Abstract
In 1974, Mario Molina and F. Sherwood Rowland warned that chlorofluorocarbons (CFCs) could destroy the stratospheric ozone layer that protects Earth from harmful ultraviolet radiation. In the decade after, scientists documented the buildup and long lifetime of CFCs in the atmosphere; found the proof that CFCs chemically decomposed in the stratosphere and catalyzed the depletion of ozone; quantified the adverse effects; and motivated the public and policymakers to take action. In 1987, 24 nations plus the European Community signed the Montreal Protocol. Today, 25 years after the Montreal Protocol was agreed, every United Nations state is a party (universal ratification of 196 governments); all parties are in compliance with the stringent controls; 98% of almost 100 ozone-depleting chemicals have been phased out worldwide; and the stratospheric ozone layer is on its way to recovery by 2065. A growing coalition of nations supports using the Montreal Protocol to phase down hydrofluorocarbons, which are ozone safe but potent greenhouse gases. Without rigorous science and international consensus, emissions of CFCs and related ozone-depleting substances (ODSs) could have destroyed up to two-thirds of the ozone layer by 2065, increasing the risk of causing millions of cancer cases and the potential loss of half of global agricultural production. Furthermore, because most ODSs are also greenhouse gases, CFCs and related ODSs could have had the effect of the equivalent of 24–76 gigatons per year of carbon dioxide. This critical review describes the history of the science of stratospheric ozone depletion, summarizes the evolution of control measures and compliance under the Montreal Protocol and national legislation, presents a review of six separate transformations over the last 100 years in refrigeration and air conditioning (A/C) technology, and illustrates government–industry cooperation in continually improving the environmental performance of motor vehicle A/C.
The comforts and conveniences of modern life are largely taken for granted. When purchasing a product, consumers are usually not concerned with how or why it works, often assuming the product is safe to use and safe for the environment. This critical review addresses why such general public acceptance and complacency is not always the best policy. The paper explains how early warnings given by vigilant scientists highlighted the dangers of ODS and calls for action and boycotts by concerned citizens 35 years ago and regulatory actions taken by governments worldwide 25 years ago successfully phased out ODSs and avoided global catastrophe. It also highlights new opportunities for the Montreal Protocol to further protect against climate change. The implication is that scientific vigilance, public policy, and citizen action have protected and can protect Earth for future generations.
Supplemental Materials: Supplemental materials are available for this paper. Go to the publisher's online edition of the Journal of the Air & Waste Management Association.
Introduction
Without a protective ozone layer in the atmosphere, animals and plants could not exist, at least upon land. It is therefore of the greatest importance to understand the processes that regulate the atmosphere's ozone content. (Royal Academy of Sciences, announcing the 1995 Nobel Prize for Chemistry for Paul Crutzen, Mario Molina, and F. Sherwood Rowland)
Ozone is naturally present in the atmosphere and has the chemical formula O3. About 10% of ozone is in the troposphere and the remaining ozone (90%) resides in the stratosphere, primarily between the top of the troposphere and about 50 km (31 miles) altitude. The large amount of ozone in the stratosphere is often referred to as the “ozone layer.” In the stratosphere, approximately 16–50 km (10–31 miles) above Earth's surface, ozone forms a thin invisible shield protecting life below from the sun's ultraviolet (UV) radiation. In the troposphere, near Earth's surface, ozone is produced by chemical reactions of naturally occurring gases and gases from fossil fuel combustion and other pollution sources. Tropospheric ozone is a human health concern and also damages animals and plants. Stratospheric ozone absorbs the shorter wavelengths (UV-C: 100–280 nm) completely and transmits only a small fraction of the middle wavelengths (UV-B: 280–315 nm). Nearly all of the longer wavelengths (UV-A: 315–400 nm) are transmitted to Earth where they cause skin aging and degrading of outdoor plastics and paint. Of the two types of UV radiation reaching ground level, UV-B is the most harmful to humans and other life forms.
Manufactured ozone-depleting substances (ODSs) now controlled by the Montreal Protocol include approximately 100 chemicals containing chlorine and bromine once used in about 240 different applications, but are now phased out in 98% of uses, with the exception of feedstock and process agent use, which are exempt from controls as long as emissions are de minimis. lists a number of halogen source gases and some of their properties relevant to this discussion. Nitrous oxide (N2O) is the largest remaining anthropogenic threat to the stratospheric ozone layer not yet controlled by the Montreal Protocol, but it is controlled under the 1997 Kyoto Protocol as a potent greenhouse gas (GHG) (CitationKanter et al. 2013). ODSs controlled under the Montreal Protocol include chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) used widely as aerosol product propellants, refrigerants, foam blowing agents, and solvents; halons used for fire protection; carbon tetrachloride used as a solvent and fire extinguishing agent; methyl chloroform used as a solvent; and methyl bromide used as a pesticide and fire extinguishing agent. Once emitted, ODSs accumulate in the atmosphere and are transported by wind and convection to the stratosphere, where they are chemically decomposed by UV-B, releasing chlorine and bromine atoms that destroy ozone. As ozone is depleted, increased transmission of UV-B radiation endangers human health and the environment by increasing skin cancer and cataracts, weakening human immune systems, and damaging crops and natural ecosystems (CitationFahey and Hegglin, 2011; CitationSlaper et al., 1998; CitationUNEP, 2010a). The most conspicuous health effects are melanoma, basal-cell carcinoma and squamous-cell carcinoma (CitationChang et al., 2009; de Gruiji et al., 2003; CitationKripke, 1974; CitationNorval et al., 2007; CitationSwaminathan and Lucas, 2012; Citationvan Hattem et al., 2009; Citationvan der Leun and de Gruijl, 2002; Citationvan der Leun et al., 2008), and cataracts (CitationAyala et al., 2007; CitationMeyer et al. 2008; CitationOriowo et al., 2001; CitationNorval et al., 2007; CitationVojnikovic et al. 2007; CitationRivas et al., 2009). The most uncertain health effects are the suppression of the human immune system (CitationDamian et al., 1998; CitationNarbutt et al., 2005; CitationNorval et al., 2007; CitationWang et al., 2008). The most economically damaging impact might have been to agricultural and natural ecosystems (CitationCaldwell, 1971; CitationCaldwell et al., 1986; CitationCaldwell et al., 2007). Approximately two out of three commercial plant species appear sensitive to UV-B, and sensitivity also differs among cultivars of the same species. For example, the Essex cultivar of soybean exhibited 19 to 25% yield reduction while the Williams cultivar was unaffected by increased radiation (CitationTeramura and Sullivan, 1991; CitationTevini, 1998). Most ODSs are also “greenhouse gases” that contribute to global warming leading to effects of climate change, including sea-level rise, intensification of storms, and changes in precipitation and temperature distributions (CitationRamanathan, 1975; CitationRamanathan et al., 1985; CitationVelders et al., 2007; CitationSchneider et al., 2007; CitationIPCC 2012).
Table 1 . Atmospheric lifetimes, global emissions, ozone depletion potentials, and global warming potentials of some halogen source gases and HFC substitute gases
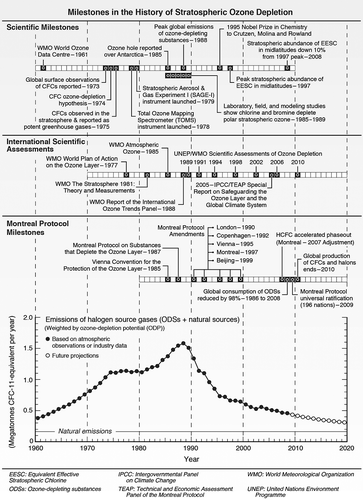
The narrative that follows describes the 225-year history of stratospheric ozone science and policy response, followed by a critical review of six separate transformations over the last 100 years in refrigeration and air conditioning (A/C) technology; it illustrates government–industry cooperation in continually improving the environmental performance of motor vehicle A/C. is a 1960 to 2010 timeline of milestones in ozone science, assessment, Montreal Protocol controls, and the dramatic reduction in integrated ODS emissions projected to 2020 (Fahey and Hegglin, 2011).
Figure 1. Major milestones in the history of stratospheric ozone depletion (Fahey and Hegglin, 2011).
ODSs are often powerful GHGs, but they are not within the group of six gases controlled by the Kyoto Protocol: carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6). ODSs were already scheduled for phase-out under the Montreal Protocol, and their inclusion in the Kyoto agreement would have provided an advantage to countries with large, easy-to-halt ODS emissions in uses such as CFC aerosol products that had already been banned in many other countries (CitationBenedick, 1998; CitationAndersen and Sarma, 2002). However, the Kyoto Protocol does include HFCs, which are ozone-safe substitutes for ODSs in refrigeration, air conditioning (A/C), and thermal insulating foam and for SF6 and PFCs, which are minor substitutes for ODSs in medical, fire protection, and some other applications. The Kyoto Protocol group also includes N2O, which is an ozone-depleting GHG not yet controlled by the Montreal Protocol (CitationRavishankara 2012 and CitationKanter et al., 2013).
Looking back, it is fortunate that (1) basic science was in place as the foundation of stratospheric ozone depletion theory, (2) enough ecological deterioration and disasters had occurred to make global environmental effects credible public concerns, (3) at least some scientists were confident and concerned enough to confront corporate stakeholders who denied the science and used sometimes ruthless tactics to discredit the scientists, (4) stratospheric ozone monitoring networks had been collecting data long enough to be credible, and (5) a sufficient number of countries were ready and willing to work with the United Nations Environment Program (UNEP) on a treaty based on the precautionary principle to avoid irreversible effects from ozone depletion predicted by a theory, but not yet proven to the satisfaction of the political and corporate interests who would accomplish an ODS phase out (CitationBenedick, 1998; CitationAndersen and Sarma, 2002).
It was also important that business and military organizations heeded an environmental warning based on complex atmospheric science and that the phase-out of chemicals suspected to deplete the stratospheric ozone layer began with public boycotts, corporate pledges, and action in a few countries long before the Montreal Protocol controls restricted ODS production and consumption (U.S. EPA and Department of Defense [DoD], 2008; CitationSingh et al., 2009).
Early Science Links Ozone to Ultraviolet Exposure
The smell of ozone was mentioned in the Iliad and the Odyssey in 850 BC (Homer, 850 BC), but the science of the ozone aloft began not much more than two centuries ago, atmospheric ozone monitoring began just 90 years ago, stratospheric ozone depletion theories were published just 40 years ago, the first national regulations of ODSs were instituted just 30 years ago, and the first international ozone treaty was 25 years ago (CitationUnited Nations Environment Programme [UNEP] 2012a; CitationAndersen and Sarma, 2002). CFCs were invented 85 years ago and were phased out globally in 2010, with most other ODSs already phased out or scheduled for phase-out in developed countries by 2030 and by 2040 in developing countries.
In 1785 when Martinus van Marum replicated the ozone smell by passing electric sparks through oxygen (O2), he termed it the “electrical odour” (CitationStolarski, 1999). In 1840, Swiss chemist Christian Schönbein associated this odor with a chemical component of the lower atmosphere, not electricity, and named it “ozone,” from the Greek word ozein, “to smell” (CitationLeeds, 1879). A few years later, J. L. Soret of Switzerland identified ozone as an unstable form of O2 composed of three atoms of oxygen.
In 1879, the Parisian Marie-Alfred Cornú measured the sun's spectrum with newly developed techniques for UV spectroscopy and found that the intensity of the sun's UV radiation decreased rapidly at wavelengths below about 300 nm (CitationCornú, 1879). He demonstrated that the wavelength of the “cutoff” increased as the sun set and the light passed through more atmosphere on its path to Earth and surmised that the cutoff was the result of an atmospheric substance absorbing light at UV wavelengths. W. N. CitationHartley (1880) concluded that this substance filtering UV radiation was ozone (CitationHartley, 1880). Hartley and Cornú soon attributed the absorption of solar radiation between wavelengths of 200 and 320 nm to ozone, and concluded that most of the ozone must be in the upper atmosphere. The first International Polar Years (IPYs), from 1881 to 1884, involved a dozen nations (CitationLuedecke, 2004).
Robert John Strutt (fourth Baron of Rayleigh) (1918) was unable to measure the absorption by ozone from a light source located ˜6.4 km across a valley and concluded that “there must be much more ozone in the upper air than in the lower.”
In 1924, CitationDobson and Harrison (1926) invented a spectrophotometer, the first instrument for routinely monitoring total ozone, called a Féry spectrometer, which made its measurements by examining the spectra of solar ultraviolet radiation using photographic plates. Dobson, Harrison, and colleagues discovered day-to-day and seasonal variations in the ozone amount over Oxford, England. Dobson hypothesized that these variations in ozone might be related to atmospheric pressure. To test this idea, he constructed more spectrophotometers and distributed them throughout Europe. These measurements demonstrated ozone variations with the passage of weather systems (CitationDobson, 1968).
F. W. P. CitationGötz (1931) worked with Dobson's Féry spectrometer at Arosa, Switzerland, measuring the intensity ratio of two wavelengths at the zenith sky throughout the day. He found that these ratios decreased as the sun set but increased just as the sun was near the horizon. He named this the Umkehr (turnaround) effect and devised the Umkehr method for measuring the vertical distribution of ozone, thereby showing that the ozone concentration reaches a maximum below an altitude of 25 km (CitationNASA, 2000).
Sydney CitationChapman (1931) was first to identify the (stratospheric) ozone “layer” and to develop a photochemical theory of stratospheric ozone formation and destruction, based on the chemistry of pure O2 with sunlight generating O3 when absorbed by molecular oxygen (O) in the atmosphere. In the 1930s, scientist Dorothy CitationFisk (1934) described the critical role the ozone layer plays as a global sunscreen that allows enough UV-B for beneficial exposure. The combined work of Chapman, Fisk, and Charles Abbott from the Smithsonian Institution inspired the scientific appreciation that the ozone layer protects living organisms from shortwave UV light (CitationCagin and Dray, 1993) The second IPY, 1932 and 1933, involved 44 nations.
The Chapman mechanism is shown as follows:
O3 is produced by the photodissociation of O2 by solar UV radiation:
The following reaction sequence then recycles O3 back into O2:
In preparation for the International Geophysical Year (July 1957 to December 1958), a worldwide network was developed to measure ozone profiles and the total column abundance of ozone using the equipment and standard quantitative procedure pioneered by Dobson. The World Meteorological Organization (WMO) established the framework for ozone-observing projects and related research and publications; this network eventually became the Global Ozone Observing System (GOOS), with 140 stations. In 1957 the British Antarctic Survey and Japanese Scientific Stations in Antarctica installed Dobson ozone monitors, which eventually recorded the depletion of the ozone that was later called the Antarctic Ozone Hole.
Scientists Identify Threats to the Ozone Layer
In 1970 Paul Crutzen called attention to the fact that nitric oxide (NO) and nitrogen dioxide (NO2) react in a catalytic cycle that destroys ozone, without being consumed themselves, thus lowering the steady-state amount of ozone. “Natural” nitrogen oxides are formed in the lower atmosphere through chemical reactions involving N2O that originates from microbiological transformations at the ground as a result of both natural and human activities (CitationKanter et al., 2013). Therefore, Crutzen warned, increasing atmospheric concentration of nitrous oxide that can occur through the use of agricultural fertilizers might lead to reduced ozone levels (CitationCrutzen, 1970). His hypothesis was that “NO and NO2 concentrations have a direct controlling effect on the ozone distributions in a large part of the stratosphere, and consequently on the atmospheric ozone production rates.”
N2O provides nitrogen oxides (NOx) for catalyzed O3 destruction in the stratosphere. A typical set of reactions is:
Johnson (1971) showed that NO and NO2 produced in the high-temperature SST exhaust could contribute to ozone loss by releasing NOx directly into the stratospheric ozone layer. CitationMcDonald (1971) theorized that even a small change in the abundance of stratospheric ozone could increase UV radiation at the surface of the Earth. Johnson estimated that “the operation of SSTs at the now-estimated fleet levels predicted for 1980–1985 could so increase transmission of solar UV radiation as to cause something on the order of 5–10,000 additional skin cancer cases per year in just the U.S. alone” (CitationMcDonald, 1971; CitationJohnston, 1971).
CitationCrutzen (1972) presented estimates of the ozone reduction that could result from the operation of SSTs. CitationWofsy and McElroy (1974) estimated that “nitric oxide emitted by SSTs would lead to a significant reduction in the concentration of atmospheric ozone.” Concern over stratospheric ozone was a contributing factor when the U.S. House of Representatives voted not to continue funding development of the Boeing SST, and Japan Air Lines, Pan Am, Qantas, and TWA canceled their orders for Concorde SSTs. The Union of Soviet Socialist Republics (USSR) abandoned commercial flights of the Tupolev Tu-144d SST after a crash inside Russia in 1978 (an earlier model Tu-144 had crashed at the 1973 Paris Air Show). Only British Airways and Air France operated the Concorde SST (CitationAndersen and Sarma, 2002). Abandonment of large fleets of SSTs perhaps avoided a major ozone depletion disaster as occurred from emissions of ODSs (CitationDubey, 1997).
Chlorine threats to the ozone layer and climate
CitationLovelock (1971) measured CFCs in air samples collected aboard a research vessel in the North and South Atlantic and warned that there may be consequences of these long-lived manufactured gases. CFCs were detected in every sample, “wherever and whenever they were sought” (CitationLovelock et al., 1973). He concluded that CFC gases had already spread globally.
Responding to Lovelock's findings, the DuPont Company formed a panel on the ecology of fluorocarbons for the world's CFC producers in 1972 (CitationGlas, 1989). The invitation letter stated:
Fluorocarbons are intentionally or accidentally vented to the atmosphere worldwide at a rate approaching one billion pounds per year. These compounds may be either accumulating in the atmosphere or returning to the surface, land or sea, in the pure form or as decomposition products. Under any of these alternatives, it is prudent that we investigate any effects which the compounds may produce on plants or animals now or in the future. (CitationMcCarthy, 1972)
Nineteen companies formed the Chemical Manufacturers Association Fluorocarbon Program Panel, a research group that eventually funded at least US$20 million in research at academic and government facilities worldwide.
In 1972, Stolarski and Cicerone concluded that hydrogen chloride spread as exhaust along the Space Shuttle's launch trajectory would deplete ozone, but that the global impact would be negligible given the low frequency of planned launches.
CitationMolina and Rowland (1974), in the first to study the atmospheric fate CFCs (then referred to as chlorofluoromethanes, CFMs), were first to warn that CFCs could deplete stratospheric ozone. Molina and Rowland hypothesized that CFCs that are highly unreactive would accumulate in the troposphere and would migrate to the stratosphere where UV radiation would cause them to decompose and release chlorine atoms, which in turn become part of a chain reaction where a single chlorine atom could destroy as many as 100,000 molecules of O3, thereby depleting stratospheric ozone. They warned that stratospheric ozone depletion would increase the intensity of UV radiation at the earth's surface, increasing skin cancer and other health and environmental effects (CitationMolina and Rowland, 1974).
A typical mechanism for ozone depletion by CFCs is:
and again potentially for thousands of times until air containing reactive halogen gases returns to the troposphere where they are removed by moisture in clouds and rain.
CitationMolina and Rowland (1974) concluded:
Chlorofluoromethanes are being added to the environment in steadily increasing amounts. These compounds are chemically inert and may remain in the atmosphere for 40–150 years, and concentrations can be expected to reach 10 to 30 times present levels. Photo-dissociation of the chlorofluoromethanes in the stratosphere produces significant amounts of chlorine atoms, and leads to the destruction of atmospheric ozone. … It seems quite clear that the atmosphere has only a finite capacity for absorbing Cl atoms produced in the stratosphere, and that important consequences may result. This capacity is probably not sufficient in steady state even for the present rate of introduction of chlorofluoromethanes. (pp. 810, 812)
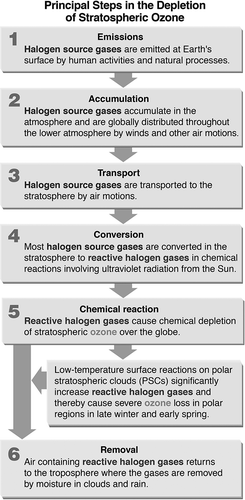
CitationMolina and Rowland (1974) estimated that “if industry continued to release a million tons of CFCs into the atmosphere each year, atmospheric ozone would eventually drop by 7 to 13 percent.” At that time, global production of CFCs was approximately 500,000 metric tonnes per year, with about 70% being used as aerosol propellants. Molina and Rowland did not anticipate that ozone depletion would occur first in the Antarctic due to annual polar stratospheric ice clouds and in the Arctic during unusual winters. A simple schematic representation of the principal steps in stratospheric ozone depletion by halogen source gases is depicted in
Later in 1974, Molina and Rowland presented their findings at a meeting of the American Chemical Society and held a press conference warning that:
If CFC production rose at the then-current rate of 10 percent a year until 1990, and then leveled off, up to 50 percent of the ozone layer would be destroyed by the year 2050. Even a 10 percent depletion, he said, could cause as many as 80,000 additional cases of skin cancer each year in the United States alone, along with genetic mutations, crop damage, and possibly even drastic changes in the world's climate.
If nothing was done in the next decade to prevent further release of chlorofluorocarbons, the vast reservoir of the gases that would have built up in the meantime would provide enough chlorine atoms to insure continuing destruction of the ozone layer for much of the twenty-first century. They urged that the use of the compounds as aerosol propellants be banned. (CitationBrodeur, 1986)
CitationRamanathan (1975) confirmed that CFCs are also powerful greenhouse gases, adding significantly to the scientific justification to control CFCs (CitationRamanathan, 1975; Ramanathan et al., 1985).
1975 to 1985: Validating the Molina–Rowland ozone depletion theory
Between 1975 and 1985, scientists debated but strongly supported the Molina–Rowland theory of stratospheric ozone depletion, although estimates of likely ozone depletion from CFCs and other ODSs were far from certain. Molina and Rowland persisted in both their advocacy of ozone layer protection and in bolstering the scientific foundation and validating the hypothesis. For example, in 10 years between their original paper in 1974 and the signing of the Vienna convention in 1985, Molina and Rowland were co-authors on a dozen ozone science journal articles and Mario Molina was co-author of 17 more. A small number of professional science skeptics challenged the Molina–Rowland theory with both plausible and fanciful explanations that were eventually disproven (CitationOreskes and Conway, 2010). Before 1987, the aerosol products, refrigeration, and A/C industry questioned the science of stratospheric ozone depletion as lacking evidence to support the theory, and argued that CFC alternatives would be flammable, toxic, and expensive. However, after the Montreal Protocol was signed in 1987, most industries accepted the theory and turned their attention to phase-out. One measure of the strength of the stratospheric ozone science is that after the Montreal Protocol was signed in 1987, there was virtually no complaints or challenges to the science by industry associations and business worldwide. The mid-1990s challenge by the U.S. pesticide industry questioning the ozone-depletion potential (ODP) of methyl bromide was unsuccessful, as the science was confirmed and the industry accepted the consensus (CitationParson, 2003).
In 1975, the National Academy of Sciences (NAS) and U.S. Department of Transportation concluded that nitrogen oxides from SSTs were a threat but that atmospheric levels of chlorine from CFCs would deplete the ozone layer six times more efficiently than oxides of nitrogen from SSTs, and that ozone depletion would consequently increase the intensity of UV light at ground level. The portion of the report covering environmental effects concluded that increases in ground-level UV light would adversely impact plant growth and animal health.
This report was the first integrated assessment of any environmental risk and included the mandate to consider both climate change and stratospheric ozone depletion. Six teams studied the causal relationships from jet engine emissions to atmospheric impacts, to environmental effects, and to social and economic consequences. Agricultural economists predicted that shortening of the frost-free growing season and increases in UV light would reduce agricultural yields (CitationNRC, 1975).
CitationWofsy et al. (1975) confirmed Rowland and Molina's scientific calculations:
“Freons® [CFCs] are a potential source of stratospheric chlorine and may indirectly cause serious reductions in the concentration of ozone. … Allowing for reasonable growth in the Freon industry, ˜10 percent per year, the reduction in ozone could be 2 percent by 1980 and, if left unchecked, could grow to the disastrous level of 20 percent by the year 2000.” Even if Freon® use were terminated as early as 1990, “it could leave a significant effect which might endure for several hundred years.” (CitationWofsy et al., 1975)
The newly created U.S. Federal Interagency Task Force on Inadvertent Modification of the Stratosphere heard testimony from McElroy, who said that bromine and bromine compounds—including halons used in fire protection and methyl bromide used in pest control and chlorobromomethane and n-propyl bromide uses as solvents and to manufacture plastics—appear “to be so effective at ozone depletion that [they] could be used as a weapon” (CitationSullivan, 1975).
The National Research Council of the National Academy of Sciences (CitationNRC, 1976) summarized results of an expert panel that examined existing atmospheric and laboratory measurements, as well as the mathematical models used to assess the impact of such pollutants on stratospheric ozone and to make recommendations on studies needed to improve understanding of the processes involved, concluding that “All the evidence that we examined indicates that the long-term release of CFC-11 and CFC-12 at present rates will cause an appreciable reduction in the amount of stratospheric ozone.” Noting that CFCs were produced and used around the world, the NRC advised:
Clearly, although any action taken by the USA to regulate the production and use of CFMs (CFCs) would have a proportionate effect on the reduction in stratospheric ozone, such action must become worldwide to be effective in the long run. (Andersen and Sarma, 2002, p. 10)
In 1977 the United Nations Environment Programme (UNEP) established a Coordinating Committee on the Ozone Layer (CCOL) that included 13 industrialized countries, 3 developing countries, 5 United Nations and international organizations, the European Economic Community, the Organization for Economic Cooperation and Development (OECD), the Chemical Manufacturers Association, and the International Council of Scientific Unions. Later, any interested country was allowed to participate (CitationAndersen and Sarma, 2002).
The CCOL met first in 1977 and yearly thereafter until 1986. The UNEP Ozone Layer Bulletins (1978 to 1985) recorded the conclusions of the world community on the science and environmental impacts of ozone depletion as they evolved, and provided the basic input to the diplomatic negotiations that were initiated in 1982. At each of its sessions, the committee examined research results on stratospheric ozone depletion, and the health and environmental impacts of depletion and the socioeconomic consequences, and presented its reports to the Governing Council meetings of UNEP and the negotiating groups.
