Abstract
This study synthesized multiwall carbon nanotube (MWNT)–titania (TiO2) composites and examined their characteristics and photocatalytic performance for the cleaning of gas-phase benzene, toluene, ethyl benzene, and o-xylene (BTEX) under simulated indoor conditions. Optical and spectral surveys of the as-synthesized composite confirmed that the TiO2 nanoparticles were bound intimately to the MWNT networks. The photocatalytic performance was evaluated using an annular-type reactor inner-coated with MWNT–TiO2 or Degussa P25 TiO2. The composite revealed gas removal ability superior to that of stand-alone TiO2. This composite was also less affected by humidity during toluene decomposition compared to the previous result obtained from a stand-alone TiO2. Unlike another previous result obtained from the TiO2, the performance of the composite was not affected by changes in input concentration (IC) within a simulated indoor air quality range (0.1–1.0 ppm) but it decreased significantly when the IC was increased to 5 and 10 ppm. As the flow rate was decreased from 4.0 to 1.0 L min−1, the average efficiency for the target compounds increased to 95% or ∼100%. The MWNT–TiO2 composite could be applied effectively to the decomposition for BTEX under certain simulated indoor conditions.
Unlike water applications, there are few reports of gas-phase applications of multiwall carbon nanotubes (MWCNT)–TiO2 composites. This study found that MWCNT–TiO2 composites showed performance in the removal of toxic gaseous aromatic superior to that of stand-alone TiO2. In addition, the pollutant degradation efficiency of the composite was less affected by humidity than for a stand-alone TiO2 unit within a simulated indoor relative humidity range. Moreover, unlike the TiO2 unit, the composite's performance was not affected by variations in the input concentrations within the simulated indoor air quality (IAQ) range. In addition, the decomposition efficiencies increased to 100% with decreasing flow rate.
Introduction
Recently, several studies have attempted to enhance the photocatalytic properties of titania (TiO2) for the decomposition of a range of environmental pollutants by coupling with an adsorbent supporter (CitationAo and Lee, 2005; CitationYao et al., 2010). The Langmuir–Hinshelwood mechanism, which best models the main photocatalytic decomposition process, requires the adsorption of species before the decomposition process (CitationValente et al., 2006; CitationAnotai et al., 2012). Therefore, the increased adsorption sites for the TiO2–adsorbent composite likely result in enhancing photocatalytic activity of TiO2. Granular-type activated carbon (GAC) was used initially as a support for TiO2 in the photocatalytic decomposition of water and air pollutants (CitationAo and Lee, 2005; CitationLiu et al., 2007; CitationLi Puma et al., 2008). Later, activated carbon (AC) fibers (ACFs) were suggested to be another type of photocatalyst-supporting material, consisting of nanographite (CitationYao et al., 2010). Compared to GAC, ACF has a larger specific surface area, a superior adsorption and desorption rate, and a faster attainment of adsorption equilibrium (CitationDas et al., 2004). In TiO2–adsorbent composite systems, the adsorbents are likely to support the TiO2 powders by concentrating the pollutants and intermediates around the powders, followed by the subsequent migration of these species to the surface of the photocatalyst via diffusion (CitationShan et al., 2010).
Carbon nanotubes (CNTs) have been suggested to be a more promising coupler of TiO2 on account of their unique structural, electrical, adsorption, mechanical, and thermal properties (CitationLeary and Westwood, 2011). CNTs can also improve the TiO2 photocatalytic activity by acting as a photosensitizer, thereby extending photocatalysis into the visible range (CitationOu et al., 2006). Therefore, several studies have applied CNT–TiO2 composites to the purification of certain environmental pollutants. These studies reported that CNT–TiO2 composites displayed superior photocatalytic performance to pure TiO2 photocatalysts for decomposition of environmental pollutants. On the other hand, previous studies were carried out primarily in the aqueous phase for the treatment of water pollutants, such as phenol (CitationYao et al., 2008), azo dye (CitationKuo et al., 2009), methylene blue (CitationOh et al., 2009), and methyl orange (CitationHu et al., 2007). Unlike water applications, there are few reports on the gas-phase applications of CNT–TiO2 composites. Both photon absorbance and reaction kinetics of environmental pollutants differ between liquid–solid and gas–solid interfaces. This suggests that the photocatalytic activity obtained from the water–solid photocatalytic process might not be applicable to that for the air–solid photocatalytic process, highlighting the need to confirm the feasibility of CNT–TiO2 composites for air purification applications.
