Abstract
Gas chromatography–mass spectrometry, olfactometry, and other related methods were applied for the qualitative and quantitative analysis of the characteristics of odorous gases in the pretreatment workshop. The composition of odorous gases emitted from municipal food waste was also investigated in this study. The results showed that the tested gases are mainly composed of aromatic gases, which account for 49% of the total volatile organic compounds (VOC) concentrations. The nitrogenous compounds comprise 15% of the total concentration and the other gases comprise the remaining 36%. The level of odor concentration ranged from 2523 odor units (OU) m−3 to 3577 OU m−3. The variation of the total chemical composition ranged from 19,725 µg m−3 to 24,184 µg m−3. Among the selected four sampling points, the discharge outlet was detected to have the highest concentration in terms of odor, total chemical, sulfur compounds, and aromatics. The correlation analysis showed that the odor concentrations were evidently related to the total chemical composition, sulfur compounds, and aromatics (P < 0.05, n = 5). The odor activity value analysis identified the top three compounds, hydrogen sulfide (91.8), ethyl sulfide (35.8), and trimethylamine (70.6), which contribute to air pollution complaint of waste materials.
Currently, the amount of food waste has rapidly increased, which leads to difficulty in waste management and more odorous gases released as air pollution. In processing of food wastes by anaerobic fermentation, odorous gases are generated, which significantly affect the workers and occupants in the plant. In the pretreatment workshop for anaerobic decomposition, the odorous gases are generated because of the stacking and decomposition of food wastes. The gases emitted mainly consist of organic gases because the food wastes are mainly organic materials. The other odors that comprise 1% of the gases are S-compounds, aromatics, esters, alkanes, and limonene, which result in unpleasant odors that are harmful to the health.
Introduction
To date, the amount of food waste disposal has rapidly increased, which leads to difficulty in waste management and more odorous gases being released as air pollution (CitationKim et al., 2008; CitationWu et al., 2010). The key methods for food waste management are landfill, compost, and anaerobic decomposition to produce biogas (CitationFilippi et al., 2002; CitationMao et al., 2006; CitationLevis et al., 2010; CitationDas et al., 2003). Biogas fermentation is a new treatment process that is gradually applied in most cities in China. Unlike landfill and compost treatment processes, food waste should be pretreated, such as by separation and beating, before entering the anaerobic fermentation tank. Odorous gases are generated during the processing of food wastes, which come in direct contact with the workers and occupants in the plant. Therefore, the composition of these odorous gases and their adverse effect on human health should be investigated.
Odorous gases are generated in the pretreatment workshop due to the stacking and decomposition of food wastes. The gases emitted mainly consist of organic gases because of the organic content of food wastes. Currently, there are no reports published for the chemical composition of odorant. The existing research studies have imitated the main contents of gases from the studies of landfill, that is, methane and carbon dioxide. The odor compounds like S-compounds, aromatics, esters, alkanes, and limonene comprise only 1% of the emitted gases (CitationDincer et al., 2006). However, these minority-content odorants make a great contribution to the unpleasant sensing that may be harmful to the health (CitationATDSR, 2007; CitationHurst et al., 2005; CitationLeong et al., 2002; CitationSarkar et al.,2003; CitationZou et al., 2003).
There are basically two types of method used for odor detection: olfactometry and instrumental chemical detection. Olfactometry is the most commonly used measurement (CitationASTM, 1997; CitationCEN, 2003; CitationJapan Ministry of the Environment, 2003; CitationTaiwan EPA, 1994). It employs a panel of sensory-trained experts to determine the concentration of diluted odor gases. However, this method is not able to quantify and qualify the composition and content of gases. The gas chromatography–mass spectrometry (GC-MS) technique is considered one of the most effective chemical detection methods and produces a list of substances involved and their concentration. Dincer and Davoli monitored the composition of odor gases in a landfill with the GC-MS technique (e.g., CitationDincer et al., 2006; CitationDavoli et al., 2003). Both solid-phase extraction and GC-MS techniques were adopted in the Defoer and Tsai et al. studies to investigate the composition of odor gases in a composting plant (CitationDefoer et al., 2002; CitationTsai et al., 2008).