The National Aeronautics and Space Administration (NASA) in October 1978 launched the Nimbus 7 satellite, and with it, two instruments that started to record ozone levels: the Total Ozone Mapping Spectrometer (TOMS) and the Solar Backscatter Ultraviolet (SBUV). The first TOMS provided data from 1978 to 1993; the second, launched on a USSR Meteor 3 spacecraft, provided data from 1991 to 1994; the third, launched on the Japanese Advanced Earth Observing Satellite (ADEOS), provided data from 1996 to 1997; and the fourth (Earth Probe TOMS) provided data from 1996 until 2006. The first SBUV operated until 1990; similar instruments have flown on several National Oceanic and Atmospheric Administration (NOAA) satellites since then (CitationHoff and Christopher, 2009; CitationHidy et al., 2009).
In 1979, the NRC followed up its earlier findings with a report by the Committee on Impacts of Stratospheric Change, and the Committee on Alternatives for the Reduction of Chlorofluorocarbon Emissions, which concluded that eventual ozone depletion would be significant despite a temporary leveling off of global CFC emissions due to the U.S. ban on nonessential aerosol propellant use (CitationNRC, 1979).
Discovering and Measuring the Antarctic Ozone “Hole”
In 1981, total ozone measurements from Japanese, British, and other Antarctic research stations using Dobson spectrophotometers recorded a 20% reduction in stratospheric ozone levels in October above Antarctica. None of the Antarctic scientists published their 1981 results or consulted other stations to confirm their observations. Joseph Farman, head of the Geophysical Unit of the British Antarctic Survey, “could only assume that something had gone wrong with his Halley Bay apparatus. He knew, of course, about the Molina–Rowland theory and the scientific debate over the relationship between man-made chemicals and ozone depletion, but the Dobson reading was simply too low to suggest anything but an instrument malfunction” (CitationCagin and Dray, 1993). Furthermore, none of the scientists concerned about stratospheric ozone depletion had suspected that it would be evident first in the Antarctic, so they were looking elsewhere for the first observational evidence.
In the Antarctic spring of 1982, low ozone levels were found at the British Antarctic Survey and other stations. At the same time, the ozone-measuring devices aboard the Nimbus 7 satellite had also registered low ozone levels. Again, none of the Antarctic ground stations or satellite scientists published the observations of ozone depletion and none raised alarm among colleagues, perhaps because the low values had not yet been linked to CFCs in the atmosphere.
In 1982, the CitationNRC (1983) concluded that squamous-cell skin cancer could be doubled if CFC production were increased at prevailing growth rates (CitationNRC, 1982) and in 1983 it concluded that “most plants, including crop plants, are adversely affected by UV-B radiation. Such irradiance stunts growth, cuts down total leaf area, reduces production of dry matter, and inhibits photosynthesis in several ways.”
CitationChubachi (1984) of the Japanese Meteorological Research Institute in Ibaraki was first to report seasonal ozone depletion over Antarctica, but Chubachi and his colleagues failed to appreciate the significance of their findings and took no actions to bring them to the attention of policymakers. Because activist scientists more directly involved in policy on stratospheric ozone had not anticipated the Antarctic ozone hole, they were not carefully monitoring the Antarctic reports.
Estimates of future worldwide ozone depletion continued to vary. CitationPrather et al. (1984) concluded that an increase in the concentration of inorganic chlorine in the stratosphere could “cause a significant change in the chemistry of the lower stratosphere leading to a reduction potentially larger than 15 percent in the column density of ozone. This could occur, for example, by the middle of the next century, if emissions of man-made chlorocarbons were to grow at a rate of 3 percent per year.”
In May 1985, scientists from the British Antarctic Survey (CitationFarman et al., 1985) sounded the alarm that ozone levels above Antarctica had been significantly depleted every Antarctic spring since at least 1981. Although their own research and the research of other scientists had no proof of causation, their warning went beyond the evidence and attributed the Antarctic ozone depletion to CFCs. Joseph C. Farman was quick to organize news conferences and interviews, explaining the significance of ozone depletion and confidently putting the blame on CFCs (CitationPearce, 2008; CitationBrysse, 2009).
The phenomenon of ozone depletion over Antarctica quickly became known as the “ozone hole,” a phrase first used in published media accounts by Rowland, and was frequently illustrated with images created by NASA that depicted levels of reduced column ozone amounts that appeared as circular regions centered near and around the South Pole.
Seven international agencies teamed up in 1985 to write an assessment of the “state of the ozone layer” (CitationNASA et al., 1985). The chemicals of interest to the agencies were NOx from subsonic and supersonic aircraft; nitrous oxide from agricultural practices and energy production; chlorofluorocarbons used as aerosol propellants, foam-blowing agents, solvents, and refrigerants; brominated compounds, including halons used to extinguish fires and suppress explosions; carbon monoxide and carbon dioxide from combustion processes; and CH4 from a variety of sources, including natural and agricultural wetlands, tundra, biomass burning, and enteric fermentation in ruminants. “It is now clear that these same gases are also important in the climate issue,” the report concluded.
The report warned that if there were a doubling of the 1985 CFC release rate, “the one-dimensional models predict that there will be 3 percent to 12 percent reduction of the ozone column, regardless of realistically expected increases in carbon dioxide, nitrous oxide, and methane.”
CitationSolomon et al. (1986) concluded that the “remarkable depletions in the total atmospheric ozone content in Antarctica … are largely confined to the region from about 10 to 20 km, during the period August to October.” They suggested that chlorine compounds might react on the surfaces of polar stratospheric clouds, providing a reaction site for heterogeneous reactions that could greatly accelerate ozone loss in the Antarctic lower stratosphere:
A unique feature of the Antarctic lower stratosphere is its high frequency of polar stratospheric clouds, providing a reaction site for heterogeneous reactions. A heterogeneous reaction between HCl and ClONO2 is explored as a possible mechanism to explain the ozone observations. This process produces changes in ozone that are consistent with the observations, and its implications for the behaviour of HNO3 and NO2 in the Antarctic stratosphere are consistent with observations of those species there, providing an important check on the proposed mechanism. (CitationSolomon et al., 1986, p. 755)
In August 1986, four teams of U.S. researchers arrived in Antarctica as part of the first National Ozone Expedition to study the ozone hole over Antarctica. The NOAA Aeronomy Laboratory team, led by Solomon, made ground-based visible absorption measurements; the University of Wyoming team, led by David Hofmann, carried out balloon-based ozone and aerosol particle measurements; the State University of New York at Stony Brook team, led by Robert de Zafra, made ground-based microwave emission measurements; and the Jet Propulsion Laboratory team, led by Crofton Farmer, made ground-based solar infrared absorption measurements. All four of the teams successfully measured the formation and strengthening of the ozone hole, confirming the phenomenon. Their measurements and findings, according to NASA, strongly suggested that “perturbed chlorine chemistry was involved.” But there was still no conclusive proof that chlorine was to blame for the ozone hole, whether the hole was a natural phenomenon having to do with changes in temperature and air circulation, or whether it was caused by chlorine compounds contributed by man-made chemicals. (CitationNASA, 1996).
Two months later, in October 1986, NASA formed an International Ozone Trends Panel in collaboration with UNEP, the U.S. Federal Aviation Administration (FAA), NOAA, and WMO. The panel was a response to “two important reports of changes in the atmospheric ozone” that occurred in 1985. “The first report was of a large, sudden, and unanticipated decrease in the abundance of springtime Antarctic ozone over the last decade. The second report, based on satellite data, was of large global-scale decreases since 1979 in both the total column content of ozone and in its concentration near 50 km altitude” (CitationNASA et al., 1988, p. 3).
The NASA (1996) Airborne Antarctic Ozone Experiment “determined that the cause of the Antarctic ozone hole was chlorine chemistry. Large quantities of chlorine monoxide were found which were co-located with areas of ozone depletion. The aerosol data gathered was consistent with processing on polar stratospheric clouds. But the theories which said that it was a natural phenomenon due to atmospheric dynamics were found to be inconsistent with the new data.” The experiment's data showed an inverse correlation between ozone and chlorine monoxide, according to NASA:
Because chlorine monoxide is produced by the process in which man-made chlorine destroys ozone, the large quantities observed provide strong evidence that man-made chemicals are involved in the Antarctic ozone loss process. … The data obtained during the Antarctic mission show[ed] the lowest ozone levels ever recorded and directly implicate[d] man-made chemical compounds, chlorofluorocarbons, in the enormous ozone loss over this remote region in the Southern Hemisphere. (NASA et al., 1996)
Health and agricultural scientists strengthen findings
In April 1987, Margaret Kripke, a skin cancer expert at the University of Texas, told the U.S. White House Domestic Policy Council that although ozone depletion was expected to increase the number of skin cancers, there were other impacts with far greater global consequences, particularly the potential impact on the global food supply and the human diseases due to the effects of UV radiation on the human immune systems (CitationCagin and Dray, 1993).
At a U.S. Senate hearing in May 1987, Alan Teramura of the University of Maryland testified that the potential of UV radiation to damage crops and plants was indisputable (CitationCagin and Dray, 1993).
Signing and strengthening the Montreal Protocol and its Vienna Convention
In March 1985, 34 countries agreed on the Vienna Convention for the Protection of the Ozone Layer, which established the framework for a protocol. The obligations of the parties to the convention were to cooperate in research, observations, and information exchange, and to adopt policies to control human activities that might modify the ozone layer. The only mention of CFCs came in Annex 1 as one of the many substances “thought to have the potential to modify the chemical and physical properties of the ozone layer” (CitationBenedick, 1998; CitationAndersen and Sarma, 2002; CitationParson and Greene, 1995; CitationYoung, 1999; CitationParson, 2003).
During 14–16 September 1987, a conference of the United Nations Environment Programme Plenipotentiaries created the Montreal Protocol on Substances that Deplete the Ozone Layer, which was signed at that meeting by 24 countries and the European Economic Community (CitationWeisskopf, 1987).
Subsequently, the Montreal Protocol's internal assessment panels provided the scientific basis for the necessity of strengthening of the Montreal Protocol and the technical and economic basis for scheduling the phase-out for each group of ODSs. It is significant that the Montreal Protocol assessments were first to bring to the attention of the parties many of the opportunities to strengthen ozone layer protection and to avoid regulatory train wrecks. For example, it was the Scientific Assessment Panel (SAP) that highlighted the importance of controlling methyl bromide, bromochloromethane, n-propyl bromide, and nitrous oxide (only n-propyl bromide and nitrous oxide are still uncontrolled), and it was the Montreal Protocal Technology and Economic Assessment Panel (TEAP) that recommended early halon phase-out to avoid overproduction, recommended collection and destruction of ODS contained in products, and crafted and advised on Essential Use Exemptions and Critical Use Exemptions that allow continued use after phase-out for applications considered important to parties (CitationAndersen and Sarma, 2002; CitationAndersen et al. 2007). Summaries of annual assessments and associated Amendments and Adjustments, showing steady progress in the evolution of the protocol, the science and understanding of ODSs, and the positive results of their control can be found in the supplementary materials online.
1998–1999: The Intergovernmental Panel on Climate Change (IPCC) and TEAP first cooperation.
“Because changes in ozone affect the Earth's climate and changes in temperature, greenhouse gases and climate affect the ozone layer” and “because HFCs included in the basket of gases of the Kyoto Protocol (CO2, CH4, N2O, PFCs, and SF6) are significant substitutes for some ODSs controlled under the Montreal Protocol” and because the choice of alternatives and substitutes for ODSs “may significantly influence energy usage” (CitationUNEP, 1999), IPCC/TEAP concluded that Life Cycle Climate Performance (LCCP) is the comprehensive metric for judging the impacts of ODS substitution, that the Kyoto Protocol need not interfere with the Montreal Protocol provided that HFC were available to replace ODSs where other options are not viable, and that the Montreal Protocol need not interfere with the Kyoto Protocol provided that implementation of the Montreal Protocol avoided HFCs where other viable alternatives to ODSs are available (UNEP, 1999).
2005: IPCC/TEAP Safeguarding the ozone layer and the global climate system.
“Safeguarding the Ozone Layer and the Global Climate System” (CitationIPCC, 2005) was far more comprehensive and detailed than the 1999 IPCC/TEAP and TEAP task force reports. It made significant progress in:
| 1. | Providing the atmospheric scientific framework for choosing alternatives and substitutes to ODSs, including the stratospheric chemistry and dynamics and their coupling to climate change, the radiative forcing of the relevant ODS, HFC, and other gases, and their roles in tropospheric chemistry and air quality. | ||||
| 2. | Collecting available data on historic and ongoing specific ODS and HFC production; estimating the annual use and emissions by sector and application, and the inventory of ODS and HFCs carried forward in chemical inventory and contained in refrigeration, air conditioning, and fire protection products; and reconciling top-down observations of ODS and HFC concentrations in the atmosphere and bottom-up estimates of annual ODS and HFC emissions. | ||||
| 3. | Summarizing available methodologies to characterize or compare the environmental performance of alternatives and substitutes for refrigeration, air conditioning, and thermal insulating foam, including total equivalent warming impact (TEWI) and life-cycle climate performance (LCCP). | ||||
| 4. | Describing technical options to ODSs HFCs, and PFCs, including consideration of process improvements in applications, improved containment, recovery and recycling during operation, servicing, and end-of-life, and including detailed consideration of technical performance, environmental health and safety, cost and availability, and total energy and resource efficiency. | ||||
2007: Estimates of the importance of the Montreal Protocol for climate protection.
CitationVelders et al. (2007) quantified the carbon-equivalent climate forcing that was avoided by the phase-out of ozone-depleting greenhouse gases, considering (1) time-dependent scenarios of annual ODS production, emissions, concentrations, and associated radiative forcing; (2) the time dependence of CO2 emissions and associated radiative forcing; and (3) the offsets of climate protection by ODSs caused by stratospheric ozone depletion and the use of ODS substitute gases.
Radiative forcing is the difference between the incoming solar radiation energy reaching the earth and the outgoing thermal radiation energy in a given climate system. The impact of GHG emissions in the atmosphere is to “trap” outgoing thermal radiation and therefore increase the earth's temperature. The increase in atmospheric GHG concentrations since the industrial revolution has resulted in an unbalanced climatic system with constant increases in radiative forcing due to such emissions.
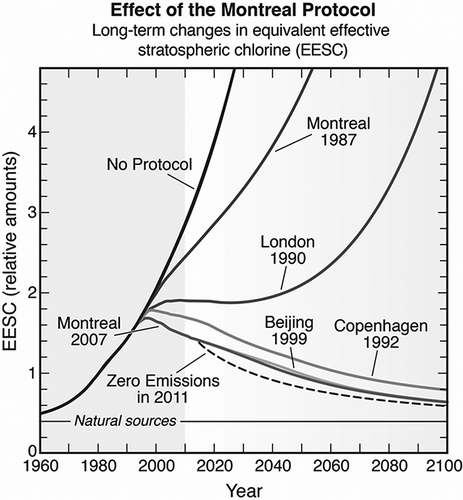
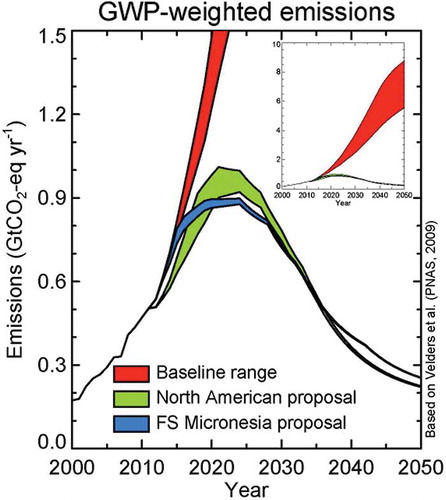
The Velders study estimated that the climate benefits of actions under the Montreal Protocol in 2010 is about 11 gigatonnes CO2-equivalent per year, which is 5–6 times the reduction target of the first commitment period (2008–2012) of the Kyoto Protocol, as illustrated in The Montreal Protocol net reduction in ODS radiative forcing in 2010 is equivalent to about 7–12 years of growth in radiative forcing of CO2 from human activities. In addition, the study estimated that it is technically feasible to further protect the ozone layer while reducing global GHG emissions by up to 5% for 10 years or more through collection and destruction of surplus or contaminated ODS, by an acceleration of the HCFC phase-out in developed countries, by adoption of technologies that are both ozone and climate safe wherever feasible, and by accelerated ODS phase-out in developing countries.
Figure 3. CO2-equivalent emissions under various scenarios. The red line represents historic and predicted future global CO2 emissions. The green area represents the CO2-eq of ODS emissions that would have occurred if Molina and Rowland had not warned the world about CFCs (could have been greater than CO2!). The blue area represents the CO2-eq of ODS emissions without the Montreal Protocol. The area below the blue line represents the total climate protection provided by the Montreal Protocol, estimated at ˜11 Gt CO2-eq (CitationVelders et al., 2007). The black line is the actual CO2-eq ODS emissions as reduced by the Montreal Protocol.
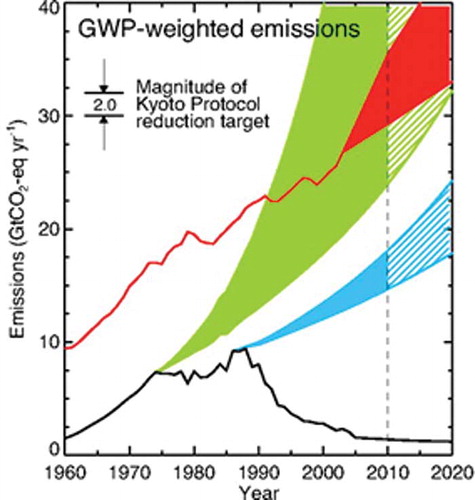
The findings of the Velders science team energized diplomats to use the Montreal Protocol to protect the climate by accelerating the phase-out of HCFCs, mindful that an earlier phase-out would also further protect the climate (Andersen et al., 2007). As a consequence, in 2007 the parties amended the Montreal Protocol to accelerate the HCFC phase-out in both developed and developing countries. The dramatic effect of the Montreal Protocol and its subsequent amendments is shown in
Protecting the stratospheric ozone layer also benefits global climate
Periodically, teams of scientists make updated estimates of the possible consequences if ODS production and consumption had increased at annual rates of 3–5%, rather than being phased out (CitationMorgenstern et al., 2008):
Nearly two-thirds of Earth's [stratospheric] ozone is gone—not just over the poles, but everywhere. The infamous ozone hole over Antarctica, first discovered in the 1980s, is a year-round fixture, with a twin over the North Pole. The ultraviolet (UV) radiation falling on mid-latitude cities like Washington, D.C., is strong enough to cause sunburn in just five minutes. DNA-mutating UV radiation is up more than 500 percent, with likely harmful effects on plants, animals, and human skin cancer rates. (CitationNASA, 2009, p. 1)
Radiative forcing from the combined effects of carbon dioxide, ODSs, other non-CO2 greenhouse gases and black carbon would have already pushed the climate past the temperatures that would melt glaciers and sea ice causing drastic sea level rise, increase the incidence of violent storms, release methane greenhouse gases from previously frozen soils, and further warming the Earth by solar absorption on land and water surfaces that are darker than when covered with snow and ice. (CitationUNEP and WMO, 2011)
The benefits of ozone layer protection far exceeded the global costs (CitationDoniger, 1988; CitationDudek et al., 1990; CitationDeCanio and Lee, 1991), and many companies phased out far more rapidly than required by the Montreal Protocol and often at a lower cost than originally projected by industry and government alike (CitationMiller and Mintzer, 1986; CitationCook, 1996; CitationLe Prestre et al., 1998; CitationAndersen and Sarma, 2002; CitationAndersen et al., 2007):
By the year 2165, actions to protect and restore the ozone layer will save an estimated 6.3 million U.S. lives that would have otherwise been lost to skin cancer ... [and] will produce an estimated $4.2 trillion in societal health benefits in the United States over the period 1990 to 2165. (CitationU.S. EPA, 2007, p. 2)
The stratosphere and climate have been protected from ODS emissions but global temperature increase from greenhouse gas emissions continues, with inclusion of growing contribution from HFCs, which are being used to replace the ozone-depleting refrigerants and foam-blowing agents being phased out by the Montreal Protocol. Emissions of HFCs are now the fastest growing of all greenhouse pollutants in the United States and in many other countries (CitationNOAA et al., 2011). Scientists have calculated that even under the most conservative assumptions of the growth in population and income, climate forcing from projected HFC emissions is likely to exceed the climate-protecting benefits of the ODS phase-out by mid-century (CitationVelders et al., 2012). The radiative forcing of HFC emissions could contribute as much as 20% of that from CO2 by 2050 if CO2 emissions continue along business-as-usual projections, and equal up to 40% if CO2 is constrained to a 450 ppm scenario (CitationUNEP, 2011a). Furthermore, scientists are unsure of the combined impacts of atmospheric feedback from projected changes in the atmosphere composition, such as colder stratospheric and warmer tropospheric temperatures; accelerated atmospheric circulation; warming and shifting surface wind, water, and storm tracks on the Antarctic Peninsula and Southern Ocean; and global ozone above its “natural state” as a consequence of increasing greenhouse gases (CitationForster et al., 2007).
Governments have proposed to take action both to preserve the climate benefits achieved by the Montreal Protocol and to leverage those benefits by phasing down the HFCs that replaced ODSs and replacing them with chemicals that have low global warming potentials (GWP) and are energy efficient. Every year since 2009, the Federated States of Micronesia have proposed an Amendment to the Montreal Protocol to phase down high-GWP HFCs, with the United States, Canada, and Mexico offering similar amendments since 2010 (CitationUNEP, 2012b, Citation2012c, 2013, 2013a). As shown in , both the Micronesian and North American proposals would reduce HFC production and consumption 85–90%, providing climate mitigation of 87–146 Gt CO2-eq. by 2050 () (CitationVelders et al., 2009; CitationMolina et al., 2009; Velders et al., 2012; CitationZaelke et al., 2012).
Figure 5. Cumulative decrease of direct GWP-weighted emissions of HFCs under the proposed Micronesian and North American Amendments to the Montreal Protocol.
Support for a phase-down of HFCs under the Montreal Protocol is steadily growing. In the Rio + 20 declaration, The Future We Want, more than 100 heads of state recognized the climate damage from HFCs and called for the gradual phase-down of their production and consumption (UN, 2012). In addition, by the time of its closing in 2012, 108 countries had joined the Bangkok Declaration calling for the use of low-GWP alternatives to CFCs and HCFCs (CitationUNEP, 2011). Through November 2012, 105 parties had provided written support to the Bali Declaration on Transitioning to Low Global Warming Potential Alternatives to Ozone Depleting Substances (CitationUNEP, 2012d).
Many national governments are already taking action on HFCs outside of the Montreal Protocol, including developing national inventories of new and old equipment utilizing HFCs, implementing mandatory refrigerant leakage checks for refrigeration and air conditioning equipment, and establishing producer responsibility schemes requiring producers and suppliers of HFCs to take back recovered bulk HFCs for further recycling, reclamation, and destruction (CitationSchwarz et al., 2011). Private companies are also taking voluntary action to limit their use of HFCs. The Consumer Goods Forum, a global network of more than 650 retailers, manufacturers, service providers, and other stakeholders from more than 70 countries, has pledged to begin phasing out HFCs in new equipment beginning in 2015 (CGF, 2012). A global partnership of companies made up of Coca-Cola, McDonalds, Pepsico, Red Bull, and Unilever, and supported by Greenpeace and UNEP, has pledged to phase out the purchase of new HFC equipment on accelerated schedules as new technology is commercialized (CitationRefrigerants, Naturally!, 2012).
Attenuating the growth in HFCs is a component of any comprehensive climate change mitigation strategy to limit temperature projected rise to below 2°C over preindustrial temperatures, the goal of international climate negotiations. Rapid reductions in a group of short-lived climate pollutants (SLCP) including black carbon soot (BC), CH4, tropospheric ozone, and HFCs can help limit global peak temperature when combined with necessary reductions to CO2 (CitationRamanathan and Xu, 2010; UNEP and WMO, 2011; CitationShindell et al., 2012; CitationIGSD, 2013). These four climate pollutants are known as SLCPs because of their relatively shorter atmospheric lifetimes compared to CO2, approximately 25% of which remains in the atmosphere for millennia (CitationSolomon et al., 2007). BC remains in the atmosphere for weeks, CH4 for approximately 12 years, tropospheric ozone for hours to days, and the average atmospheric lifetime of the current mix of HFCs by weight is 15 years (CitationZaelke et al., 2012; Velders et al., 2012).
The rapid combined mitigation of all four SLCPs can avoid as much as 0.6°C of additional warming by midcentury, with the mitigation of HFCs contributing an estimated 17% of the avoided warming by 2050 (CitationRamanathan and Xu, 2010). This would cut the estimated current rate of global warming by half, and the rate of warming in the Arctic, which has warmed at twice the global average since 1980, by two-thirds (UNEP and WMO, 2011; CitationAMAP, 2011; Hu et al., 2013). These studies show that fast action to reduce these major climate forcers can potentially forestall some of the worst predicted impacts of climate change.
Unfortunately, human emissions of greenhouse gases, aerosols, and BC are moving in the wrong direction. According to the WMO, the atmospheric concentration of CO2, the most important greenhouse gas, reached 390.9 ppm in 2011, or 140% of the preindustrial level of 280 ppm (CitationWMO, 2012). Climate models indicate that limiting global temperatures below 2°C will require limiting the atmospheric concentration of CO2 to 450 ppm or less by 2100 (CitationMeinshausen et al., 2009; CitationLuderer, 2012). Emissions of CH4 from human activities increased atmospheric concentrations to a new high of about 1813 parts per billion (ppb) in 2011, or 259% of the preindustrial levels (WMO, 2012). If left unchecked, emissions of CH4 may increase by as much as 40% by 2030 (CitationRamanathan and Xu, 2010). Emissions of BC, the second most powerful anthropogenic climate forcer behind CO2, are also rising (CitationBond et al., 2013; CitationU.S. EPA, 2012). In 1996, global emissions of BC were estimated at 8.0 million tonnes, and rose to 8.4 Mt by 2000. BC emissions are expected to increase by 15% by 2015 (WMO, 2012). N2O, which is long-lived in the atmosphere and not included in the preceding analysis, is a powerful greenhouse gas whose atmospheric concentrations reached approximately 324 ppb in 2011, 1 ppb above the previous year and 120% of the preindustrial level. N2O also depletes stratospheric ozone (WMO, 2012).