This study examined the surface and morphological characteristics and the heterogeneous photocatalytic performance of a multiwall CNT–TiO2 (MWNT–TiO2) composite to clean gas-phase aromatic hydrocarbons using an annular-type Pyrex reactor. The photocatalytic performance was examined under a range of simulated indoor conditions because the photocatalytic activities in air media can vary with real environmental conditions, such as the types and concentrations of gaseous pollutants and humidity (CitationDemeestere et al., 2007). For simulated indoor environmental conditions, the tested humidity range covered both dried and humidified environmental conditions, and the surveyed concentrations included the indoor air quality (IAQ) concentration levels (≤1 ppm). The target compounds included four organic vapors (benzene, toluene, ethyl benzene, and xylene [BTEX]) that are toxic or potentially toxic to humans (CitationChen et al., 2008). These compounds are one of the volatile organic compound (VOC) groups that are typically detected at higher concentrations in indoor air compared to outdoor air (CitationOhura et al., 2009). Typical indoor BTEX concentrations ranges were 0.0007–0.05 ppm, 0.002–0.44 ppm, 0.0004–0.03 ppm, and 0.0005–0.06 ppm for the four components, respectively (CitationZuraimi et al., 2006; CitationOhura et al., 2009). The photocatalytic performance of commercially available Degussa P25 TiO2 was also assessed under the same experimental conditions for comparison with that of the MWNT–TiO2.
Methods
Preparation, coating, and characterization of photocatalysts
A sol–gel process was used to synthesize MWNT–TiO2 composite photocatalysts (CitationYen et al., 2008). In this process, the MWNTs purchased from the Carbon Nano-Material Technology, Korea were acid treated and washed with distilled water to remove the impurities and form MWNT–carboxyl functional groups. After this acid treatment, 1.0 g of MWNT (95.9%,) was added and sonicated in 65 mL ethanol (99.9% Merck) for 20 min to disperse it. Subsequently, 20 mL of tetrabutyl orthotitanate (TBOT, Sigma-Aldrich) was added to the MWNT–ethanol solution. After sonicating this mixture for 30 min to form a better mix, it was stirred vigorously for 30 min. Subsequently, 6 mL of nitric acid (69 wt%, Junsei) and 25 mL of deionized water were added to this solution to catalyze the hydrolysis and condensation. The resulting mixture was then stirred vigorously until a homogeneous gel was formed. This gel was aged in air for 4 days at room temperature, and the xerogel was crushed into a fine powder and dried at 80°C in an oven for 12 hr. The powders were calcined at 400°C for 5 hr to obtain the MWNT–TiO2 nanocomposites.
The as-synthesized MWNT–TiO or Degussa P25 TiO2 powders were coated on the inner surface of the Pyrex reactor (CitationJo et al., 2002). Briefly, MWNT–TiO powder (0.5 g) or Degussa P25 TiO2 (0.5 g) was added to 2 mL of an aqueous 0.1 M ethylenediamine tetraacetic acid solution and ground to produce a viscous paste. The paste was diluted by adding 1 mL of water slowly. Subsequently, 0.1 mL of Triton X-100 was added and the paste was smeared on the inner surface of the Pyrex reactor. The coated reactor was then dried for 1 hr at room temperature and baked at 400°C for 30 min.
The synthesized MWNT–TiO2 powders, along with uncoupled acid-treated MWNTs, were characterized by x-ray diffraction (XRD, Rigaku D/max-2500), thermogravimetric analysis (TGA, TA Instrument SDT Q600 TG/DTA), field emission scanning electron microscopy (FE-SEM, Hitachi S-4300 and EDX-350), x-ray photoelectron spectroscopy (XPS, PHI Quantera SXM), and -ransform infrared (FTIR, PerkinElmer Spectrum GX and AutoImage spectrophotometer) spectroscopy. The XRD patterns were determined using Cu Kα radiation operated at 40 kV and 100 mA in the range of 20–80° (2θ) at a scanning rate of 3° min−1. The particle morphology was observed by FE-SEM at an acceleration voltage of 15 kV. XPS was carried out using Al Kα radiation (hν 1486.6 eV), and the angle between the x-ray source and the detector was 40°. FTIR analysis was performed at a resolution of 0.25 cm−1 in the spectral range of 400–4000 cm−1 using a potassium bromide pellet for sample preparation.
Survey protocol
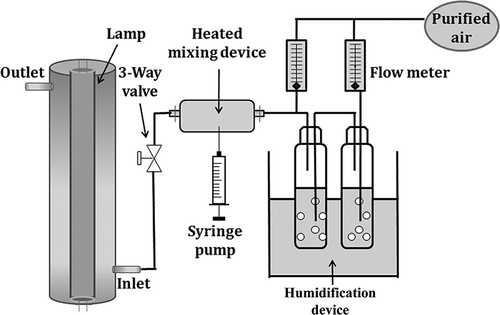
The schematic diagram of the experimental setup is shown in The photocatalytic reactor used in this study had an annular geometry. The reactor consisted of a Pyrex tube (ID 3.5 cm and length 26.5 cm) coated on the inner surface with a thin film of MWNT–TiO2. For comparison, another annular-type reactor was prepared by inner surface coating with commercially available Degussa P25 TiO2. A conventional cylindrical lamp was inserted inside the reactor, whose outer surface was used as the inner surface of the annular reactor. The gas flowed through the annular region. The humidity was adjusted by passing zero-grade air through a charcoal filter, followed by a humidification device in a water bath. The relative humidity (RH) was measured in front of the photocatalytic reactor inlet using a humidity meter (T & D Thermo Recorder TR-72S). The stream flow rate (SFR) measurements were carried out using identical flowmeters. A standard gas was prepared with a precision of <5% by injecting the target compounds into a mixing bulb through a syringe pump (KdScientific Model 210), which was then fed into the reactor.
