particular, the NH4 + contribution decreases substantially after GAC treatment. This could be related to a drying out of particles, outgassing, and potentially the interaction of NH3 with GAC. Overall, the inorganic ion composition resembles that of an urban aerosol which suggests that, at least for the CAB, part of the aerosol might be recirculated outside air.
Airborne sterols
A class of wastewater components of concern is sterols. The target species in this study are sterols detected in urban atmospheres and at WWTPs (CitationRadke and Herrmann, 2003; CitationRadke, 2005), including campesterol, cholesterol, β-sitosterol, coprostan-3-ol, stigmastanol, and stigmasterol. Only cholesterol and coprostanol have been detected in all size fractions in the present study and hence have size distribution data available. The other species were detected only occasionally in individual size fractions. The result is consistent with work by CitationRadke (2003), who observed for these species concentrations in the range of several tens of picograms per cubic meter to 1 ng m−3. In this study the detection limits for individual size ranges were in the range of 0.05–0.5 ng m−3. The concentrations observed in individual samples ranged from below the detection limit to 0.68 ng m−3 for sitosterol and 0.28 ng m−3 for campesterol. Although cholesterol is typically found in the urban atmosphere and has been associated with cooking emissions (CitationSchauer et al., 1996), coprostanol is a fecal sterol that is produced only in the digestive tract of mammals (CitationWells, 1957) and hence is unambiguous proof of sewage aerosolization.
The size distribution shows that coprostanol is emitted in both coarse and fine fractions of the PM and is even present in submicrometer particles, which is consistent with aerosolization of PM over a wide particle size range (see ). At the aeration basin, the majority of coprostanol is associated with the larger particle sizes, whereas at the fence site the coprostanol observed is associated with smaller sizes. The short distance and hence short travel time make it unlikely that the difference is a result of larger particles settling out (which is also not seen in PM mass concentrations). These results are consistent with dispersion and drying of aerosols. Note that relative humidity was low during the sampling, as it averaged 39% (range 8–82%). Finally, the presence of coprostanol at the fence site is clear evidence that small aerosolized sewage particles can be transported beyond the treatment facility and could impact neighboring areas.
Figure 3. Size distribution of 3-coprostanol in aerosol observed at the open aeration basin (OAB) plant at curb and fence sites.
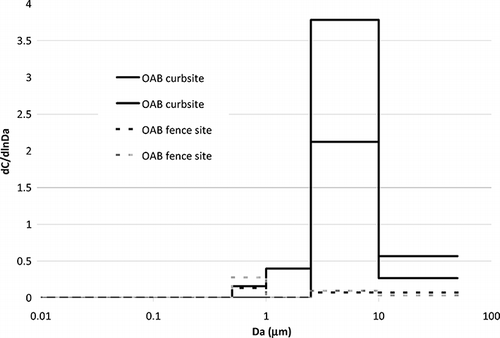
The size distributions observed in the present study are consistent with those of CitationRadke and Hermman (2003), who also reported that most coprostanol was present in the coarse particle range. In that study, samples were collected directly above the aeration basin in high humidity conditions.
Ammonia emissions
Aeration basins are known emitters of nitrous oxide gases, and some data are available on their greenhouse gas emissions (e.g., CitationAhn et al., 2010), but little is known about NH3 emissions. NH3 concentrations above the aeration basin and NH3 emission fluxes were investigated in this study. presents the NH3 concentrations observed. Each measurement is a duplicate of two separate denuder channels. The analytical precision is high with an average standard deviation of 9%.
Figure 4. Gas-phase NH3 collected by denuders at the closed aeration basin (CAB) site and open aeration basin (OAB) site.
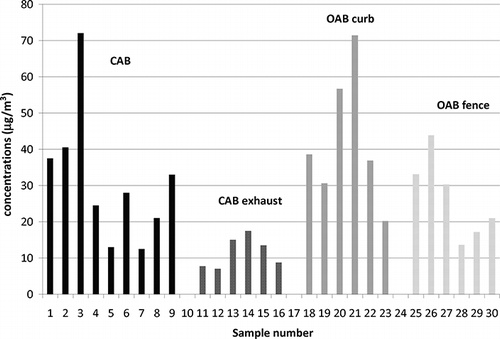
NH3 concentrations were highest at the CAB facility above the aeration basin, where they ranged from 13 to 72 μg m−3. Lower concentrations were observed downstream of the odor control unit at the CAB (7 to 18 μg m−3). The data show a large variability of a factor of seven between different days. This could be linked to differences in the nitrogen content of the wastewater received by the treatment plant. Although no simultaneous measurements were made upstream and downstream of the odor control system, comparison of the data sets from inside the CAB and downstream of the odor control treatment suggests that the ammonia concentrations were significantly lower (two sided t-test, confidence level 95%) by approximately 62%.