Technical, Health, and Environmental History of Refrigeration and Air Conditioning
After a brief summary to put this section into perspective and show its relationship to the Montreal Protocol, a historical review of refrigeration and air conditioning throughout the ages is provided. This is followed by the case history of one specific application, the development and barriers encountered in mobile air conditioning.
This section presents an abbreviated history of six distinct market transformations in refrigeration and A/C that were driven by scientific concern for health and environment and, in the case of ODSs, by the Montreal Protocol and national implementation in all countries. It includes a discussion of the latest market transformation to protect climate, which is just getting underway.
The six market transformations are: (1) from primitive refrigeration to global markets for harvested ice; (2) from harvested ice to manufactured ice using toxic and flammable refrigerants; (3) from point-of-use ice to distributed mechanical refrigeration, still using toxic and flammable refrigerants; (4) from toxic and flammable refrigerants to CFCs that are ozone-depleting greenhouse gases; (5) from ozone-depleting greenhouse gas refrigerants to ozone-safe greenhouse gases; and (6) from greenhouse gas refrigerants to climate-safe refrigerants with near-zero emissions and high energy efficiency (CitationNagengast, 1988; CitationDonaldson and Nagengast, 1994; CitationHarry, 1999; CitationCalm 2012). The six transitions overlap to different degrees within and between countries due to differences in regulatory mandates, wealth, cost of and access to new technology, and other factors.
The first three transitions were driven by an improved understanding of the health and economic benefits of cold storage for food. Safety concerns, such as flammability and toxicity, were generally viewed as an acceptable trade-off for improvements in food safety and security. Details are found in the next section.
The fourth refrigerant transition, after 1930 to CFCs, was driven by the apparent safety and health advantages of CFCs, a lack of understanding of the ozone depletion and climate change risks, and the competitive cost of the refrigerant. However, history has shown that the transition to CFCs would have destroyed the stratospheric ozone layer and altered the climate if scientific warnings had been as little as 25 years later or if the public and policymakers had acted less quickly to initiate what became the fifth transition. This might be considered the first major unintended consequence of what “seemed a good idea at the time,” which it truly was.
The fifth refrigerant transition, away from ozone-depleting greenhouse gas refrigerants (such as CFCs) to ozone-safe greenhouse gases (primarily HFCs, with temporary use of HCFCs where other options were not available), was undertaken despite uncertainty in both the atmospheric science and the availability of new technology. Heeding the early scientific warnings about the effect of CFCs on the stratospheric ozone layer, policymakers acted quickly to implement domestic control measures and begin global treaty negotiations. This is noteworthy because a delay of as little as 25 years theoretically would have destroyed the stratospheric ozone layer and altered climate, as discussed in detail earlier in this review.
The science would have been delayed by at least 25 years (1) if ozone monitoring stations had not been part of the 1957 Geophysical Year initiatives; (2) if the U.S. NASA and other space agencies had not placed earth monitoring satellites in orbit; and (3) if Mario Molina and Sherwood Rowland had been less confident in their science or less persistent in their political activism. The U.S. ozone monitoring satellite that was critical to proving ozone depletion was placed in orbit by a Soviet rocket after U.S. scientists lost access to space as a consequence of the 2½-year grounding of the Space Shuttle fleet after the Challenger exploded during launch in January 1986. The offer by the USSR to transport the U.S. satellite to orbit was prompted by a sense of urgency during the diplomatic negotiations leading up to the Montreal Protocol agreement (CitationKhattatov, 2002).
In order to provide guidance to the reader, lists the major chemicals used as automotive refrigerants in the current market and some relevant properties.
Table 2 . Mobile air conditioning refrigerants discussed in this review
While the aforementioned transitions, away from CFCs, averted the high risk of destruction of stratospheric ozone and contributed to climate protection with lower GWP refrigerants and higher energy efficiency, the climate forcing of the substitutes proved too large, particularly because emissions of CO2 and other major climate forcers have not been reduced. The greatest emphasis was on immediate phase-out of CFCs, and their quick replacement with substitutes that were cost-competitive, easy to manufacture, and that could be substituted with minimal technical changes to existing equipment.
The sixth refrigerant transition, which is just getting underway, seeks to phase down the production and consumption of HFCs and replace them with climate- and ozone-safe energy efficient alternatives. HFCs are the greenhouse refrigerants most commonly used as ozone-friendly replacements for CFCs and HCFCs. A phase-down in HFCs would provide climate mitigation—up to 146 billion tonnes of CO2-eq reductions by 2050 (CitationVelders et al., 2009; CitationVelders et al., 2007). Additional climate mitigation would also be realized through avoided growth in electricity use because refrigeration and A/C systems using low-GWP alternatives to HFCs are able to achieve equal or superior energy efficiency (up to 30% improvements) over those currently employing HFCs (Schwarz et al., 2011).
Fast action on the prevention of increasing use and emissions of HFCs and reduction of other SLCPs, such as BC, CH4, and tropospheric ozone, can cut the rate of global warming in half for the next several decades. Action to address SLCPs can also cut the rate of warming over the elevated regions of the Himalayan–Tibetan Plateau by at least half and reduce the rate of warming in the Arctic by two-thirds over the next 30 years, while reducing health and ecological risks (UNEP and WMO, 2011). Despite the projected benefits of an HFC phase-down, success with reducing CO2 emissions is also necessary to have a reasonable probability of limiting global temperate rise to 2°C compared with preindustrial levels through 2100 (CitationRamanathan and Xu, 2010).
Brief history of air conditioning and refrigeration, prehistoric to 1800s
Simple air conditioning was achieved in ancient cultures with a variety of innovative techniques:. Egyptians cooled rooms using flowing cold water or evaporating water on porous surfaces. Romans circulated cold water from aqueducts through walls. Persiana and Indiana used cisterns and passive wind towers, and Chinese used manual- and water-powered rotary fans with evaporative fountains to cool indoor spaces (CitationNeedham, 1991). In the 17th century, Cornelis Drebbel “turned Summer into Winter” for King James I of England by adding salt to water and ice (CitationLaszlo, 2001). In the summer of 1881, a fan and ice system (200 kg ice per hour) cooled U.S. President James A. Garfield (CitationBillings, 1893; CitationNagengast, 1999). Air conditioning, even as simple as cold towels and ice packs, was recognized for the health and medical benefits in treating heat exhaustion and reducing core body temperature from fevers.
From prehistoric times, refrigeration of perishable foods has been accomplished in caves, cellars, wells, and artesian “springhouses.” As indicated in , natural ice and snow were collected locally or brought down from nearby mountains and stored in pits or insulated chambers for warm-weather use. In the mid-1500s, food and wine were refrigerated in containers placed in water baths cooled by adding chemicals such as sodium nitrate and potassium nitrate. In the 1600s, wine was cooled using rotating bottles in water with dissolved saltpeter.
Table 3 . History of refrigeration and air conditioning
1800 to 1900: Harvested ice replaced with manufactured ice and mechanical on-site refrigeration for breweries and meat packing plants
In the first half of the 1800s, American Frederick Tudor invented insulated ice storage and American Nathaniel Wyeth invented tools and methods for efficiently cutting uniform blocks of ice from frozen ponds. These inventions facilitated the large-scale shipment of ice globally on sailing ships. In the 1840s rail cars refrigerated with ice were used to transport milk and butter and seafood by 1860. In 1867, American J. B. Sutherland patented the first railroad car refrigerated by ice that controlled temperature by adjusting the amount of air passing over ice and placing products requiring the coldest temperatures nearest the ice. During this time period, ice markets held their own while engineers struggled to make mechanical refrigeration reliable and safe.
In 1748, William Cullen of Scotland first demonstrated the basic method of mechanical refrigeration by boiling diethyl ether in a partial vacuum, which absorbed heat from the surrounding air. In 1758, American Benjamin Franklin and Britain John Hadley produced freezing temperatures by evaporating highly volatile liquids such as alcohol and ether (CitationFranklin, 1758). In 1805, American Oliver Evans described, but did not build, the first mechanical refrigeration equipment designed to compress a refrigerant, expanding it in an “evaporator coil” and then discharging the heat in a second heat exchanger before the refrigerant was recompressed and recirculated. In 1820, the British scientist Michael Faraday invented a machine based on the Evans design that achieved cooling by evaporating ammonia and other gases that had been compressed and liquefied. In 1834, Jacob Perkins built a working refrigeration system and obtained the first vapor-compression refrigeration patent. In 1842, American physician John Gorrie demonstrated a closed-cycle compressor technology similar to the machine invented by Oliver Evans to create ice that he used to cool yellow fever patients being treated in a Florida hospital. Gorrie was granted a patent in 1851 (CitationGladstone, 1998).
American Alexander C. Twinning obtained patents in 1850 and 1853 and was the first to profitably commercialize refrigerant equipment for the brewing and meatpacking industries beginning in 1856. In 1854, Australian James Harrison constructed a large-scale vapor-compressor ice-making machine using ether, which was granted a patent in 1855. He built numerous commercial systems in the 1860s and beyond. In 1859, Ferdinand Carré of France commercialized a more complex system for brewing and meatpacking applications using ammonia, which has the advantage of higher cooling capacity as a result of its low boiling temperature. In 1866, T. C. Lone made the first recorded use of CO2 as a refrigerant, and in the 1870s S. Liebmann's Sons Brewing Company in Brooklyn, New York, first used absorption refrigeration. In 1876, German engineer Carl P. G. Linde patented the process of liquefying gas that is part of basic vapor-compression refrigeration technology.
In 1882, William Soltau Davidson fitted a compression refrigeration unit to the New Zealand vessel Dunedin, which shipped meat and dairy products from Australasia and South America. In 1886 John Hall of Dartford, England, outfitted the SS Selembria with a vapor compression system to bring mutton to market from the Falkland Islands (CitationPalmer, 1973; CitationJ & E Hall International, 2012). Refrigerated rail and ocean shipping facilitated the globalization of meat and dairy markets, bringing high profits to producers in remote locations with low production costs but insufficient wealthy local customers.
By the 1890s, nearly every large brewery and meat packing plant was equipped with refrigeration machines, typically using toxic and flammable refrigerants. Air conditioning found early applications in hospitals, dining rooms, theatres, print shops, office buildings, retail stores, and schools.
In addition, harvested ice became a health problem because most rivers and lakes were increasingly contaminated with human sewage, agricultural waste, and chemical pollution. The solution to contaminated harvested ice was to manufacture ice using mechanical refrigeration from pure water sources, which also had the advantage of “just-in-time” production and no risk of a warm winter or a hot summer unbalancing the supply and demand for ice. However, there were substantial worker safety risks associated with mechanical refrigeration, including accidents caused by flammable and toxic refrigerants, resulting in injury and sometimes death. Toxic, nonflammable methyl bromide and carbon tetrachloride, although infrequently used as refrigerants, were eventually discovered to be potent ozone-depleting substances (ODSs) and were later controlled by the Montreal Protocol.
1900 to 1930: Ice competes with mechanical refrigeration using toxic and flammable refrigerants
From about 1900 until the 1930s, the use of toxic and flammable refrigerants, primarily sulfur dioxide, ethers, ammonia, and hydrocarbons (isobutane and propane), was widespread. Butane, carbon disulfide, carbon dioxide, carbon tetrachloride, dichlorethylene, ethane, ethylamine, ethyl bromide, gasoline, methyl bromide, methyl formate, methylene chloride, methylamine, methyl chloride, naphtha, nitrous oxide, trichloroethylene, and trimethylamine were also used during this time period (CitationNagengast, 1988; CitationBodinus, 1999; CitationCalm, 2012). Carbon dioxide saw widespread application for cargo refrigeration on ships from 1890 until about 1930, with continued use on British ships until 1940 (CitationBodinus, 1999). Carbon dioxide was also used in breweries, packing plants, and other large cold storage applications as well as smaller cold storage and display counters in public spaces such as food markets, hotels, and hospitals, where it was promoted as a safer alternative to sulfur dioxide and ammonia (CitationBodinus, 1999). At the 1904 St. Louis World's Fair, Brunswick Refrigeration company introduced the first self-contained mechanical refrigerator using ammonia and cooled with water; in 1914 Fred Wolf Company marketed the first air-cooled, electric, self-contained refrigerator; and in 1916 Alfred Mellowes introduced an improved refrigerator design that General Motors bought in 1918 and produced under its Frigidaire brand. By 1923, Kelvinator, first with automatic temperature control, had 80% of the market. In 1927 General Electric introduced the first mass-produced completely sealed system with separate temperature control for frozen and refrigerated food and quickly dominated the market, while Electrolux marketed the gas-fired absorption refrigerator (using ammonia) for homes not yet electrified (CitationNagengast, 1988, 1997; CitationNAE, 2013). Refrigerators in the 1920s and early 1930s used a variety of refrigerants, including ethyl chloride (Allison, Holmes), sulfur dioxide (General Electric, Westinghouse), and methyl chloride (Williams) (CitationNagengast 1997).
Air conditioning was introduced in the first hospital in 1906 (Boston Floating Hospital), in the first hotel (Congress Hotel Chicago), and in numerous other public and private buildings. In 1928 Carrier introduced the “Weathermaker” home air conditioner, and in 1929, Frigidaire marketed the first room A/C using sulfur dioxide as the refrigerant (NAE, 2013).
This transition to mechanical ice making was driven by a combination of convenience, concern about the health risks of ice made from polluted water, and the unreliability of ice due to hot weather events and delivery interruptions. Harvested and manufactured ice successfully competed with mechanical refrigeration in commercial and residential applications until the 1930s and 1940s, when CFCs made the operation of small mechanical refrigerators safe. The remaining uses of ice for refrigeration are in remote locations lacking electrification and in specialized applications where ice has a technical advantage, such as in keeping fish both refrigerated and humid.
Mechanical refrigeration was considered more convenient than ice, which could not consistently provide safe temperatures for food refrigeration, and required more frequent delivery in hot weather. However, mechanical refrigeration had serious safety drawbacks. In many locations the electric power supply was unreliable and leaks of the most common refrigerants—sulfur dioxide and ammonia—typically required rapid evacuation of homes and buildings. People who came into contact with toxic refrigerants suffered from vomiting, burning eyes, and painful breathing. Accidents with sulfur dioxide and ammonia rarely resulted in death, but accidents with methyl chloride refrigerant were frequently fatal.
Commercial and residential customers were well informed of the known trade-offs of ice versus mechanical refrigeration. Ice refrigeration suppliers promoted the simplicity and reliability of ice and warned against the hazards of poisonous and flammable refrigerants. Mechanical refrigeration suppliers, on the other hand, cautioned customers about the possibility of contaminated ice and pointed to the inconvenience and lack of reliability associated with ice refrigeration.
Although this flammability versus toxicity risk trade-off discussion was highly informed, not enough was known at that time about the chronic toxicity effect of repeated exposures to toxic refrigerant leaks over a long period of time. Minor use of methyl bromide and carbon tetrachloride, highly toxic ozone-depleting substances, continued in the first half of the 20th century. However, the portion of carbon tetrachloride used as a refrigerant was minor in comparison to that used as a solvent, fire extinguishing agent, and chemical feedstock. The quantity of methyl bromide used as a refrigerant was also minor compared with the quantities used as pesticides, fire extinguishing agents, and anesthetics. All together, the release of all ODSs used before 1930 posed little to no risk to the ozone layer at that time. Similarly, because these substances have a relatively short atmospheric lifetime, their pre-1930 use did not contribute in a meaningful way to the serious ozone depletion experienced in the 1970s and later. summarizes flammable and toxic refrigerants in use before CFCs were introduced.
Table 4 . Flammable and toxic refrigerants in use before CFCs (CitationAndersen and Sarma, 2002)
1930 to 1990: CFCs and HCFCs replace most toxic and flammable refrigerants
In late 1928, executives from General Motors (GM) and its refrigerator manufacturing division, Frigidaire, assigned Thomas Midgley and a small team of engineers at the GM Research Laboratory the task of inventing a nontoxic, nonflammable, and noncorrosive refrigerant. Midgley determined that elements with boiling points appropriate for refrigeration were clustered on the Langmuir periodic table, which is arranged according to the number of vacancies in the outer shell of electrons. Working with Albert Henne and Robert McNary, Midgley ruled out unstable and inert elements. This left for consideration carbon, nitrogen, oxygen, sulfur, hydrogen, and the halogens fluorine, chlorine, and bromine. Others had dismissed fluorine because chemical substances containing fluorine are often toxic and/or corrosive (CitationKauffman, 1989).
Midgley and Henne, however, were familiar with Belgian chemist Frédéric Swarts's theory that the toxicity of fluorine could be negated if it were strongly bonded with chemicals that had complementary valences (CitationAndersen and Sarma, 2002). Within two or three days of receiving their research assignment, they had identified chlorofluorocarbons (CFCs) as prime candidates and synthesized dichloromonofluoromethane (CFC 21) from carbon tetrafluoride. Within months, Midgley and his team satisfied the existing criteria for confirmed that CFCs were nonflammable, nonexplosive, noncorrosive, very low in toxicity, and odorless, and that their vapor pressures and heats of vaporization made them suitable for refrigeration applications.
Within a year, GM patented the family of CFCs and perfected the manufacturing process for the first commercial substances trichlorofluoromethane (CFC-11) and dichlorodifluoromethane (CFC-12). The Midgley research notes also identified other refrigerants, such as HFC-134a (1,1,1,2-tetrafluoroethane), that were unappreciated at the time because the stratospheric ozone depletion and climate forcing were not yet identified (CitationAndersen and Sarma, 2002). On 27 August 1930, GM and DuPont formed a joint stock company, the Kinetic Chemical Company, to manufacture and market CFCs (CitationAndersen and Sarma, 2002).
In the 1930s air conditioning gradually became more common as companies produced systems large enough to cool occupied spaces using large CFC refrigerant charges that would be prohibitively dangerous if toxic and flammable. In 1947 engineer Henry Galson perfected a low-cost residential air conditioner design that was manufactured under license by a number of manufacturers (National Academy of Engineering, 2013). By 1970, more than half of U.S. cars and most new homes had central air conditioning, with window A/C affordable for homes without ductwork.
CFCs and HCFCs rapidly replaced other refrigerants in all but applications where companies accepted the increased risk of flammable and toxic refrigerant releases or in applications where the existing technologies were more energy efficient. For example, ammonia continued to be used in cold storage, ice making, ice rinks, and absorption refrigerators using gas flame as an energy source. Hydrocarbons continued to be used in industrial refrigeration, particularly at oil and chemical facilities (CitationAndersen and Sarma, 2002).
CFC-12 soon became the dominant refrigerant in most small appliances, refrigerated storage applications, and many other new applications. CFC-11 became dominant in A/C applications until the 1950s, when HCFC-22 was applied in commercial refrigeration, room A/C, and building A/C. From the 1940s onward to the 1990s, CFCs and HCFCs were also marketed as aerosol propellants, solvents, foam blowing agents, and more.
1975 to 1980: CFC aerosol propellant boycotts, bans, and voluntary agreements
Until early 1974, CFCs and HCFCs were considered perfect in every known way because neither stratospheric ozone depletion nor climate alteration was well understood or anticipated. Refrigeration and air-conditioning equipment manufacturers and their customers came to think of CFCs and HCFCs as “wonder gases.” Because these were relatively inexpensive, near-absolute containment was not a design priority for equipment manufacturers or users. Refrigerants were typically vented at service to avoid any risk of damage to equipment from refrigerants that might be contaminated with air, acids, water, or metal filings. Energy efficiency of refrigerators and air conditioning was mostly unmeasured and unregulated and considered by manufacturers to be unimportant to sales, and therefore not worth the extra engineering and manufacturing costs to achieve.
Following the call to action resulting from the findings of Molina, Rowland, Crutzen, and others in the early 1970s, hydrocarbons became substitute propellants for cosmetic and convenience aerosol products. Not-in-kind alternatives for aerosol hairspray include pumps, sprays, and creams; deodorant alternatives include pumps, sprays, sticks, and roll-ons. Once industry made the transition to hydrocarbon aerosol products, the consumer cost was far less than when CFCs were used, and the lower cost of hydrocarbon aerosol propellants increased the profits to cosmetic product manufacturers.
By 1975, consumer boycotts of CFC hairspray and deodorant were penetrating the American market and influencing sales in Canada, Europe, and Japan. In June 1975, S.C. Johnson Company announced that it would phase out CFCs as aerosol product propellants. One month later the Sherwin-Williams Company, Bristol Meyers, and Mennen joined S.C. Johnson in aggressively advertising alternatives, including pumps, sprays, creams, and sticks. In 1978, the United States banned the use of CFC propellants in products considered nonessential, which accounted for more than 80% of all U.S. CFC aerosol products.
[By 1978] Canada had achieved a 50 per cent decrease in aerosol production by voluntary agreement with industry, and had announced its intention to issue regulations in 1979. Sweden banned the manufacture and import of aerosol products containing CFC aerosol propellants, effective 1 July 1979. The Netherlands issued regulations that required a warning label on all aerosol products containing fluorocarbons sold after 1 April 1979 and further provided that a ban would be imposed on non-essential aerosol use if similar action were taken by the main producing nations. Germany was pursuing a cooperative approach with industry directed at reducing use of fluorocarbons in aerosols by 30 per cent by 1979. Norway recognized the gravity of the risks despite uncertainties, and called for international action. In March 1980, the Council of the European Economic Community legislated a 30 per cent cutback in CFC aerosol use from 1976 levels. (CitationAndersen and Sarma, 2002, p. 51)
The European Commission (EC) reduction was enacted under Decision 80/372/EEC, in March 1980.
Hydrocarbon aerosol products have flammability risks not experienced with CFC aerosol products, and there was at least one costly commercial fire before warehouse and transportation fire codes were modified to account for the additional flammable ingredients in hydrocarbon aerosol products (CitationIndustrial Hydrocarbons, Inc. [IH], 2012). In the first 10 years after the CFC aerosol product ban (1978–1987) there was an average of 3,922 accidents per year reported to the Consumer Product Safety Commission (CPSC), which amounted to one incident per 606,000 cans sold. In the last 10 years (2001–2010), the average has been 4,388 per year, which amounted to an average of one incident per 832,000 cans sold (IH, 2012).
1990 to 2015: HFCs replace most CFC and HCFC refrigerants, with flammable and toxic “natural refrigerants” making a slow comeback
When the Montreal Protocol was instituted in 1987, there was no time to wait for new technology, despite the success of the head start on phase-out of aerosol products by some countries. Sales of ODSs were increasing rapidly and stratospheric ozone depletion was recognized. ODS replacements had to be found immediately. Hydrocarbon refrigerants were quickly proposed to replace CFCs, but the typical leak rates and service venting practices would have been unsafe in many applications, particularly in equipment with large refrigerant charges. Additionally, no one was confident of how quickly containment, detection, and isolation technology could be implemented to mitigate flammability.
As soon as the Montreal Protocol was signed, in 1987, the fluorocarbon chemical industry and its refrigerant customers moved rapidly to market existing HCFC-22 and HCFC-142b to replace CFCs, to commercialize HFC-134a to replace CFC-12, and to commercialize HCFC-123 to replace CFC-11. HFC-134a and HCFC-123 had been identified decades earlier and patented in the 1970s (CitationAndersen and Sarma 2002). New chemicals—including HCFC-225, HFC-143a, and HFC-124—were invented to replace ODSs in applications other than refrigeration. By the time Gustav Lorentzen and colleagues filed for their first modern patent for carbon dioxide refrigeration systems in 1989 (granted in 1993), this technology was too late to capture any of the market for the CFC phase-out, although it is currently replacing HFCs in many stationary applications (CitationLorentzen and Pettersen, 1993).
To speed commercialization of new fluorocarbon refrigerants, industry formed the Programme for Alternative Fluorocarbon Toxicity Testing (PAFT) in 1988 and the Alternative Fluorocarbon Environmental Acceptability Study (AFEAS) in 1989 with the strategy of robust cooperative financing of research into toxicity and environment impacts by the most respected laboratories and other research institutes.
Advocates of nonfluorocarbon refrigerants organized around the concept of “natural refrigerants,” defined as naturally occurring and existing in nature, but modified their list of candidates to rule out many of the naturally occurring toxic and flammable refrigerants used before the invention of CFCs. Now natural refrigerants usually include only ammonia (R-717), hydrocarbons (isobutane [R-600a] and pentane [R-290]), carbon dioxide (R-744), and sometimes water (R-718) and air (R-729).
Natural refrigerants staged a comeback in the 1990s that continues to gain market share. In 1992, Greenpeace inspired European government, industry, and consumer support for the use of hydrocarbons in domestic refrigerators. Within one year, a hydrocarbon-based domestic refrigerator was introduced in Germany, and soon hydrocarbon refrigerators gained market dominance in Europe and penetrated markets in Asia, including Japan, but, so far, not the United States. Meanwhile, suppliers of equipment using ammonia as a natural refrigerant recaptured market share in HCFC cold storage and food freezing subsectors. They also made limited progress in applying ammonia to commercial refrigeration and A/C using secondary loops for safety. At the same time, European researchers with the support of European vehicle manufacturers, particularly the German automobile manufacturers, pursued carbon dioxide for use in mobile air conditioners (CitationAndersen and Zaelke, 2003). Later in this paper, the transitions in mobile air conditioners are introduced.
In the majority of cases, the replacement of ODSs was accomplished with no compromise in any measure of environmental risk, and manufacture of equipment and safety performance are maintained or improved. Not-in-kind alternatives replacing about 85% of ODS use and emissions were benign, such as the elimination of testing, training, and accidental discharge of halon fire extinguishing agents or the elimination of CFCs solvents with no-clean soldering and metal forming. HFC metered-dose inhalers (MDIs) used in treatment of asthma and chronic obstructive pulmonary disease (COPD) more accurately dispense the medicine than the CFC products they replace, and sterilization without CFCs is superior in every way (CitationAndersen and Sarma, 2002).