The composition of food waste varies by season and region, which results in uncertainty of the chemical composition of odorous gases emitted by food waste during the pretreatment processes. This study aims to detect the composition of odorous gases and to characterize the pollutant content using human sensing technologies and GC-MS. The main pollution factors and rules are also analyzed. Moreover, this study would provide basic guidelines for the reduction of odorous gases during the recycling process of food wastes and the prevention of air pollution.
Materals and Methods
Site description
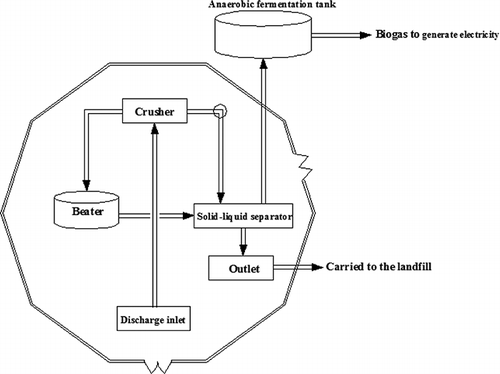
The food waste disposal plant in this study is one of earliest established plants in Beijing. It was first utilized in 2010 and disposes of 200 tons of wastes per day. Moreover, this plant uses anaerobic decomposition technology for food waste treatment and utilizes biogas as an energy source to generate electricity. Given the chemical complexity of food waste (), pretreatment is necessary before anaerobic decomposition to eliminate the dopants that cannot be fermented. shows the procedure of the technological process; the discharge inlet, crusher, beater, solid–liquid separator, and discharge outlet are located in the pretreatment plant, whereas the anaerobic fermentation tank is positioned outside the plant. The fermentable components were filtered, crushed, and beaten in the pretreatment plant before being sent to the anaerobic fermentation tank. The pretreatment plant is a relatively enclosed space and the odorous smell accumulates during the processing of waste.
Table 1. Main composition of food waste and weather condition survey during sampling
Sampling and analysis method
Olfactometry and solid-phase microextraction (SPME), followed by GC-MS, were used to determine the odor and VOC concentrations of the collected samples.
Sampling method.
The sampling points were set at the discharge outlet, inlet, belt openings, and waste accumulation compartments. These four points were selected because they are exposed during the food waste disposal and become the critical pollution sources that emit odorous smells. Furthermore, the sampling points were set at 1.5 m above the floor, which is the average height of an adult's inhaling level when standing. Sampling was carried out once in every month from March to July 2012. The food waste source, composition, and ambient environmental conditions were recorded for the corresponding sampling. The air in the sampling points was taken by an atmospheric sampling instrument (HL-1, Eesonline, China) and collected in 5-L Tedlar sampling bags (S-5L, PL Company, USA) for both GC-MS and olfactometry analyses. To avoid the contamination of samples, the odor bag is placed inside of a sealed container evacuated by a vacuum pump. The sealed container is connected to the inlet of sampling instrument while the flow rate is set at 0 to 1 mL/min. When the sampling instrument is started, the sealing container will be pumped to vacuum, while the sampling bags will automatically absorb the gas from sampling points under the condition of negative pressure. Finally, hydrogen sulfide, ammonia, and trimethylamine were collected separately using the solution absorption method, and were analyzed via spectrophotometry. Sampling bags and glass sample tube displaced three times use nitrogen gas before sampling, to ensure the purity of the acquired gas.
Chemical analysis.
