Abstract
Quantifying non-methane hydrocarbons (NMHC) from animal feeding operations (AFOs) is challenging due to the broad spectrum of compounds and the polar nature of the most abundant compounds. The purpose of this study was to determine the performance of commercial NMHC analyzers for measuring volatile organic compounds (VOCs) commonly emitted from AFOs. Three different NMHC analyzers were tested for response to laboratory generated VOCs, and two were tested in the field at a commercial poultry facility. The NMHC analyzers tested included gas chromatography/flame ionization detector (GC/FID), photoacoustic infrared (PA-IR) and photoionization detector (PID). The GC/FID NHHC analyzer was linear in response to non-polar compounds, but detector response to polar oxygenated compounds were lower than expected due to poor peak shape on the column. The PA-IR NMHC instrument responded well to the calibration standard (propane), methanol, and acetone, but it performed poorly with larger alcohols and ketones and acetonitrile. The PA-IR response varied between compounds in similar compound classes. The PID responded poorly to many of the most abundant VOCs at AFOs, and it underreported alcohols by>70%. In the field monitoring study, total NMHC concentrations were calculated from sum total of VOC determined using EPA Methods TO-15 and TO-17 with GC-MS compared to results from NMHC analyzers. NMHC GC/FID values were greater than the values calculated from the individual compound measurements. This indicated the presence of small hydrocarbons not measured with TO-15 or TO-17 such as propane. The PA-IR response was variable, but it was always lower than the GC/FID response. Results suggest that improved approaches are needed to accurately determine the VOC profile and NMHC emission rates from AFOs.
Commercial nonmethane hydrocarbons (NMHC) analyzers that monitor volatile organic compounds (VOCs) will underreport true concentrations of VOCs if the compound profiles have significant levels of polar compounds. Laboratory experiments showed that the commercial instruments accurately measured nonpolar compounds, but polar compounds were being underreported by NMHC analyzers with known standards. Field experiments showed that laboratory instruments underreported true concentration in the field due to the fact that the most abundant NMHC associated with animal feeding operations were polar VOCs. This report recommends not using NMHC analyzers for quantifying VOCs at animal feeding operations.
Introduction
Modern agriculture has fundamentally changed the way we produce our foods. Farms are no longer diversified and raising multiple crops and species of animals, but rather agriculture operations have grown increasingly specialized, focusing on narrow market segments. This is exemplified in animal agriculture, which has seen considerable consolidation and expansion of large production facilities over the past 20 years. Consolidation has been driven by the need for improved efficiency and economy of scale in production, and it has led to the integration of growers with large meat production companies (CitationMacDonald and McBride, 2009). Over the last 20 years production levels of both pork and broiler operations have increased by 114 and 104%, respectively, while the total number of facilities have declined significantly over that time period (U.S. Department of Agriculture Citation[USDA], 2007). As a result, production facilities described as medium to large concentrated animal feeding operations (CAFOs) (Citation Federal Register, 2003) today represent more than 95% of the swine and poultry production in the United States (USDA, 2007).
Growth in the number and size of modern AFOs has led local residents and environmental groups to push for enforcement of existing laws with the U.S. Environmental Protection Agency (EPA) (i.e., Clean Air Act). However, existing laws were developed to regulate well-characterized emissions from industrial operations using extensively tested methodologies. The scientific and regulatory communities have not come to a consensus on the best way to quantify emissions from animal feeding operations (AFOs), given both the nature of the compounds and uncertainty associated with emissions from nonpoint sources (CitationNational Research Council [NRC], 2003).
In 2005, the U.S. Environmental Protection Agency entered into a voluntary consent agreement with the AFO industry to conduct a 2-year study of air emissions from selected animal feeding operations across the country, called the National Air Emissions Monitoring Study (NAEMS) (U.S. EPA, 2011; Citation Federal Register, 2005; CitationHeber et al., 2006). One of the stated objectives of the study was to promote a national consensus on methodologies for estimating emissions from AFOs. The agreement outlined the methodologies that would be used for measuring emissions from AFOs. One class of pollutants included in the study was volatile organic compounds (VOCs), since these compounds have both health and nuisance issues (i.e., odor) (CitationSchiffman and Williams, 2005; NRC, 2003). The agreement outlined that VOCs would be continuously monitored using a flame ionization detector (FID) analyzer (U.S. EPA Method 25A). Total carbon results from continuous monitoring using Method 25A were to be multiplied by the molecular weight/carbon weight ratio derived from the gas chromatography-mass spectrometry (GC-MS) or GC-FID speciation in order to calculate the total mass of VOCs.
Method 25A has historically been used to quantify gaseous nonpolar hydrocarbons from urban industrial sources (i.e., alkanes, alkenes, etc.), whereas VOC emissions from AFOs are biogenic and have been found to be dominated by polar oxygenated compounds (CitationSchiffman, Bennett, and Raymer, 2001; CitationFilipy et al., 2006; CitationNgwabie et al., 2008; CitationFeilberg et al., 2010; CitationTrabue et al., 2010; CitationRumsey, Aneja, and Lonneman, 2012). It was noted in the consent agreement that some error was anticipated due to the imprecise response of Method 25A to oxygenated compounds (Federal Register, 2005), since FID detectors have a greater sensitivity toward aliphatic hydrocarbons than toward polar oxygenated compounds (Sternberg, Gallow, and James, 1962; CitationDietz, 1967; CitationJorgensen et al., 1990; CitationKallai, Veres, and Balla, 2001; CitationFaiola et al., 2012).