The HFCs that replaced CFCs had generally lower GWP and equal or higher energy efficiency. For example, HFC-134a (1430 times as potent as CO2) has replaced CFC-12 (GWP = 10,900) in many refrigeration and A/C applications while achieving up to 30% improvement in energy efficiency and 30% reduction in refrigerant leak rates. But the introduction of new technology is not without its challenges. In some cases the new technology has higher or new risk, primarily in manufacturing and servicing, such as the higher risk of worker exposure to ammonia refrigerant or the fire risk in servicing equipment charged with butane and pentane hydrocarbon refrigerants. In still other cases, the new technology may be environmentally superior to the one it replaced, yet arguably still not acceptable under increasingly stringent environmental standards. Thus, high-GWP HFCs are now proposed for phase-down under the Montreal Protocol because of unanticipated emissions in developing countries and because environmentally superior low-GWP alternatives are now available. As a further example, some low-GWP HFC refrigerants whose atmospheric degradation by-products may be unsustainable over the long run may need further review (CitationMcCulloch, 1999; CitationLuecken et al., 2010; CitationHenne et al., 2012). Atmospheric degradation of some HFCs produces trifluoroacetic acid (TFA; CF3COOH) via hydrolysis of trifluoroacetyl fluoride (CF3COF). TFA is a mildly phytotoxic (CitationBoutonnet et al., 1999) strong organic acid (CitationHenne and Fox, 1951) with no known degradation mechanism in water. However, risk assessments indicate that deposition of TFA from current emissions of HFCs does not pose a risk to aquatic ecosystems, and technology is available to reduce emissions to near-zero levels (CitationLuecken et al., 2010; CitationMetz et al., 2005).
Support by industry leaders hastened the transition from CFC to HFC refrigerants. The mobile air conditioning (mobile A/C) sector was first, in 1988, to agree to recover and recycle refrigerant and was the first in 1990 to announce plans to replace CFC-12 with HFC-134a. At that time, mobile A/C accounted for up to half of all nonaerosol CFC sales and emissions. The early mobile A/C commitment to HFC-134a gave chemical manufacturers the confidence to invest in full-scale production, even before toxicity testing and government approval was completed (CitationAndersen and Morehouse, 1997). HFC-134a was quickly embraced by other refrigeration and A/C applications because it was similar to CFC-12, nonflammable, nontoxic, proven compatible with specific lubricants, competitively priced, and widely available. Coca-Cola, the world's largest customer for refrigerator cases and vending machines, also made an early worldwide commitment to HFC-134a, which encouraged its suppliers in both developed and developing countries to take ozone layer protection seriously and to move quickly with the CFC-12 phase-out (CitationAndersen and Sarma, 2002). Now Coca-Cola is fully committed to CO2 for vending machines, refrigerated display cases, and drink dispensers and will phase out HFC-134a in new equipment purchases.
As of the end of 2009, the parties to the Montreal Protocol had phased out the consumption of 98% of all of the chemicals controlled by the Protocol. In addition to providing critical ozone layer protection, the agreement also produced substantial climate protection benefits. Because ozone depleting substances are also global warming gases, the reduction in ozone depleting substances between 1990, when they reached peak levels, and the year 2000 has yielded a net integrated reduction of approximately 25 billion tonnes of CO2-equivalent global warming gases. This figure does not include the additional CO2 emissions that were avoided due to improvements in the energy efficiency of refrigeration and A/C systems when the global CFC phaseout ushered in a new generation of equipment. As refrigeration and A/C manufacturers retooled their products to accommodate new ozone safe refrigerants, many also took the opportunity to make improvements in overall operating efficiency. At this time, many national governments were beginning to adopt efficiency standards and incentive programs and customers were becoming increasingly concerned about appliance electricity cost.
The U.S. Environmental Protection Agency (EPA) estimated the gains to customers and society of the replacement of old air-conditioning equipment when 44% of existing chillers were converted or displaced by non-CFC chillers. (Chillers in large buildings exhibited energy efficiency improvements of up 40% over older models.) Thus, U.S. EPA estimated that building owners who install new chillers saved almost $500 million and 7 billion kilowatt hours of electricity in 1999, representing the annual electrical needs of 740,000 U.S. households. These savings avoid emissions of more than 4 million tons of carbon dioxide and 34,000 tons of sulfur dioxide (SO2). The reduction in SO2 represents the annual emissions of one and one-half large coal-fired power plants. Similarly, projects involving CFC chiller replacement in India resulted in 30% improvement in energy efficiency with average savings for each chiller replaced of 214,000 kWh/yr or ˜$12,000/yr (also CO2 equivalent benefits—0.82 kg CO2 equivalent /kWh) and a reduction of load capacity of ˜130 kW/chiller.
2010 and continuing: Natural refrigerants and low-GWP HFCs compete on the basis of life-cycle climate performance (LCCP)
Policymakers, public interest organizations, and companies worked hard to select alternatives and substitutes to CFCs with the lowest possible environmental impact. It was widely recognized that the climate impact of any refrigeration or A/C technology should be comprehensively accounted over the lifetime of the product, taking into account the direct emissions of greenhouse gas refrigerants, the indirect emissions of energy used to power the technology, and the embodied emissions from the manufacture, distribution, and disposal of the technology at end of product life.
The concept of TEWI is used to calculate carbon equivalent life-cycle greenhouse gas emissions (U.S. Department of Energy [DOE], 1991): TEWI = life-cycle direct chemical greenhouse gas emissions + lifecycle indirect CO2 energy emissions (CitationFischer et al., 1991). Because TEWI neglects emissions associated with product manufacturing, including HFC-23 byproduct greenhouse gas emissions during HCFC-22 refrigerant manufacturing, it was extended in 1997 as life cycle warming impact (LCWI): LCWI = TEWI (direct and indirect) + chemical production + atmospheric breakdown products + transport (CitationPapasavva and Moomaw, 1998).
In 1999 LCWI was expanded into “life-cycle climate performance” (LCCP): LCCP = LCWI (direct + indirect + chemical production + atmospheric breakdown products + transport) + component manufacturing (CitationAndersen, 1999).
Finally, in 2003–2006, scientists who developed LCWI and LCCP, through global consensus, developed an LCCP model for motor vehicle A/C called “GREEN-MAC-LCCP,” which is an SAE International (previously called the Society of Automotive Engineers, SAE) standard and hosted on the U.S. EPA website (CitationHill and Papasavva, 2005; CitationPapasavva et al., 2008; CitationPapasavva et al., 2010; CitationPapasavva and Andersen, 2011).
Today, natural refrigerants compete favorably with fluorocarbon refrigerants in an increasing variety of applications and systems. Hydrocarbon natural refrigerants are competitive in systems with relatively small charges, but only in a very small number of systems with large charges. Ammonia is increasingly competitive in industrial and commercial refrigeration, but has not penetrated A/C applications. In Japan, there is considerable success with carbon dioxide heat-pump water heaters in markets where the only water heating competition is electric resistance heating (CitationMetz et al., 2005).
The carbon footprint for refrigerant emissions depends on the GWP of the refrigerant and the emissions during manufacture, use, service, and at the end of product lifetime. The carbon footprint for electric refrigeration and A/C equipment depends on electric generation fuel source, efficiency in power generation, distribution processes, and electricity transmission losses.
Refrigerant GHG emissions for electrically powered refrigeration and A/C can be up to about 95% of the carbon footprint for equipment powered entirely by sources like hydroelectric, but are more commonly 10 to 30% for equipment powered by a typical mix of coal, oil, or biomass. The portion of refrigerant carbon footprint is lower in climates with long hot and humid cooling seasons.
The carbon footprint for motor vehicle A/C using fossil fuels depends on the fuel efficiency of the engine, the emission rate of the refrigerant, and the total hours of A/C operation. In climates with little cooling or dehumidifying, the refrigeration emissions dominate the carbon footprint, while in climates with long cooling and dehumidifying seasons the energy consumption dominates. In the United States, mobile A/C refrigerant emissions are about one-third of A/C fuel emissions, while in climates like Europe with less air conditioner use the refrigerants are about half of the A/C carbon footprint.
Each refrigerant has a potential energy efficiency that depends on its chemical and physical properties and a realized energy efficiency that depends on factors including design, component efficiency, and system controls. Design features such as a secondary cooling loop requiring an additional heat exchanger and long cooling lines, necessary for safe use of toxic and flammable refrigerants, have an energy penalty.
The Montreal Protocol TEAP reports that systems using low-GWP alternatives achieve equal or superior energy efficiency in a number of sectors, including domestic refrigeration, commercial refrigeration, and some types of air-conditioning systems. For example, hydrocarbon and ammonia systems are typically 10–30% more energy-efficient than conventional high-GWP HFC systems. Sectors achieving superior energy efficiency with low-GWP refrigerants include mobile A/C, small room air-conditioning, and small and large reciprocating, scroll, and screw chillers (UNEP, 2009).
Schwarz et al. (2011) identified technically feasible and cost-effective low-GWP alternative technologies capable of achieving equal or better energy efficiency in 26 refrigeration and A/C subsectors. About half of the technology identified as superior was capable of achieving 30% higher energy efficiency than the high-GWP equipment it replaced.
Additional improvements in overall system energy efficiency are likely to occur as HFCs are gradually replaced or contained. As A/C and refrigeration manufacturers retool their products to accommodate a new refrigerant, they will redesign them to improve efficiency, as witnessed during the CFC phase out. The extent to which additional improvements are made will depend on consumer demand, cost, safety, policy incentives or disincentives, and other drivers.
CitationMcNeil et al. (2008) identified a cost-effective opportunity to avoid 304 Mt CO2 by 2030 in residential refrigeration and 214 Mt CO2 in commercial space cooling. CitationIEA (2012) suggests that improving energy efficiency could give the world another 5 years to change course and begin the transition to renewables and other low-carbon energies.
Unresolved are questions of regarding adverse health effects of air conditioning. At issue is whether air conditioning itself is healthy, how to avoid “sick building” symptoms (headache, fatigue, and sensory irritation) most often attributed to indoor air quality, and how to avoid more serious illness, such as Legionnaire's disease, caused by bacterial contamination propagated and dispersed by air conditioning (CitationWheeler, 1999).
Case Study of the Transition from CFC-12 to HFC-134a with Lessons for the ongoing Transition to HFO-1234yf
This portion of the critical review examines in depth 25 years of extraordinary cooperation exhibited between automobile manufacturers, automobile air conditioner service organizations, governmental environmental authorities, and environmental nongovernmental organizations (NGOs) to reduce refrigerant emissions through manufacturing practices, containment, recovery, recycling, and destruction at the end of vehicle life and the complementary cooperative efforts to continuously improve the environmental performance of vehicle A/C in both direct refrigerant emissions and energy efficiency (CitationAtkinson, 2008). A timeline for the use of refrigerants in mobile air conditioning is given in .
Table 5 . Mobile A/C timeline
Table 6 . U.S. market penetration of factory-installed vehicle A/C (CitationBhatti, 1999)
The first mechanical motor vehicle air conditioners
For a long time, opening windows, including “wind wing” side windows and hinged or roll-up windshields, provided the only automobile ventilation. Some automobiles were vented by picking up air from the base of the windshield or on the side of the car, and in locations with low humidity, evaporative coolers hung from a side window offered some relief. However, it was not until the 1960s that rear vents were added to create “flow-through” ventilation with the windows closed (CitationHoffpauir, 2012). Fresh air heaters were introduced in the 1940s that also used the blower to increase ventilator airflow.
In 1930 the Kelvinator Company customized a Cadillac with an air conditioner hung like a trunk above the rear bumper and powered by a separate gas engine, in 1933 Popular Science featured floor-mounted AC from an unspecified manufacturer, in 1934 Houde Engineering and Carrier demonstrated the first self-contained automobile conditioner, and 1935 A/C systems designed by McCord Radiator & Manufacturing were tested on buses (CitationBhatti, 1999; CitationAnonymous, 1933). In 1937 the Kelvinator Company installed air conditioning on a White Research Coach. GM engineers developed a prototype trunk-mounted car A/C 1939 (CitationBhatti, 1999). The first factory-installed mechanical motor vehicle A/C was offered on the 1940 model year Packard and the second system was on a 1941 Cadillac (CitationThe Online Imperial Club, 2001). Chrysler DeSoto offered factory-installed A/C in 1942 (CitationHoffpauir, 2012). Air conditioning was suspended during World War II and was not revived in volume until 1953, when Chrysler, GM, and Packard offered systems on luxury models with components under the hood and in the trunk; Chrysler used HCFC-22 and GM and Packard used CFC-12. In 1954, GM developed the A/C self-contained system still used today with evaporators in the passenger compartment and all other components under the hood.
Through the 1950s and 1960s, motor vehicle A/C was offered either as a factory-installed or as a dealer-installed accessory on selected motor vehicles and as a “hang-on” accessory installed by enterprising automobile service companies. The hang-on A/C system was sold as a kit with standardized parts including an interior console (with evaporator, expansion valve, fan, and controls), compressor, condenser, and the custom parts necessary to fit the system to a particular vehicle. The model-specific parts include a drive pulley, a compressor drive belt, hose assemblies, and an assortment of brackets to align the pulleys and attach the various components. The console hung under the dash, the compressor was bolted to the engine, the compressor drive pulley bolted to the crankshaft damper, and the condenser was mounted below or in front of the radiator. Most kit air conditioners also include the necessary lubricant, CFC-12 refrigerant, and charging hoses. In hot climates, the engine cooling system was often up-sized or fitted with larger cooling fans.
In the 1970s, A/C was increasingly offered as standard equipment on motor vehicles or as an option. By 1987, when the Montreal Protocol was signed, A/C was installed on more than 90% of vehicles sold in North America and on about 50% of vehicles sold in Europe and Japan. By 2000, A/C was standard on nearly all vehicles sold in Europe, Japan, and North America, partly because new European and other safety standards for demisting and defrosting could only be achieved by cooling cabin intake air to dehumidify and then heating the air to provide comfort and defrosting. By 2010, vehicle A/C was standard worldwide on almost all four-wheel vehicles and available as an option on the few vehicles where it was not standard. Today, vehicle A/C consumes 3 to 20% of motor fuel, depending on climate conditions. Market penetration of mobile air conditioning in the United States is shown in .
Table 7 . ASHRAE refrigerant safety groups
This A/C fuel use contributes to low fuel economy, local pollution, and global GHG emissions with an estimated 5.5%, 3.5%, and 3.2% of total motor fuel consumed in the United States, European Union (EU), and Japan, respectively. The United States annually consumes about 26 billion liters of fuel for vehicle A/C, emitting 62 billion kg of CO2 annually (CitationRugh et al., 2004). In India, A/C consumes about 20% of automobile fuel as a consequence of heavy traffic congestion throughout long, hot, and often humid cooling seasons and where drivers prefer windows up to block sound, dust, and pollution (CitationChaney et al., 2007). Worldwide, refrigerant emissions accounted for about half of the air-conditioned motor vehicle life-cycle carbon-equivalent emissions in the 1980s as a consequence of the high GWP of CFO-12 (10,700), leaky design, and service without recovery and recycle (CitationBhatti, 1998, 1999). The redesign for lower leaks, the new HFC-134a (GWP = 1430) refrigerant, and service using precision leak detection and refrigerant recovery and recycle equipment reduced the A/C carbon footprint to less than 8%. As discussed in the following, the transition to HFO-1234yf alone will reduce that to about 5.5% and a pledged 30% improvement in A/C energy efficiency will reduce it to about 3.5% (CitationRugh et al., 2004).
CitationAndersen et al. (2007) describe the sense of community and the many reasons for cooperation in forming the Montreal protocol:
| • | The theoretical and observational clarity of the science, particularly the Antarctic Ozone Hole. | ||||
| • | The ambition and urgency to protect Earth for future generations as a precaution against the risk of irreversible consequences. | ||||
| • | The Clean Air Act requirement and the U.S. EPA unequivocal regulatory authority. | ||||
| • | The financial reality that phase-out regulations would be enforced against government operations and infrastructures, including critical uses in military weapon systems and scientific research laboratories. | ||||
| • | The recognition that government manufacturing standards prescribed ODSs use for military, aerospace, telecommunication, and environmental compliance. | ||||
| • | The importance of protecting markets, jobs, and prosperity during extraordinary market transitions. | ||||
1974 to 1978 Mobile A/C community leadership: Reducing A/C system leakage
After CitationMolina and Rowland (1974), U.S. motor vehicle manufacturers quietly responded by reducing the refrigerant charge, improving the leak-tightness of A/C systems with better hoses and seals, and reducing CFC emissions from leak testing and refrigerant charging during vehicle manufacture.
At the same time, CFC manufacturers confidentially investigated chemical alternatives to CFCs. Half a dozen companies patented chemical processes to produce refrigerant HFC-134a, and Allied Signal and Delphi built and road-tested HFC-134a systems, but companies put the technology on the shelf when regulations only targeted aerosol products (CitationBhatti, 1999).
1987 to 1988 Mobile A/C community leadership: Commercializing refrigerant recycling under warranty
Automakers argued that the recycled refrigerant should be as clean as newly manufactured CFC. This could require service equipment to recover the refrigerant, shipment to an off-site purification facility for column distillation, and shipment back to service facilities. Because CFC-12 was inexpensive (˜$2/kg) this scenario presented a difficult or impossible business case.
Service equipment companies with a head start on recycling equipment design wanted federal regulations to compel its use. But without industry support, it was difficult to reach agreement on federal regulations, and a limited number of recycling equipment suppliers provided inadequate competition to serve the public interest.
The Mobile Air Conditioning Society and its members wanted equipment certified to satisfy warranty repair as a signal that recycling was not a compromise in system durability and reliability, and they wanted environmental organizations to endorse recycling to help technicians sell the now more expensive A/C service to their customers.
The U.S. EPA wanted recycling to be simple enough to be implemented in both large and small service facilities. The agency shared the concern of automakers and service organizations on system durability and reliability because it would be short-sighted to implement recycling if contaminated refrigerant caused components to leak and fail.
An important early technical breakthrough occurred when Parker Hannifin Corporation (a high respected supplier of A/C filter dryers) scientifically proved to the satisfaction of automobile makers that a new filter would remove any damaging residues. The implication to recycling was that simple filtration and moisture removal would be adequate and that more elaborate processing, such as redistillation, would be unnecessary. The research team discovered that refrigerant was mildly contaminated in relatively new vehicles, possibly because of manufacturing quality control or because of the metal residues when new parts are broken in by operation.
The strategy was to agree first on criteria that would satisfy all stakeholders, and later on the technical performance satisfying these criteria and the specific test method validating that performance:
| • | Vehicle manufactures would agree to accept under new car warranty refrigerant with contamination no greater than that existing in normally operating A/C systems in automobiles only a few months old. | ||||
| • | Recycling equipment manufacturers would agree to test the ability of their machines against a highly contaminated test sample typical of the worst contamination found in vehicles with failed air conditioners. | ||||
| • | Underwriters Laboratory (UL) would agree to certify recycling equipment that could clean the contaminated test sample to the agreed standard of purity when operated in shop-like circumstances. | ||||
| • | NGOs would agree to promote the new technology to the public. Once these agreements were in place, the plan was to have U.S. EPA propose regulations compelling use of recycling equipment and the certification of technicians using the equipment. | ||||
This agreement was revolutionary because at the time vehicle manufacturers had never before accepted used parts or fluids in warranty repair, UL had never certified the performance of any product (only its safety), and the U.S. EPA had never made informal agreements with industry that a regulation would be agreed by consensus.
The strategy had the advantage of a global team of experts all working with a common purpose and each contributing to product development. For example, the original agreement on the testing of the contaminated sample had not anticipated that the recycling equipment would need a system to purge noncondensable gases (air) from the refrigerant. In service, without recycling, the A/C system is merely subjected to a strong vacuum that removes all the air from the system and then it is recharged with new refrigerant with no air contamination.
Recycling equipment draws both refrigerant and air that has leaked into the A/C system through the filters and dryers to purify the refrigerant/air mix. To accomplish this, an air-purge system is needed to discharge the air in its vapor stage while containing the refrigerant in its liquid stage. Because even a small amount of air left in the system will diminish cooling capacity and increase energy use, it was important to err on the side of purity. But erring on the side of refrigerant purity meant that U.S. EPA and the public would have to accept that some CFC would be discharged into the environment. Fortunately, everyone saw the advantage of fast action and agreed to abide by the agreement to not compromise the integrity of the system but to continue cooperative work to further improve the equipment after first commercialization.
Over a period of one year, the team:
| • | Conducted statistically significant sampling of refrigerant in normally operating new vehicles (which was validated by confidential sampling by several of the vehicle manufacturers and service equipment companies). | ||||
| • | Built and tested a wide variety of recycling equipment designs with different sized filters, dryers, vacuum pumps, and controls. | ||||
| • | Authored an SAE J-Standard specifying the performance and safety of recycling equipment used on vehicles. | ||||
At the same time, UL wrote the test procedure to certify equipment to the SAE J-Standards and the U.S. EPA, industry, and NGOs worked with Congress to specify that the SAE standard and UL certification would be the basis of legislation and regulation compelling recycling. All in all, millions of dollars were spent perfecting the recovery and recycling equipment.
Environmental organizations fully supported recycling, state and federal agencies announced proposed regulations making recovery/recycling mandatory, and many automobile manufacturers required their dealers to purchase at least one recycling machine. The service equipment companies that had participated in the cooperative effort sold more than US$2 billion worth of recycling equipment within the first two years ($1.5 billion U.S. sales and $0.5 Canadian and global sales), UL more than recovered the cost of developing the test procedures, the Mobile Air Conditioning Society made substantial progress with its goal of professionalizing A/C service, and consumers became willing to do their part to protect the ozone layer. Friends of the Earth was the driving force behind environmental NGO support for both recovery and recycling and for the rapid transition from CFC-12 to HFC-134a.
Refrigerant recycling, restricting the sale of small cans of CFCs to certified technicians, and retrofit of some older vehicles to HFC-134a allowed a faster phase-out of CFCs with a continuous supply available to those buyers willing to pay the price. CFC-12 prices increased from $2.00/kg in 1987 to $70/kg at the price peak around 2000 and then declined to about $35/kg after 2005, when few vehicles manufactured with CFC-12 were still on the road. HFC-134a cost about $13/kg during the 1990 to 1994 transition and then declined to about $3.50 to $6/kg between 1995 through 2005 (CitationAtkinson, 1989). Higher refrigerant prices discouraged recharge without repair and encouraged reduced refrigerant charge in new vehicles, which was achieved using heat exchangers with smaller internal volume and shorter and smaller diameter refrigerant hoses.
The intense engineering efforts to commercialize recycling had associated benefits in improvements in both A/C design and service procedures. Prior to the cooperative effort, vehicles were often charged using a sight glass in the tube from the compressor to the evaporator that allowed the technician to see bubbles in the refrigerant flow. The belief was that presence of bubbles indicated an undercharged system, or conversely that a bubble-free fluid would show that the system was properly charged. The research team determined that sight glasses were unreliable indicators of proper charge and that the recycling equipment could automatically recover whatever charge was in the vehicle and then recharge the amount specified for that vehicle. The proper charge increases equipment life, reduces energy use, and avoids unnecessary emissions caused when overcharged systems are vented through pressure relief valves. For manufacturers, removing the sight glass eliminated one system component and two connections that could leak.
1987 to 1990 Mobile A/C community leadership: Testing and selecting HFC-134a
Simultaneously with the work on the mobile A/C recycling agreement, the chemical industry and its refrigerant customers moved rapidly to commercialize HFC-134a, which had been identified and patented more than a decade earlier. It may be noted that the potentially environmentally preferable options of HFC-152a, CO2, and HFO-1234yf (2,3,3,3-tetrafluoropropene) are only now becoming technically feasible. At that time, CO2 was not considered suitable for use as a refrigerant in vehicle A/C systems, partly because Gustav Lorentzen kept his technology confidential from 1989 when he filed for a patent until 1993 when the patent was granted. Flammable refrigerants, including hydrocarbons and HFC-152a, were not considered because leak rates were too high and discharge of a flammable refrigerant during service was far too dangerous without fully implemented refrigerant recovery and recycling. At that time HFO-1234yf would have been considered far too costly, particularly because there was little political appreciation of climate change and little prospect of near-term restriction on the GWP of refrigerants.
HFC-134a was considered to be the most technically feasible choice to replace CFC-12 refrigerant in most A/C and refrigeration applications because it was not flammable and had toxicity, pressure, and temperature properties very similar to the CFC-12 it replaced. Additionally, its price was only 3–5 times higher than CFC-12, which was a refrigerant cost increase of as little as $2/vehicle. HFC-134a is ozone safe and has the environmental advantage of a 100-yr GWP of 1430—eight times lower than the 100-yr 10,900 GWP of the CFC-12 it replaced.
The 1987 Montreal Protocol required only a 50% reduction in CFC use, which could have been accomplished in countries with unregulated CFC aerosol products with little technical innovation. The first technical assessment for the Montreal Protocol listed HFC-134a as a potent greenhouse gas likely to be less energy efficient and thus contribute indirectly to greenhouse gas emissions from fuel burned to power refrigeration and A/C equipment (UNEP, 1989). In addition, workshops held by UNEP in preparation for the negotiations of the 1985 Vienna Convention and the 1987 Montreal Protocol also emphasized climate change topics. The 1991 TEAP report presented recently published estimates of TEWI, which combine the direct impacts of refrigerant greenhouse gases and the indirect impacts of the fuel emissions necessary to power the equipment (UNEP Ozone Secretariat, 2012).
The American Refrigeration Institute (ARI), the American Society of Heating, Refrigerating, and Air Conditioning Engineers (ASHRAE), the International Institute of Refrigeration (IIR), and the Oak Ridge National Laboratory agreed on a full spectrum of refrigerant properties and performance testing and made the information publicly available.
However, a more daunting technical challenge was the necessity to test the materials and lubricant compatibility of HFC-134a. Lubricant compatibility with CFC-12 had been through trial and error decades earlier, there was no accepted scientific method for testing compatibility, and the few existing compatibility testing experts were spread among competing organizations that had no history of cooperation.
Corporate members of the U.S. Motor Vehicle Manufacturers Association (MVMA) appreciated the value of cooperation in avoiding duplication of effort and in achieving a critical mass of focused engineering talent, but corporate attorneys warned against any collaboration that might raise antitrust concerns. The MVMA solution was to use the National Cooperative Research Act (NCRA) of 1984 (NCRA, P.L 98–462) to reduce potential antitrust liabilities of research joint ventures. The idea of the NCRA was to guarantee that the “rule of reason” standard would be a mitigating factor in any antitrust investigation if the purpose and conduct of the research were clearly in the broad public interest (CitationLink and Scott, 2001; CitationScott, 2008).
