Abstract
Spraying slightly acidic electrolyzed water (SAEW) has been considered as a potential approach to reduce airborne bacteria in laying-hen houses. In this study, the effects of spraying SAEW on airborne bacterial reduction were investigated in a laying-hen house as compared with using diluted didecyl dimethyl ammonium bromide (DDAB). Averaged air temperature reduced by approximate 1 °C and average relative humidity increased by 3% at a stable ventilation rate (about 2.5 m3 hr−1 per bird) in the laying-hen house 30 min after spraying (120 mL m−2). Compared with the control without spraying, the airborne bacterial concentration was reduced by about 0.70 and 0.37 log10 colony-forming units (CFU) m−3 in the 4 hr after spraying 120 mL m−2 SAEW (available chlorine concentration [ACC] of 156 mg L−1) and diluted DDAB (active compound concentration of 167 mg L−1), respectively. Compared with spraying diluted DDAB, spraying SAEW was determined to be more effective for reducing airborne bacterial in laying-hen houses. The effects of spraying SAEW and diluted DDAB on airborne bacterial reduction in the laying-hen house increased with the increasing available chlorine concentrations for SAEW (156, 206, 262 mg L−1) and increasing active compound concentrations for diluted DDAB (167, 333, 500 mg L−1), respectively. Spraying SAEW and diluted DDAB with two levels of spraying volumes (120 and 90 mL m−2) both showed significant differences on airborne bacterial reduction in the laying-hen house (P < 0.05).
It is difficult to effectively reduce airborne bacteria in laying-hen houses. This work describes the application of spraying slightly acidic electrolyzed water as a new approach for reducing airborne bacteria in a laying-hen house. The effects of active compound concentrations and spray volumes on the airborne bacterial reductions by spraying SAEW were also investigated. This study provided a new effective and environmentally friendly approach to reduce the airborne bacteria in poultry houses, contributing to bird housing environment management and improving bird health.
Introduction
In recent years, the health of chickens is becoming an increasing concern in egg production. Airborne bacteria and other disease-causing microorganisms can spread throughout a hen house and cause bird diseases (CitationWhyte, 2002; CitationMitchell et al., 2004). Airborne bacteria such as Salmonella and Staphylococcus aureus can be transmitted between laying birds through air and cause bird diseases, or introduced into the food chain from breeding birds to eggs (CitationLeach et al., 1999; CitationHajmeer et al., 2006).
Spraying disinfectant is an approach to reduce airborne bacteria in poultry houses, with regard to avoiding the application of antibiotic additives in feedstuffs and to minimizing the necessity to use therapeutic drugs (CitationBiihm, 1998). Spraying disinfectant during production can reduce airborne pathogenic microorganisms and improve the housing environment in poultry houses (CitationZheng et al., 2010, 2012b). Spraying is carried out without dampening the floor and chicken feather. A variety of chemical disinfectants are used for spraying in poultry houses in China, of which quaternary ammonium compounds (such as didecyl dimethyl ammonium bromide) are widely used. Didecyl dimethyl ammonium bromide (DDAB) is reported being hazardous to cause skin, eye, or respiratory tract irritation by the manufacturers. Acidic electrolyzed water (AEW) with lower pH values (<2.7), also known as electrolyzed oxidizing water (EOW), has been regarded as a potential alternative to environmentally friendly microbial decontamination. AEW has been proven to reduce pathogens on vegetables, eggs, poultry, and pork (CitationKoseki et al., 2004; CitationRussell, 2003; CitationPark et al., 2002; CitationKim et al., 2005; CitationFabrizio and Cutter, 2005). However, the utilization of AEW has limited potential for spraying in poultry houses. AEW can easily dissolve to Cl2 gas due to volatilization, which causes chlorine loss, thus decreasing AEW bactericidal activity with time (Len et al., 2000; CitationCui et al., 2009). The strong acidity (pH <2.7) of AEW can cause corrosion of equipment (CitationGuentzel et al., 2008). Slightly acidic electrolyzed water (SAEW) (pH 6.0–6.5) with a higher pH may be considered as a novel disinfectant for spraying in poultry houses. SAEW (pH 6.0–6.5) has an equivalent bactericidal efficacy compared with AEW and can keep its available chlorine during storage under shaded and sealed conditions (CitationKoide et al., 2009). SAEW improves bactericidal activity by maximizing the use of hypochlorous acid, reducing corrosion of surfaces, and minimizing Cl2 off-gassing (CitationGuentzel et al., 2008). SAEW was compared with NaClO solution and AEW for inactivation of Salmonella enteritidis and its contaminated shell eggs. The results indicated that SAEW was a promising disinfectant agent for the shell egg washing processing (CitationCao et al., 2009). SAEW is considered to be a novel, highly effective, healthy, and environmentally friendly disinfectant in food industry (CitationKoide et al., 2009; CitationQuan et al., 2010; CitationAbdulsudi et al., 2011). However, limited information is available on the application of SAEW as a disinfectant for spraying during production in poultry houses.