The diversity of compounds associated with AFOs is large (more than 400 compounds), and previous research has shown that concentrations are highly variable (CitationYasuhara, 1987; Filipy et al., 2006; CitationNgwabie et al., 2008; CitationFeilberg et al., 2010; CitationTrabue et al., 2011). Our research group recently published a study monitoring the VOCs from a poultry facility (CitationTrabue et al., 2010). In this study, the most abundant compounds were confirmed to be polar oxygenated hydrocarbons, and VOC concentration levels were highest in areas that housed animals. The dominant VOC chemical classes included alcohols, volatile fatty acids (VFAs), and ketone compounds, and these classes of compounds accounted for between 65 and 85% of all VOCs (CitationTrabue et al., 2010). However, what was not previously reported but monitored was the simultaneous measurement of total nonmethane hydrocarbons (NMHC) using NMHC analyzers during the same parallel sampling. The purpose of this study was to compare and evaluate the response of several NMHC analyzers with known concentrations of oxygenated compounds that were most abundant in CitationTrabue et al. (2010), along with various other reference standards including hexane, TO-15 62-compound mixture, and reduced sulfur compounds and compound mixtures. In addition, results of field sampling using U.S. EPA methods TO-15 and TO-17 with GC-MS analysis were compared with field NMHC analyzers measurements. Three different types of commercially available NMHC analyzers were tested using different types of detectors, and these included gas chromatography/flame ionization detector (GC/FID), photoacoustic infrared (PA-IR), and photoionization detector (PID). This work represents the first critical evaluation of these commonly used detectors in the measurement of VOCs associated with AFO emissions.
Experimental Methods
Laboratory validation of NMHC analyzers
The three different NMHC analyzers compared in this study included a GC/FID model 55C (Thermo Scientific, Franklin, MA), a PA-IR model 1412 (INNOVA model 1412, INNOVA AirTech Instruments A/S, Denmark), and a PID model ppbRAE 3000 (RAE Systems, Inc., San Jose, CA). The GC/FID NMHC analyzer uses a mini-column to separate methane from nonmethane total hydrocarbons. The sampling cycle for the GC/FID instrument was approximately 3 min, and this included a 75-sec total response time for both methane and NMHC. The GC/FID instrument was calibrated using a single propane standard with a 25 ppmv standard. The PA-IR was used to analyze NMHC, along with NH3, CH3, CO2, and water vapor. The analysis cycle time was approximately 26 sec. The PA-IR instrument was calibrated by the manufacturer 2 months prior to the study. The PID instrument is a hand held device with a 3-sec response time. The PID was calibrated following manufacturer's recommendations using a 100-ppm gas standard of isobutylene.
Gas standards of both individual and mixtures were either generated statically in Tedlar bags (SKC, Inc., Eighty Four, PA) or dynamically with either gas cylinders connected to a diluter (model 4020, Environics, Inc., Tolland, CT) or certified permeation tubes and permeation oven system (model 491M-B, Kin-Tek Laboratories, Inc., La Marque, TX). gives a list of the gas standards used in this study. All compounds used to generate gas standards were purchased from Aldrich (Sigma-Aldrich, St. Louis, MO) with a minimum purity of 98% or greater. All gas cylinder standards and mixtures were prepared in oxygen-free nitrogen gas and were certified with an accuracy of at least ±5% and a tolerance blend of ±5%.
Table 1. List of compounds used to evaluate nonmethane hydrocarbon analyzers, the system utilized to prepare the standard, and the type of test conducted
Gas standards generated statically were made in 10-L Tedlar bags that were filled with ultrahigh-purity (UHP) nitrogen gas (Matheson Trigas, Des Moines, IA) prior to adding target compound and compound mixtures. Individual compounds and compound mixtures were initially diluted in water (high-performance liquid chromatography [HPLC] grade, Fisher Scientific, Fair Lawn, NJ) to known concentrations and directly added to the bags through the septum with a glass syringe (600 series, Hamilton Company, Reno, NV). Gas standards for both individual and mixtures were targeted at 0.1–10 ppmv carbon equivalents of propane. Bags were allowed to equilibrate for 0.5 hr prior to sampling with NMHC analyzers. Each individual gas standard was tested at three different concentrations, and each test was run in duplicate at a minimum. NMHC analyzers were directly attached to the Tedlar bags and each bag was sampled to approximately quarter full. Only one NHMC analyzer could analyze any one bag at a given time, but multiple analyzers could sample the same bag sequentially.
Dynamically generated gas standards were made one of two ways: Either they were purchased from Scott Specialty Gases (Plumsteadville, PA) or they were generated from permeation oven systems (Kin-Tek Laboratories, Inc.). All dynamically generated standards used UHP nitrogen gas (Matheson Trigas) for dilution to 0.2–10 ppmv carbon equivalences of propane for both individual compounds and compound mixtures. Individual compounds were run at a minimum of three different carbon equivalence levels except for acetic acid, which was run at only two different levels. Gases purchased from Scott's included three different mixture and three individual sulfur standards. The three gas mixtures included: (1) TO-15 62 compound mixture () with all compounds at 1 ppmv; (2) polar compound mixture 1 containing acetone, acetonitrile, 2-butanone, n-butanol, ethanol, methanol, propanol, and propene (all at 10 ppmv); (3) polar compounds mixture 2 containing acetone, methanol, ethanol, n-propanol, and n-butanol (all at 10 ppmv); and (4) sulfur compound mixture containing hydrogen sulfide, methanethiol, carbon disulfide, carbonyl sulfide, and dimethyl sulfide (all at 10 ppmv). Permeation tube standards were purchased and calibrated from Kin-Tek Laboratories, Inc., and included both acetic and butanoic acids. In dynamic systems, NMHC analyzers were attached to the exit tube of the diluters, which were maintained at flow rates greater than the NHMC analyzers sampling rate. Depending on availability of NMHC analyzers, all were attached to the exit tube of the diluters at approximately the same time. Sample time for analysis was approximately 15 min, which is approximately five cycles for the GC/FID NMHC-based instruments.