On 30 July 1987, the MVMA and the U.S. EPA were registered as a technology cooperation consortium under the NCRA for “Fluorocarbon-134a lubricants for mobile A/C systems” and subsequently the consortium was able to conduct successfully guided and coordinated testing (CitationGibson and Smilor, 1992).
The MVMA strategy was so legally impressive that the aerospace and electronics industries created the Industry Cooperative for Ozone Layer Protection (ICOLP) and the fire protection industries created the Halon Alternatives Research Consortium (HARC) under the NCRA. Noteworthy is that MVMA, ICOLP, and HARC included foreign companies that were able to cooperate in ways that may not have been allowed in their home countries.
In 1990, vehicle manufacturers in Europe, Japan, and North America made coordinated announcements that HFC-134a would be the refrigerant of choice for mobile A/C. One measure of the urgency of ozone layer protection and the confidence of industry and environmental authorities in HFC-134a is that, at that time, toxicity studies were not yet complete, HFC-134a was not approved under the U.S. EPA Significant New Alternative Policy (SNAP) program, and there were no HFC-134a manufacturing plants under construction (CitationAtkinson, 1989). Within a month of the global mobile A/C choice of HFC-134a, five global chemical manufacturers announced commercialization and began HFC-134a manufacturing plant construction. These announcements encouraged manufacturers of stationary refrigeration and A/C equipment to accept HFC-134a, partly because the mobile A/C market was large enough to assure economies of scale and competitive prices.
By 1995, the mobile A/C sector accomplished the first global sector phase-out of the use of CFC-12 in refrigeration and A/C equipment, with conversion completed for the vast majority of new car manufacture in almost most countries. The last companies in China and India halted the use of CFCs in new vehicle A/C in 2002 and 2003, respectively.
Rapid commercialization and market domination of HFC-134a not only eliminated a large portion of ODS emissions, but also built contagious confidence in the ability for new technology to be invented, demonstrated, approved by regulatory authorities, and welcomed into markets. Entrepreneurs within the fluorocarbon industry faced such stiff competition from non-fluorocarbon solutions that, in the end, only 15% of processes and products sold today that once depended on ODSs now depend on fluorocarbon solutions.
The 1993 disclosure of the Lorentzen CO2 system captured the imagination of engineers at Daimler and a few other vehicle companies who wanted to eliminate HFC refrigerants without adversely affecting cooling performance or fuel efficiency. In 1994, CO2 advocates found strong support from the European Community Industrial and Materials Technology Program, which funded the “Refrigeration and Automotive Climate under Environmental Aspects” (RACE) program.
The consortium adopted CO2 as the most technically and economically feasible option, and in just three years, RACE advanced the technology and built two working prototypes for a BMW 520i and a VW Polo (CitationECCRDIS, 1997). One outcome of the cooperation was a joint patent by BMW and Daimler for a CO2 vehicle A/C system that was designed to cool and also to heat vehicles lacking waste heat from internal combustion engines, such as plug-in hybrid, electric, or fuel cell vehicles. The heat pump technology would also heat conventionally powered vehicles faster than waiting for the engine to warm up.
In 1997, RACE presented its results at the Earth Technology Forum in Washington, DC, and, in 1998 brought the prototypes to Phoenix, AZ, for an informal meeting with global industry leaders. The Phoenix “ride and drive” testing confirmed cooling performance. In Phoenix, the RACE member companies transformed the European consortium into the “Phoenix Forum” with SAE sponsorship, global participation, and a wider agenda that created healthy competition among several options:
| • | Enhancing the existing HFC-134a systems. | ||||
| • | Choosing the low-GWP HFC-152a refrigerant as a near drop-in replacement. | ||||
| • | Taking the revolutionary leap to the entirely new CO2 technology. | ||||
At the 1999 meeting of the Phoenix Forum, participants organized the Mobile Air Conditioning Climate Protection Partnership (MACCPP) (CitationU.S. EPA, 1999b) with a mission statement to reduce refrigerant greenhouse gas emissions by at least 50% (achieving less than 20 g per year emissions) and A/C fuel consumption by at least 30%. The partnership teamed up with the SAE's Alternative Refrigerants Cooperative Research Program (CRP), and with U.S. EPA and the U.S. Department of Energy's National Renewable Energy Laboratory (NREL) on cooperative projects to enhance the environmental performance HFC-134a mobile A/C.
In 2000, European national and EC environmental authorities began serious consideration of controls on HFCs and five other GHGs (CO2, CH4, N2O, PFCs, and SF6). The EC saw the advantage of harmonized global regulation and invited participation of environmental and industry associations, notably the U.S. EPA, SAE, and the Mobile Air Conditioning Society. This collaboration led to several improvements in the proposal including allowing HFC-152a (GWP 124), shifting the phase-out date to 2017, and consideration of energy efficiency in addition to a refrigerant GWP limit.
Meanwhile, by 2001, Daimler engineers were confident enough in CO2 technology to recommend it to management for the redesigned S-Class Mercedes Benz, which already had many of the sophisticated monitors and controls necessary to operate the more complicated CO2 system. However, in late 2002, Daimler chose to delay introduction because the system suppliers bid higher than the company reservation price and because, despite lobbying for HFC regulation by Daimler, the European Commission (EC) was unable to confirm its intentions either to control HFC-134a or to reward HFC-free systems (CitationAndersen and Zaelke, 2003). However, in December 2002, Toyota's fuel-cell hybrid vehicle was launched for street testing with a Denso electrically driven, hermetically sealed air conditioner and heat pump using CO2 refrigerant.
In February 2003, executives of the EC concluded after two days of technical presentations that “HFC-134a is an unsustainable option for mobile A/C that could be phased out within the decade.” The final EC “F-gas Directive” proposed in August 2003, and agreed in May 2006 (Directive 2006/40/EC of the European Parliament and the Council), that air conditioners sold on new “type” vehicles in the E.U. after 2010 and all vehicles sold in the E.U. after 2017 use refrigerants with 100-yr GWP < 150.
The original EC proposal included additional incentives and flexibility that were removed at the request of European vehicle manufacturers. These incentives included an elegant combination of transferable quotas and credits and a “safety valve” that would have allowed vehicle manufacturers to pay 100 euros for each vehicle sold if they were unable to meet the deadline for particular models. One HFC-134a credit was to have been earned for every two “Enhanced 134a Systems” sold prior to 2014, with enhanced systems defined as having less than 20 g emissions for single evaporator systems and less than 25 g emissions for systems with a double-evaporator; one credit is earned for each “alternative system” (CO2, 152a, or HC) sold prior to the start of phase-out.
At the time of the 2006 EC announcement, the only publicly disclosed refrigerants satisfying the criteria of GWP < 150 were hydrocarbons (GWP ˜5), CO2 (GWP = 1), and HFC-152a (GWP ˜124). However, within weeks of the EC F-gas Directive, more than five international chemical manufacturers—including Asahi, Arkema, DuPont, Honeywell, Ineos, and Sinochem—announced new fluorocarbon refrigerants with GWP below 150. Soon it became clear that the most suitable of the candidate fluorocarbons was hydrofluoroolefin (HFO)-1234yf, which has a GWP ˜4 and is comparable in toxicity to CFC-12 and HFC-134a, but is mildly flammable. Refrigerants are classified by American National Standards Institute/American Heating, Refrigerating, and Air-Conditioning Engineers (CitationANSI/ASHRAE, 2007; CitationANSI/ASHRAE, 2010) into a matrix diagram of safety groups, considering flammability and toxicity. shows the definition of ASHRAE toxicity and flammability groups. HFC-32 and HFO-1234yf are group A2L (lower flammability and toxicity), while HC-290 and HC-600 are group A3 (higher flammability and lower toxicity).
Table 8 . Best-in-class and worst-in-class mobile A/C refrigerant leakage (g/yr) by vehicle type
For the next five years, CO2 and HFC-152a competed with HFO-1234yf. Despite their low GWP and very low cost, hydrocarbons were never seriously considered by any major automaker, and advocates of hydrocarbons never organized the technical teams necessary to achieve U.S. EPA SNAP approval. HFC-152a was first to be SNAP listed for mobile A/C in June 2008 (CitationU.S. Federal Register, 2008), HFO-1234yf was SNAP listed for mobile A/C in March 2011 (CitationU.S. Federal Register, 2011), and CO2 was SNAP listed for mobile A/C in June 2012 (CitationU.S. Federal Register, 2012).
Because HFC-152a is mildly flammable and HFO-1234yf is mildly flammable, SNAP requires that safety hazards be mitigated in accordance with SAE design standards, which limit refrigerant charge and isolate potential leaks from sources of ignition. Because CO2 can asphyxiate or diminish driver capacity, SNAP requires that system designs ensure that a short-term concentration of 15 minutes or more is <3000 ppm CO2 and that 4000 ppm CO2 is never reached, and that risk be mitigated in accordance with SAE design standards, which limit refrigerant charge and keep refrigerant from occupied spaces.
HFC-152a has the advantages of a low GWP (124), energy efficiency significantly higher than HFC-134a, low price from existing chemical manufacturing facilities, and its patents have expired so it can be competitively produced by a wide range of suppliers. The challenge was to quantify the risk of the refrigerant reaching flammable concentrations in the passenger compartment. U.S. Army experts on the team had faced similar analytical challenges in designing armored vehicles to reduce the risk of fuel and other flammable fluids during normal operations and combat. They adapted computational fluid dynamics (CFD) models to calculate the characteristics of the worst-case refrigerant-air mixture for a plume from an A/C evaporator under the vehicle dashboard. This work confirmed that a conventional A/C system would be unsafe with HFC-152a refrigerant but that a redesigned system would be safe if the refrigerant were kept out of the passenger compartment or if the quantity and rate of discharge into the passenger compartment were kept within safe limits.
The HFC-152a design team identified several mitigation strategies for the safe use of HFC-152a. The safety mitigation strategy—called a “secondary loop”—uses an under-hood heat exchanger where the flammable refrigerant cools a nonflammable fluid that is then circulated in the second loop to an under-dash heat exchanger. In this design the refrigerant can leak only into the engine compartment, which is heavily vented by the cooling fan and vehicle movement. The second safety mitigation strategy places a reliable electronic sensor in the chamber around the evaporator to detect refrigerant leakage and trigger a valve that discharges the refrigerant charge harmlessly away from the vehicle occupants. Both strategies require that A/C components in the engine compartment be isolated from ignition sources.
The secondary loop safety strategy has the advantage of being a fail-safe design that requires no action by the driver or passenger. The leak detector safety system has the disadvantage of requiring a reliable leak detector that accurately identifies a serious release, but avoids false positives. The secondary loop system has the advantage of using the thermal ballast of the secondary loop fluid to continue cooling the vehicle when the engine is stopped to save fuel at intersections or in congested traffic. The “idle stop” feature on hybrid and conventional vehicles is usually active when temporary loss of cooling is acceptable to passengers.
HFO-1234yf has the advantage of very low GWP, energy efficiency comparable to HFC-134a, and low toxicity. It has the disadvantages of high price, slight flammability, limited production facilities, and patents by Honeywell claiming exclusive right to manufacture or license the manufacture despite at least five chemical companies having patented chemical pathways in manufacture.
HFO-1234yf is so difficult to ignite that it need not be kept from occupied spaces where there are no high-energy sources of ignition, but A/C components, including hoses, in the engine compartment must be physically separated from sources of ignition. Refrigerant from a leak must not contact hot exhaust parts or electrical sparks from the ignition system or relays. HFO-1234yf is similar in toxicity to CFC-12, HFC-134a, and HFC-152a. HFC-1234yf also has the undesirable atmospheric fate of producing trifluoroacetic acid (TFA), a naturally occurring substance, which will be increased in concentration unless HFO-1234yf refrigerants are contained without leakage. The scientific assessment panels of the Kyoto and Montreal Protocols have judged that future concentrations of TFA will not pose a significant environmental risk, even if HFO-1234yf replaces all current applications of HFC-134a (CitationMetz et al., 2005; CitationSAP, 2010).
CO2 has the advantages of low GWP, nonflammability (in fact, a fire-extinguishing agent), and wide availability at low cost. It has the disadvantage of low cooling capacity and high energy consumption when operating at high ambient temperatures when A/C is most needed. There are few specific patents on overall design and on specific components. CO2 at low concentrations may cause neurotoxin effects such as drowsiness, distraction, and increased reaction time that diminish driver capacity in ways similar to alcohol or drug use. In the United States and many other countries health and environmental authorities consider both asphyxiation and neurological effects, but the EC only officially considers asphyxiation risk.
The CO2 cooperative design team identified several safety mitigation strategies, including detection and discharge and the use of a stenching gas. As in the case of HFC-152a, the leak detection must sense release without false positives. This is complicated in the case of CO2 because normal air contains 350 ppm CO2 with substantial variability from nearby emitters, including other engines and human exhalation.
The stenching gas strategy (based on distinctive unpleasant odor) faced uncertain regulatory hurdles in proving it was safe, proving that it was compatible with lubricants and materials, and proving that people would respond appropriately to the smell (roll the windows down and stop and exit the vehicle). The stenching proposal failed because all the stenching agents tested were absorbed in the A/C lubricating oil and lost the telltale aroma within weeks.
U.S. barriers to the adoption of low-GWP energy efficient refrigerants proved indicative of the situation in most developed countries:
| • | Department of Transportation accumulator pressure requirements. | ||||
| • | State bans on toxic and flammable auto A/C refrigerants. | ||||
| • | Occupational Safety and Health Administration (OSHA) requirements for training, personal protective equipment, safe handling, pressure relief devices, equipment inspection, worker exposure, ventilation, and refrigerant storage. | ||||
| • | U.S. EPA Significant New Alternative Program (SNAP) and Toxicity Program review. | ||||
| • | National Highway Traffic and Safety Administration (NHTSA) requirements. | ||||
In some cases barriers were removed by administrative decision based on technical information provided by the MACCPP team and reviewed by government authorities. The U.S. Department of Transportation waived the accumulator pressure requirement with an interpretation: “[Air conditioning systems] are an integral component of a motor vehicle and necessary for the operation of the vehicle” and “Based on the information you provided, the air conditioning system … is not subject to the Hazardous Materials Regulation” (Gale, 2006).
In another application of administrative decision, OSHA declared that SAE J-Standards for servicing CO2, HFC-152a, and HFO-1234yf mobile A/C systems would satisfy requirements for training, personal protective equipment, safe handling, pressure relief devices, equipment inspection, worker exposure, ventilation, and refrigerant storage. One advantage of OSHA citing the SAE standard is that SAE updates in response to accident experience or revised engineering calculations immediately have the full effect of law.
In 2006, 18 U.S. States and the District of Columbia had laws that banned flammable refrigerants, often without defining or specifying what was toxic or flammable, and 12 states banned toxic refrigerants. It was important to remove state bans on toxic and flammable refrigerants because HFC-152a and HFO-1234yf are flammable and CO2 is toxic. These state bans were mostly enacted in the early 1990s when unethical and unscrupulous entrepreneurs were trying to take advantage of the public desire to protect the stratospheric ozone by marketing highly flammable and sometimes toxic refrigerants for recharge of vehicle air conditioners designed for CFC-12.
The strategy in removing these state barriers was to assemble a government–industry team of respected authorities to create safety standards that would allow the safe use of refrigerants listed under the U.S. EPA SNAP regulation. The Alliance of Automobile Manufacturers and the Association of International Automobile Manufacturers (now renamed as the Association of Global Automakers) were particularly influential. The team developed a comprehensive list of states with specific barriers, determined which could be removed by administrative ruling and which would require new legislation, identified one or more key points of contact, and assembled the technical and environmental justification for the proposed changes.
One by one, over a period of about three years, these outright bans were either removed or replaced. The typical regulation replacing the outright prohibition used a combination of U.S. EPA and SNAP approval adherence to SAE J-Standards for health and safety, specific exemption from the ban on flammability for HFC-152a and HFO-1234yf, or clarification that the ban on flammable refrigerants applied only to hydrocarbons.
The mobile A/C LCCP system model (CitationHill and Papasavva, 2005) became the starting basis for the SAE Interior Climate Control Committee working group that developed and disseminated a global template for corporate, government, and public use. The LCCP harmonization activity was opened to the entire automotive industry in 2006 with strong participation of SAE, Verband der Automobilindustrie (VDA, the German Association of the Automotive Industry), and Japan Automobile Manufacturers Association (CitationPapasavva et al., 2008). Papasavva and Hill—with a global team of 50 world experts from 32 industries representing governmental and nongovernmental organizations, national laboratories, and academia—perfected and continuously improved their LCCP model, with input and data from automobile manufacturers and suppliers and by including state-of-the-art cabin comfort conditions using modeling results provided by participants from the NREL (CitationJohnson, 2002).
The public domain model—named GREEN-MAC-LCCP—uses globally harmonized data and assumptions, and provides a more realistic application of the engineering data obtained from bench tests because it applies them to various driving cycle engine conditions. Most of the input data are fixed and based on the harmonization process (CitationU.S. EPA, 2009). This prevents manipulation of the modeling process to influence results to benefit a particular technology and makes sure that comparisons are fair. Only a small amount of input data is required to run the model, which is unique to each refrigerant such as its GWP value, its system efficiency, and so on. The model allows the user to enter coefficient of performance (COP) and evaporator cooling capacity data obtained by laboratory bench-test experiments that were confirmed by consensus of the SAE Strategic Alliance, and the SAE Interior Climate Control Committee (SAE, 2004).
GREEN-MAC-LCCP analyzes up to six alternative refrigerants and compares them with a fixed HFC-134a baseline. Model output results provide annual and lifetime LCCP CO2-equivalent per vehicle for 67 world cities and lifetime LCCP CO2-equivalent for global fleets in various world regions. For each city, the user can select among three different vehicles (small, midsize, sport-utility), and four alternative fuels (gasoline, diesel, ethanol, and methanol). The model is also flexible to analyze LCCP GHG emissions from global fleets running with A/C on, for any given year during 2008–2017.
GREEN-MAC-LCCP has become the global standard methodology for assessing the climate impact of mobile A/C systems and has been approved as an SAE technical standard (SAE J-2766).
To reduce refrigerant emissions it was necessary to first estimate those emissions—what gets measured is what gets managed. Previous analysis by the IPCC/TEAP had estimated the portion of global HFC emissions from vehicle A/C, and previous analysis by the EC had estimated vehicle refrigerant leak rates.
The IPCC/TEAP had used a “top-down” approach that reconciled atmospheric concentrations of HFC-134a against the reported annual global manufacture of HFC-134a and allocated emissions by sector using best available judgment (CitationMetz et al., 2005). Analysis commissioned by the German Ministry of Environment (MoE) and the EC had used a “bottom-up” approach that subtracted the amount of refrigerant recovered from an A/C system from the charge amount at the time of manufacture, and divided by the age of the vehicle to yield annual leak rate (CitationSchwartz, 2001; CitationSiegl and Wallington, 2002; CitationSchwartz and Harnisch, 2003). The German MoE and the EC study used unrepresentative samples, questionable assumptions about vehicle repair history, and uncalibrated recovery equipment and scales, all of which likely biased the results.
The partnership undertook a more scientific “bottom-up” approach by analyzing confidential records of warranty repair for new vehicles and service records for large vehicle fleets, and by surveying Mobile Air Conditioning Society members, to report the refrigerant use in service for vehicles no longer receiving warranty repair from manufacturers. In addition, an engineer from GM designed an experiment to precisely charge used vehicle A/C systems to the amount recommended by the manufacturer and later subtract the amount of refrigerant recovered from the system to quantify the amount that had leaked out.
As part of the test protocol, it was discovered that recovery machines did not recover the full amount of refrigerant just charged into a completely empty A/C system. These results were confirmed on variety of vehicles using different recovery machines, including brand new equipment. The explanation was that a portion of the refrigerant charge remains in the system—absorbed in the oil and trapped in system components even after being subject to the strong vacuum of a recovery machine. The implication was that the EC analysis overestimated the emission rates by the amount not recovered and that the recovery equipment standard needed to be revised to provide a stronger vacuum for a longer period of time.
The new HFC-134a recovery/recycling/recharging (RRR) equipment standard SAE J-2788 requires 95% refrigerant recovery, compared to 50–75% recovery from the older equipment designed to SAE J-2210 (CitationSAE, 2013). The new SAE standard for electronic leak detectors increases their leak detection sensitivity from 14 g per year to 4 g per year, which allows technicians to confirm that repairs are as leak-tight as possible.
The SAE J-2834 standard for HFO-1234yf RRR equipment requires not only high efficiency of refrigerant recovery but also a new two-step process to avoid recharge without repair. In the quest for improved HFC-134a systems, SAE also developed the J-2727 standard for estimating new vehicle refrigerant leak rates based on the quality of components, permeability and length of refrigerant hoses, the number of connections and ports, and seal design. Manufacturers publish the estimated leak rate for all vehicles sold in Minnesota (CitationMinnesota, 2008; CitationMPCA, 2011). Later, California required J2727 as the basis for achieving a fleet average refrigerant leak rate of less than 9 g per year in compliance with “LEV III A/C Requirement 2: Fleet Average Leak Rate ≤ 9 g/yr” (CitationCARB, 2012). MPCA (2011) data indicate dramatic improvements between 2009 and 2012 best-in-class leak rates for minivans and passenger automobiles but little progress in pickup trucks, sport-utility vehicles (SUVs), and full-sized vans. This is shown in . The MPCA data indicate that if all manufacturers achieved the leak rates of the best-in-class, total fleet emissions could be reduced by about half.
Until recently, energy use for A/C, power steering, and alternators, as well as rolling resistance and aerodynamics, has not been part of automobile fuel efficiency tests and has not been factored into fleet mileage standards or fuel mileage labels. With no specific testing of A/C refrigerant use, manufacturers had little incentive to spend more on improved A/C systems. Outsourcing contracts for A/C systems specified cooling capacity, reliability, noise, and vibration, but not energy efficiency. Many A/C systems were designed to operate at full cooling capacity and to control comfort by reheating the air, as needed, rather than controlling the system to provide the desired cooling comfort with the least amount of energy.
Increasing mobile A/C fuel efficiency can make a considerable contribution to reducing CO2 emissions because 3 to 20% of vehicle fuel use is for A/C, demisting, and defrosting. If automakers achieved the MACCPP goal of a 30% increase in fuel efficiency worldwide, the annual savings would be 26 billion liters (7 billion gallons) of fuel in the United States, 6.9 billion liters (1.8 billion gallons) in Japan, and 20 billion liters or more in the rest of the world.
When increased fuel efficiency became a priority, engineers identified new technologies including microchannel heat exchangers, computer-controlled electronic expansion valves, better airflow, and component optimization.
Toyota engineers determined that a variable displacement compressor controlled by the engine control module could save energy with “regenerative cooling,” which increases compressor displacement during deceleration to cool the evaporator and controls interior comfort by adjusting the vent fan speed. When the vehicle is not decelerating, the compressor only provides cooling if the evaporator is too warm to maintain comfort levels with the engine control module programmed to increase compressor displacement when the engine can provide the power with the least amount of fuel (for example at constant vehicle speed rather than during acceleration). Toyota has achieved up to 50% reduction in A/C fuel use with this strategy alone.
GM engineers determined that window defogging could be achieved with less fuel use by controlling the evaporator temperature to more efficiently dehumidify the air being directed to the windows.
NREL applied integrated modeling, assessments of optimized techniques to deliver conditioned air to vehicle occupants, thermophysiological modeling, and studies of waste-heat cooling and heating opportunities (CitationRugh and Farrington, 2008), and the following approaches emerged:
| • | Reduce the thermal load by using solar-reflective glass or shades and parked-car ventilation. | ||||
| • | Incorporate low mass, naturally ventilated seating (or active climate-control seating). | ||||
| • | Consider advanced cabin insulation and solar reflective paint to minimize solar heat gain. | ||||
| • | Incorporate the most efficient refrigerant and A/C components available. | ||||
| • | Maximize the use of recirculated air, considering air quality, dehumidification, and safety issues, while avoiding window condensation. | ||||
| • | Eliminate the overcooling and subsequent reheating of air with automatic control systems. | ||||
Delphi engineers determined that the improved energy efficiency of the refrigerant-to-fluid heat exchanger located in the engine compartment in a secondary-loop A/C system could offset the energy required for the additional liquid-to-air heat exchanger in the passenger compartment. They also figured out that the thermal mass of the secondary loop fluid can be used to extend the interval of cooling during idle stop, thus avoiding restarting the engine only to provide A/C.
GREEN-MAC-LCCP determined that HFO-1234yf and HFC-152a systems would provide lower life-cycle carbon footprints for vehicles operating in all but the mildest climates, where CO2 systems had a slight carbon footprint advantage.
The MACCPP (Weisler 2009) concluded in 2008 that HFO-1234yf is the refrigerant of choice to replace HFO-134a in motor vehicle A/C and in 2009 announced “cooperation aimed at accelerating the commercial introduction of improved A/C systems in U.S. and global markets using refrigerant HFO-1234yf which can significantly reduce the carbon-equivalent emissions of greenhouse gases while increasing vehicle fuel efficiency” (CitationVASA, 2009). The decision came just two years before the first “new type” vehicles would require a refrigerant with GWP below 150 in order to be sold in the EU.
In spite of some potential advantages, the motor vehicle industry did not select CO2 as the new refrigerant of choice because of higher energy requirements, difficult leak testing in air with 350+ ppm CO2, poor reliability, and high cost. CO2 has much higher operating pressures and that would require a complete reengineering of mobile A/C systems to deal with the high pressures—at great cost to the industry both for initial introduction and as a continuing manufacturing requirement.
In the United States, the U.S. EPA now offers a credit for a low-GWP refrigerant like HFO-1234yf toward Corporate Average Fuel Economy (CAFE). In Japan, the quantities of HFC-134a available to vehicle manufacturers are restricted, which creates incentives to minimize system mobile A/C system charge, and recovery at vehicle disposal is paid from a deposit paid on new car sales (like recycling deposits on the sale of new tires and batteries).
