Abstract
The performance of two direct-reading organic vapor monitors (monitors) when calibrated at different environmental conditions was compared with charcoal tube results. Three MIRAN SapphIRe portable ambient air analyzers (SAP) and three Century portable toxic vapor analyzers (TVAs) were evaluated. Prior to sampling, the monitors were calibrated per the manufacturer's instructions using methane for the TVA flame ionization detector (FID) and isobutylene for the photoionization detector (PID), whereas the SapphIRe instruments were zeroed and the instrument's manufacturer-supplied library was used. For the first series of tests (“Part 1—Same condition”), the monitors were calibrated under the same environmental conditions as those present during sampling. They were then challenged with four cyclohexane concentrations (30, 150, 300, and 475 ppm) under two extreme environmental conditions: 5 °C and 30% relative humidity (RH) (same/cold) and 38 °C and 90% RH (same/hot). For the second series of tests (“Part 2—Different condition”), the monitors were calibrated at approximately normal indoor environmental conditions (21 °C and 50% RH) and sampled at extreme environmental conditions (different/cold and different/hot). The monitor readings from the two methods were compared with the actual cyclohexane concentration determined from charcoal tubes using ratios and root mean square errors. A number of monitor failures, both below detection limit values in the presence of a known challenge concentration and erroneously high measurements, occurred in each part: same condition 20.7% (149/720) and different condition 42.4% (305/720), with a majority of the failures (>78%) during the hot and humid conditions. All monitors performed best at the same/cold, followed by the same/hot, in terms of closeness to the reference standard method and low within-monitor variability. The ranked choice of monitors for same/cold is PID > SAP > FID; for different/cold FID > PID > SAP; for same/hot SAP > PID > FID; and for different/hot PID > SAP (FID not included due to 100% failure rate).
Direct-reading organic vapor monitors are used for assessing the concentrations of volatile organic compounds in the air at varying environmental conditions. Typical calibration is performed at laboratory temperature and pressure. The monitors may be used in atmospheres that differ from that during calibration. An understanding of the effect of calibration environment on monitor performance may provide valuable information on the reliability and appropriateness of certain monitor types for industrial hygienists, emergency responders, and exposure assessment practitioners. Results of the study indicate monitor calibration should be performed at the same environmental conditions as sampling.
Introduction
Direct-reading organic vapor monitors (monitors) are used by industrial hygienists and emergency responders for surveying volatile organic compound (VOC) concentrations. They have utility in identifying source locations, planning compliance sampling locations, and identifying safe zones in emergency response scenarios. For the most part, these monitors are calibrated in a temperature- and relative-humidity-controlled environment such as a laboratory or office setting where environmental conditions may be different from the actual sampling location. The monitors are assumed to have the same response to chemicals regardless of calibration environment. The goal of the current research was to test the validity of this assumption.
Real-time detection of VOCs can be performed by a variety of techniques such as photoionization, flame ionization, infrared absorption, photoacoustic, electrochemical, and colorimetric (CitationTodd, 2003). Each of these has advantages and disadvantages such as cost, dynamic range, portability, and detection limits. Photoionization has documented difficulties with high humidity conditions and can only reliably measure VOCs with an ionization potential less than the lamp energy, which is typically 10.6 eV. Flame ionization requires hydrogen for flame operation and measures a wider range of compounds than photoionization, and when coupled to a gas chromatograph the flame ionization detector (FID) can provide quantification of VOC species. However, the FID in this study is only capable of total VOC measurement. Infrared may be used for selective detection of a specific volatile organic compound based on the frequency of absorption, but will not measure total VOCs. Infrared is the only detection technique studied that will provide both quantitative and qualitative information in near-real time.