NH3 concentrations at the OAB at the curb of the aeration basin (20 to 71 μg m−3) were in the same range as those inside the CAB. Concentrations at the fenceline were, on average, 32% lower (14 to 44 μg m−3) than at the edge of the basin; however, this difference is not significant at the 95% confidence level, likely because of the large scatter in the data.
Ammonia concentrations at the WWTP facilities were within the range observed for urban sources. The observations are an order of magnitude higher (10–70 μg m−3) than those of an urban background such as Los Angeles (1.8 μg m−3; CitationFraser and Cass, 1998). Ammonia concentrations in specific environments, such as tunnels, were slightly higher (91–135 μg m−3) than our observations (CitationAllen et al., 2001).
Daily NH3 emission rates were calculated for the CAB facility using the daily air flow and the average observed concentrations before and after odor control treatment. The results are substantially lower than literature reports for manure or slurries (e.g., CitationBalsari et al., 2007) (see ).
Figure 5. Daily ammonia emissions. Cattle and pig slurry data come from CitationBalsari et al. (2007) and are scaled to the same-size slurry as the covered aeration basin (CAB).
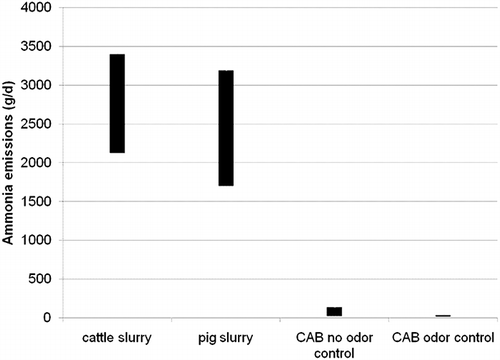
The highest daily NH3 emission rate by the CAB is 136 g NH3 day−1, which is dwarfed by overall emissions of ∼41,500 g NH3 day−1 in Maricopa County (2008 Maricopa County PM10 Emission Inventory) or even the estimate for WWTPs within this inventory of 3700 g day−1.
Summary
Wastewater aeration basin emissions of PM2.5, PM10, and NH3 have been characterized and evaluated. PM mass and OC aerosol concentrations at the WWTPs are similar to the concentrations in the general urban areas, and concentrations in treated off-gases are lower.
Daily PM2.5 emissions of the aeration basins at a medium-sized plant (18 MGD) are low (∼43 g day−1) compared with mobile or stationary sources. Surface emission flux was ∼0.5 μg m−2 s−1, which is more than two orders of magnitude lower than in laboratory experiments on sea salt (CitationTyree and Allen, 2008). The odor control system (GAC bed) investigated in the present study was able to reduce PM emissions by ∼80%. The ionic composition of the PM is similar to that of urban aerosols. However, substantial sterol concentrations were observed. Cholesterol and coprostanol were detected in both coarse and fine PM. Most coprostanol was in the coarse fraction, but a small amount was detected in the fine PM fraction. The presence of coprostanol, a nonvolatile fecal steroid, is clear evidence of the aerosolization of wastewater material. Coprostanol in fine PM at the fence site, distant from the aeration basin, is a clear indication of export of aerosolized wastewater beyond WWTPs.
NH3 concentrations at the WWTPs are within the range seen in urban environments, higher than the urban background but similar to that for specific environments such as tunnels. The daily emission rates in terms of emissions per surface area are more than two orders of magnitude lower than for manure storage and similar activities. Even considering the large surface area of aeration basins, they are not significant point sources of NH3.
This study shows that aerosolization of wastewater occurs. On one hand, aeration is not an important source of PM mass, organic carbon or NH3 emissions in an urban environment. On the other hand, a concern is that aerosolization of wastewater material occurs in the fine PM fraction (PM2.5), as evidenced by coprostanol measurements, which allows for longer range transport and residence time that result in exposure beyond the facilities themselves.
Acknowledgments
This project was funded by Science Foundation Arizona grant CAA 0284-08. The authors thank the staff of the two anonymous utilities in the Phoenix area for their cooperation. They are grateful to Prof. Matt Fraser and Andrea Clements for assistance with sample analysis and access to their facilities.