In Australia, there is a new tax on the imports of greenhouse gas refrigerants in bulk or contained in products that acts as an incentive to minimal use and emissions and as a source of funding for recovery, reuse, and destruction.
Progress and setbacks
Unlike the four-year global transition from CFC-12 to HFC-134a, the transition from HFC-134a to HFO-1234yf has been fraught with complications, even as public and policy support grows for a global transition to HFO-1234yf.
Honeywell legal claims of patent monopoly slow HFO-1234yf uptake, but its monopoly is gradually weakened by litigation.
A legal complication, which slowed technical progress, was initiated in 2009 by Honeywell claiming “application patents” in Europe (EP 2 163 592 A2) and the United States (CitationU.S. Patent, 2004) for the use of HFO-1234yf as a refrigerant. If granted, such claims could result in monopoly profits and inadequate supply that could slow the adoption and use worldwide while increasing costs.
U.S. environmental NGOs petition U.S. EPA to remove HFC-134a from the list of acceptable refrigerants for mobile A/Cs.
In May 2010, the Natural Resources Defense Council (NRDC), the Institute for Governance & Sustainable Development (IGSD), and the Environmental Investigation Agency (EIA) petitioned U.S. EPA under the SNAP program to remove HFC-134a from the list of acceptable substitutes for CFC-12 for use in motor vehicle air conditioners. On February 14, 2011, U.S. EPA announced that the petition was complete for new passenger cars and light duty vehicles and that U.S. EPA has the authority to revise this list on its own, or in response to a petition, to remove a substitute previously listed as acceptable. A likely outcome would to be a U.S. phase-down no faster than prescribed by the EC F-gas Directive.
The Japanese earthquake and tsunami destroy the Asahi HFO-1234yf production facility supplying HFO-1234yf to Honeywell/DuPont customers and a cumbersome regulatory process slows the construction of the Chinese DuPont/Honeywell HFO-1234yf facility.
In late 2011, DuPont and Honeywell—the only chemical suppliers legally allowed to supply HFO-1234yf under the Honeywell monopoly application patents—notified the EC that they were unable to supply the quantities of HFO-1234yf necessary for companies to comply with mobile A/C F-gas Directive because the refrigerant production facility in Japan had been disrupted by the earthquake and tsunami, and the facility for mass production in China had been delayed by an unexpectedly cumbersome regulatory approval process. In March 2012, the EC decided to allow vehicles to use HFC-134a until adequate quantities of HFO-1234yf became available, and with a definitive limitation on 31 December 2012, if refrigerant HFO-1234yf is not available, provided that vehicles are fitted with mobile A/C systems that are compatible with Directive 2006/40/EC (CitationECEID-G, 2012).
The high prices and unreliable supply of HFO-1234yf had consequences to the automobile manufacturers that were forced to slow product introduction; to the suppliers of mobile A/C systems and components; to the suppliers of custom recycling equipment, leak detectors, and other tools only useful in servicing HFO-1234yf systems; and, of course, to the protection of the climate. The suspension of enforcement of the EC mobile A/C Directive as a consequence of refrigerant shortage weakened the confidence of companies depending on clarity and reliability of regulation and reliable chemical supply.
Daimler Announces Mercedes-Benz automobiles unsafe with HFO-1234yf.
On September 25 CitationDaimler (2012) reported that a new vehicle crash study demonstrated that HFO-1234yf ignited when discharged onto hot exhaust pipes. Daimler went on to announce that HFO-1234yf will not be used in its automobiles and that it intended to revert to HFC-134a for the immediate future, which after 1 January 2013 will be a violation of the F-gas Directive. On September 28, 2012, Mercedes-Benz announced a voluntary safety-related recall of U.S. vehicles equipped with HFO-1234yf systems (CitationMACS, 2012).
As part of its normal ongoing product testing, DAG (Daimler AG) subjected a vehicle not sold in the U.S. to a test program designed to replicate worst-case conditions which exceed normal industry and governmental test standards. During this testing, DAG became aware that in the event of a severe frontal impact that would also cause a rupture of the refrigerant line, there is a possibility that the refrigerant may ignite in the engine compartment under these worst-case conditions. Under normal temperature and operation conditions the refrigerant does not ignite. (CitationMercedes-Benz, 2012)
The Daimler announcement is inconsistent with the unequivocal safety endorsement presented two weeks earlier “on behalf of the German manufacturers and VDA” that endorsed HFO-1234yf as “a new, safe and environmentally friendly refrigerant” for mobile A/Cs and went on to elaborate:
| none | “Ignition of R1234yf is nearly impossible in the engine compartment considering operating conditions of the vehicle.” | ||||
| none | “Ignition of R1234yf is impossible inside the vehicle cabin in reality.” | ||||
| none | “Ignites only at presence of an open flame or high energy spark.” | ||||
| none | “Inflammability observed only when sprayed on hot surfaces above 900°C (pure R1234yf) (or) 700°C (with 3 percent PAG lubricant)” (CitationHammer et al., 2012). | ||||
At the time of the Daimler announcement, five automakers were reported to each have at least one model with HFO-1234yf systems and two other companies nearing production. DuPont, Honeywell, and GM publically defended the safety of HFO-1234yf, but other automakers have been mostly silent (CitationGaved, 2010; CitationGM 2010). In October, SAE launched a new Cooperative Research Program (CRP) to further analyze the safety of HFO-1234yf. CRP participants include Audi, BMW, Chrysler, Daimler, Ford, GM, Honda, Hyundai, Jaguar Land Rover, Mazda, PSA, Renault, and Toyota.
In early December 2012, SAE reaffirmed that its 2009 review had concluded that HFC1234yf “is a safe and acceptable alternative refrigerant for mobile air conditioning systems that can be used to meet new environmental and consumer needs” and reported that “the majority of the OEMs involved in the new CRP do not believe that any of the new information reviewed will lead to a change in the overall risk assessment” (CitationSAE, 2012).
Later in December, the EC reaffirmed its conclusion that HFO-1234yf is safe to use and rejected Daimler's request for a delay in the transition to refrigerants with GWP less than 150:
Detailed risk assessments and standardisation processes were conducted with this objective, involving all manufacturers, which concluded that the risk of the use of this gas was equivalent or inferior of other flammable fluids used in vehicles, including gasoline. (CitationEC, 2012)
In March, the EC reiterated its intention to strictly enforce the MAC Directive and strengthened its resolve to enforce law, stating, “According to Framework Directive 2007/46/EC, it is not possible for motor vehicles to be registered and marketed in the EU if they are not in conformity with the relevant legislation” (CitationEC, Citation2013b).
On April 23, 2013 the SAE concluded that: “…the refrigerant release testing conducted by Daimler is unrealistic…The Daimler testing did not include any actual vehicle collisions or the mitigating factors that occur in an actual collision. These factors include the quenching effect of front end compartment deformation, the extinguishing effect of steam released due to radiator breakage, and dispersion of the refrigerant from the condenser outside the engine compartment. Daimler's refrigerant release apparatus and nozzle does not represent actual crash-damaged refrigerant lines, and was found to be artificial.” (SAE, 2013a).
Currently, most motor vehicle manufacturers, the U.S. EPA, SAE International, and a number of environmental NGOs all agree that HFO-1234yf is safe. Manufacture is under way, and plans to use it as the “new MAC refrigerant” are moving ahead.
Lessons and Conclusions from Refrigerant Transitions in Motor Vehicle A/C
Without the Montreal Protocol and the national actions that preceded it, an estimated two-thirds of the ozone layer would be depleted by 2065 and climate change would have been accelerated. The Montreal Protocol is succeeding at protecting the stratospheric ozone layer and in helping protect the climate because of global public and political support, stringent treaty controls and national legislation, financing of the incremental costs of phase-out in developing countries, and extraordinary technical progress fostered by strong industry–government cooperation.
Environmentally acceptable alternatives and substitutes have been globally commercialized and used for each of approximately 240 industry and military sectors that were once dependent on ODSs. Every United Nations state is a member of the Montreal Protocol and every country is in full compliance. Ninety-eight percent of ODS production and consumption of nearly 100 industrial chemicals has been phased out to the satisfaction of public, corporate, and government stakeholders. The transition has been so smooth that science skeptics are silent, few scholars find fault with the treaty or its implementation, companies and military organizations are proud of what has been accomplished, and consumers have hardly noticed.
These stratospheric ozone protection and reduction of climate forcing gas emissions successes were accomplished in many sectors of industry. Automobile manufacturers and service associations worldwide have been strong supporters of stratospheric ozone protection for more than 25 years of both breakthrough and continuous improvement in leak-tightness, refrigerant transition, and best service practices. United States-based organizations—particularly SAE, the Mobile Air Conditioning Society, and the automobile manufacturers associations—have been technology pathfinders to the world. Industry–government cooperation was critical in recovery and recycling equipment development, approval, and commercialization; fast introduction of HFC-134a; and redesign of A/C systems for lower total charge, and lower annual and life-cycle leakage. Restricting the sales of CFC-12 to certified technicians reduced emissions by at least 50% and increased the reliability and operating energy efficiency of the new systems.
Unanticipated consequences of the Montreal Protocol include the current recognition of the need to phase out HFC-134a only 20 years after its introduction and acceptance as the “ultimate” A/C refrigerant gives cause for concern. There were also conspicuous failures to minimize HFC-134a emissions and to prevent refrigerant contamination during recycling. Worldwide, emissions of HFC-134a would be far less if environmental authorities required and enforced the use of best available control technology (BACT) for refrigerant components and seals, required and enforced frequent upgrades to the most efficient recovery equipment and most sensitive leak detection, and if the price of HFC-134a were high enough to be an effective incentive in repairing leaky systems prior to recharge and high enough to reward auto disassembly enterprises for recovery and recycle. In the United States and other countries with untrained and do-it-yourself (DIY) repair of vehicle air conditioners, HFC-134a emissions could be much reduced if refrigerants were sold only to certified technicians.
Furthermore, the carbon footprint of mobile A/Cs can be further reduced by the rapid transition to low-GWP refrigerants and high energy efficiency using the technology already identified and demonstrated by the mobile A/C industry–government partnerships and by incentivizing further innovation.
In the United States, certified technicians using leak detectors and recycle/recovery equipment service 90% of the fleet of vehicles with the same amount of refrigerant used by DIY car owners to service 10% of the vehicle fleet. Lack of technician training is also a contributing factor for higher emissions, especially in cases of contamination of vehicle A/C systems with the wrong refrigerant, failure to identify and repair leaks, and overcharging of systems. In the EC, HFC-134a emissions could be significantly reduced if technicians were required to use the latest recovery/recycle/recharge equipment. In all countries, ozone-depleting and greenhouse gas refrigerants could be collected and destroyed and the transition to refrigerants with low GWP and high energy efficiency could be accelerated.
The success of the Montreal Protocol proves that global cooperation can work, that there is synergy in jointly protecting the ozone layer and climate, that government–industry cooperation can complement conventional regulations, and that global economies of scale in new technology can make environmental protection more affordable than ever (CitationKauffman, 1997; CitationNorman, et al., 2008). The challenge is to continue using the best available science, to involve the wider environmental community in pursuing energy efficiency and health co-benefits, and to becoming more agile in taking fast action.
Looking to the Future: The Next 25 years of the Montreal Protocol
HFC phase-down: Will the Montreal Protocol complement the Kyoto Protocol?
HFCs are almost exclusively used as alternatives to ODS, but are no longer needed in many applications. A phase-down of HFCs would produce an immediate benefit for climate protection due to their high GWPs and short atmospheric lifetimes. The Kyoto Protocol controls the emissions of HFCs, but the Montreal Protocol would control the production and consumption.
N2O, n-propyl bromide and beyond: Will the Montreal Protocol control neglected ODSs?
N2O is the largest identified anthropogenic threat to the stratospheric ozone layer and it is also a climate greenhouse gas. n-Propyl bromide (nPb) and RC-316c (1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane, CAS 356-18-3) are ODS solvents with unknown market potential not controlled by the Montreal Protocol. The Montreal Protocol could be amended to control known ODSs listed by name with appropriate phase-down schedules, and to control unknown ODSs listed by chemical description and a preemptive control schedule (say, capping production and consumption at 100 kg). This would allow research quantities but require an adjustment to the protocol when commercialization was contemplated.
HCFC phase-out: Faster and with exemption of HCFC-123 for building chillers?
The phase-out of HCFC is currently scheduled to reduce HCFCs by 99.5% in developed countries by 2020 and by 97.5% by 2030 in developing countries, with 10 more years each before 100% phase-out to allow for servicing of existing equipment. Because most HCFCs have high GWPs, it is likely that 2020 and 2030 dates will be moved forward.
The intriguing exception to an HCFC phase-out is that there is little, if any, environmental merit in phase-out of HCFC-123 for use in building air conditioner chillers. HCFC-123 has a low GWP100yr of 77 and a low ODP of 0.02. HCFC-123 is a liquid at atmospheric temperature and pressure, which allows near-zero emissions, and it achieves higher energy efficiency than any other currently available refrigerant. Of course, the GWP and ODP are environmentally irrelevant if there are no emissions. So the question is whether the Montreal Protocol by Adjustment or global Essential Use Exemption will allow for its continued use if no superior technology is commercialized.
New emphasis on treaty synergy and environmental co-benefits?
The Montreal Protocol incidentally improved the environmental performance of products once dependent on ODSs. This occurred through the influence of environment agencies like U.S. EPA that had authority to approve or disapprove alternatives and substitutes and through the global network of ozone offices and financing organizations that collaborated on technology choice. A more deliberate and synergistic approach could capture co-benefits of energy efficiency, clean air, local sourcing, and sustainability.
Continued exemption for feedstock and process agents?
Because Feedstock and Process Agent uses are exempt from Montreal Protocol controls, there has been little incentive to commercialize chemical pathways to produce the same final goods and services avoiding ODSs or to commercialize alternatives and substitutes for the products made with ODS. For example, there is an aqueous alternative to the manufacture of ballistic armor currently manufactured using CFC-113 as a process agent and there are many alternatives to products made with polytetrafluoroethylene (PTFE, commonly known as Teflon). HCFC-22 feedstocks are not significantly emitted in the manufacture of PTFE, but HFC-23 (GWP100yr =14,800) is emitted as an unwanted by-product of HCFC-22 production if it is not captured and destroyed.
What does the future hold for mobile air conditioning?
With the exception of German companies, HFO-1234yf is the global industry choice as a replacement for HFC-134a and will be the choice for German vehicles for the immediate future if the EC enforces its regulation. The questions are: (1) Will HFO-1234yf be implemented on the same schedule in developing and developed countries? (2) Will CO2 vehicle A/C be offered in temperate climates where energy efficiency and fuel costs are low priority? (3) Will fundamental changes in vehicle design (electric, fuel cell, and hybrid) allow more toxic, flammable, or high-pressure refrigerants to be safely contained in hermitic systems?
Will environmental, health, and safety authorities stop trade in counterfeit and controlled refrigerants and DIY service?
The life-cycle benefits of environmentally superior refrigerants are diminished in systems that are recharged with obsolete low-cost refrigerants. The illegal trade in ODS refrigerants jeopardizes the success of stratospheric ozone protection. The use of counterfeit refrigerants, often containing toxic and explosive ingredients, jeopardizes health and safety and the profitability of recycling when contamination prevents reuse. The solution requires coordinated actions of customs and environmental authorities, chemical suppliers and distributors, waste recovery, recycle and destruction enterprises, and others.
Will chemists and engineers continue the pace of technical progress?
If the past is prelude to the future, technical progress will continue on refrigeration and air conditioning. The near future includes new refrigerants and refrigerant blends, as well as more efficient heat exchangers and system controls. The more distant future includes new compressor configurations, better seals, oil-free designs, magnetic bearings, and high-pressure containment. The far future is anyone's guess.
2013 Critical Review Presentation Slides
Download Zip (20.6 MB)Supplementary Material
Download MS Word (16.8 KB)Acknowledgment
The authors acknowledge significant contributions from their colleagues at the Institute for Governance and Sustainable Development: Durwood Zaelke, Dennis Clare, Danielle Grabiel, and Xiaopu Sun; from scientist David Fahey and science writer Lani Sinclair; from automotive experts John Cabaniss, Paul DeGuiseppi, Elvis Hoffpauir, John Rugh, and Jim Taylor; and Montreal Protocol authorities Gilbert Bankobeza and Megumi Seki.
Harvard University Environmental Science and Public Policy Archives (ESPPA) has large collections of photographs, personal notes, and previously confidential information such as records of government negotiating positions and corporate strategy donated by Stephen O. Andersen, K. Madhava Sarma, and Edward A. Parson. Additional donations are welcome. http://hcl.harvard.edu/libraries/lamont/collections/environment.
References
- American National Standards Institute (ANSI) and American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) . 2007 . ASHRAE Standard: Designation and Safety Classification of Refrigerants. ANSI/ASHRAE Addendum ak to ANSI/ASHRAE Standard 34-2007 https://www.ashrae.org/File%20Library/docLib/Public/20090224_ad342007x_y_ aa_ab_ac_ad_ae.pdf (accessed May 1, 2013).
- American National Standards Institute/American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ANSI/ASHRAE) . 2010 . ASHRAE Standard: Designation and Safety Classification of Refrigerants. ANSI/ASHRAE Addendum ak to ANSI/ASHRAE Standard 34-2007 https://www.ashrae.org/.../docLib/.../20100309_34_2007_ak_final.pdf (accessed May 1, 2013).
- Andersen , S.O. 1999 . HFC and PFC Task Force of the Technology and Economic Assessment Panel, Montreal Protocol Ozone Secretariat , Nairobi Kenya : UNEP .
- Andersen, S.O., and E.T. Morehouse. 1997. The ozone challenge: Industry and government learned to work together to protect the environment. ASHRAE J. 39(9): 29–31 bookstore.ashrae.biz/journal/download.php?file=Ozone Challenge.pdf (http://bookstore.ashrae.biz/journal/download.php?file=Ozone Challenge.pdf) (Accessed: December 31, 2012 ).
- Andersen , S.O. and Sarma , K.M. 2002 . Protecting the Ozone Layer: The United Nations History , London , , UK : Earthscan Press (official publication of the United Nations Environment Programme) .
- Andersen , S.O. , Sarma , K.M. and Taddonio , K.N. 2007 . Technology Transfer for the Ozone Layer: Lessons for Climate Change , London , , UK : Earthscan Press (official publication of the Global Environment Facility and the United Nations Environment Programme) .
- Andersen , S.O. and Zaelke , D. 2003 . “ Daimler-Chrysler: CO2, the champagne of natural refrigerants ” . In Industry Genius: Inventions and People Protecting the Climate and Fragile Ozone Layer , 53 – 69 . Sheffield , , UK : Greenleaf .
- Andersen, S.O., G.J.M. Velders and P. Canan. 2007. How Science Guides Industry Choice of Alternatives to Ozone-Depleting Substances,” Twenty Years of Ozone Decline: Proceedings of the Symposium for the 20th Anniversary of the Montreal Protocol. New York, NY: Springer Science
- Anonymous. 1933. First air-conditioned auto. Popular Science Monthly http://books.google.com/books?id=7CcDAAAAMBAJ&pg=PA30&dq=Popular+Science+1933+plane+%22Popular+Science%22&hl=en&ei=QeBUTc_ hNsH-8AaqrZyPBw&sa=X&oi=book_result&ct=result&resnum=2&ved=0 CDQQ6AEwAQ#v= onepage&q=Popular%20Science%201933%20plane%20%22Popular%20Science%22&f=true (http://books.google.com/books?id=7CcDAAAAMBAJ&pg=PA30&dq=Popular+Science+1933+plane+%22Popular+Science%22&hl=en&ei=QeBUTc_ hNsH-8AaqrZyPBw&sa=X&oi=book_result&ct=result&resnum=2&ved=0 CDQQ6AEwAQ#v= onepage&q=Popular%20Science%201933%20plane%20%22Popular%20Science%22&f=true)
- Arctic Monitoring and Assessment Programme (AMAP) . 2011 . Snow, Water, Ice and Permafrost in the Arctic , Oslo , , Norway : AMAP Secretariat .
- Atkinson , W. 1989 . A look at the future with HFC-134a . ACtion Magazine. , December: 3.
- Atkinson , W. 2008 . From Montreal to Kyoto: Two Decades of Change in the Mobile A/C Industry , Lansdale , PA : Mobile Air Conditioning Society .
- Ayala , M. , Strid , H. , Jacobsson , U. and Soderberg , P.G. 2007 . Expression and apoptosis in the lens after ultraviolet radiation exposure . Invest. Ophthalmol. Vis. Sci. , 48 : 4187 – 91 . doi: 10.1167/iovs.06-0660
- Benedick , R. 1998 . Ozone Diplomacy: New Directions in Safeguarding the Planet, enlarged edition , Cambridge , MA : Harvard University Press .
- Bhatti , M.S. November 9–11 1998 . Global Warming Impact of Automotive Air Conditioning Systems. Paper no. 982929. Society of Automotive Engineers, VII International Mobility Technology Conference and Exhibition November 9–11 ,
- Bhatti , M.S. 1999 . Evolution of automotive air conditioning: Riding in comfort . ASHRAE Journal , September : 44 – 50 . doi: 10.4271/1999-01-0870
- Billings , J.S. 1893 . “ Ventilating and heating ” . In Engineering Record (Report of Officers of the Navy on Ventilating and Cooling Executive Mansion), Vol. 8 , Washington , DC : U.S. Government Printing Office . http://archive.org/stream/ventilationheati00billuoft/ventilationheati00billuoft_djvu.txt
- Bodinus , W.S. 1999 . The rise and fall of carbon dioxide systems . ASHRAE J. , : 37 – 42 .
- Bond , T.C. , Doherty , S.J. , Fahey , D.W. , Forster , P.M. , Berntsen , T. , DeAngelo , B.J. , Flanner , M.G. , Ghan , S. , Karcher , B. , Koch , D. , Kinne , S. , Kondo , Y. , Huinn , P.K. , Sarofim , M.C. , Schultz , M. G. , Sculz , M. , Venkataraman , C. , Zhang , H. , Zhang , S. , Belouin , N. , Guttikunda , S.K. , Hopke , P.K. , Jacobson , M.Z. , Kaiser , J.W. , Klimont , Z. , Lohmann , U. , Schwarz , J.P. , Shindell , D. , Storelvmo , T. , Waren , S.G. and Zender , C.S. 2013 . Bounding the role of black carbon in the climate system: A scientific assessment . J. Geophys. Res. Atmospheres. , doi: 10.1002/jgrd.50171
- Boutonnet , J.C. , Bingham , P. , Calamari , D. , deRooij , C. , Franklin , J. , Kawano , T. , Libre , J.M. , McCulloch , A. , Malinverno , G. and Odom , J.M. 1999 . Environmental risk assessment of trifluoroacetic acid . Hum. Ecol. Risk Assess. Int. J. , 5 ( 1 ) : 59 – 124 . doi: 10.1080/10807039991289644
- Brodeur, P. 1986. Annals of chemistry. The New Yorker http://www.newyorker.com/archive/1975/04/07/1975_04_07_047_TNY_CARDS_000311657 (http://www.newyorker.com/archive/1975/04/07/1975_04_07_047_TNY_CARDS_000311657) (Accessed: December 31, 2012 ).
- Brysse, K. 2009. Oral History Transcript—Dr. Joseph Farman. Niels Bohr Library & Archives, American Institute of Physics, College Park, MD http://www.aip.org/history/ohilist/33642.html (http://www.aip.org/history/ohilist/33642.html) (Accessed: December 31, 2013 ).
- Cagin , S. and Dray , P. 1993 . Between Earth and Sky: How CFCs Changed our World and Endangered the Ozone Layer , New York , NY : Pantheon Books .
- Caldwell , M.M. 1971 . “ Solar UV irradiation and the growth and development of higher plants ” . In Photophysiology, Current Topics in Photobiology and Photochemistr , Edited by: Giese , A.C. Vol. VI , New York , NY : Academic Press .
- Caldwell , M.M. , Bornman , J.F. , Ballaré , C.L. , Flint , S.D. and Kulandaivelu , G. 2007 . Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors . Photochem. Photobiol. Sci. , 6 : 131 – 77 . doi: 10.1039/b700019g
- Caldwell , M.M. , Camp , L.B. , Warner , C.W. and Flint , S.D. 1986 . “ Action spectra and their key role in assessing biological consequences of solar UV-B radiation change ” . In Stratospheric Ozone Reduction Solar Ultraviolet Radiation and Plant Life , Edited by: Worrest , R.C. and Caldwell , M.M. 87 – 111 . Berlin , , Germany : Springer .
- California Air Resources Board (CARB). 2012. LEV III Greenhouse Gas Non-Test Cycle Provisions Technical Support Document. Sacramento, CA http://www.arb.ca.gov/regact/2012/leviiighg2012/levappr.pdf (http://www.arb.ca.gov/regact/2012/leviiighg2012/levappr.pdf) (Accessed: March 12, 2013 ).
- Calm , J. M. and 29–30 , October . 2012 . Refrigerant Transitions…Again, Moving Towards Sustainability , Gaithersburg , MD : Proceedings of the ASHRAE-NIST Refrigerants Conference .
- Chaney , L. , Thundiyil , K. , Andersen , S. O. , Chidambaram , S. and Abbi , Y.P. 2007 . India Fuel Savings and Emission Reductions from Next-Generation Mobile Air Conditioning Technology in India , Golden , CO : U.S. Department of Energy National Renewable Energy Laboratory .
- Chang , Y.M. , Barrett , J.H. , Bishop , D.T. , Armstrong , B.K. , Bataille , V. , Bergman , W. , Berwick , M. , Bracci , P.M. , Elwood , J.M. , Ernstoff , M.S. , Gallagher , R.P. , Green , A.C. , Gruis , N.A. , Holly , E.A. , Ingvar , C. , Kanetsky , P.A. , Karagas , M.R. , Lee , T.K. , Le Marchand , L. , Mackie , R.M. , Olsson , H. , Osterlind , A. , Rebbeck , T.R. , Sasieni , P. , Siskind , V. , Swerdlow , A.J. , Titus- Ernstoff , L. , Zens , M.S. and Newton-Bishop , J.A. 2009 . Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls . Int. J. Epidemiol. , 38 : 814 – 30 . doi: 10.1093/ije/dyp166
- Chapman, S. 1931. Some phenomena of the upper atmosphere. Proc. R. Soc. London Ser. A 132(820): 353–374. doi:10.1098/rspa.1931.0105 http://www.jstor.org/stable/95664 (http://www.jstor.org/stable/95664) (Accessed: December 31, 2012 ).