This study examined the heterogeneous photocatalytic performance of a MWNT–TiO2 for the cleaning of gas-phase pollutants under different experimental conditions. These experimental conditions were established based on the RH, SFRs, and initial concentrations (ICs). The RH range for these experiments was 20–95% (20, 45, 70, and 95%). The SFR was adjusted to 1.0, 2.0, 3.0, or 4.0 L min−1. Four different ICs (0.1, 0.5, 1.0, and 2.0 ppm) were surveyed. For each parameter test, the other parameters were fixed to their representative values: RH, 45%; SFR, 4.0 L min−1; and IC, 0.1 ppm. The hydraulic diameter (defined as the internal diameter of the annular reactor tube minus the external diameter of the lamp) of the photocatalytic reactor was 10 mm. The ultraviolet (UV) radiation supplied by an 8-W fluorescent black light (Sankyo Denki F8T5/BLB) with a maximum spectral intensity at 352 nm was used to compare the photocatalytic activities of MWNT–TiO2 and UV-efficient Degussa P25 TiO2. The photocatalytic activity of the Degussa P25 TiO2 unit was examined under the same representative operational conditions.
Air measurements
Air measurements were conducted periodically at the inlet and outlet of the photocatalytic reactor to determine its decomposition efficiency with regard to the target compounds. Air samples were collected by filling an evacuated a 5-L Tedlar bag at a constant flow rate. The air from this bag was then drawn through a 0.64-cm OD and 10-cm-long SS sorbent trap containing 0.2 g of Tenax TA using a constant flow-sampling pump (SKC Aircheck Sampler model 224-PCXR8). The sampling times were varied from 1 to 5 min depending on the flow rate. All samples were taken at ambient room temperature (19–23°C). The gas-phase compounds collected on the sorbent trap were analyzed by coupling a thermal desorption system (Perkin Elmer ATD 400) to a gas chromatograph (Agilent GC 7890A) in conjunction with a flame ionization detector using a capillary column (Agilent, DB-1). The quality control program included laboratory blank traps and spiked samples. For each day, a lab blank trap and an external standard was analyzed to check for any trap contamination and quantitative response, respectively. When the quantitative response differed from that estimated by the specified calibration curve by more than 10%, a new calibration curve was obtained. The method detection limits of the target compounds ranged from 0.0002 to 0.0007 ppm.
Results and Discussion
Characteristics of MWNT and MWNT–TiO2
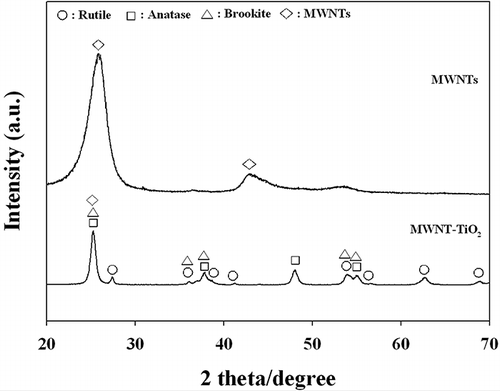
The characteristics of the stand-alone MWNT and as-synthesized MWNT–TiO2 powders were analyzed by XRD, TGA, SEM, XPS, and FTIR spectroscopy. shows the XRD patterns of both the MWNT and MWNT–TiO2 composite. XRD of MWNT revealed peaks at 26.0 and 43.5° 2θ, which are characteristic of CNTs (Lee and Sigmund, 2003). In contrast, the MWNT–TiO2 composite yielded a small peak at 26.0° 2θ, but there was no peak at 43.5° 2θ. This is consistent with that reported by CitationWang et al. (2007), even though their MWNT–TiO2 powders had been synthesized using a hydrothermal process, whereas a sol–gel method was used in the present study. The peak at 26.0° 2θ observed for the MWNT–TiO2 composite was assigned to the overlap of an MWNT peak with the anatase and brookite peaks for TiO2 coupled with MWNTs. XRD patterns revealed the MWNT–TiO2 composite to contain major amounts of anatase of TiO2 with minor amounts of brookite and rutile phases.