References
- Ahn , J.H. , Kim , S. , Park , H. , Rahm , B. , Pagilla , K. and Chandran , K. 2010 . N2O emissions from activated sludge processes, 2008–2009: Results of a national monitoring survey in the United States . Environmental Science and Technology , 44 : 4505 – 4011 . doi: 10.1021/es903845y
- Allen , J.O. , Mayo , P.R. , Hughes , L.S. , Salmon , L.G. and Cass , G.R. 2001 . Emissions of size-segregated aerosols from on-road vehicles in the Caldecott Tunnel . Environmental Science and Technology , 35 : 4189 – 4197 . doi: 10.1021/es0015545
- Balsari , P. , Airoldi , G. , Dinuccio , E. and Gioelli , F. 2007 . Ammonia emissions from farmyard manure heaps and slurry stores—Effect of environmental conditions and measuring methods . Biosystems Engineering , 97 : 456 – 463 . doi: 10.1016/j.biosystemseng.2007.03.033
- Bauer , H. , Fuerhacker , M. , Zibuschka , F. , Schmid , H. and Puxbaum , H. 2002 . Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants . Water Research , 36 : 3965 – 3970 . doi: 10.1016/S0043-1354(02)00121-5
- Beck , M. and Radke , M. 2006 . Determination of sterols, estrogens and inorganic ions in wastewater and size-segregated aerosol particles emitted from wastewater treatment . Chemosphere , 64 : 1134 – 1140 . doi: 10.1016/j.chemosphere. 2005.11.063
- Birch , M.E. and Cary , R.A. 1996 . Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust . Aerosol Science and Technology , 25 : 221 – 241 . doi: 10.1080/02786829608965393
- Carducci , A. , Morici , P. , Pizzi , R. , Battistini , R. , Rovini , E. and Verani , M. 2008 . Study of the viral removal efficiency in an urban wastewater treatment plant . Water Scence and Technology , 58 : 893 – 897 . doi: 10.2166/wst.2008.437
- Clark , C.S. 1987 . Potential and actual biological related health risks of wastewater industry employment . Journal of the Water Pollution Control Federation , 59 : 999 – 1008 .
- Demokritou , P. , Kavouras , I.G. , Ferguson , S.T. and Koutrakis , P. 2002 . Development of a high volume cascade impactor for toxicological and chemical characterization studies . Aerosol Science and Technology , 36 : 925 – 933 . doi: 10.1080/02786820290092113
- Fannin , K.F. , Vana , S.C. and Jakubowski , W. 1985 . Effect of an activated sludge wastewater treatment plant on ambient air densities of aerosols containing bacteria and viruses . Applied Environmental Microbiology , 49 : 1191 – 1196 .
- Fraser , M.P. and Cass , G.R. 1998 . Detection of excess ammonia emissions from in-use vehicles and the implications for fine particle control . Environmental Science and Technology , 32 : 1053 – 1057 . doi: 10.1021/es970382h
- Glassmeyer , S.T. , Furlong , E.T. , Kolpin , D.W. , Cahill , J.D. , Zaugg , S.D. , Werner , S.L. , Meyer , M.T. and Kryak , D.D. 2005 . Transport of chemical and microbial compounds from known wastewater discharges: Potential for use as indicators of human fecal contamination . Environmental Science and Technology , 39 : 5157 – 5169 . doi: 10.1021/es048120k
- Gray , N.F. 1989 . Biology of Wastewater Treatment , New York , NY : Oxford University Press .
- Grisoli , P. , Rodolfi , M. , Villani , S. , Grignani , E. , Cottica , D. , Berri , A. , Picco , A.M. and Dacarro , C. 2009 . Assessment of airborne microorganism contamination in an industrial area characterized by an open composting facility and a wastewater treatment plant . Environmental Research , 109 : 135 – 142 . doi: 10.1016/j.envres.2008.11.001
- Heng , B.H. 1994 . Prevalence of hepatitis A virus among sewage workers in Singapore . Epidemiology and Infection , 113 : 121 – 128 . doi: 10.1017/S0950268800051530
- Leclerc , H. , Mossel , D.A. , Edberg , S.C. and Struijk , C.B. 2001 . Advances in the bacteriology of the coliform group: Their suitability as markers of microbial water safety . Annual Reviews in Microbiology , 55 : 201 – 234 . doi: 10.1146/annurev.micro.55.1.201
- Präzmo , Z. , Krysiñska-Traczyk , E. , Skórska , C. , Sitkowska , J. , Cholewa , G. and Dutkiewicz , J. 2003 . Exposure to bioaerosol in a municipal sewage treatment plant . Annals of Agricultural and Environmental Medicine , 10 : 241 – 248 .