- Chubachi, S. 1984. Preliminary Result of Ozone Observations at Syoma Station from February 1982 to January 1983. Memoirs of National Institute of Polar Research Special Issue No. 34, Proceedings of the Sixth Symposium on Polar Meteorology and Glaciology http://ci.nii.ac.jp/vol_issue/nels/AA00733561/ISS0000026297_ja.html (http://ci.nii.ac.jp/vol_issue/nels/AA00733561/ISS0000026297_ja.html) (Accessed: December 31, 2012 ).
- Consumer Goods Forum . 2012 . Better Lives Through Better Business http://www.theconsumergoodsforum.com/PDF/CGF_Corporate_Brochure.pdf (accessed May 1, 2013).
- Cook , E. 1996 . Ozone Protection in the United States: Elements of Success , Washington , DC : World Resources Institute .
- Cornú , M. A. 1879 . Sur la Limite Ultra-Violette du Spectre Solaire . Proc. R. Society London , 29 : 47 – 55 . doi: 10.1098/rspl.1879.0011
- Crutzen , P. J. 1970 . The influence of nitrogen oxides on the atmospheric ozone content . Q. J. R. Meteorol. Society , 96 ( 408 ) : 320 – 325 . doi: 10.1002/qj.49709640815
- Crutzen , P. J. 1972 . SST's: A threat to the earth's ozone shield . Ambio , 1 ( 2 ) : 41 – 51 .
- Daimler. 2012. New findings concerning the risks of the new R1234yf refrigerant: Mercedes-Benz wishes to continue using the tried-and-tested R134a refrigerant in passenger cars. Press release. September 25, 2012 http://media.daimler.com/deeplink?cci=2232953 (http://media.daimler.com/deeplink?cci=2232953) (Accessed: December 31, 2012 ).
- DeCanio , S.J. and Lee , K.N. 1991 . Doing well by doing good: Technology transfer to protect the ozone . Policy Stud. J. , 19 : 137 – 148 . doi: 10.1111/j.1541-0072
- Damian , D.L. , Halliday , G.M. , Taylor , C.A. and Barnetson , R.S. 1998 . Ultraviolet radiation induced suppression of Mantoux reactions in humans . J. Invest. Dermatol. , 110 : 824 – 27 . doi: 10.1046/j.1523-1747.1998.00176.x
- de Gruijl , F.R. , Longstreth , J. , Norval , M. , Cullen , A.P. , Slaper , H. , Kripke , M.L. , Takizawa , Y. and van der Leun , J.C. 2003 . Health effects from stratospheric ozone depletion and interactions with climate change . Photochem. Photobiol. Sci. , 2 : 16 – 28 . doi: 10.1039/b211156j
- Dobson , G.M.B. 1968 . Forty years’ research on atmospheric ozone at Oxford: A history . Appl. Optics , 7 ( 3 ) : 387 – 405 . doi: 10.1364/AO.7.000387
- Dobson , G.M.B. and Harrison , D.N. 1926 . Measurements of the amount of ozone in the earth's atmosphere and its relation to other geophysical conditions . Proc. R. Society London Ser. A , 110 ( 756 ) : 660 – 93 . doi: 10.1098/rspa.1926.0040
- Donaldson , B. and Nagengast , B. 1994 . Heat and Cold: Mastering the Great Indoors: A Selective History ASHRAE Code 40303 , Atlanta , GA : American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc .
- Doniger , D. 1988 . Politics of the ozone layer . Issues Sci. Technol. , 4 ( 3 ) : 86 – 92 .
- Dubey , M.K. , Smith , G.P. , Hartley , W.S. , Kinnison , D.E. and Connell , P.S. 1997 . Rate parameter uncertainty effects in assessing stratospheric ozone depletion by supersonic aviation . Geophys. Res. Lett. , 24 ( 22 ) : 2737 – 40 . doi: 10.1029/97GL02859
- Dudek , D.J. , Leblanc , A.M. and Sewall , K. 1990 . Cutting the cost of environmental policy: Lessons from business response to CFC regulation . Ambio , 19 ( 6/7 ) : 324 – 28 .
- European Commission . 2012 . Declaration by the European Commission regarding Point 9 of the agenda of the 31st meeting of the ‘Technical Committee—Motor vehicles’ (TCMV): State of Play of the EU Mobile Air-Conditioning directive (2006/40/EC) http://ec.europa.eu/enterprise/sectors/automotive/files/environ ment/mac/note-macs-december-2012_en.pdf (accessed May 1, 2013).
- European Commission. 2013a. Automotive Mobile Air-Conditioning Systems (MACs): The European Directive on Mobile Air-Conditioning Systems (MACs) Aims at Reducing Emissions of Specific Fluorinated Greenhouse Gases in the Air-Conditioning Systems Fitted to Passenger Cars (Vehicles of Category M1) and Light Commercial Vehicles (Category N1, Class 1) http://ec.europa.eu/enterprise/sectors/automotive/environment/macs/index_en.htm. (http://ec.europa.eu/enterprise/sectors/automotive/environment/macs/index_en.htm.) (Accessed: March 4, 2013 ).
- European Commission. 2013b. Implementation of Directive 2006/40/EC—Questions and Answers. Brussels http://ec.europa.eu/enterprise/sectors/automotive/files/environment/mac/q-and-a_en.pdf (http://ec.europa.eu/enterprise/sectors/automotive/files/environment/mac/q-and-a_en.pdf) (Accessed: March 4, 2013 ).
- European Commission Community Research and Development Information Service. 1997. Refrigeration and Automotive Climate Systems Under Environmental Aspects http://cordis.europa.eu/search/index.cfm?fuseaction=proj.document&PJ_LANG=EN&PJ_RCN=710487 (http://cordis.europa.eu/search/index.cfm?fuseaction=proj.document&PJ_LANG=EN&PJ_RCN=710487) (Accessed: December 31, 2012 ).
- European Community Enterprise and Industry Directorate-General (ECEID-G). 2012. Note to the Attention of the Members of the Technical Committee on Motor Vehicle http://ec.europa.eu/enterprise/sectors/automotive/files/environ ment/mac/note-macs-april-2012_en.pdf (http://ec.europa.eu/enterprise/sectors/automotive/files/environ ment/mac/note-macs-april-2012_en.pdf) (Accessed: December 31, 2012 ).
- Fahey , W.D. and Hegglin , M.I. 2011 . Twenty Questions and Answers About the Ozone Layer: 2010. Scientific Assessment of Ozone Depletion. Reprinted from World Meteorological Organization. 2011. Scientific Assessment of Ozone Depletion: 2010. Global Ozone Research and Monitoring Project Report No. 52 , Geneva , , Switzerland : World Meteorological Organization . (coordinating lead authors)
- Farman , J.S. , Gardiner , B.G. and Shanklin , J.D. 1985 . Large losses of total ozone in antarctic reveal seasonal ClOx/NOx interaction . Nature , 315 : 207 – 10 . doi: 10.1038/315207a0
- Fischer , S.K. , Hughes , P.J. , Fairchild , P.D. , Kusik , C.L. , Dieckmann , J.T. , McMahon , E.M. and Hobday , N. 1991 . Energy and Global Warming Impacts of CFC Alternative Technologies , Washington , DC : U.S. EPA .
- Fisk , D. 1934 . Exploring the Upper Atmosphere , Oxford , , UK : Oxford University Press .
- Forster , P. , Ramaswamy , V. , Artaxo , P. , Berntsen , T. , Betts , R. , Fahey , D. W. , Haywood , J. , Lean , J. , Lowe , D. C. , Myhre , G. , Nganga , J. , Prinn , R. , Raga , G. , Schulz , M. and Van Dorland , R. 2007 . “ Changes in atmospheric constituents and in radiative forcing ” . In Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Cambridge , , UK : Cambridge University Press .
- Franklin , B. June 17, 1758 1758 . Cooling by Evaporation. Letter to John Lining June 17, 1758 , London http://www.waughfamily.ca/Montgomery/benfranklin1758.htm
- Gaved, A. 2010. General Motors to Use New Car Refrigerant from 2013 http://www.racplus.com/news/refrigeration/general-motors-to-use-new-car-refrigerant-from-2013/8603813.article (http://www.racplus.com/news/refrigeration/general-motors-to-use-new-car-refrigerant-from-2013/8603813.article) (Accessed: December 31, 2012 ).
- Gibson , G.V. and Smilor , R.W. 1992 . Technology Transfer in Consortia and Strategic Alliances , Lanham , MD : Rowman & Littlefield .
- Gladstone , J. 1998 . John Gorrie, The visionary . ASHRAE J. , December : 29 – 35 .
- Glas, J. P. 1989. Protecting the ozone layer: A perspective from industry. In Technology and Environment, 137–155. Washington, DC: National Academy Press http://www.nap.edu/openbook.php?record_id=1407&page=137 (http://www.nap.edu/openbook.php?record_id=1407&page=137) (Accessed: December 31, 2012 ).
- Gale A. J. 2006. PHMSA Interpretation #06-0097, PHMSA Response Letter 11 July, 2006. http://www.phmsa.dot.gov/portal/site/PHMSA/menuitem.ebdc7a8a7e39f2e55cf2031050248a0c/?vgnextoid=381ac0515d544110VgnVCM1000009ed07898RCRD&vgnextchannel=aa8cd3c1af814110VgnVCM10 00009ed07898RCRD&vgnextfmt=print (http://www.phmsa.dot.gov/portal/site/PHMSA/menuitem.ebdc7a8a7e39f2e55cf2031050248a0c/?vgnextoid=381ac0515d544110VgnVCM1000009ed07898RCRD&vgnextchannel=aa8cd3c1af814110VgnVCM10 00009ed07898RCRD&vgnextfmt=print)
- GM. 2010. GM First to Market Greenhouse Gas-Friendly Air Conditioning Refrigerant in U.S. Inside News Forum http://www.gminsidenews.com/forums/f12/gm-first-market-greenhouse-gas-friendly-air-conditioning-refrigerant-u-s-93968/ (http://www.gminsidenews.com/forums/f12/gm-first-market-greenhouse-gas-friendly-air-conditioning-refrigerant-u-s-93968/) (Accessed: December 31, 2012 ).
- Götz , F.W.P. 1931 . Das Atmospharische Ozon . Ergebnisse der KomschenPhysik , 1 : 180 – 235 .
- Götz, F.W.P., A.R. Meetham, and G.M.B. Dobson. 1934. The vertical distribution of ozone in the atmosphere. Proc. R. Society London Ser. A 145(855): 416–46. doi:10.1098/rspa.1934.0109 http://www.jstor.org/stable/2935512 (http://www.jstor.org/stable/2935512) (Accessed: December 31, 2012 ).
- Hammer, H., I. Lazaridis, S. Morgenstern, J. Wertenbach, and S. Wöhrl. 2012. R1234yf—A new, safe and environmentally friendly refrigerant for mobile air conditioning www.rivoiragas.it/wp-content/uploads/pdf/Presentazione%20Euro pean%20Automotive%20AC%20Convention.pdf (http://www.rivoiragas.it/wp-content/uploads/pdf/Presentazione%20Euro pean%20Automotive%20AC%20Convention.pdf) (Accessed: December 31, 2012 ).
- Harrison , H. 1970 . Stratospheric ozone with added water vapour: Influence of high-altitude aircraft . Science , 170 ( 3959 ) : 734 – 36 . doi: 10.1126/science.170.3959.734
- Harry , H.W. 1999 . The First Century of Air Conditioning. ASHRAE Code 90415 , Atlanta , GA : American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc .
- Hartley , W.N. 1880 . On the probable absorption of the solar ray by atmospheric ozone . Chemical News , 268 November 26
- Henne , A.L. and Fox , C.J. 1951 . Ionization constants of fluorinated acids . J. Am. Chem. Soc. , 73 ( 5 ) : 2323 – 25 . doi: 10.1021/ja01149a122
- Henne , S. , Shallcross , D.E. , Reimann , S. , Xiao , P. , Brunner , D. , ’Doherty , S. O and Buchmann , B. 2012 . Future emissions and atmospheric fate of HFO-1234yf from mobile air conditioners in Europe . Environ. Sci. Technol. , 46 ( 3 ) : 1650 – 58 . doi: 10.1021/es2034608
- Hidy , G.M. , Brook , J.R. , Chow , J.C. , Green , M.C. , Husar , R.B. , Lee , C. , Scheffe , R.D. , Swanson , A. and Watson , J.G. 2009 . Remote sensing of particulate pollution from space: Have we reached the promised land?: A critical review . J. Air Waste Manage. Assoc. , 59 ( 10 ) : 1130 – 39 . doi: 10.3155/1047-3289.59.10.1130
- Hill , R.W. and Papasavva , S. 2005 . Life Cycle Analysis Framework; A Comparison of HFC-134a, HFC-134a Enhanced, HFC-152a, R744, R744 Enhanced, and R290. SAE Technical Paper 2005-01-1511 , doi: 10.4271/2005-01-1511
- Hoff , R.M. and Christopher , S.A. 2009 . Remote sensing of particulate pollution from space: Have we reached the promised land? A critical review . J. Air Waste Manage. Assoc. , 59 ( 6 ) : 645 – 75 . doi: 10.3155/1047-3289.59.6.645
- Hoffpauir , E. 2012 . Personal communication with author , Lansdale , PA : Mobile Air Conditioning Society Worldwide (MACS) .
- Homer. 850 BC . The Iliad and The Odyssey. Reprinted 2007 , Blacksburg , VA : Wilder Publications . ISBN-10:1934451460
- Hu, A., Y. Xu, C. Tebaldi, W. M. Washington, and V. Ramanathan 2013. Mitigation of short-lived climate pollutants slows sea-level rise, Nature Climate Change. doi:10.1038/NCLIMATE1869
- Institute for Governance & Sustainability . 2013 . Primer on Short-Lived Climate Pollutants , Washington , DC : IGSD .
- Industrial Hydrocarbons, Inc. 2012. Aerosol Product Storage and Warehousing www.industrialhydrocarbons.com/storage.htm (http://www.industrialhydrocarbons.com/storage.htm) (Accessed: December 31, 2012 ).
- Intergovernmental Panel on Climate Change . 2005 . Safeguarding the Ozone Layer and the Global Climate System , Cambridge , , UK : Cambridge University Press .
- Intergovernmental Panel on Climate Change. 2012. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change http://www.ipcc-wg2.gov/SREX/images/uploads/SREX-All_FINAL.pdf (http://www.ipcc-wg2.gov/SREX/images/uploads/SREX-All_FINAL.pdf) (Accessed: December 31, 2012 ).
- International Energy Agency . 2012 . World Energy Outlook 2012 , Paris , , France : International Energy Agency .
- J & E Hall International. 2012. History: More Than 200 Years of Progress and Achievement http://www.jehall.co.uk/about/history.jsp (http://www.jehall.co.uk/about/history.jsp) (Accessed: December 31, 2012 ).
- Johnson , V.H. 2002 . Fuel Used for Vehicle Air Conditioning: A State-by-State Thermal Comfort-Based Approach . SAE Technical Series Paper 2002-01-1957 , doi: 10.4271/2002-01-1957
- Johnston , H.S. 1971 . Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust . Science , 173 ( 3996 ) : 517 – 22 . doi: 10.1126/science.173.3996.517
- Kanter , D. , Mauzerall , D.L. , Ravishankara , A.R. , Daniel , J.S. , Portmann , R.W. , Grabiel , P.M. , Moomaw , W.R. and Galloway , J.N. 2013 . A post-Kyoto partner: Considering the stratospheric ozone regime as a tool to manage nitrous oxide . Proc. Natl. Acad. Sci. USA. , doi: 10.1073/pnas.12222231110
- Kauffman , G.B. 1989 . Midgley: Saint or serpent? . Chemtech Magazine , 19 ( 12 ) : 717 – 25 .
- Kauffman , J.M. 1997 . “ Domestic and international linkages in global environmental politics: A case-study of the Montreal Protocol ” . In Internationalization of Environmental Protection , Edited by: Schreurs , M.A. and Economy , E. Cambridge , , UK : Cambridge University Press .
- Khattatov , V. 2002 . “ How common concern for the environment led to cooperation in space ” . In Protecting the Ozone Layer: The United Nations History , Edited by: Andersen , S.O. and Sarma , K.M. London , , UK : Earthscan Press (official publication of the United Nations Environment Programme) .
- Kripke , M.L. 1974 . Antigenicity of murine skin tumors induced by ultraviolet light . J. Natl. Cancer Inst. , 53 : 1333 – 36 .
- Laszlo , P. 2001 . Salt: Grain of Life. Translated from the 1998 original by Mary Beth Mader , New York , NY : Columbia University Press .
- Le Prestre , P.G. , Reid , J.D. and Morehouse , E.T. Jr. 1998 . Protecting the Ozone Layer: Lessons, Models and Prospects , Boston , MA : Kluwer Academic .
- Leeds , A.R. 1879 . Lines of discovery in the history of ozone, with an index of its literature, and an appendix upon the literature of peroxide of hydrogen . Ann. NY Acad. Sci. , 1 ( 1 ) : 363 – 91 . doi: 10.1111/j.1749-6632.1879.tb55134.x
- Link , A.N. and Scott , J.T. 2001 . Public/private partnerships: Stimulating competition in a dynamic market . Int. J. Ind. Organization , 19 ( 5 ) : 763 – 94 . doi: 10.1016/S0167-7187
- Lorentzen , G. and Pettersen , J. 1993 . A new, efficient and environmentally benign system for car air-conditioning . Int. J. Refrigeration , 16 ( 1 ) : 4 – 12 . doi: 10.1016/0140-7007(93)90014-Y
- Lovelock , J.E. 1971 . Atmospheric fluorine compounds as indication of air movements . Nature , 230 ( 5293 ) : 379 doi: 10.1038/230379a0
- Lovelock , J.E. , Maggs , R.J. and Wade , R.J. 1973 . Halogenated hydrocarbons in and over the Atlantic . Nature , 241 : 194 – 96 . doi:10.1038/241194a0 (as cited in Kowalok, M.E. 1993. Common threads: Research lessons from acid rain, ozone depletion, and global warming. Environment 35(6):12–38. doi:10.1080/00139157.1993.9929107
- Luderer , G. , Bosetti , V. , Jakob , M. , Leimbach , M. , Steckel , J. C. , Waisman , H. and Edenhofer , O. 2012 . The economics of decarbonizing the energy system: Results and insights from the RECIPE model intercomparison . Climatic Change , 114 ( 1 ) : 9 – 37 . doi: 10.1007/s10584-011-0105-x
- Luecken , D.J. , Waterland , R.L. , Papasavva , S. , Taddonio , K.N. , Hutzell , W.T. , Rugh , J.P. and Andersen , S.O. 2010 . Ozone and TFA impacts in north america from degradation of 2,3,3,3-tetrafluoropropene (HFO-1234yf), a potential greenhouse gas replacement . Environ. Sci. Technol. , 44 ( 1 ) : 343 – 48 . doi: 10.1021/es902481f
- Luedecke , C. 2004 . The First International Polar Year (1882–83): A big science experiment with small science equipment . Proc. Int Commission on History of Meteorology. , http://www.meteohistory.org/2004proceedings1.1/pdfs/06luedecke.pdf (accessed May 1, 2013).
- McCarthy , R. 1972 . DuPont business correspondence
- McCulloch , A. 1999 . CFC and halon replacements in the environment . J. Fluorine Chem. , 100 ( 1–2 ) : 163 – 73 . doi: 10.1016/S0022-1139(99)00198-0
- McDonald , J.E. 1971 . Relationship of skin cancer incidence to thickness of ozone layer. Statement submitted to hearings before the House Subcommittee on Transportation Appropriations (Mar 2, 1971) . Cong. Rec. , 117 : 7252
- McNeil , M.A. , Letschert , V.E. and de la Rue du Can , S. 2008 . Global Potential of Energy Efficiency Standards and Labeling Programs , Berkeley , CA : Lawrence Berkeley National Laboratory Environmental Energy Technologies Division .
- Meinshausen , M. , Meinshausen , N. , Hare , W. , Raper , S.C.B. , Frieler , K. , Knutti , R. , Frame , D.J. and Allen , M.R. 2009 . Greenhouse-gas emission targets for limiting global warming to 2°C . Nature , 458 : 1158 – 62 . doi: 10.1038/nature08017
- Mercedes-Benz. 2012. Re: Part 573 Defect Information Report. Letter from Mercedes-Benz to the U.S. National Highway Traffic Safety Administration Division, Washington, DC, dated 28 September 2012 http://www-odi.nhtsa.dot.gov/acms/cs/jaxrs/download/doc/UCM427727/RCDNN-12V478-4113.pdf (http://www-odi.nhtsa.dot.gov/acms/cs/jaxrs/download/doc/UCM427727/RCDNN-12V478-4113.pdf) (Accessed: December 31, 2012 ).
- Metz , B. , Kuijpers , L. , Solomon , S. , Andersen , S.O. , Davidson , O. , Pons , J. , de Jager , D. , Kestin , T. , Manning , M. and Meyer , L. 2005 . Safeguarding the Stratospheric Ozone Layer and the Global Climate: Issues Relating to Hydrofluorocarbons and Perfluorocarbons , Cambridge , , UK : Cambridge University Press .
- Meyer , L.M. , Dong , X. , Wegener , A. and Soderberg , P. 2008 . Dose dependent cataractogenesis and maximum tolerable dose (MTD. (2.3:16)) for UVR 300 nm- induced cataract in C57BL/6J mice . Exp. Eye Res. , 86 : 282 – 89 . doi: 10.1016/j.exer.2007.10.019
- Miller , A. and Mintzer , I. 1986 . The Sky Is the Limit: Strategies for Protecting the Ozone Layer , Washington , DC : World Resources Institute .
- Minnesota Pollution Control Agency. 2011. Climate Change: Mobile Air Conditioners http://www.pca.state.mn.us/index.php/topics/climate-change/regulatory-initiatives-programs-and-policies/climate-change-mobile-air-conditioners.html?menuid=&redirect=1 (http://www.pca.state.mn.us/index.php/topics/climate-change/regulatory-initiatives-programs-and-policies/climate-change-mobile-air-conditioners.html?menuid=&redirect=1) (Accessed: December 31, 2012 ).
- Minnesota Session Laws. 2008. Mobile Air Conditioner Leakage Rates; Disclosure. 2008 c 296 art 1 s 23. https://www.revisor.leg.state.mn.us/statutes/?id=216H&view=chapter#stat.216H.12 (http://https://www.revisor.leg.state.mn.us/statutes/?id=216H&view=chapter#stat.216H.12)
- Mobile Air Conditioning Society 2012. The Bumpy Road to R-1234yf. ACtion November/December http://www.nxtbook.com/nxtbooks/macs/action_2012 1112/#/0 (http://www.nxtbook.com/nxtbooks/macs/action_2012 1112/#/0)
- Molina , M. and Rowland , F.S. 1974 . Stratospheric sink for chlorofluoromethanes: chlorine atom-catalyzed destruction of ozone . Nature , 249 ( 5460 ) : 810 – 12 . doi: 10.1038/249810a0
- Molina , M. , Zaelke , D. , Sarma , K.M. , Andersen , S.O. , Ramanathan , V. and Kaniaru , D. 2009 . Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions . Proc. Natl. Acad. Sci. USA , 106 ( 49 ) : 20616 – 21 . doi: 10.1073/pnas.0902568106
- Montreal Protocol Technology and Economic Assessment Panel. 2009. Task Force Decision XX/8 Report: Assessment of Alternatives to HCFCs and HFCs and Update of the TEAP 2005 Supplement Report Data http://ozone.unep.org/teap/Reports/TEAP_Reports/teap-may-2009-decisionXX-8-task-force-report.pdf (http://ozone.unep.org/teap/Reports/TEAP_Reports/teap-may-2009-decisionXX-8-task-force-report.pdf) (Accessed: December 31, 2012 ).
- Montreal Protocol on Substances that Deplete the Ozone Layer . 25–29 November 2002 . Decision XIV/10 UNEP/Ozl.Pro.14/9 , 25–29 November , Rome , , Italy : Fourteenth MOP to the Montreal Protocol .
- Morgenstern , O. , Braesicke , P. , Hurwitz , M.M. , ’Connor , F.M. O , Bushell , A.C. , Johnson , C.E. and Pyle , J.A. 2008 . The world avoided by the Montreal Protocol . Geophys. Res. Lett. , 35 ( L16811 ) : 5 doi: 10.1029/2008GL034590
- Nagengast , B. 1997 . History of sealed refrigeration systems . ASHRAE J. , January : S44 – 52 .
- Nagengast , B. 1988 . A historical look at CFC refrigerants . ASHRAE J. , XX : 37 – 39 .
- Nagengast , B. 1999 . Comfort from a block of ice: A history of comfort cooling using ice . ASHRAE J. , 41(2)
- Narbutt , J. , Lesiak , A. , Skibinska , M. , Wozniacka , A. , van Loveren , H. , Sysa-Jedrzejowska , A. , Lewy-Trenda , I. , Omulecka , A. and Norval , M. 2005 . Suppression of contact hypersensitivity after repeated exposures of humans to low doses of solar simulated radiation . Photochem. Photobiol. Sci. , 4 : 517 – 22 . doi: 10.1039/b503166d
- National Aeronautics and Space Administration. 2009. New Simulation Shows Consequences of a World Without Earth's Natural Sunscreen.” (accessed May 1, 2013) http://www.nasa.gov/topics/earth/features/world_avoided.html (http://www.nasa.gov/topics/earth/features/world_avoided.html)
- National Academy of Engineers. 2013. Greatest Engineering Achievements of the 20th Century: Air Conditioning and Refrigeration History http://www.greatachievements.org/?id=2958 (http://www.greatachievements.org/?id=2958) (Accessed: February 23, 2013 ).
- National Aeronautics and Space Administration, Federal Aviation Administration, U.S. National Oceanic and Atmospheric Administration, United Nations Environment Programme, World Meteorological Organization, Commission of the European Communities, and Bundesministerium für Forschung und Technologie . 1985 . Atmospheric Ozone 1985: Assessment of our Understanding of the Processes Controlling Its Present Distribution and Change , Washington , DC : National Aeronautics and Space Administration .