SPME and GC-MS (Trace DSQ, Finnigan, USA) were used for qualitative analysis, wherein the GC detector served as a flame ionization detector (FID) and the chromatographic column was set at DB-5 (diameter = 0.25 mm, column height = 30 m). The starting temperature in the GC was 40°C and was maintained for 3 min. After this, the temperature was increased to 250°C at the rate of 20°C /min and was maintained for 5 min. The temperature at the inlet and ion sources was 250°C. Moreover, the split ratio was 1:10 and nitrogen (carrier gas) was provided at 10 mL/min. Given that the FID was unable to test the sulfide content in the sample, a flame photometric detector (FPD) was utilized. In this case, the chromatographic column used was GAS-PRO (diameter = 0.32 mm, column length = 30 m). The starting temperature inside the GC was 40°C, which was held for 1 min. The temperature was then increased to 200°C at 20°C/min, and was maintained for 10 min. Finally, the inlet temperature was 250°C, with an initial oven temperature of 250°C. Nitrogen was provided at 1.2 mL/min without split flowing. VOC was quantified by external standard method. This method uses the relationship between peak areas and five analytic concentrations. The calibration curve is constructed after multiple, identical injections of a number of standard solutions of known concentration into the GC-MS. Methylene blue spectrophotometry was used to test the concentration of hydrogen sulfide in the sample (CitationChina Ministry of Health, 1989). The hydrogen sulfide in the air was absorbed by alkaline hydrogen cadmium oxide suspension, generating cadmium sulfide precipitation. In the sulfuric acid solution, hydrogen sulfide, p-aminodimethyl aniline solution, and ferric chloride solution reacted to produce methylene blue. The absorbance was tested at around 665 nm based on the colors in the colorimetric analysis. The composition of hydrogen sulfide in the air was calculated using the measured absorbance. Finally, Nessler's reagent spectrophotometry was used to test the composition of ammonia (CitationChina EPA, 2009). In this study, acid spectrophotometric measurement was used to test the trimethylamine concentration (CitationHan and Lin, 2005).
Olfactometry analysis.
The odor concentration of the samples and threshold was measured using a dynamic dilution instrument (AC'SCENT, St. Croix Sensory, USA). The analysis method was based on approved standards (CitationChina EPA, 1993). Importantly, the six assessors selected to determine the odor in the samples were all sensory-trained professionals. In the step-by-step dilution using the dynamic dilution instrument, a threshold will be reached when the odorant cannot be perceived.
Statistical analysis.
Statistical evaluation of the data was performed using SPSS Version 8. In academic research, the Pearson correlation coefficient is commonly used to compare the linear correlation between two variables (CitationNeter et al., 1996). It is adopted in this study to analyze the relationship of odor concentration and total chemical concentration, as well as the single variable or multivariate linear correlation between odor concentrations and VOCs.
Results and Discussion
Composition and concentration of odorous gases
Different compounds were found in the odorous samples collected from the four sampling points. 29 VOCs were measured (), which were classified as S-compounds (e.g., hydrogen sulfide and ethyl sulfide), N-compounds (e.g., ammonia and trimethylamine), aromatics (toluene and naphthalene), alkanes (e.g., tetradecane), esters (e.g., dibutyl phthalate), acids (e.g., 4-(para-tolyl)-butyric acid), and other compounds (e.g., decanal, phenol, and limonene). The measured concentrations of several compounds were dramatically higher than those in the landfill, especially the concentration of aromatics, which are all above 1500 μg m−3, much higher than in the landfill site (CitationDing et al., 2012; CitationDavoli et al., 2003; CitationDincer et al., 2006; CitationFang et al., 2012; CitationZou et al., 2003). This could result from the slower production with the covered landfills, wherein the odors emitted by biodegradable substances are released slowly. As compared, the food waste treatment workshop in this study was relatively open, which led to high concentrations of odorous gases in the air.
Table 2. Odor gases composition and content for food waste in the pretreatment workshop (μg m− 3)
The tested compounds and their concentrations were categorized as follows: aromatics (49% of the total concentration), other compounds (28%), N-compounds (15%), volatile fatty acids (4%), alkanes (2%), S-compounds (1%), and esters (1%). The substances with highest concentrations in each category were naphthalene (1651 μg m−3), limonene (3735 μg m−3), dimethylacetamide (2404 μg m−3), 4-(para-tolyl)-butyric (1581 μg m−3), docosane (504.9 μg m−3), ethyl sulfide (281.3 μg m−3), and phthalate (157.7 μg m−3). In the pretreatment workshop, the composition of the aromatic compounds was relatively higher and more varied than that of the other compounds, which could be attributed to the common use of plastic bags. The generated limonene in the test is attributed to the use of air cleaners, washing liquids, and the presence of vegetables (CitationZou et al., 2003).