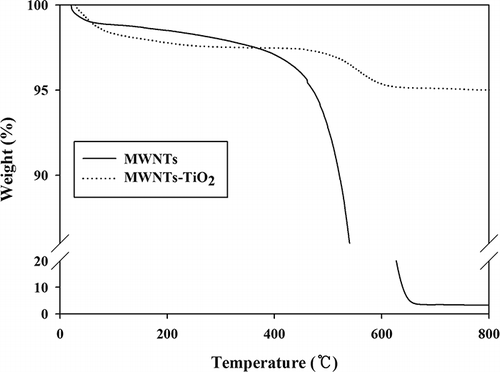
TGA of the MWNT and MWNT–TiO2 composite was conducted to evaluate their thermal stability and determine the TiO2 content combined on the network of MWNTs. shows the TGA results of the MWNT and MWNT–TiO2 composite. Three-step weight losses were observed in the temperature range 20–800°C. For the MWNTs, the maximum gasification rate was observed at approximately 600°C, which is similar to that of a previous study (CitationBom et al., 2002). A 94.9% weight loss was observed in the second step, suggesting the destruction of most MWNTs. For the MWNT–TiO2 composite, a weight loss (0.6%) with an endothermic process was observed between 20 and 500°C. This weight loss was attributed to the release of residual TiO2 precursor and organic solvent. The second sharp weight loss (2.4%) was observed between 502 and 597°C and was attributed to thermal decomposition of MWNTs and the crystallization of amorphous TiO2 (CitationWang et al., 2007). Almost no weight loss occurred between 650 and 800°C, suggesting that TiO2 became the major component over this temperature range. Therefore, the amount of TiO2 coupled with MWNTs was approximately 95 wt%.
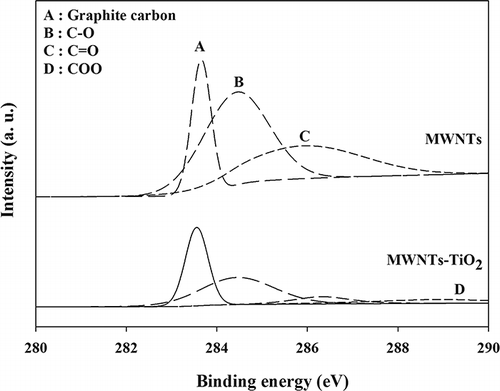
XPS was used to examine the C 1s regions for both the MWNT–TiO2 composite and acid-treated MWNTs. As shown in , the spectrum of the MWNTs revealed four peaks at 283.7, 284.2, 284.4, and 285.9 eV. The peaks at 283.7 and 284.2 eV were assigned to the carbide and graphite carbon atoms, respectively, and the other peaks at 284.4 and 285.9 were attributed to–C–O and –C=O, respectively. For the MWNT–TiO2 composite, however, another peak appeared at 288.9 eV corresponding to a –COO bond, which would be formed by the oxidation of MWNT surfaces during the composite synthesis process (CitationYen et al., 2008). The carbide peak was not observed in the XP spectra of the MWNT–TiO2 composite, and the intensities of the other peaks were lower than for the stand-alone MWNT. The MWNT–TiO2 composite was calcined at 400°C, whereas the MWNT was just acid treated without calcination. Therefore, the different XP spectra between the composite and the stand-alone MWNT were attributed to destruction of certain chemical species, which would have occurred during calcination of the MWNT–TiO2 composite.
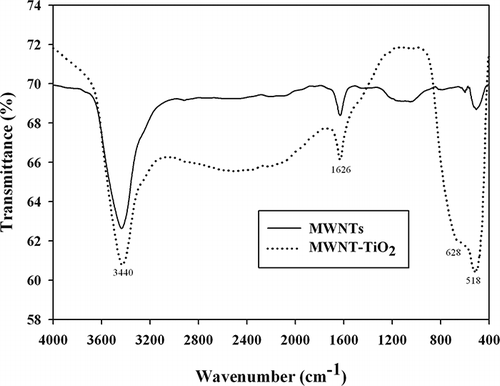
shows the FTIR spectra of the acid-activated MWNT and MWNT–TiO2 composite. Both spectra revealed a small peak at 1626 cm−1, which was assigned to the MWNT–COOH spectrum (CitationWang et al., 2007). This peak confirmed the presence of a carboxyl group formed during the acid treatment of MWNTs. The band at 3440 cm−1 was assigned to the O–H stretching vibration of adsorbed water molecules (CitationDai et al., 2009). The broad band below 1000 cm−1 observed for the MWNT–TiO2 composite corresponds to the titania crystal lattice vibration (CitationWei et al., 2008); this band was not observed for the stand-alone MWNTs.
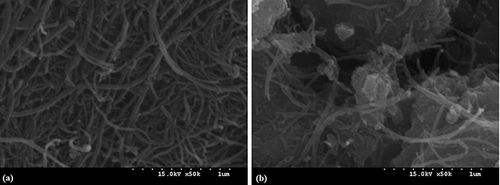
shows SEM images of the MWNT and MWNT–TiO2 composite. The latter image clearly indicates that TiO2 nanoparticles are bound intimately to the MWNT networks. This result is similar to those of MWNT–TiO2 synthesized by CitationYen et al. (2008) and Dai et al. (2009) using different hydrothermal processes. Consequently, the spectral and optical results including these SEM images confirmed that for the as-synthesized MWNT–TiO2 composite, MWNTs could be coupled with TiO2 using the sol–gel route applied to the current study.