Field study
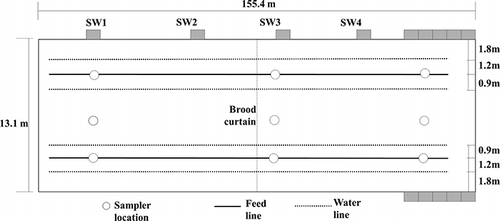
Air monitoring of VOCs was carried out at a commercial broiler production facility in the southeastern United States. Details regarding the facility, time of year samples were taken, the air sampling methodology, and the frequency and number of samples taken can be found in CitationTrabue et al. (2010). In brief, gas samples were collected in winter (production cycle) and early spring (empty building) at a commercial broiler production house that was mechanically ventilated, with a capacity of approximately 25,000 birds per flock. Samples were analyzed for individual VOCs and for total NMHC. Samples were collected from inside the production building between production cycles with decaked litter present; from inside the building during a production cycle) and from the exhaust of two different side wall ventilation fans (SW1 and SW3); and from the tunnel ventilation fan during bird production (). During the production cycle sampling, the birds were still in the brooding stage (16 days old) and were confined to only half the building with the use of a brood curtain (). Fused silica-lined canisters (U.S. EPA Method TO-15) and thermal desorption tubes (U.S. EPA Method TO-17) were used to capture and analyze gas samples for speciation of VOCs using GC/MS.
Total NMHC were determined with GC/FID (model 200, VIG Industries, Inc., Anaheim, CA) with a dual FID to measure both methane and NMHC component(s) in the air sample. The response time of the model 200 NMHC analyzer was 70 sec and NMHC reading was updated every 3 min (CitationLi et al., 2008). In addition, a PA-IR instrument (INNOVA model 1412, INNOVA AirTech Instruments A/S, Denmark) equipped with NMHC filter was programmed with a 1-sec sampling integration time and fixed flushing time of 2 sec for the chamber and 3 sec for the tubing; the required time to complete one sampling cycle for four gases including both NMHC and dewpoint temperature measurements was approximately 30 sec. All NMHC instruments in the field were calibrated with 5 ppmv propane gas.
Results and Discussion
Laboratory validation
Individual compound standards.
The compounds included in the present study were previously reported as significant in two other studies conducted at poultry facilities (CitationTrabue et al., 2008; CitationTrabue et al., 2010). Target compound classes included VFAs, alcohols, nonpolar hydrodcarbons, and reduced sulfur compounds. The initial phase of the laboratory evaluation included testing the average response of each instrument with individual compound standards (). A theoretical predicted FID response for each compound was calculated using previously published effective carbon number reduction values () (CitationSternberg et al., 1962; CitationDietz, 1967; CitationJorgensen et al., 1990; Yan, Wang, and Barlow, 1992). In this “theoretical” prediction, a compound in the alcohol class would have an effective reduction of 0.4 carbon units in response to a similar nonpolar hydrocarbon, whereas carboxyl or carbonyl compounds have an effective reduction of 1.0 carbon unit compared to a similar nonpolar hydrocarbon. Consequently, a compound such as propane will have a response of 3 carbon units on an FID, but n-propanol or acetone would have a response of 2.4 and 2.0 carbon units, respectively, on an FID. Not all individual compound standards were tested for all three instruments due to equipment availability. However, seven compounds ranging from 1 to 4 carbon units including hydrocarbons, alcohols, ketones, and nitrile compounds were tested using all three instruments (). These results do provide important insights into the limitations of the three instruments for quantifying total VOCs.
Table 2. Results of laboratory evaluation of nonmethane hydrocarbon analyzers with individual compound standards ranging from 0.2 to 10 ppmv carbon equivalents; measured results represent average values measured across the concentration range
Table 3. Previously published values of GC/FID effective carbon number reduction by functional group
Figure 2. Comparison response between known VOC concentrations of individual compounds (C Equiv), expected FID response (Exp FID), and calculated response for GC-FID, PA-IR, and PID NMHC analyzers. All concentrations are given in ppmv carbon, and error bars are the standard error of the mean.