Odor and total chemical concentrations
The results of the olfactory and chemical analyses for the four different sampling points in the pretreatment workshop are shown in . The total concentration is the sum concentration of all the tested compounds. The tested odor concentrations ranged from 2523 OU m−3 to 3577 OU m−3, with an average of 3050 OU m−3 (OU m−3 is based on EN13725:2003-3 standard).
Table 3. Concentration of different type of compounds at sampling sites (odor concentration unit: OU m− 3; chemical concentration unit: μg m− 3)
First, the range of total concentration fluctuated from 19,725 μg m−3 to 24,184 μg m−3, with an average of 21,954 μg m−3. In the four sampling points, the odor concentration, total concentration, S-compounds, and aromatics were relatively higher at the discharge outlet, the most seriously polluted site. These might due to the increase in specific surface area after the waste materials were crushed and beaten, which led to a faster release rate of odorous gases, which increase the average concentration of the compounds. The highest concentration of acid was in the waste accumulation compartment because the accumulation of trash promoted the buildup of acid compounds.
Second, the odor concentration was tested by olfactometry. This method indicates the human perception and ranking of odors; hence, it is a suitable standard for determining the effect of odor control. The experimental measurement shows that the concentration level of odorant was highest at the charge outlet. Furthermore, among the seven tested compounds, three were concentrated at this sampling point. These three compounds were mainly S-compounds and aromatics, which had the most intense and harmful effect on the human body.
Finally, the odor concentration can be a useful and indicative standard to a certain degree. The conditions of severely polluted areas should be further evaluated in the future, particularly during ventilation design for the workshop and operation of plants or workshops, to reduce the adverse influence of high concentrations of odorous gases to human health.
This study tested eight types of aromatics (). The concentrations of these compounds were close to each other and were relatively high. Styrene, pentamethylbenzene, naphthalene, and methylnaphthalene were detected at the four sites. Methylnaphthalene was detected from the samples associated with the use of insecticides (CitationZou et al., 2003). Toluene, ethylbenzene, and o-xylene were not detected at the inlet, which can be attributed to the gradually emission of these compounds that occurred in the pretreatment process. The compounds in the samples can result in human poisoning and are harmful to human health after prolonged exposure. For example, styrene is a suspected carcinogen that simulates narcosis in the human body. The tested styrene concentration was higher than the threshold level of 390 μg m−3 in air, but lower than the limit of 50 mg m−3 specified in the “Workplace Harmful Factor Occupational Exposure Limit” (CitationChina Ministry of Health, 2007).
Figure 2. Chemical concentration horizontal distribution of aromatics (a), S-compounds (b), and N-compounds (c).
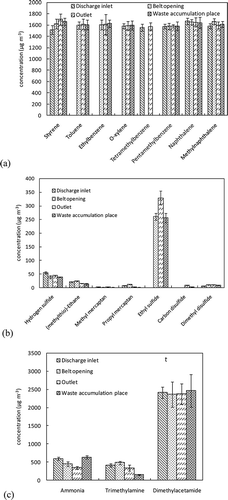
At the sampling sites, seven S-compounds were tested and analyzed qualitatively and quantitatively. shows that the diethyl sulfide concentrations at the conveyor, outlet, and waste accumulation compartments are 59.9, 328.9, and 255.1 μg m−3, respectively. Hydrogen sulfide was the second highest compound detected at all the four sampling sites because of rich protein content in food waste (up to 15.6%), which generates hydrogen sulfide (Ting et al., 2010). Thus, hydrogen sulfide is released abundantly in pretreatment workshops.
Furthermore, although the composition of (methylthio)ethane (12.6–23.7 μg m−3) and dimethyl disulfide (5.3–9.6 μg m−3), perceived as cacosmia smell, accounts for a minority portion, these were widely distributed in the workshop and were detected at the four sampling sites. Therefore, sulfide is a key compound in the pretreatment for anaerobic decomposition of food wastes and has evident smell characteristics, while the reduction of sulfide in the workshop is as important as that in landfills.