Although photoionization, flame ionization, and infrared absorption techniques have been characterized in the literature for specific applications under a limited range of environmental conditions, robust assessments of the effect of calibration methods and environmental conditions have only recently been conducted (CitationBarsky et al., 1985; CitationCoffey et al., 2009; CitationCoy et al., 2000; CitationDrummond, 1997; CitationLongworth et al., 1999, Citation2000; CitationPoirot et al., 2004). The performance of these three monitor groups under varying environmental conditions and calibration methods was recently investigated in a study conducted at the National Institute for Occupational Safety and Health (NIOSH) (CitationCoffey et al., 2012). The researchers found that the manufacturer's recommended span calibration was just as effective as a five-point chemical-of-interest (cyclohexane) calibration. They also observed that inter- and intramonitor variabilities were significant, but did not investigate the effect of calibrating a monitor at a different temperature and relative humidity than the planned sampling conditions.
The purpose of the current study was to assess the impact of sampling at different temperature and relative humidity than the calibration environment on the performance of direct-reading organic vapor monitors. Recommendations for the best-performing monitors are presented based on their ability to reproduce the same concentration as the standard reference method (charcoal tubes).
Experimental Methods
Because the methods have been fully described elsewhere, only a brief description is given here (Coffey et al., 2012).
Monitors
This study evaluated three MIRAN SapphIRe (SAP) portable ambient air analyzers (series 205B, model XL; Thermo Fisher Scientific, Inc., Franklin, MA) and three Century portable toxic vapor analyzers (TVAs; model TVA-1000B; Thermo Fisher Scientific) equipped with both a flame ionization detector (FID) and a photoionization detector (PID). The TVA monitors were equipped with a water trap probe filter in the sampling probe (CitationThermo Electron Corporation, 2003).
Test setup
All testing was conducted in a 22-m3 walk-in environmental chamber (Nor-Lake Enviroline; Nor-Lake Scientific, Hudson, WI). Temperature and humidity conditions were monitored in the environmental chamber by a model HX94V relative humidity/temperature transmitter, duct style (Omega Engineering, Inc., Stanford, CT), which was calibrated prior to the start of testing with a HX90-CAL (Omega Engineering) humidity calibration kit. A 0.4-m3 Rochester-style (exposure) chamber was placed inside the environmental chamber.
Challenge chemicals
Four concentrations (30, 150, 300, and 475 ppm) of cyclohexane (certified ACS grade; catalog number C556-1; Fisher Scientific, Pittsburgh, PA) were used to challenge the monitors. Tests at a 0 ppm concentration (i.e., clean air) were conducted as a control and the data were processed separately to investigate false-positive prevalence rates. The challenge vapor inside the exposure chamber was generated by pumping the liquid cyclohexane from an enclosed reservoir to a three-necked flask on the inlet of the exposure chamber using a Cheminert M50 liquid handling pump (Valco Instruments Company Inc., Houston, TX) and an insulated liquid transfer line heated to approximately 32 °C where it was evaporated. To introduce the cyclohexane into the exposure chamber, air was removed from the bottom of the exposure chamber using a standard overhung multistage centrifugal blower (model 075-1/3; The Spencer Turbine Company, Windsor, CT). Prior to the blower, the air passed through a single fan filter housing (model FS4000; Flow Sciences, Wilmington, NC) equipped with an organic solvent bed filter (model FS4251; Flow Sciences) to remove the cyclohexane before entering the blower and returning to the environmental chamber. The 0 ppm condition was produced by placing an activated carbon bed on the exposure chamber inlet to remove any organic vapors present in the environmental chamber. No cyclohexane was added to the intake air.
A data automation system, consisting of LabVIEW 2009 (National Instruments Corporation, Austin, TX) and a National Instruments NI cDAQ-9174 CompactDAQ chassis, controlled the exposure chamber cyclohexane concentration via continuous feedback using a Miran 1A general-purpose gas analyzer (model 1A-AA1; The Foxboro Company, Bridgewater, MA). The MIRAN 1A was calibrated using a closed loop system (model 071-5707; Foxboro) before each series of replicates. The data automation system also recorded the outputs from the test monitors and the environmental conditions.