Photocatalytic performance of MWNT–TiO2 composite and Degussa P25 photocatalyst
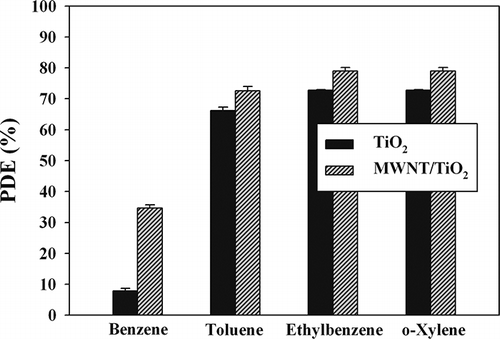
The heterogeneous photocatalytic performance of a MWNT–TiO2 composite was evaluated for the purification of gas-phase BTEX under a range of conditions, including simulated indoor conditions based on the RHs and ICs. In addition, the photocatalytic performance of Degussa P25 TiO2 was examined for a comparison with that of the MWNT–TiO2 composite. shows the average BTEX photocatalytic decomposition efficiencies determined using the UV/MWNT–TiO2 unit and UV/Degussa P25 TiO2 under the same operational conditions. For all target compounds, the decomposition efficiencies determined by the MWNT–TiO2 unit were higher than those of the Degussa P25 TiO2 unit. The mean efficiencies determined by the MWNT–TiO2 for BTEX were 35, 73, 79, and 80%, respectively, whereas those of the commercial TiO2 powders were 8, 66, 72, and 73%, respectively. Consequently, the MWNT–TiO2 showed superior performance in the photocatalytic decomposition of toxic gas-phase aromatic pollutants to the commercially available reference photocatalyst, Degussa P25 TiO2.
Figure 7. Mean photocatalytic decomposition efficiency (PDE) and standard deviation of BTEX determined using MWNT– TiO2 and Degussa P25 TiO2.
The superior performance of the MWNT– TiO2 composite was attributed to the synergistic effect of the electrical properties and adsorption capacity of the MWNT–TiO2 composite (CitationLeary and Westwood, 2011). As CNTs have large electron-storage capacity with one electron per 32 carbon atoms (CitationKongkanand and Kamat, 2007), they might store photon-excited electrons in the TiO2–CNT composite, which would retard electron–hole recombination. The CNT–TiO2 barrier, where there is a space-charge separation region, might increase the recombination times for the photon-generated electron–hole pairs, thereby increasing the photocatalytic activity of the MWNT–TiO2 nanomaterials (CitationLeary and Westwood, 2011). MWNTs also have adsorption capacity with a surface area >150 m2 g−1, where BTEX molecules can be adsorbed, even though their specific surface area is less than that of typical GAC or ACF (CitationLeary and Westwood, 2011). Adsorption in a MWNT bundle can occur in the aggregated pores, inside the tube, or on the external walls (CitationSerp et al., 2003).
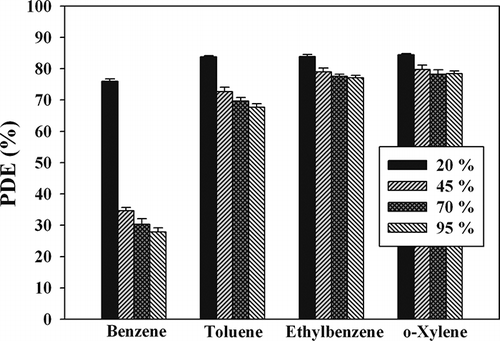
shows the effect of humidity on the MWNT–TiO2 performance for the photocatalytic decomposition of BTEX. For all target compounds, the lowest decomposition efficiency was observed under the lowest RH (20%) condition. For benzene, the decomposition efficiency decreased gradually with increasing RH within the RH range of 20–95%. On the other hand, the decomposition efficiencies of the other three target compounds were similar at the three highest RHs (45, 70, and 95%). For example, the mean decomposition efficiency for toluene was 85% for a RH of 25%, which decreased to 73% for a RH of 45%, but remained relatively constant within the RH range of 45–95%. Therefore, the effects of humidity on the performance of MWNT–TiO2 can vary with chemical types. With respect to high RH conditions, excessive water molecules would preferably occupy active sites on the photocatalyst surface, causing a decrease in the reaction rate. On the other hand, the changes in the toluene decomposition efficiencies with the RH for the MWNT–TiO2 composite were different from those of stand-alone TiO2 reported by CitationSleiman et al. (2009). Using TiO2 as a photocatalyst under similar experimental conditions (0.02–0.4 ppm), one study reported that the toluene decomposition efficiency decreased with increasing RH within the RH range of 55–95%, whereas in the present study it did not vary significantly within a similar RH range. Therefore, the photocatalytic decomposition efficiency of the MWNT–TiO2 composite was affected less by humidity than that of stand-alone TiO2.