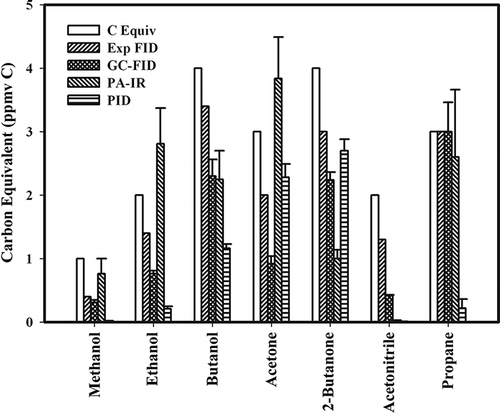
Results of this test indicate that none of the three NMHC analyzers tested had a universal response to all classes of compounds on a carbon equivalent basis. The best results were with the GC/FID and PA-IR based instruments for propane, where the response of both detectors was essentially equal to the predicted value. The GC/FID also performed well with hexane and methanethiol (). Propane is often used as a calibration gas for NMHC analyzers, so it was not surprising that both instruments responded well. The lower concentration range of propane standards tested was 0.1 ppmv. This low level was well below the calibration standard used for each instrument (25 ppmv). This result demonstrated the linearity of the two instruments over several orders of magnitude despite using a single high-calibration point. The PID instrument responded poorly to the propane standards. Photoionization detectors have been available for many years as GC detectors (CitationPrice et al., 1968), and these detectors can be used for aliphatic hydrocarbon analysis. However, the characteristics of the PID lamp and the atmospheric conditions during analysis can drastically affect measured response. Published correction factors from the manufacturer of the instrument used in the present study indicate no response for a propane standard using the 10.6-eV lamp (CitationRae Systems, 2010). Therefore, the hand-held PID detector utilized in the present study does not appear to be useful for the analysis of propane.
Previous researchers have found that the FID detector performed poorly in the analysis of polar compounds compared to aliphatic hydrocarbons (CitationSternberg et al., 1962; CitationScanlon and Willis, 1985; Jorseng et al., 1990; CitationFaiola et al., 2012). Poor performance was also observed in the present study where responses for polar compounds measured using the GC/FID NMHC analyzer were lower than predicted values (). Measured average FID results were 35 to 60% lower for volatile fatty acids, 13 to 30% lower for alcohols, 15 to 37% lower than predicted for ketones. Response for acetonitrile was 51% lower than expected. However, the less polar ethyl acetate was only 2% below the predicted value. The predicted values have already been adjusted for effective carbon number reduction; therefore, additional factors were influencing detector response.
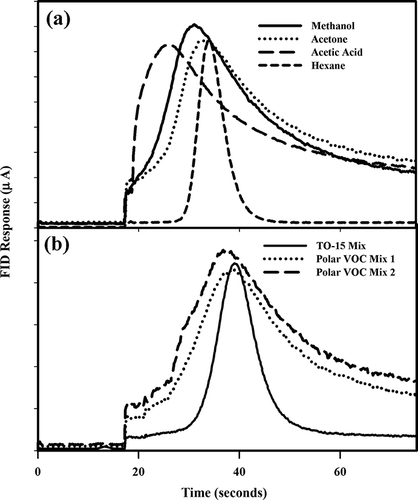
Chromatograms for compounds with the poorest performance were examined, and problems with the mini-column in the instrument were identified (). All the polar oxygenated compounds that exhibited response values lower than expected also had poor peak shape, with peaks still emerging from the column at the end of the chromatogram. R. Mouradian (Thermo Fisher Scientific, personal communication, July 30, 2012) observed that highly polar compounds sometimes did not back-flush completely during the analysis cycle and sometimes created low, wide, and/or oddly shaped peaks that are difficult to integrate. In the present study, compounds that matched their expected FID response had chromatograms with baseline resolution in both the front and tail of the peak (). Compounds that had peaks with baseline resolution on the mini-column had responses that fell within 5% of expected FID signal and averaged 101% of expected values. However, the polar oxygenated compounds with poorer chromatograms averaged only 55% of expected FID response values. This reduction in response was not uniform for each compound class, but performance generally decreased as the length of the carbon chain in a particular class increased. Results indicate that the most polar compounds with the highest vapor pressure will perform poorly on the GC/FID analyzer.
Figure 3. Response of the GC/FID based NMHC analyzer for individual compounds and compound mixtures: (a) individual compounds and (b) mixtures of compounds.
The PA-IR NMHC analyzer did detect all of the alcohols and ketone standards, but it exhibited a very low signal for acetonitrile. For the other polar compounds included in the present study, the PA-IR performed relatively well with methanol, ethanol, and acetone (), measuring with values falling within ±15% difference of the expected value. However, the PA-IR responses for n-propanol and 2-butanone were more than 30% below the expected values (). CitationHodgson (1995) also found great variability in PA-IR for response to different chemical classes varying by 6 to 560%. The PA-IR NMHC analyzer is based on an assumption that hydrocarbon compounds in a certain mid-infrared region, 2950 cm−1, absorb similarly. The PID performed poorly in detecting the alcohols and acetonitrile, but came close to expected values for ketone compounds (i.e., acetone and 2-butanone). As expected, the PID responded differently to hydrocarbons based on their ionization energies. Methane, methanol, and water vapor are known to reduce PID response if they are present in high concentrations. Since the alcohols are important contributors to the emissions from AFOs (CitationTrabue et al., 2010; CitationHoward et al., 2010), and methane concentrations are likely to be high, the use of PIDs for AFO emission characterization should be avoided.
Of the three sulfur compounds tested, only methanethiol exhibited a measureable signal using the FID NMHC analyzer. The compound separated well on the mini-column (data not shown), and the response was similar to an aliphatic hydrocarbon as seen by other researchers () (CitationJorgensen et al., 1990). The FID did not respond to the carbonyl sulfide or carbon disulfide standards, which is a result of the two compounds having no ionizable carbons. The small signal associated with carbon disulfide may have resulted from an enriched flow of hydrogen fuel into the FID (CitationDressler, 1986). In the present study, none of the sulfur compounds were tested with the PA-IR.