For each replicate, cyclohexane vapor was sampled using two Anasorb coconut shell charcoal sorbent tubes (catalog number 226-01; 6 × 70 mm size, two-section, 50/100 mg sorbent; SKC, Eighty Four, PA) connected to personal sampling pumps (model GilAir-5 with 800518 Low Flow module; Sensidyne LP, Clearwater, FL) set to a flow rate of 0.1 L min−1. The pumps were calibrated at the start and end of each day using a National Institute of Standards and Technology (NIST)-traceable primary calibrator (model 4146 primary calibrator; TSI, Incorporated, Shoreview, MN). The pumps were operated on a charger controlled by the test automation system.
Charcoal tube analysis
The charcoal tubes were the standard reference method for comparing with the monitor measurements. Tubes were analyzed in-house using NIOSH Method 1500 Hydrocarbons, BP 36°-216°C, modified as described previously (CitationNIOSH, 2003; Coffey et al., 2012). Two five-point response factor calibration curves (both r 2 = 1) for cyclohexane were developed (3.05–61.1 and 61.1–763.7 ng/sample) to encompass the range of concentrations studied. The limit of detection for the modified method was considered to be 3.05 ng.
Calibration
Monitors were calibrated at the same environmental conditions (Part 1—Same) at which sampling occurred or at different environmental conditions (Part 2—Different). For the Same condition, temperature and relative humidity (RH) were cold and dry at 5 °C and 30% RH (same/cold) or hot and humid at 38° C and 90% RH (same/hot). For the Different condition, the monitors were calibrated at normal laboratory temperature and RH (21 °C and 40% RH); they were subsequently challenged at cold and dry (different/cold) or hot and humid (different/hot) conditions identical to the first series of experiments. For each combination of test conditions, there were 10 replicates conducted.
Regardless of the calibration condition, all six monitors were calibrated by the manufacturer prior to the study. In addition, they were calibrated daily at either the same or different environmental conditions as sampling occurred. The built-in library supplied with the SapphIRe monitors was used and has the following parameters: gas high-range limit (HRL) of 500 ppm, a detection limit of 6 ppm, wavelength of 11.156 μm, and a path length of 12.5 m (CitationThermo Electron Corporation, 2004). They were zeroed everyday using a zero particulate filter (part number TR101ZU; Thermo Fisher Scientific) and a zero gas chemical filter (TR101PU; Thermo Fisher Scientific).
The TVA monitors were calibrated by filling separate 100-L Tedlar polyvinyl fluoride bags (CEL Scientific Corporation, Santa Fe Springs, CA) with a zero gas (zero grade air; Butler Gas Products, McKees Rocks, PA) and span gases (500 [±2%] ppm methane in air [Scotty Specialty Gases, Plumsteadville, PA] for the FID and 1000 [±2%] ppm isobutylene in air [Scotty Specialty Gases] for the PID). The TVA monitors were challenged with the zero gas. After zeroing, they were challenged with the span gases to obtain a second calibration point. As an indication of the success of the daily field calibration of the TVA instruments, the “zero” (counts expected when a zero gas is applied to the detector) and span (counts expected when a span gas of known concentration is applied to the detector) detector counts were recorded.
Data analysis
Summary statistics and distribution testing were performed in JMP (SAS Institute, Cary, NC). An average of the 3600 data points from each monitor was calculated for a 30-min time-weighted average. The TVA monitor results were multiplied by the appropriate response factor for cyclohexane provided by the instrument manufacturer. The standard approach for sampling cyclohexane concentrations is an active sampling method using coconut shell charcoal sorbent tubes by gas chromatography with a FID (NIOSH, 2003). Therefore, the results of each of the nine monitors were ratioed with the average of two charcoal tubes for each of the tests and multiplied by 100 to produce percentage charcoal tube values (%CT). This enabled comparisons across concentrations in terms of monitor bias.