Figure 8. Mean photocatalytic decomposition efficiency (PDE) and standard deviation of BTEX according to the relative humidity.
shows the BTEX photocatalytic degradation efficiency determined using the MWNT–TiO2 unit according to the ICs. The decomposition efficiencies did not vary significantly with increasing IC within the range of 0.1–1.0 ppm. For example, the average decomposition efficiencies for BTEX for an IC of 0.1 ppm were 34, 73, 79, and 80%, respectively, and those of an IC of 1.0 ppm were similarly 33, 71, 78, and 80%, respectively. This suggests that, within this concentration range, photocatalytic decomposition would occur on the MWNT–TiO2 surface through a Langmuir–Hinshelwood mechanism via first-order reaction kinetics. In contrast, CitationJo and Yang (2009) reported that the BTEX degradation efficiencies determined using the UV/Degussa P25 TiO2 unit decreased gradually with increasing IC from 0.1 to 1.0 ppm. This concentration range (0.1–1.0 ppm) covers the typical IAQ levels (CitationOhura et al., 2009). This suggests that, unlike the stand-alone TiO2, the photocatalytic performance of TiO2 coupled with MWNTs for the decomposition of toxic gas-phase pollutants is not affected by changes in concentration within the simulated IAQ concentration range.
Figure 9. Mean photocatalytic decomposition efficiency (PDE) and standard deviation of BTEX according to the initial concentrations.
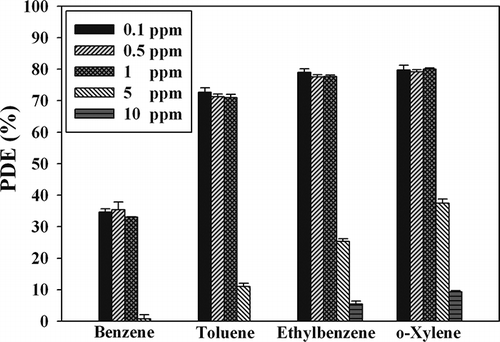
Nevertheless, when the IC was increased to 5.0 ppm, the mean degradation efficiencies for BTEX determined by the MWNT–TiO2 unit decreased to approximately 0, 11, 25, and 38%, respectively. Furthermore, the mean degradation efficiencies decreased to approximately 0% for both benzene and toluene, and 6 and 9% for ethyl benzene and o-xylene, respectively, when the IC was increased to 10 ppm. Other studies (CitationPengyi et al., 2003; CitationBouzaza et al., 2006) that tested certain stand-alone TiO2 units under hundreds of parts per million IC conditions also reported a similar IC dependence on the decomposition efficiency of monocyclic aromatic hydrocarbons to the present study. This IC dependence was attributed to a competitive adsorption rate between contaminant molecules on the catalyst surface because this adsorption rate is an important parameter for the photocatalytic degradation efficiency (Demeestere et al., 2007).
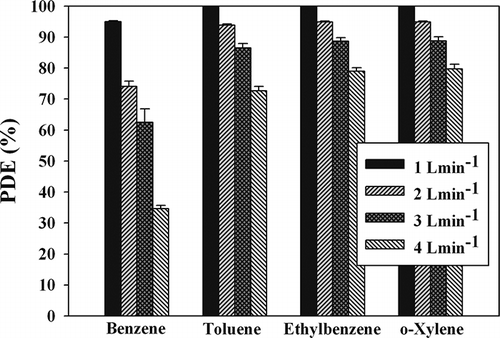
shows the BTEX photocatalytic decomposition efficiency determined using the MWNT–TiO2 unit for four different SFRs (1.0, 2.0, 3.0, and 4.0 L min−1). Similar to a previous TiO2 study (CitationKontos et al., 2012), the decomposition efficiencies of all the target compounds increased with decreasing SFRs. As the SFR was decreased from 4.0 to 1.0 L min−1, the average efficiencies for BTEX increased from 35 to 95%, 73 to approximately 100%, 79 to approximately 100%, and 80 to approximately 100%, respectively. This suggests that SFR is still an important parameter for the photocatalytic BTEX decomposition mechanism of the MWNT–TiO2 unit. Decreased SFRs would result in a decrease in the bulk mass transport of target compounds from the gas phase to the surface of the catalyst particle due to convection and diffusion, which is an important heterogeneous catalytic reaction process (CitationKontos et al., 2012). In this case, the removal rate would decrease with decreasing SFR, indicating that BTEX removal is limited to the catalyst surface. On the other hand, this pattern is inconsistent with that obtained from the current study. At higher SFRs, the gas residence time in the continuous-flow photocatalytic reactor might be too short to allow sufficient BTEX transfer from the gas phase to the catalyst surface (CitationKontos et al., 2012). The residence times in the present study, which were calculated by dividing the reactor volume by SFR, were 7.6, 3.8, 2.5, and 1.9 sec for SFRs of 1.0, 2.0, 3.0, and 4.0 L min−1, respectively. Accordingly, the lower efficiencies at higher SFRs suggest that an insufficient reactor residence time effect outweighs the bulk mass transport effect on BTEX decomposition on the catalyst surfaces.