Mixed standards.
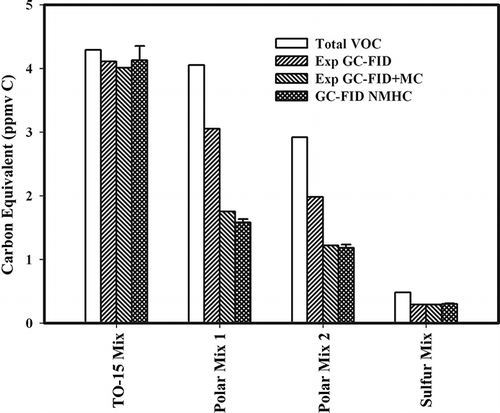
In a follow-up to the individual compounds, gas mixtures were created and their concentrations were measured on a GC/FID NMHC analyzer (). The measured values were compared to expected values based on effective carbon number reduction values from the literature for FID instruments (). The nonpolar TO-15 mix contained compounds that performed well on the mini-column and agreed to within 98% of expected values based only on effective carbon number reduction corrections. Hogdson (1995) also found that known gas mixtures of aliphatic and aromatic compounds responded well (93%) with GC/FID NMHC. In the sulfur compound mixture, both methanethiol and dimethyl sulfide separated well on the mini-column, and the response agreed with expected values (; Yan et al., 1991). However, mixtures that did not chromatograph well resulted in NMHC analyzer values that were approximately 20–30% too low. The effect of the column on compound mixtures is similar to individual compounds with polar oxygenated mixtures still emerging from the column at the end of the chromatograms (). Using the FID response values generated with reference standards from and including reduction in carbon numbers based on literature for compounds not tested (), a GC/FID NMHC mini-column (Exp FID + MC) correction factor was developed that incorporated both correction factors (). This correction significantly improved agreement between expected (Exp GC -FID + MC) and measured values (GC-FID NMHC, ). However, application of this approach in reality is unrealistic since it would require a prior knowledge of the composition of the VOC mixture. It should also be pointed out that this correction is not for accurate measurement of VOCs, but rather to explain the poor performance of GC/FID NMHC analyzers.
Field comparison of GC-MS methods with GC/FID and PA-IR
U.S. EPA Methods TO-15 and TO-17 with GC-MS analysis were used to characterize the NMHC profile at a poultry production building. Samples were collected from inside the building between production cycles with decaked litter present; inside the building during a poultry production cycle; and from the sidewall and tunnel ventilation fan outlets during the production cycle. Production cycle samples were taken during the brooding stage of the birds (16 days) when they were confined to only half of the building (). The average VOC profile observed was dominated by contributions from alcohols/phenol, ketones/carbonyl and acids (), with only minor contributions from nonpolar aliphatic and aromatic compounds (CitationTrabue et al., 2010). Results from the GC-MS analysis methods were normalized to total carbon equivalents for comparison with results from simultaneous measurements from GC/FID and PA-IR NMHC analyzers. Expected GC/FID concentration values were calculated based on the GC-MS profile results, and expected GC/FID concentration values were further corrected for expected losses on the GC/FID mini column (). The average GC-MS VOC concentration in the building was 2.75 ppmv C between animal production cycles and 3.15 ppmv C during early production. The highest average GC-MS VOC concentration was found at sidewall 1 (6.13 ppmv C) and the lowest was at the tunnel fan (0.98 ppmv C).
Figure 5. Results of TO-15 and TO-17 analyses of VOCs measured at a poultry production facility presented in carbon equivalence, with data originally from CitationTrabue et al. (2010). All concentrations are given in ppmv carbon and error bars are the standard error of the mean.
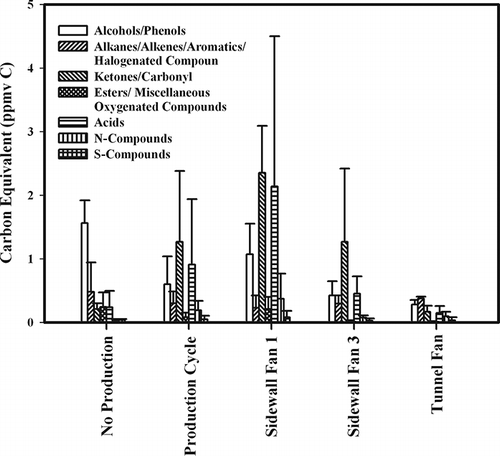
Figure 6. Results of field comparison between average total VOC concentrations measured using TO-15 and TO-17 methods with GC-MS (GC-MS); expected FID response based on measured GC-MS results (Exp FID); expected FID response based on measured GC-MS results plus mini column correction factors (Exp FID + MC); average measured GC/FID NMHC analyzer concentration; and average measured PA-IR NMHC analyzer concentration. All concentrations are given in ppmv carbon, and error bars are the standard error of the mean.