False positives and false negatives were investigated. False positives are defined as occurrences when the monitors measures a concentration greater than the detection limit during the 0 ppm control condition (n = 30 per condition). False negatives, or below detection limit (BDL) values, are defined as occurrences when the monitors measure a concentration less than the detection limit in the presence of a challenge concentration (i.e., 30, 150, 300, and 475 ppm) (n = 120 per condition). A number of monitors also displayed failures in terms of erroneously high measurements (e.g., reporting 100,000 ppm). Outlier analyses were performed to identify rogue data points. A subjective cutoff criterion was applied to remove data points that would skew the modeling efforts: when the monitor values were more than 2 times the challenge concentration or a BDL value, the data point was removed. These failure rates were tracked and reported; all subsequent analysis used the data set with outliers removed. It should be noted that the entire FID group at different/hot and all concentrations is absent from all analyses due to unidentifiable instrument failures at this condition. Also, the SAP group at different/hot and 30 ppm concentration is absent due to failures.
Root mean square error (RMSE) was used as a measure of accuracy in order to have a single metric assessing bias and precision. RMSE was calculated according to Equationeq 1:
where bias = (%CT − 100). RMSE and %CT values were used to compare instrument performance across concentrations and environmental conditions.
Results and Discussion
Overall, total failures occurred in each part: same condition 20.7% (149/720) and different condition 42.4% (305/720), with a majority of the failures (>78%) during the hot and humid environmental conditions; therefore, monitor measurements were more reliable when calibrated and sampled at the same environmental condition and when it is cold/dry.
False-positive, false-negative, and erroneously high measurements and total failures as percentage of trials are displayed in . False positives were most prevalent during different/hot condition. False negatives were most prevalent at same/cold and same/hot conditions for the FID group and different/cold and different/hot for the SAP group.
Table 1. False-positive, false-negative, and erroneously high measurements as percentage of trials for monitor groups
Summary statistics by part (Part 1—Same and Part 2—Different) and nominal cyclohexane concentration are displayed in . Briefly, the FID group had the highest failure rates (%F = 50–70%) for “Part 2—Different” condition, supporting the recommendation for calibrating monitors at the same environmental condition as sampling occurs. These failure rates may not be compared with those given in because they were calculated with pooled data from cold/hot conditions at a given cyclohexane concentration. As noted in Coffey et al. (2012), variability at the nominal 30 ppm concentration condition may have been influenced by the low volumetric flow rate of the M50 liquid handling pump, which in turn had the potential to affect the failure and false-positive rates observed in this study. The PID group had lower failure rates (22% and 10%) than the SAP group (35% and 33%) at 150 and 300 ppm, respectively. The relationship reversed at 30 and 475 ppm, where the SAP group had slightly lower failure rates than the PID.
Table 2. Summary statistics for monitor measurements by part (Part 1—Same and Part 2—Different) and nominal cyclohexane concentration
Means and 95% confidence interval error bars of percent charcoal tube values (%CT) for individual monitors by part and environmental condition are displayed in Bias is visually observed as the closeness of the mean to 100%. Relatively low within-instrument variability is evidenced by the 95% confidence interval error bar approaching zero. Underprediction and overprediction of the monitor measurements are evidenced by 95% confidence intervals completely below or above 100%, respectively. When the 95% confidence interval includes 100%, no determination may be made in regards to underprediction or overprediction (i.e., the monitor is assumed to agree with the standard reference method).
Figure 1. Means and 95% confidence intervals of percent charcoal tube values (%CT) for individual monitors when calibration and sampling occurs at (A) cold or (B) hot conditions at either the same or different environmental conditions.