- National Aeronautics and Space Administration, Goddard Space Flight Center. 1996. The Airborne Antarctic Ozone Experiment http://hyperion.gsfc.nasa.gov/Analysis/aircraft/aaoe.html (http://hyperion.gsfc.nasa.gov/Analysis/aircraft/aaoe.html) (Accessed: December 31, 2012 ).
- National Aeronautics and Space Administration, Jet Propulsion Laboratory, USA. 2000. Ozone Milestones. ( http://atmos.jpl.nasa.gov/milestones.htm (http://atmos.jpl.nasa.gov/milestones.htm) (Accessed: December 31, 2012 ).
- National Oceanic and Atmospheric Administration (NASA), Federal Aviation Administration (FAA), World Meteorological Organization (WMO), and United Nations Environment Programme (UNEP) . 1988 . Report of the International Ozone Trends Panel 1988 , Washington , DC : National Aeronautics and Space Administration .
- National Oceanic and Atmospheric Administration, National Aeronautics and Space Administration, United Nations Environment Programme, World Meteorological Organization, and European Commission. 2011. Scientific Assessment of Ozone Depletion: 2010 http://ozone.unep.org/Assessment_Panels/SAP/Scientific_Assessment_2010/01-Contents_Preface.pdf (http://ozone.unep.org/Assessment_Panels/SAP/Scientific_Assessment_2010/01-Contents_Preface.pdf) (Accessed: December 31, 2012 ).
- National Research Council . 1975 . Environmental Impact of Stratospheric Flight , Washington , DC : National Academy of Sciences .
- National Research Council . 1976 . Halocarbons: Effects on Stratospheric Ozone , Washington , DC : National Academy of Sciences .
- National Research Council . 1979 . Protection against Depletion of Stratospheric Ozone by Chlorofluorocarbons , Washington , DC : National Academy of Sciences .
- National Research Council . 1982 . Causes and Effects of Stratospheric Ozone Reduction: An Update , Washington , DC : National Academy of Sciences .
- National Research Council . 1983 . Causes and Effects of Changes in Stratospheric Ozone: Update 1983 , Washington , DC : National Academy of Sciences .
- Needham , J. 1991 . Science and Civilization in China, Volume 4: Physics and Physical Technology, Part 2, Mechanical Engineering , Cambridge , , UK : Cambridge University Press .
- Nielsen , O.J. , Javadi , M.S. , Sulbaek-Andersen , M.P. , Hurley , M.D. , Wallington , T.J. and Singh , R. 2007 . Atmospheric chemistry of CF3CFCH2: Kinetics and mechanisms of gas-phase reactions with Cl atoms, OH radicals, and O3 . Chem. Phys. Lett. , 439 ( 1–3 ) : 18 – 22 . doi: 10.1016/j.cplett.2007.03.053
- Newman , P.A. , Oman , L.D. , Douglass , A.R. , Fleming , E.L. , Frith , S.M. , Hurwitz , M.M. , Kawa , S.R. , Jackman , C.H. , Krotkov , N.A. , Nash , E.R. , Nielsen , J.E. , Pawson , S. , Stolarski , R.S. and Velders , G.J.M. 2009 . What would have happened to the ozone layer if chlorofluorocarbons (CFCs) had not been regulated? . Atmos. Chem. Phys. , 9 ( 6 ) : 2113 – 28 . doi: 10.5194/acp-9-2113-2009
- Norman , C.S. , DeCanio , S.J. and Fan , L. 2008 . The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection . Global Environ. Change Hum. Policy Dimens. , 18 ( 2 ) : 330 – 40 . doi: 10.1016/j.gloenvcha.2008.03.003
- Norval , M. , Cullen , A.P. , de Gruijl , F.R. , Longstreth , J. , Takizawa , Y. , Lucas , R.M. , Noonan , F.P. and van der Leun , J.C. 2007 . The effects on human health from stratospheric ozone depletion and its interactions with climate change . Photochem. Photobiol. Sci. , 6 : 232 – 51 . doi: 10.1039/b700018a
- Oreskes , N. and Conway , E.M. 2010 . Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming , New York , NY : Bloomsbury Press .
- Oriowo , O.M. , Cullen , A.P. , Chou , B.R. and Sivak , J.G. 2001 . Action spectrum and recovery for in vitro UV-induced cataract using whole lenses . Invest. Ophthalmol. Vis. Sci. , 42 : 2596 – 602 .
- Palmer , M. 1973 . William Soltau Davidson: A pioneer of New Zealand estate management . N. Z. J. History , 7 ( 2 ) : 148 – 164 .
- Papasavva , S. , Hill , W.R. and Brown , R.O. 2008 . GREEN-MAC-LCCP©: A Tool for Assessing Life Cycle Greenhouse Emissions of Alternative Refrigerants. SAE Technical Series Paper 2008-01-0829 , doi: 10.4271/2008-01-0828
- Papasavva , S. , Hill , W.R. and Andersen , S.O. 2010 . GREEN-MAC-LCCP©: A tool for assessing the life cycle climate performance of MAC systems . Environ. Sci. Technol. , 44 ( 19 ) : 7666 – 72 . doi: 10.1021/es100849g
- Papasavva1 , S. and Andersen , S.O. 2011 . GREEN-MAC-LCCP©: Life-Cycle Climate Performance Metric for Mobile Air Conditioning Technology Choice . Environ. Prog. Sustain. Energy , 30 ( 2 ) : 234 – 47 . doi: 10.1002/ep.10465
- Papasavva , S. and Moomaw , W.R. 1998 . Life-cycle global warming impact of CFCs and CFC-substitutes for refrigeration . J. Ind. Ecol. , 1 ( 4 ) : 71 – 91 . doi: 10.1162/jiec.1997.1.4.71
- Parson , E.A. 2003 . Protecting the Ozone Layer: Science and Strategy , New York , NY : Oxford University Press .
- Parson , E.A. and Greene , O. 1995 . The complex chemistry of the international ozone agreements . Environment , 37 ( 2 ) : 16 – 20 . 35–43 doi: 10.1080/00139157.1995.9929219
- Pearce , F. 2008 . Who really discovered the ozone hole? . New Scientist , 2674: 46–47.
- Prather , M.J. , McElroy , M.B. and Wofsy , S.C. 1984 . Reductions in ozone at high concentrations of stratospheric halogens . Nature , 312 : 227 – 31 . doi: 10.1038/312227a0
- Ramanathan , V. 1975 . Greenhouse effect due to chlorofluorocarbons: Climatic implications . Science , 190 : 50 – 52 . doi: 10.1126/science.190.4209.50
- Ramanathan , V. 1985 . Trace gas trends and their potential role in climate change . J. Geophys. Res. , 90 ( D3 ) : 5547 – 66 . doi: 10.1029/JD090iD03p05547
- Ramanathan , V. and Xu , Y. 2010 . The Copenhagen Accord for Limiting Global Warming: Criteria, constraints, and available avenues . Proc. Natl. Acad. Sci. USA , 107 ( 18 ) : 8055 – 62 . doi: 10.1073/pnas.1002293107
- Ravishankara , A.R. 11 November 2012 . Emerging issues: HFCs and Nitrous Oxide, Montreal Protocol Seminar: Protecting Our Atmosphere, Meeting of the Parties to the Montreal Protocol 11 November , Geneva , , Switzerland
- Refrigerants, Naturally! 2012. What We Do http://www.refrigerantsnaturally.com/about-us/what-we-do.htm (http://www.refrigerantsnaturally.com/about-us/what-we-do.htm) (Accessed: January 3, 2013 ).
- Rivas , M. , Araya , M.C. , Duran , V. , Rojas , E. , Cortes , J. and Calaf , G.M. 2009 . Ultraviolet light exposure and skin cancer in the city of Arica, Chile . Mol. Med. Rep. , 2 ( 4 ) : 567 – 72 . doi: 10.3892/mmr_00000138
- Rugh , J. , Hovland , V. and Andersen , S.O. April 15 2004 . Significant Fuel Savings and Emission Reductions by Improving Vehicle Air Conditioning , April 15 , Washington , DC : Presentation at the 15th Annual Earth Technologies Forum and Mobile Air Conditioning Summit .
- Rugh , J.P. and Farrington , R. 2008 . Vehicle Ancillary Load Reduction Project Close-Out Report: An Overview of the Task and a Compilation of the Research Results, NREL Technical Report , Golden , CO : U.S. Department of Energy National Renewable Energy Laboratory .
- International. 2004. Cooperative Research Program. SAE Alternate Refrigerant Cooperative Research Project Phase I http://www.sae.org/standardsdev/tsb/cooperative/altsummary-ph1.pdf (http://www.sae.org/standardsdev/tsb/cooperative/altsummary-ph1.pdf) (Accessed: December 31, 2012 ).
- SAE International. 2012. SAE International Cooperative Research Project Offers Update on R1234yf Refrigerant. Press release, December 14 http://www.sae.org/servlets/pressRoom?OBJECT_TYPE=PressReleases&PAGE=showRelease&RELEASE _ID=1941 (http://www.sae.org/servlets/pressRoom?OBJECT_TYPE=PressReleases&PAGE=showRelease&RELEASE _ID=1941)
- SAE International. 2013. HFC-134a (R-134a) Recovery/Recycle/Recharging Equipment for Mobile Air-Conditioning Systems http://standards.sae.org/j2788_201301 (http://standards.sae.org/j2788_201301)
- SAE. 2013a. SAE International CRP1234-4 Analysis of R-1234yf Nears Completion. (accessed May 2, 2013) http://www.sae.org/servlets/pressRoom?OBJECT_TYPE= PressReleases&PAGE=showRelease&RELEASE_ID=2063 (http://www.sae.org/servlets/pressRoom?OBJECT_TYPE= PressReleases&PAGE=showRelease&RELEASE_ID=2063)
- Schneider , S.H. , Semenov , S. , Patwardhan , A. , Burton , I. , Magadza , C.H.D. , Oppenheimer , M. , Pittock , A.B. , Rahman , A. , Smith , J.B. , Suarez , A. and Yamin , F. 2007 . “ Assessing key vulnerabilities and the risk from climate change ” . In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , 779 – 810 . Cambridge , , UK : Cambridge University Press .
- Schwartz, W. 2001. Emissions of Refrigerant R-134a from Mobile Air Conditioning Systems, prepared for the German Federal Environment Office http://www.umweltdaten.de/publikationen/fpdf-l/3110.pdf (http://www.umweltdaten.de/publikationen/fpdf-l/3110.pdf) (Accessed: December 31, 2012 ).
- Schwartz, W., and J. Harnisch. 2003. Final Report on Establishing Leakage Rates of Mobile Air Conditioners, prepared for the European Commission (DG Environment) http://ec.europa.eu/clima/policies/eccp/docs/leakage_rates_final_report_en.pdf (http://ec.europa.eu/clima/policies/eccp/docs/leakage_rates_final_report_en.pdf) (Accessed: December 31, 2012 ).
- Schwarz, W., B. Gschrey, A. Leisewitz, A. Herold, S. Gores, I. Papst, J. Usinger, D. Oppelt, I. Croiset, P.H. Pedersen, D. Colbourne, M. Kauffeld, K. Kaar, and A. Lindborg. 2011. Preparatory Study for a Review of Regulation (EC) No 842/2006 on Certain Fluorinated Greenhouse Gases: Final Report. European Commission http://ec.europa.eu/clima/policies/f-gas/docs/2011_study_en.pdf (http://ec.europa.eu/clima/policies/f-gas/docs/2011_study_en.pdf) (Accessed: December 31, 2012 ).
- Scientific Assessment Panel . 2010 . The 2010 Assessment of the Scientific Assessment Panel , Nairobi , Kenya : Montreal Protocol Ozone Secretariat .
- Scott , J.T. 2008 . The National Cooperative Research and Production Act . Issues Competition Law Policy , 2 : 1297 – 317 . ABA section of Antitrust Law
- Seidel , S.R. 1996 . “ Keeping cars cool ” . In Ozone Protection in the United States: Elements of Success , Edited by: Cook , E. Washington , DC : World Resources Institute .
- Shindell , D. , Kuylenstierna , J.C.I. , Vignati , E. , van Dingenen , R. , Amann , M. , Klimont , Z. Anenberg , S.C. 2012 . Simultaneously mitigating near-term climate change and improving human health and food security . Science , 335 : 183 doi: 10.1126/science.1210026
- Singh , A.K. , Kumar , M. and Bagai , Atul . 2009 . Ozone Protection and National Security—A Military Perspective Toolkit for Defence Forces , Nairobi , Kenya : UNEP .
- Siegl , W.O. and Wallington , T.J. 2002 . R134a emissions from vehicles . Environ. Sci. Technol. , 36 ( 4 ) : 561 – 66 . doi: 10.1021/es011108x
- Slapper , H. , Velders , G.J.M. and Matthijsen , J. 1998 . Ozone depletion and skin cancer incidence: A source risk approach . J. Hazard. Mater. , 61 ( 1–3 ) : 77 – 84 . doi: 10.1016/S0304-3894(98)00110-1
- Solomon , S. , Garcia , R.R. , Rowland , F.S. and Wuebbles , D.J. 1986 . On the depletion of Antarctic ozone . Nature , 321 : 755 – 58 . doi: 10.1038/321755a0
- Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K.B. , Tignor , M. and Miller , H.L. 2007 . Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Cambridge , , UK : Cambridge University Press .
- Stolarski, R.S. 1999. History of the Study of Atmospheric Ozone http://hyperion.gsfc.nasa.gov/Personnel/people/Stolarski_Richard_S./history.html (http://hyperion.gsfc.nasa.gov/Personnel/people/Stolarski_Richard_S./history.html)
- Strutt , R.J. 1918 . Ultra-violet transparency of the lower atmosphere, and its relative poverty in ozone . Proc. R. Society London Ser. A , 94 ( 660 ) : 260 – 68 . doi: 10.1098/rspa.1918.0012
- Sullivan , W. 1975 . Ozone depletion seen as a war tool . New York Times , February 28: 20
- Swaminathan , T. P. A. and Lucas , R. M. 2012 . Climate change and health with an emphasis on interactions with ultraviolet radiation: a review . Global Change Biology , 18 ( 8 ) : 2392 – 2405 . doi: 10.1111/j.1365-2486.2012.02706.x
- Teramura , A.H. and Sullivan , J.H. 1991 . “ Potential impacts of increased solar UV-B on global plant productivity ” . In Photobiology , Edited by: Riklis , E. New York , NY : Plenum Press .
- Tevini , M. and Morehouse , E.T. 1998 . “ Effects of UV-B on plants and terrestrial ecosystems ” . In Protecting the Ozone Layer: Lessons, Models, and Prospects , Edited by: Le Prestre , P.G. and Reid , J.D. New York , NY : Springer .
- The Online Imperial Club, 2001. Airtemp: Chrysler's Airy Solution to the Long, Hot Summer http://www.imperialclub.com/Repair/Air/airtemp.htm (http://www.imperialclub.com/Repair/Air/airtemp.htm) (Accessed: December 31, 2012 ).
- 2008 . Protection of the Stratospheric Ozone: Alternatives for the Motor Vehicle Air Conditioning Sector Under the Significant New Alternatives Policy (SNAP) Program; Final Rule. 40 CFR Part 82 U.S. Federal Register
- 2011 . Protection of Stratospheric Ozone: New Substitute in the Motor Vehicle Air Conditioning Sector Under the Significant New Alternatives Policy (SNAP) Program; Final Rule. 40 CFR Part 82 U.S. Federal Register
- 2012 . Protection of Stratospheric Ozone: Alternative for the Motor Vehicle Air Conditioning Sector Under the Significant New Alternatives Policy (SNAP) Program; Final Rule. 40 CFR Part 82 U.S. Federal Register
- Nations , United . 2012 . Resolution adopted by the General Assembly: The Future We Want, A/res/66/288 http://www.uncsd2012.org/thefuturewewant.html (accessed May 1, 2013).
- United Nations Environment Programme . 1978 . Ozone Layer Bulletin , Nairobi , Kenya : UNEP (UN Document number Na 77-5494, January) .
- United Nations Environment Programme . 1978 . UNEP Ozone Layer Bulletin 2 , Nairobi , Kenya : UNEP (July) .
- United Nations Environment Programme . 1979 . UNEP Ozone Layer Bulletin 3 (Volume II, no. 1) , Nairobi , Kenya : UNEP (UN Document number Na 78-4873, January) .
- United Nations Environment Programme . 1979 . UNEP Ozone Layer Bulletin 4 (Volume II, no. 2) , Nairobi , Kenya : UNEP (July) .
- United Nations Environment Programme . 1980 . UNEP Ozone Layer Bulletin 5 , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 1981 . UNEP Ozone Layer Bulletin 6 , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 1982 . UNEP Ozone Layer Bulletin 7 , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 1984 . UNEP Ozone Layer Bulletin 8 , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 1985 . UNEP Ozone Layer Bulletin 9 , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 1989 . Technical Progress on Protecting the Ozone Layer; Report of the Technology Review Panel Pursuant to Article (6) of the Montreal Protocol on Substances That Deplete the Ozone Layer Under the Auspices of the United Nations Environment Program , Nairobi , Kenya : UNEP. ISBN: 9280712470 .
- United Nations Environment Programme . 1999 . The Implications to the Montreal Protocol of the Inclusion of HFCs and PFCs in the Kyoto Protocol , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . Decision XXII/Anx III: Annex III -2010. Declaration on The Global Transition Away From Hydrochlorofluorocarbons (HCFCs) and Chlorofluorocarbons (CFCs) , Nairobi , Kenya : UNEP (UN Document number UNEP/Ozl.Pro.22/9) .
- United Nations Environment Programme . 2010a . Environmental Effects of Ozone Depletion And Its Interactions With Climate Change: 2010 Assessment , Nairobi , Kenya : UNEP .
- United Nations Environment Programme . 2011 . Report of the Combined Ninth Meeting of the Conference of the Parties to the Vienna Convention on the Protection of the Ozone Layer and the Twenty-Third Meeting of the Parties to the Montreal Protocol on Substances that Deplete the Ozone Layer , Nairobi , Kenya : UNEP (UN Doc UNEP/Ozl.Pro.23/11, 8 December 2011) .
- United Nations Environment Programme . 2011a . HFCs: A Critical Link in Protecting Climate and the Ozone Layer , Nairobi , Kenya : UNEP .
- United Nations Environment Programme (UNEP) . 2012a . Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer , Nairobi , Kenya : Montreal Protocol Ozone Secretariat .
- United Nations Environment Programme . 2012b . Proposed Amendment to the Montreal Protocol, Submitted by the United States, Canada and Mexico (UN Doc. UNEP/OzL.Pro.WG.1/32/6, 11 May) (‘Proposed Amendment by the U.S., Canada and Mexico’)
- United Nations Environment Programme . 2012c . Proposed Amendment to the Montreal Protocol, Submitted by the Federated States of Micronesia (UN Doc. UNEP/OzL.Pro.WG.1/32/5, 11 May) (‘Proposed Amendment by Micronesia’)
- United Nations Environment Programme . 2012d . Report of the Twenty-Fourth Meeting of the Parties to the Montreal Protocol on Substances that Deplete the Ozone Layer. (UN Doc. UNEP/Ozl.Pro.24/10, 16 November 2012)
- United Nations Environment Programme and World Meteorological Organization . 2011 . Integrated Assessment of Black Carbon and Tropospheric Ozone: Summary for Decision Makers , Nairobi , Kenya : UNEP .
- United Nations Environment Programme. 2013. Proposed Amendment to the Montreal Protocol, Submitted by the Federated States of Micronesia (UN Doc. UNEP/OzL.Pro.WG.1/33/4) 16 April (‘Proposed Amendment by Micronesia’)
- United Nations Environment Programme. 2013a. Proposed Amendment to the Montreal Protocol, Submitted by Canada, Mexico and the United States of America (UN Doc. UNEP/OzL.Pro.WG.1/33/3) 16 April (‘Proposed Amendment by Canada, Mexico and the USA’)
- U.S. Environmental Protection Agency . 1999a . The Benefits and Costs of the Clean Air Act, 1990–2010 , Office of Air and Radiation. EPA 4W-R-99-001 . http://www.epa.gov/air/sect812/1990-2010/fullrept.pdf
- U.S. Environmental Protection Agency . 1999b . The Importance of Motor Vehicle Air Conditioning in Climate Protection , Washington , DC : U.S. EPA .
- U.S. Environmental Protection Agency . 2007 . Achievements in Stratospheric Ozone Protection: Progress Report , Office of Air and Radiation. EPA 430-R-07-001 . http://www.epa.gov/ozone/downloads/spd-annual-report_final.pdf
- U.S. Environmental Protection Agency. 2009. Comparing the Climate Impacts of Mobile Air Conditioners http://www.epa.gov/cpd/mac/compare.htm. (http://www.epa.gov/cpd/mac/compare.htm.) (Accessed: December 31, 2012 ).
- U.S. Environmental Protection Agency . 2012 . Report to Congress on Black Carbon , Washington , DC : U.S. EPA .
- U.S. Environmental Protection Agency and Department of Defense . 2008 . The Importance of Military Organizations in Protecting the Climate , Washington , DC : IGSD .
- U.S. Patent 7,534,366 . 2003 . Filed 27 October
- U.S. Patent 7,279,451 . 2004 . Filed 29 April
- van der Leun , J.C. and de Gruijl , F.R. 2002 . Climate change and skin cancer . Photochem. Photobiol. Sci. , 1 : 324 – 26 . doi: 10.1039/b201025a
- van der Leun , J.C. , Piacentini , R.D. and de Gruijl , F.R. 2008 . Climate change and human skin cancer . Photochem. Photobiol. Sci. , 7 : 730 – 33 . doi: 10.1039/b719302e
- van Hattem , S. , Aarts , M.J. , Louwman , W.J. , Neumann , H.A. , Coebergh , J.W. , Looman , C.W. , Nijsten , T. and de Vries , E. 2009 . Increase in basal cell carcinoma incidence steepest in individuals with high socioeconomic status: results of a cancer registry study in The Netherlands . Br. J. Dermatol. , 161 : 840 – 45 . doi: 10.1111/j.1365-2133.2009.09222.x
- VASA. 2009. Hot Air Newsletter. The Mobile AC, Electrical and Cooling Technicians of Australia http://www.vasa.org.au/wp-content/hotair/hot_air_feb09.pdf (http://www.vasa.org.au/wp-content/hotair/hot_air_feb09.pdf) (Accessed: December 28, 2012 ).
- Velders , G. J. M. , Andersen , S. O. , Daniel , J. S. , Fahey , D. W. and McFarland , M. 2007 . The importance of the Montreal Protocol in protecting climate . Proc. Natl. Acad. Sci. USA , 104 ( 12 ) : 4814 – 19 . doi: 10.1073/pnas.0610328104
- Velders , G.J.M. , Fahey , D.W. , Daniel , J.S. , McFarland , M. and Andersen , S.O. 2009 . The large contribution of projected HFC emissions to future climate forcing . Proc. Natl. Acad. Sci. USA , 106 : 10949 – 54 . doi: 10.1073/pnas.0902817106
- Velders , G.J.M. , Ravishankara , A.R. , Miller , M.K. , Molina , M.J. , Alcamo , J. , Daniel , J.S. , Fahey , D.W. , Montzka , S.A. and Reimann , S. 2012 . Preserving Montreal Protocol climate benefits by limiting HFCs . Science , 335 ( 6071 ) : 922 – 23 . doi: 10.1126/science.1216414
- Vojnikovic , B. , Njiri , S. , Coklo , M. , Toth , I. , Spanjol , J. and Marinovic , M. 2007 . Sunlight andincidence of pterygium on Croatian Island Rab-epidemiological study . Coll. Anthropol. , 31 ( suppl. 1 ) : 61 – 62 .
- Wang , L. , Toda , M. , Saito , K. , Hori , T. , Horii , T. , Shiku , H. , Kuribayashi , K. and Kato , T. 2008 . Post-immune UV irradiation induces Tr1-like regulatory T cells that suppress humoral immune responses . Int. Immunol. , 20 : 57 – 70 . doi: 10.1093/intimm/dxm124
- Weisler, P. 2008. EPA Presses for Faster Switch to A/C Refrigerant R-1234yf. SAE Automotive Engineering International, News Online, 7 April http://www.sae.org/mags/aei/6137 (http://www.sae.org/mags/aei/6137) (Accessed: March 20, 2013 ).
- Weisskopf , M. 1987 . Ozone depletion worsens, is linked to man-made gas . Washington Post , October 1: a. 23
- Wheeler , A.E. 1999 . A view of IAQ (indoor air quality) as the century closes . ASHRAE J. , November : 35 – 38 .
- Wofsy , S.C. and McElroy , M.B. 1974 . HOx, NOx, and ClOx: Their role in atmospheric photochemistry . Can. J. Chem. , 52 : 1582 – 91 . doi: 10.1139/v74-230
- Wofsy , S.C. , McElroy , M.B. and Sze , N.D. 1975 . Freon consumption: Implications for atmospheric ozone . Science , 187 ( 4176 ) : 535 – 36 . doi: 10.1126/science.187.4176.535
- World Meteorological Organization. 2012. WMO Greenhouse Gas Bulletin: The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2011 www.wmo.int/pages/mediacentre/press_releases/pr_965_en.html (http://www.wmo.int/pages/mediacentre/press_releases/pr_965_en.html) (Accessed: December 31, 2012 ).
- Young , O.R. 1999 . The Effectiveness of International Environmental Regimes: Causal Connections and Behavioral Mechanisms , Cambridge , MA : MIT Press .
- Zaelke , D. , Andersen , S.O. and Borgford-Parnell , N. 2012 . Strengthening ambition for climate mitigation: The role of the Montreal Protocol in reducing short-lived climate pollutants . Rev. Eur. Commun. Int. Environ. Law , 21 ( 3 ) : 231 – 42 . doi: 10.1111/reel.12010
- Zerefos , C. , Contopoulos , G. and Skalkeas , G. 2009 . Twenty Years of Ozone Decline: Proceedings of the Symposium for the 20th Anniversary of the Montreal Protocol , Dordrecht , , The Netherlands : Springer .