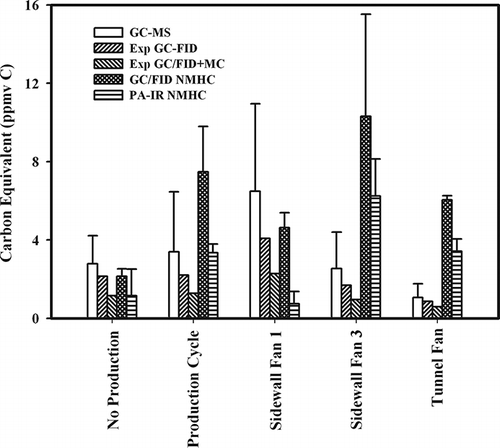
Direct comparisons of the GC-MS VOC concentration with that measured by GC/FID NMHC analyzer were in good agreement for samples collected between production cycles (no production) and from sidewall 1, with the GC-MS VOC exhibiting differences of less than 15%. However, the GC/FID exceeded the GC-MS VOC concentration by 41 to 72% for measurements made in the building during production and from the other two fans (i.e., sidewall [SW] 3, tunnel). Application of GC/FID detector effective carbon number reduction correction to the GC-MS VOC concentration (Exp GC/FID in ) improved the agreement with measured GC/FID NMHC analyzer values to within 3% for no production and to within 6% for SW1. However, this correction only worsened the agreement between predicted and measured values for the other locations. The worst agreement was between the measured GC/FID values and those with both the detector and mini column correction factors included (Exp GC/FID + MC in ). The higher values in the actual measurements for the GC/FID NMHC analyzer compared to model values from total speciated VOCs likely reflects unreported compounds not detected using either TO-15 or TO-17.
It should be noted that U.S. EPA Methods TO-15 and TO-17 do not include all possible VOCs (CitationTrabue et al., 2010; CitationTrabue et al., 2011). Major classes of compounds not quantified with EPA TO-17 and TO-15 would include small carbonyl compounds (i.e., acetaldehyde), amines, and ester compounds; however, these compound classes would probably account for less than 10% of total VOCs since their abundance is about an order of magnitude lower than alcohols or volatile fatty acids (CitationSmith, Francis, and Duxbury, 1977; CitationYasuhara, 1987; CitationNgwabie et al., 2008; CitationFeilberg et al., 2010). These two methods also do not include propane. Producers often use propane as a fuel to heat poultry houses. Contributions from propane gas could explain higher concentrations measured with the GC/FID NMHC analyzers during bird production in the winter months.
The differences between the predicted and measured values may also be a result of higher concentrations (ppmv) of individual compounds used in the laboratory validation as compared to field values. Laboratory studies were performed at ppmv levels for individual compounds, whereas concentrations of individual compounds in the field were sub-ppmv levels with most compounds at less than 0.1 ppmv. The higher concentrations used in the validation experiments for polar compounds may have affected the trapping efficiency of the mini-column, resulting in lower response of NMHC analyzers.
For the PA-IR NMHC analyer, results were mixed. Some average measurements from the PA-IR exceeded the GC-MS VOC results and some fell below (). For the barn in production, the PA-IR compared well to the GC-MS VOC value, showing only a 6% difference in average building concentrations; however, this average is deceiving since PA-IR measurements from other areas of the building (i.e., SW1, SW3, and tunnel) were not well correlated to each other. For example the SW1 area was much lower than SW3 and tunnel. The poor agreement between PA-IR and the GC-MS VOC speciation method is not surprising given results of the laboratory validation and the work of CitationHodgson (1995). The manufacturer suggested using a Nafion dryer to improve results by reducing water-vapor concentrations, but a Nafion dryer would actually remove a large number of our polar compounds, including both the volatile fatty acid and alcohol compound classes. The average PA-IR concentration was always lower than the GC/FID value. The best agreement between the GC/FID and PA-IR were from samples collected between production cycles (12% difference) when concentrations of ketones and acid concentrations were low (). During production the PA-IR values were between 24 and 38% lower than the GC/FID value except for sidewall 1, where the PA-IR was 84% lower than the GC/FID. Therefore, despite the apparent agreement between the PA-IR and GC-MS VOC values under some conditions, results of the present study indicate problems with the use of this NMHC analyzer for AFO emissions.
What are the alternatives to current commercial NMHC analyzers? CitationMaris et al. (2003) and CitationChung et al. (2003) demonstrated an instrument that had the capacity to both quantify NMHC and speciate the individual compounds. The instrument traps and purges sampled air that is then oxidized to carbon dioxide and subsequently reduced to methane for quantification with FID (CitationMaris et al., 2003). The instrument performed well in the field for quantifying total NMHC, but the compounds used for validation were nonpolar hydrocarbons, a class of compounds that GC/FID NMHC quantifies well. In addition, this instrument uses liquid nitrogen for cryotrapping, which may be difficult to transport and maintain at AFO, and procedures used to purge CO, CO2, and methane may result in the loss of the most volatile compounds. While promising, this still may have drawbacks similar to GC/FID NMHC analyzers. Another alternative technology that focuses on the quantitation of individual compounds rather than total NMHC is selective-ion-flow tube–mass spectrometry (SIFT-MS). Instruments for this are commercially available and have been used successfully at both swine and dairy operations (CitationShaw et al., 2007; CitationNgwabie et al., 2008; CitationFeilberg et al., 2010; Saha et al., 2011). The instruments use chemical ionizations for real-time measurement. The strength of these instruments is their ability to quantify compounds in real time, which enhances quantitation of reactive compounds such as acetaldehyde and hydrogen sulfide since there is no processing or storage of samples. However, these instruments do have limitation in terms of instrument mobility, power demands, and high cost, all of which limit their use at AFOs.