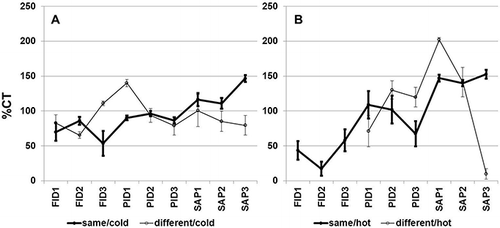
Clearly, calibrating and sampling at cold and dry conditions (i.e., same/cold) produced relatively low bias and low within-instrument variability for the PID and SAP monitors compared with sampling at different environmental conditions. Whereas calibrating and sampling at hot and humid conditions (same/hot) produced a more stable response within the SAP monitors compared with sampling at different environmental conditions (different/hot), the PID was the least affected by calibrating and sampling at cold and dry conditions (same/cold) in terms of bias and variability. PID1 showed a switch from overprediction to underprediction when comparing different/cold with different/hot, respectively. SAP was the most consistent (low variability and roughly equivalent response between monitors) at different/hot, indicating a bias correction may be applied to the SAP monitors under these conditions. The extremely low %CT of SAP3 at different/hot was presumably due to a high percentage (65%) of false negatives.
The PID is the best overall choice for sampling VOCs at varying environmental conditions based on the following: low false positives, except for different/hot where humidity appeared to negatively influence the measurement of a VOC-free atmosphere; low false negatives; and high within-monitor agreement at the same condition. The effect of hot and humid environments has previously been reported by the authors (Coffey et al., 2012) as well as others (Barsky et al., 1985). All monitors performed best at the same/cold, followed by the same/hot, in terms of closeness to the reference standard method and low within-monitor variability. This is surprising because the FID monitors should not be as affected by humidity as the PID monitors owing to the method of ionization. The electronics, however, may have been impaired by the extreme environmental conditions, as evidenced by erroneous readings in all FID measurements at the different/hot condition.
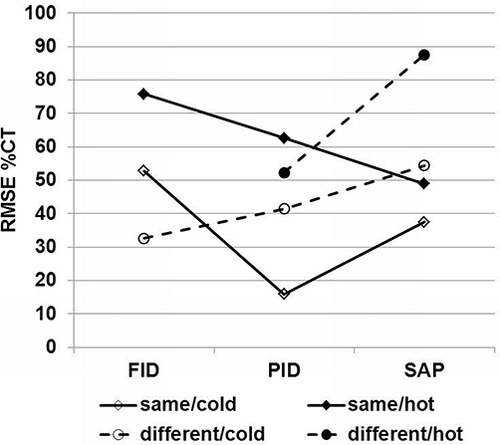
RMSEs of %CT for individual monitors by part and environmental condition indicate the PID and SAP groups performed the best at same/cold (). The SAP group at different/hot had the highest RMSE, indicating a very poor relationship with the tube values. This was presumably due to SAP3, which displayed high measurement variability (i.e., high %RSD) and high absolute bias (i.e., %CT significantly different from 100%), as seen in . The best choice of monitors for same/cold is the PID, followed by the SAP and FID. However, for different/cold, the best choice is the FID, followed by the PID and SAP. For same/hot, the best choice is the SAP, followed by the PID and FID. For different/hot, the best choice is the PID, followed by the SAP (FID not included due to 100% failure rate).
Conclusion
All monitors performed best at the same/cold, followed by the same/hot, in terms of closeness to the reference standard method (%CT values), low within-monitor variability (%RSD), and accuracy (RMSE). The ranked choice of monitors for same/cold is PID > SAP > FID; for different/cold FID > PID > SAP; for same/hot SAP > PID > FID; and for different/hot PID > SAP (FID not included due to 100% failure rate). A number of erroneously high measurements resulted from different/hot condition indicating monitors should not be calibrated at normal temperature and humidity (˜22 °C and 50% RH) when sampling is planned in a hot and humid (˜38 °C and 90% RH) environment. Monitors should be calibrated at the same environmental conditions intended for sampling.
Acknowledgment
This work was supported by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of commercial product or trade name does not constitute endorsement by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health.
The authors wish to acknowledge Michael Commodore and Judith Hudnall for conducting the environmental tests; Catherine Calvert for performing the charcoal tube analysis; and John Powers, Larry Lee, Bradley Newbraugh, and Steve Martin for their assistance in the development of the LabView program and associated hardware.