Finally, dimethylacetamide was found to be the key component in the tested N-compounds with a measured concentration of more than 2000 μg m−3 (). However, this compound had no significant odorous smell and has a low toxicity level, and the threshold level for dimethylacetamide is dramatically high (165 mg m−3). As compared, ammonia and trimethylamine have strong smells and can stimulate human discomfort. Based on the analysis of the processes, ammonia was generated during the degradation of normal food, while trimethylamine was generated by the degradation of fish and other food items with high protein content (CitationWan et al., 1993). Given that food waste in this plant mainly comes from luxury restaurants where the main component of food waste sources is fish and meat, these two tested compounds are present abundantly in the samples.
Relationship between odor and total chemical concentrations
The analysis for the relationship between odor and the total chemical concentrations of different compounds using localized tests has significant benefits. Therefore, this study investigated the association between odor concentrations and the different types of compounds.
The relationship between odor and the total chemical concentrations was analyzed using the statistical method. First, the relevance metric was determined by the values of variables. The relationship was then measured based on the Pearson correlation coefficient (). The results showed that a significant relationship exists between odor concentration and total chemical concentration of the S and N compounds (P < 0.05, n = 5).
Table 4. Correlation coefficient analysis between compounds and odors
Analysis of the total associated data between odor concentration and total chemical concentration of the five samples showed that the total chemical concentration related to the odor concentration was 87% (r 2 = 0.87, n = 5, P < 0.05). Based on the linear regression analysis of different odors and compound concentrations, the concentration of aromatic hydrocarbon and ester had the best estimated parameters, with an 85% correlation with odor concentrations (r 2 = 0.85, n = 5, P < 0.05).
Odor activity values of the typical odor gases and odor intensity analysis
The correlation analysis determines the existing relationship between odor gas concentrations and the various types of compounds. However, further studies are needed for determining the main contents of complex compounds. The odor thresholds of relatively special compounds were tested in this study (). As compared to the range of odor threshold levels tested in other studies, the monitored levels in the current study varied with the standard range of variation (CitationDevos et al., 1990; CitationNagata, 2003; CitationO'Neil, 1992; CitationRuth, 1986). However, the odor detection thresholds for propyl mercaptan and ethyl sulfide have not been determined in previous studies.
Table 5. Odor threshold levels and odor activity values of typical odor gases
The odor activity value is the ratio between the compound concentration and its threshold in the environment, which elucidates the actual influence of odors to the environment. Eight typical compounds were selected to be studied, given the high environmental sensitivity and obvious threshold that can be obtained.
shows that the odor activity levels of hydrogen sulfide, ethyl sulfide, and trimethylamine were the highest, with the values calculated at 91.77, 35.83, and 70.58, respectively, as compared with the other compounds. Therefore, hydrogen sulfide, ethyl sulfide, and trimethylamine were the main contritions to odorous smell, and play an important role in air pollution.
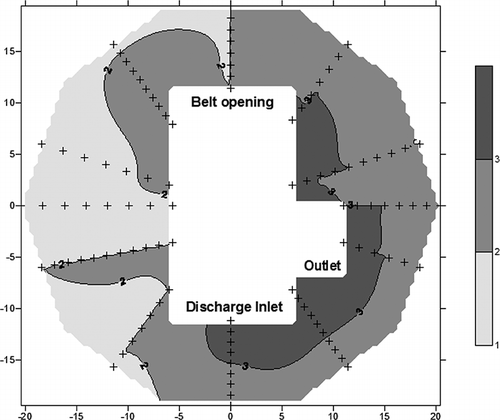
Odor intensity reflects the perception of people toward odor. Thus, odor intensity was recorded at different sites in the workplace. The average intensity of each testing site was calculated based on the collected data. Theintensity contour map was produced with assistant of SURFER, as shown in . Based on the analysis in , the odor intensity in the workshop was relatively higher at the inlet, outlet, and belt drive areas, with values higher than 3. The other areas in the workplace had values between 2 and 3. Therefore, the result was relatively similar to the total chemical concentration analysis discussed earlier.