Conclusion
The purpose of this study was to evaluate the performance of commercial NMHC analyzers in measuring total VOCs at AFOs using a combination of laboratory and field experiments. All NMHC analyzers tested underreported concentrations of polar oxygenated compounds compared to aliphatic hydrocarbons. The GC/FID-based NMHC analyzer underreported polar oxygenated compounds due to both the response of the detector toward polar compounds and the poor resolution of peaks on the mini column. While there are methods to correct the FID signal obtained with NMHC analyzers for polar compounds, these methods are only effective if the identity and concentration of VOCs are known. The PA-IR-based NMHC analyzer did not have a consistent response pattern from any chemical compounds class, and the PID NMHC analyzer quantified alcohols poorly. Based on results of the present study, none of the NMHC analyzers provided accurate, consistent total hydrocarbon concentration results when tested with VOCs typical of AFO emissions like alcohols, VFAs, and ketones. However, the use of the NMHC analyzer may give insights into areas that have the highest emissions levels and may serve as screening device for placement of samplers.
This study highlights the limitations and pitfalls related to utilizing a single method (i.e., NMHC analyzers) to quantify total VOCs emissions. In addition, this study draws into question the methodology prescribed in the NAEMS study for VOC emission quantification. Future studies on VOC emissions from AFOs should focus on true measurements of individual compounds using validated air sampling techniques.
Acknowledgment
This research was partially funded through grants supplied by Tyson Foods Corporation, and USDA NIFA AFRI 2009-35112-05244 and 2010-85112-20523. The authors thank Cynthia Swalla, Julie Steele, Catalina Olea, Srikar Middala, Kennedy Vu, Austen Scruggs, and Lucien Nana for assistance with sampling and analysis. Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and use of a name by the USDA implies no approval of the product to the exclusion of others that may be suitable.
References
- Chung , M. , Maris , C. , Krischke , U. , Meller , R. and Paulson , S. 2003 . An investigation of relationship between total non-methane organic carbon and the sum of speciated hydrocarbons and carbonyls measured by standard GC/FID: measurements in the Los Angeles air basin . Atmos. Environ. , 37: S159–S170 doi: 10.1016/S1352-2310(03)00388-1
- Dietz , W. 1967 . Response factors for gas chromatographic analyses . J. Gas Chromatogr. , 5 : 68 – 71 . doi: 10.1093/chromsci/5.2.68
- Dressler , M. 1986 . Journal of Chromatography Library Volume 36: Selective gas chromatrographic detectors , New York , NY : Elsevier Science .
- Faiola , C. , Erickson , M. , Fricaud , V. , Johnson , B. and VanReken , T. 2012 . Quantification of biogenic volatile organic compounds with a flame ionization detector using the effective carbon number concept . Atmos. Meas. Tech. Discuss. , 5 : 2415 – 2447 . doi: 10.5194/amtd-5-2415-2012
- Register , Federal . 2003 . National pollutant discharge elimination system permit regulation and effluent limitation guideline and standards for concentrated animal feeding operations (CAFOs) final rule , 68 : 7176 – 7274 .
- Register , Federal . 2005 . Animal feeding operations consent agreement and final order , 70 : 4958 – 4977 .
- Feilberg , A. , Liu , D. , Adamsen , A. , Hansen , M. and Jonassen , K. 2010 . Odorant emissions from intensive pig production measured by online proton-transfer-reaction mass spectrometry . Environ. Sci. Technol. , 44 : 5894 – 5900 . doi: 10.1021/es100483s
- Filipy , J. , Rumburg , B. , Mount , G. , Westberg , H. and Lamb , B. 2006 . Identification and quantification of volatile organic compounds from a dairy . Atmos. Environ. , 40 : 1480 – 1494 . doi: 10.1016/j.atmosenv.2005.10.048
- Heber , A. , Ni , J. , Lim , T. , Tao , P. , Schmidt , A. , Koziel , J. , Beasley , D. , Hoff , S. , Nicolai , R. , Jacobson , L. and Zhang , Y. 2006 . Quality assured measurements of animal building emissions: Gas concentrations . J. Air Waste Mange. Assoc. , 56 : 1472 – 1483 . doi: 10.1080/10473289.2006.10465680
- Hodgson , A. 1995 . A review and a limited comparison of methods for measuring total volatile organic compounds in indoor air . Indoor Air International Journal of Indoor Environment and Health , 5 : 247 – 257 . doi: 10.1111/j.1600-0668.1995.00004.x
- Howard , C.J , Kumar , A. , Malkina , I. , Mitloehner , F. , Green , P.G. , Flocchini , R.G. and Kleeman , M.J. 2010 . Reactive organic gas emissions from livestock feed contribute significantly to ozone production in central California . Environ. Sci. Technol. , 44 : 2309 – 2314 . doi: 10.1021/es902864u
- Jorgensen , A. , Picel , K. and Stamoudis , V. 1990 . Prediction of gas chromatography flame ionization detector response factors from molecular structures . Anal. Chem. , 62 : 683 – 689 . doi: 10.1021/ac00206a007
- Kallai , M. , Veres , Z. and Balla , J. 2001 . Response of flame ionization detectors to different homologous series . Chromatography , 54 : 511 – 517 . doi: 10.1007/BF02491209
- Li , H. , Burns , R. , Xin , H. , Gates , R. , Trabue , S. , Overhults , D. , Moody , L. and Earnest , J. June 29–July 3 2008 . “ Hydrogen sulfide and nonmethane hydrocarbon emissions from broiler houses in the Southeastern United States ” . In Paper 084417, ASABE Annual International Meeting , June 29–July 3 , RI : Providence .