Conclusion
This study examined the feasibility of MWNT–TiO2 composite uses for the decomposition of toxic gas-phase aromatic hydrocarbons (BTEX) under simulated indoor conditions. XRD, TGA, SEM, XPS, and FTIR spectroscopy of the as-synthesized MWNT–TiO2 composite confirmed that TiO2 nanoparticles had been coupled successfully to the networks of the MWNTs via the sol–gel synthesis route applied in this study. The MWNT–TiO2 composite unit showed superior performance for the decomposition of the target compounds compared to the stand-alone TiO2 unit. Moreover, the composite was less affected by humidity than the stand-alone TiO2 unit. Furthermore, unlike the result obtained from the TiO2 unit, the composite's performance was not affected by changes in input concentration within the simulated IAQ concentration range. On the other hand, similar to the TiO2 unit, the decomposition efficiencies increased with decreasing SFR within the test range. The MWNT–TiO2 composite could be applied effectively to the decomposition of gas-phase pollutants under certain simulated indoor conditions, such as the typical IAQ concentrations and low RH and SFR conditions. Moreover, it is recommended that this photocatalyst can be applied for purification of residential and nonindustry occupational indoor air.
Acknowledgment
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2011-0027916). The author thanks a graduate student (Kun-Hwan Kim) in the Department of Environmental Engineering, Kyungpook National University, for their sample collecting and/or analyses.
References
- Anotai , J. , Jevprasesphant , A. , Lin , Y.-Y. and Lu , M.-C. 2012 . Oxidation of aniline by titanium dioxide activated with visible light . Sep. Purif. Technol. , 84 : 132 – 37 . doi: 10.1016/j.seppur.2011.09.035
- Ao , C.H. and Lee , S.C. 2005 . Indoor air purification by photocatalyst TiO2 immobilizedon an activated carbon filter installed in an air cleaner . Chem. Eng. Sci. , 60 : 103 – 9 . doi: 10.1016/j.ces.2004.01.073
- Bom , D. , Andrews , R. , Jacques , D. , Anthony , J. , Chen , B. , Meier , M.S. and Selegue , J.P. 2002 . Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry . Nano Lett. , 2 : 615 – 19 . doi: 10.1021/nl020297u
- Bouzaza , A. , Vallet , C. and Laplanche , A. 2006 . Photocatalytic degradation of some VOCs in the gas phase using an annular reactor: Determination of the contribution of mass transfer and chemical reaction steps in the photodegradation process . J. Photochem. Photobiol. A , 177 : 212 – 17 . doi: 10.1016/j.jphotochem.2005.05.027
- Chen , C.S. , Hseu , Y.C. , Liang , S.H. , Kuo , J.-Y. and Chen , S.C. 2008 . Assessment of genotoxicity of methyl-ter-butyl ether, benzene, toluene, ethyl benzene, and xylene to human lymphocytes using comet assay . J. Hazrd. Mater. , 153 : 351 – 56 . doi: 10.1016/j.jhazmat.2007.08.053
- Dai , K. , Peng , T. , Ke , D. and Wei , B. 2009 . Photocatalytic hydrogen generation using a nanocomposite of multi-walled carbon nanotubes and TiO2 nanoparticles under visible light irradiation . Nanotechnology , 20 : 125603 doi: 10.1088/0957-4484/20/12/125603
- Das , D. , Gaur , V. and Verma , N. 2004 . Removal of volatile organic compound by activated carbon fiber . Carbon , 42 : 2949 – 62 . doi: 10.1016/j.carbon.2004.07.008
- Demeestere , K. , Dewulf , J. and Van Langenhove , H. 2007 . Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air: State of the art . Crit. Rev. Environ. Sci. Technol. , 37 : 489 – 538 . doi: 10.1080/10643380600966467
- Hu , G. , Meng , X. , Feng , X. , Ding , Y. , Zhang , S. and Yang , M. 2007 . Anatase TiO2 nanoparticles/carbon nanotubes nanofibers: preparation, characterization and photocatalytic properties . J. Mater. Sci. , 42 : 7162 – 70 . doi: 10.1007/s10853-007-1609-7
- Jo , W.K. , Park , K.H. and Chun , H.D. 2002 . Photocatlytic destruction of VOCs for in-vehicle air cleaning . J. Photochem. Photobiol. A , 148 : 109 – 19 . doi: 10.1016/S1010-6030(02)00080-1
- Jo , W.K. and Yang , C.H. 2009 . Feasibility of a tandem photocatalytic oxidation–adsorption system for removal of monoaromatic compounds at concentrations in the sub-ppm range . Chemosphere , 77 : 236 – 41 . doi: 10.1016/j.chemosphere.2009.07.034
- Kongkanand , A. and Kamat , P.V. 2007 . Electron storage in single wall carbon nanotubes. Fermi level equilibration in semiconductor–SWCNT suspensions . ACS Nano , 1 : 13 – 21 . doi: 10.1021/nn700036f