Conclusion
This study investigated the composition, content, and distribution of odor gases emitted from the anaerobic fermentation of food waste. The tested odor concentrations can be considered as effective standards for indicative assessment, wherein the S-compounds were the main odor gases in the workplace, with significant odor characteristics. Twenty-nine volatile gases were determined, which were classified as S-compounds, N compounds, aromatics, alkanes, esters, acids, and other compounds. The correlation analysis showed that a close relationship existed between odor and total chemical concentrations. The correlation of hydrogen sulfide and aromatics had the best estimation values of approximately 85% variation with the concentration of odorous gases. Furthermore, based on the odor activity values, hydrogen sulfide, ethyl sulfide, and trimethylamine had greater contribution to air pollution, which coincided with the results of the odorous intensity analysis. Therefore, the test repeatability and reliability were relatively high for utilization in the analysis of similar studies.
Acknowledgment
This work was supported by the National High Technology Research and Development Program of (863) China (number 2012AA030302), the National Natural Science Foundation of the People's Republic of China (numbers 21277011 and 20877008) and Program for New Century Excellent Talents in University (number NCET-09-0218), and the Fundamental Research Funds for the Central Universities (numbesr FRF-TP-10-004B and FRF-BR-11-017B).
References
- Agency for Toxic Substances and Disease Registry. 2007accessed 2008 http://www.atsdr.cdc.gov (http://www.atsdr.cdc.gov)
- ASTM . 1997 . Method E679-91. Standard practice for determination of odor and taste thresholds by a forced-choice ascending concentration series methods of limits. ,
- CEN . 2003 . prEN 13725. Air quality—Determination of odour concentration by dynamic olfactometry. ,
- China EPA . 1993 . Air quality—Determination of odor—Triangle odor bag method
- China EPA . 2009 . Air and exhaust gas—Determination of ammonia—Nessler's reagent spetcrophotometry
- China Ministry of Health . 1989 . Standard method for hygienic examination of hydrogen sulfide in air of residential areas—Methylene blue spectrophotometric method
- China Ministry of Health . 2007 . Occupational exposure limits for hazardous agents in the workplace
- Das , K.C. , Tollner , E.W. and Eiteman , M.A. 2003 . Comparison of synthetic and natural bulking agents in food waste composting . Compost Sci. Util , 11 : 27 – 35 .
- Davoli , E. , Gangai , M.L. , Morselli , L. and Tonelli , D. 2003 . Characterisation of odorants emissions from landfills by SPME and GC/MS . Chemosphere , 51 : 357 – 68 . doi: 10.1016/S0045-6535(02)00845-7
- Defoer , N. , Bo , I.D. , Langenhove , H.V. , Dewulf , J. and Elst , T.V. 2002 . Gas chromatography–mass spectrometry as a tool for estimating odour concentrations of biofilter effluents at aerobic composting and rendering plants . J. Chromatogr. A , 970 : 259 – 73 . doi: 10.1016/S0021-9673(02)00654-4
- Devos , M. , Patte , F. , Rouault , J. , Laffort , P. and Van Gemert , L. 1990 . Standardized Human Olfactory Thresholds , New York, NY : Oxford University Press .
- Ding , Y. , Cai , C.Y. , Hu , B. , Xu , Y. , Zheng , X.J , Chen , Y.X. and Wu , W.X. 2012 . Characterization and control of odorous gases at a landfill site: A case study in Hangzhou, China . Waste Manage , 32 : 317 – 26 .
- Dincer , F. , Odabasi , M. and Muezzinoglu , A. 2006 . Chemical characterization of odorous gases at a landfill site by gas chromatography–mass spectrometry . J. Chromatogr. A , 1122 : 222 – 29 . doi: 10.1016/j.chroma.2006.04.075
- Fang , J.J. , Yang , N. and Cen , D.Y. 2012 . Odor compounds from different sources of landfill: Characterization and source identification . Waste Manage , 32 : 1401 – 10 . doi: 10.1016/j.wasman.2012.02.013
- Filippi , C. , Bedini , S. , Levi-Minzi , R. , Cardelli , R. and Saviozzi , A. 2002 . Co-composting of olive oil mill by-products: Chemical and microbiological evaluation . Compost Sci. Util , 10 : 63 – 71 .
- Han , S.X. and Lin , J. 2005 . Trimethylamine content determination in the choline chloride powder . Feed Res , 8 : 45 – 49 .