References
- Barsky , J.B. , Que Hee , S.S. and Clark , C.S. 1985 . An evaluation of the response of some portable, direct-reading 10.2 eV and 11.8 eV photoionization detectors, and a flame ionization gas chromatograph for organic vapors in high humidity atmospheres . AIHA J. , 46 : 9 – 14 . doi: 10.1080/15298668591394293
- Coffey , C. , LeBouf , R.F. , Lee , L. , Slaven , J. and Martin , S. 2012 . Effect of calibration and environmental condition on the performance of direct-reading organic vapor monitors . J. Occup. Environ. Hyg. , 9 : 670 – 680 . doi: 10.1080/15459624.2012.725015
- Coffey , C. , Pearce , T. , Lawrence , R. , Hudnall , J. , Slaven , J. and Martin , S. 2009 . Measurement capability of field portable organic vapor monitoring instruments under different experimental conditions . J. Occup. Environ. Hyg. , 6 : 1 – 8 . doi: 10.1080/15459620802514728
- Coy , J.D. , Bigelow , P.L. , Buchan , R.M. , Tessari , J.D. and Parnell , J.O. 2000 . Field evaluation of a portable photoionization detector for assessing exposure to solvent mixtures . AIHA J. , 61 : 268 – 274 . doi: 10.1080/15298660008984536
- Drummond , I. 1997 . On-the-fly calibration of direct reading photoionization detectors . AIHA J , 58 : 820 – 822 . doi: 10.1080/15428119791012333
- Longworth, T.L, J.I. Barnhouse, and K.Y. Ong. 2000. Domestic Preparedness Program—Testing of MIRAN SapphIRe Portable Ambient Air Analyzers against Chemical Warfare Agents Summary Report. Aberdeen Proving Ground, MD: Soldier and Biological Chemical Command http://www.ecbc.army.mil/downloads/reports/ECBC_miran_sapphire_analyzers.pdf (http://www.ecbc.army.mil/downloads/reports/ECBC_miran_sapphire_analyzers.pdf) (Accessed: 15 May 2012 ).
- Longworth, T.L., J.C. Cajigas, J.I. Barnhouse, K.Y. Ong, and S.A. Procell. 1999. Testing of Commercially Available Detectors Against Chemical Warfare Agents Summary Report. Aberdeen Proving Ground, MD: Soldier and Biological Chemical Command http://www.ecbc.army.mil/downloads/reports/ECBC_detectors_summary.pdf (http://www.ecbc.army.mil/downloads/reports/ECBC_detectors_summary.pdf) (Accessed: 15 May 2012 ).
- NIOSH. 2003. Manual of analytical methods: Method 1500 http://www.cdc.gov/niosh/nmam/pdfs/1500.pdf (http://www.cdc.gov/niosh/nmam/pdfs/1500.pdf) (Accessed: 15 May 2012 ).
- Poirot , P. , Subra , I. , Gerardin , F. , Baudin , V. , Grossmann , S. and Hery , M. 2004 . Determination of short-term exposure with a direct reading photoionization detector . Ann. Occup. Hyg. , 48 : 75 – 84 . doi: 10.1093/annhyg/meg079
- Thermo Electron Corporation . 2003 . TVA-1000B Toxic Vapor Analyzer Instruction Manual (P/N BK3500) , Franklin , MA : Thermo Electron Corporation .
- Thermo Electron Corporation . 2004 . Miran® 205B Series SapphIRe Portable Ambient Air Analyzers Instruction Manual (P/N BK3538) , Franklin , MA : Thermo Electron Corporation .
- Todd , L.A. 2003 . “ Direct-reading instrumental methods for determining concentrations of gases, vapors, and aerosols ” . In The Occupational Environment: Its Evaluation, Control, and Management , Edited by: DiNardi , S.R. 275 – 302 . Fairfax , VA : American Industrial Hygiene Association .