- MacDonald , J. and McBride , W. 2009 . The transformation of U.S. livestock agriculture: scale, efficiency and risk. Economic Information Bulletin 43 . Economic Research Service, U.S. Department of Agriculture. , doi: 10.2139/ssrn.1354028
- Maris , C. , Chung , M. , Lueb , R. , Krischke , U. , Meller , R. , Fox , M. and Paulson , S. 2003 . Development of instrumentation for simultaneous analysis of total non-methane organic carbon and volatile organic compounds in ambient air . Atmos. Environ. , 37 : S149 – S158 . doi: 10.1016/S1352-2310(03)00387-X
- National Research Council . 2003 . Air emission from animal feeding operations current knowledge future needs , Washington , DC : National Academies Press .
- Ngwabie , N. , Schade , G. , Custer , T. , Linke , S. and Hinz , T. 2008 . Abundance and flux estimates of volatile organic compounds from a dairy cowshed in Germany . J. Environ. Qual. , 37 : 565 – 573 . doi: 10.2134/jeq2006.0417
- Price , J.G.W. , Fenimore , D.C. , Simmonds , P.G. and Zlatkis , A. 1968 . Design and operation of a photoionization detector for gas chromatography . Anal. Chem. , 40 : 541 – 547 . doi: 10.1021/ac60259a013
- Rae Systems. 2010. Technical note TN-106, correction factors, ionization energies, and calibration characteristics http://www.raesystems.com/downloads/product-manuals (http://www.raesystems.com/downloads/product-manuals) (Accessed: 1 September 2012 ).
- Rumsey , I. , Aneja , V. and Lonneman , W. 2012 . Characterizing non-methane volatile organic compounds emission from a swine concentrated animal feeding operation . Atmos. Environ. , 47 : 348 – 357 . doi: 10.1016/j.atmosenv.2011.10.055
- Saha , C. , Feilberg , A , Zheng , G. and Adamsen , A. 2011 . Effects of airflow on odorants’ emissions in model pig house—A laboratory study using proton-transfer-reaction mass spectrometry (PTR-MS) . Sci Total Environ , 410–411 : 161 – 171 . doi: 10.1016/j.scitotenv.2011.09.017
- Scanlon , J. and Willis , D. 1985 . Calculation of flame ionization detector relative response factors using the effective carbon number concept . J. Chromatogr. Sci. , 23 : 333 – 340 . doi: 10.1093/chromsci/23.8.333
- Schiffman , S. , Bennett , J. and Raymer , J. 2001 . Quantification of odors and odorants from swine operations in North Carolina . Agric. For. Meteorol. , 108 : 213 – 240 . doi: 10.1016/S0168-1923(01)00239-8
- Schiffman , S.S. and Williams , C. 2005 . Science of odor as a potential health issue . J. Environ. Qual. , 34 : 129 – 138 .
- Shaw , S. , Mitloehner , F. , Jackson , W. , Depeters , E. , Fadel , J. , Robinson , P. , Holzinger , R. and Goldstein , A. 2007 . Volatile organic compound emissions from dairy cows and their waste as measured by proton-transfer-reaction mass spectrometer . Environ. Sci. Technol. , 41 : 1310 – 1316 . doi: 10.1021/es061475e
- Smith , M. , Francis , A. and Duxbury , J. 1977 . Collection and analysis of organic gases from natural ecosystems: application to poultry manure . Environ. Sci. Technol. , 11 : 51 – 55 . doi: 10.1021/es60124a005
- Sternberg , J. , Gallow , W. and Jones , D. 1962 . “ The mechanism of response of flame ionization detectors ” . In Gas chromatography , Edited by: Brenner , N. , Callen , J. and Weiss , M. 231 – 267 . New York , NY : Academic Press .
- Trabue , S. , Scoggin , K. , Li , H. , Burns , R. , Xin , H. and Hatfield , J. 2010 . Speciation of volatile organic compounds from poultry production . Atmos. Environ. , 44 : 3538 – 3546 . doi: 10.1016/j.atmosenv.2010.06.009
- Trabue , S.L. , Scoggin , K. , Mitloehner , F. , Li , H. , Burns , R. and Xin , H. 2008 . Field sampling method for quantifying sulfur compounds from animal feeding operations . Atmos. Environ. , 42 : 3332 – 3341 . doi: 10.1016/j.atmosenv.2007.03.016
- Trabue , S.L. , Scoggin , K. , McConnell , L. , Maghirang , R. , Razote , E. and Hatfield , J. 2011 . Identifying and tracking key odors associated with cattle feedlots . Atmos. Environ. , 45 : 4243 – 4251 . doi: 10.1016/j.atmosenv.2011.04.081
- U.S. Department of Agriculture. 2007. 2007 Census of agriculture: United States summary and state data, Vol. 1, Geographical area series, Part 51. USDA National Agricultural Statistical Services February http://www.agcensus.usda.gov/Publications/2007/Full_Report/index.asp (http://www.agcensus.usda.gov/Publications/2007/Full_Report/index.asp) (Accessed: 16 May 2012 ).
- Yan , X. , Wang , Z. and Barlow , P. 1992 . Quantitative estimation of garlic oil content in garlic oil based health products . Food Chem. , 45 : 135 – 139 . doi: 10.1016/0308-8146(92)90024-V
- Yasuhara , A. 1987 . Identification of volatile compounds in poultry manure by gas chromatrography-mass spectrometry . J. Chromatogr. , 387 : 371 – 378 . doi: 10.1016/S0021-9673(01)94539-X