- Hurst , C. , Longhurst , P. , Pollard , S. , Smith , R. , Jefferson , B. and Gronow , J. 2005 . Assessment of municipal waste compost as a daily cover material for odor control at landfill sites . Environ. Pollut , 135 : 171 – 77 . doi: 10.1016/j.envpol.2004.09.004
- Japan Ministry of the Environment . 2003 . Odor index regulation and triangular odor bag method
- Kim , J.D. , Park , J.S. , In , B.H. , Kim , D. and Namkoong , W. 2008 . Evaluation of pilot-scale in-vessel composting for food waste treatment . J. Hazard. Mater , 154 : 272 – 77 . doi: 10.1016/j.jhazmat.2007.10.023
- Leong , S.T. , Muttamara , S. and Laortanakul , P. 2002 . Applicability of gasoline containing ethanol as Thailand's alternative fuel to curb toxic VOC pollutants from automobile emission . Atmos. Environ , 36 : 3495 – 503 . doi: 10.1016/S1352-2310(02)00288-1
- Levis , J.W. , Barlaz , M.A. , Themelis , N.J. and Ulloa , P. 2010 . Assessment of the state of food waste treatment in the United States and Canada . Waste Manage , 30 : 1486 – 94 . doi: 10.1016/j.wasman.2010.01.031
- Mao , F. , Tsai , C.J. , Shen , S.H. , Lin , T.F. , Chen , W.K. and Chen , M.L. 2006 . Critical components of odors in evaluating the performance of food waste composting plant . Sci. Total Environ , 370 : 323 – 29 . doi: 10.1016/j.scitotenv.2006.06.016
- Nagata , Y. 2003 . “ Odor intensity and odor threshold value ” . In Bull. Jpn. Environ. Sanitation Center 17 – 25 .
- Neter , J. , Kutner , M.H. , Nachtsheim , C.J. and Wasserman , W. 1996 . Applied Linear Statistical Models , New York , NY : McGraw-Hill .
- O'Neill , D.H. and Phillips , V.R. 1992 . A review of the control of odour nuisance from livestock buildings: Part 3, Properties of the odorous substances which have been identified in livestock wastes or in the air around them . J. Agric. Eng. Res , 53 : 23 – 50 . doi: 10.1016/0021-8634(92)80072-Z
- Ruth , J.H. 1986 . Odour thresholds and irritation levels of several chemical substances: A review . Am. Ind. Hyg. Assoc. J , 47 : A142 – 151 . doi: 10.1080/15298668691389595
- Sarkar , U. and Hobbs , S.E. 2003 . Landfill odor: Assessment of emissions by the flux footprint method . Environ. Model. Softw , 18 : 155 – 63 . doi: 10.1016/S1364-8152(02)00071-3
- Taiwan EPA . 1994 . Malodor olfactometry—Triangular odor bag method NIEA A201.10A
- Tsai , C.J. , Chen , M.L. , Ye , A.D. , Chou , M.S. , Shen , S.H. and Mao , I.F. 2008 . The relationship of odor concentration and the critical components emitted from food waste composting plants . Atmos. Environ , 42 : 8246 – 51 . doi: 10.1016/j.atmosenv.2008.07.055
- Wan , J.R. , Hong , Y.J. and Wu , G.H. 1993 . Chemical Analysis Manual of Aquatic Food , Shanghai : Shanghai Science and Technology Press .
- Wu , T. , Wang , X.M. , Li , D.J. and Yi , Z.G. 2010 . Emission of volatile organic sulfur compounds (VOSCs) during aerobic decomposition of food wastes . Atmos. Environ , 44 : 5065 – 71 . doi: 10.1016/j.atmosenv.2010.09.019
- Zou , S.C. , Lee , S.C. , Chan , C.Y. , Ho , K.F. , Wang , X.M. , Chan , L.Y. and Zhang , Z.X. 2003 . Characterization of ambient volatile organic compounds at a landfill site in Guangzhou, South China . Chemosphere , 51 : 1015 – 22 . doi: 10.1016/S0045-6535(03)00004-3