- Kontos , A.G. , Katsanaki , A. , Likodimos , V. , Maggos , T. , Kim , D. , Vasilakos , C. , Dionysiou , D.D. , Schmuki , P. and Falaras , P. 2012 . Continuous flow photocatalytic oxidation of nitrogen oxides over anodized nanotubular titania films . Chem. Eng. J. , 179 : 151 – 57 . doi: 10.1016/j.cej.2011.10.072
- Kuo , C.-Y. 2009 . Prevenient dye-degradation mechanisms using UV/TiO2/carbon nanotubes process . J. Hazard. Mater. , 163 : 239 – 44 . doi: 10.1016/j.jhazmat.2008.06.083
- Leary , R. and Westwood , A. 2011 . Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis . Carbon , 49 : 741 – 72 . doi: 10.1016/j.carbon.2010.10.010
- Li Puma , G. , Bono , A. , Krishnaiah , D. and Collin , J.G. 2008 . Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper . J. Hazard. Mater. , 157 : 209 – 19 . doi: 10.1016/j.jhazmat.2008.01.040
- Liu , Y. , Yang , S. , Hong , J. and Sun , C. 2007 . Low-temperature preparation and microwave photocatalytic activity study of TiO2-mounted activated carbon . J. Hazard. Mater. , 142 : 208 – 15 . doi: 10.1016/j.jhazmat.2006.08.020
- Oh , W.-C. , Jung , A.-R. and Ko , W.-B. 2009 . Characterization and relative photonic efficiencies of a new nanocarbon/TiO2 composite photocatalyst designed for organic dye decomposition and bactericidal activity . Mater. Sci. Eng. C , 29 : 1338 – 47 . doi: 10.1016/j.msec.2008.10.034
- Ohura , T. , Amagai , T. , Shen , X. , Li , S. , Zhang , P. and Zhu , L. 2009 . Comparative study on indoor air quality in Japan and China: Characteristics of residential indoor and outdoor VOCs . Atmos. Environ. , 43 : 6352 – 59 . doi: 10.1016/j.atmosenv.2009.09.022
- Ou , Y. , Lin , J. , Fang , S. and Liao , D. 2006 . MWNT–TiO2: Ni composite catalyst: A new class of catalyst for photocatalytic H2 evolution from water under visible light illumination . Chem. Phys. Lett. , 429 : 199 – 203 . doi: 10.1016/j.cplett.2006.08.024
- Pengyi , Z. , Fuyan , L. , Gang , Y. , Qing , C. and Wanpeng , Z. 2003 . A comparative study on decomposition of gaseous toluene by O3/UV, TiO2/UV and O3/TiO2/UV . J. Photochem. Photobiol. A , 156 : 189 – 94 . doi: 10.1016/S1010-6030(02)00432-X
- Serp , P. , Corrias , M. and Kalck , P. 2003 . Carbon nanotubes and nanofibers in catalysis . Appl. Catal. A , 253 : 337 – 58 . doi: 10.1016/S0926-860X(03)00549-0
- Shan , A.Y. , Ghazi , T.I.M. and Rashid , S.A. 2010 . Immobilization of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review . Appl. Catal. A , 389 : 1 – 8 . doi: 10.1016/j.apcata.2010.08.053
- Sleiman , M. , Conchon , P. , Ferronato , C. and Chovelon , J.-M. 2009 . Photocatalytic oxidation of toluene at indoor air levels (ppbv): Towards a better assessment of conversion, reaction intermediates and mineralization . Appl. Catal. B , 86 : 159 – 65 . doi: 10.1016/j.apcatb.2008.08.003
- Valente , J.P.S. , Padilha , P.M. and Florentino , A.O. 2006 . Studies on the adsorption and kinetics of photodegradation of a model compound for heterogeneous photocatalysis onto TiO2 . Chemosphere , 64 : 1128 – 33 . doi: 10.1016/j.chemosphere.2005.11.050
- Wang , Q. , Yang , D. , Chen , D. , Wang , Y. and Jiang , Z. 2007 . Synthesis of anatase titania-carbon nanotubes nanocomposites with enhanced photocatalytic activity through a nanocoating-hydrothermal process . J. Nano. Res. , 9 : 1087 – 96 . doi: 10.1007/s11051-006-9199-x
- Wei , F. , Ni , L. and Cui , P. 2008 . Preparation and characterization of N-S-codoped TiO2 photocatalyst and its photocatalytic activity . J. Hazard. Mater. , 156 : 135 – 40 . doi: 10.1016/j.jhazmat.2007.12.018
- Yao , Y. , Li , G. , Ciston , S. , Lueptow , R.M. and Gray , K.A. 2008 . Photoreactive TiO2/carbon nanotube composites: Synthesis and reactivity . Environ. Sci. Technol. , 42 : 4952 – 57 . doi: 10.1021/es800191n
- Yao , S. , Li , J. and Shi , Z. 2010 . Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants . Particuology , 8 : 272 – 78 . doi: 10.1016/j.partic.2010.03.013
- Yen , C.-Y. , Lin , Y.-F. , Hung , C.-H. , Tseng , Y.-H. , Ma , C.-C. , Chang , M.-C. and Shao , H. 2008 . The effects of synthesis procedures on the morphology and photocatalytic activity of multi-walled carbon nanotubes/TiO2 nanocomposites . Nanotechnology , 19 : 045604 doi: 10.1088/0957-4484/19/04/045604
- Zuraimi , M.S. , Roulet , C.-A. , Tham , K.W. , Sekhar , S.C. , Cheng , K.W.D. , Wong , N.H. and Lee , K.H. 2006 . A comparative study of VOCs in Singapore and European office buildings . Build. Environ. , 41 : 316 – 29 . doi: 10.1016/j.buildenv.2005.01.028