- Radke , M. 2005 . Sterols and anionic surfactants in urban aerosol: Emissions from wastewater treatment plants in relation to background concentrations . Environmental Science and Technology , 39 : 4391 – 4397 . doi: 10.1021/es048084p
- Radke , M. and Herrmann , R. 2003 . Aerosol-bound emissions of polycyclic aromatic hydrocarbons and sterols from aeration tanks of a municipal wastewater treatment plant . Environmental Science and Technology , 37 : 2109 – 2113 . doi: 10.1021/es025926g
- Schauer , J.J. , Rogge , W.F. , Hildemann , L.M. , Mazurek , M.A. , Cass , G.R. and Simoneit , B.R.T. 1996 . Source apportionment of airborne particulate matter using organic compounds as tracers . Atmospheric Environment , 30 : 3837 – 3855 . doi: 10.1016/1352-2310(96)00085-4
- Sorooshian , A. , Wonaschütz , A. , Jarjour , E.G. , Hashimoto , B.I. , Schichtel , B.A. and Betterton , E.A. 2011 . An aerosol climatology for a rapidly growing arid region (southern Arizona): Major aerosol species and remotely sensed aerosol properties . Journal of Geophysical Research , 116 : D19205 doi: 10.1029/2011JD016197
- Sun , Q. , Alexandrova , O.A. , Herckes , P. and Allen , J.O. 2009 . Quantitative extraction of organic tracer compounds from ambient particulate matter collected on polymer substrates . Talanta , 78 : 1115 – 1121 . doi: 10.1016/j.talanta.2009.01.039
- Thorn , J. , Beijer , L. , Jonsson , T. and Rylander , R. 2002 . Measurement strategies for the determination of airborne bacterial endotoxin in sewage treatment plants . Annales of Occupational Hygiene , 46 : 49 – 554 . doi: 10.1093/annhyg/mef068
- Tyree , C.A. and Allen , J.O. 2008 . Foam droplet separation for nanoparticle synthesis . Journal of Nanoparticle Research , 10 : 465 – 473 . doi: 10.1007/s11051-007-9280-0
- Tyree , C.A. , Hellion , V.M. , Alexandrova , O.A. and Allen , J.O. 2007 . Foam droplets generated from natural and artificial seawaters . Journal of Geophysical Research , 112 : D12204 doi: 10.1029/2006JD007729
- Upadhyay , N. , Sun , Q. , Allen , J.O. , Westerhoff , P. and Herckes , P. 2011a . Synthetic musk emissions from wastewater aeration basins . Water Research , 45 : 1071 – 1078 . doi: 10.1016/j.watres.2010.10.024
- Upadhyay , N. , Clements , A. , Fraser , M. and Herckes , P. 2011b . Chemical speciation of PM2.5 and PM10 in South Phoenix, Arizona . J. the Air Waste Manage. Assoc. , 61 : 302 – 310 . doi: 10.3155/1047-3289.61.3.302
- U.S. Environmental Protection Agency . 1989 . EPA Design Manual: Fine Pore Aeration Systems , EPA Center for Environmental Research Information, EPA/625/8-85/10 .
- U.S. Environmental Protection Agency . 1996 . Clean Water Needs Survey , Washington , DC : U.S. EPA .
- Wells , W.W. 1957 . Coprostanol formation. 1. The effect of sodium taurocholate and tween 80 on the sterol composition of fat feces . Archives of Biochemistry and Biophysics , 66 : 217 – 225 . doi: 10.1016/0003-9861(57)90552-0
- Watson , J.G. , Chow , J.C. , Chen , L.-W.A. , Lowenthal , D.H. , Fujita , E.M. , Kuhns , H.D. , Sodeman , D.A. , Campbell , D.E. , Moosmüller , H. , Zhu , D. and Motallebi , N. 2011 . Particulate emission factors for mobile fossil fuel and biomass combustion sources . Science of the Total Environment , 409 : 2384 – 2396 . doi: 10.1016/j.scitotenv.2011.02.041