The objective of this research was to
| 1. | evaluate the reduction of airborne bacterial levels by spraying SAEW in a laying-hen house, compared with diluted DDAB; and | ||||
| 2. | investigate the effects of active compound concentrations and spray volumes on the airborne bacterial level reductions by spraying SAEW and diluted DDAB in a laying-hen house. | ||||
Materials and Methods
Experimental house and spraying system
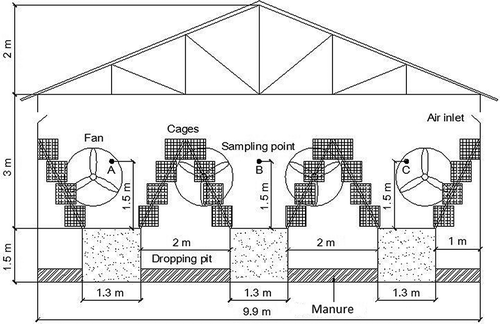
Experiments were conducted in a 10.5 × 80-m laying-hen house located at a commercial poultry farm in Sichuan Province, southwest China. The study was carried out from October to December of 2010. Eighteen thousand laying hens, half-year-old, were confined in stair-step cages with four rows and four tiers in a tunnel-ventilated house (as shown in and ). Six 1.23-m-diameter axial exhaust fans (model 1380; Qingdao Big Herdsman Mechanical Co., Ltd., Qingdao, China) were held in one of the two gable walls located at the two ends of the building (as shown and ). Two evaporative cooling pads (1500 × 9400 cm) and 38 air inlets (50 × 25 cm) whose size could be adjusted based on the temperature were distributed along the sidewalls (as shown ). Four of the six axial flow fans were operated when the lights were on, and two of them were shut down when the lights were off during the experimental period. The evaporative cooling pads were closed and air came in through the 38 air inlets, which were fully opened during the experimental period. Manure was gathered in a four dropping pit under cages and scraped out of the house daily by a mechanical manure scraper at about 9:00 a.m. Feed was distributed at 8:30 a.m., 11:00 a.m., and 4:00 p.m. every day. The light was turned on at 4:00 a.m. and off at 8:00 p.m. (16-hr on/8-hr off).
Figure 1. Longitudinal section view of the laying-hen house. Airborne bacterial measurements were performed at points A, B, and C to compare bacterial reductions by spraying disinfectants. Air temperature and relative humidity were measured at point B and outside of the building.
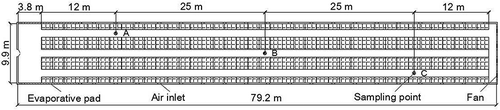
Figure 2. Cross-section view of the laying-hen house. Airborne bacterial measurements were performed at points A, B, and C to compare bacterial reductions by spraying disinfectants. Air temperature and relative humidity were measured at point B and outside of the building.
A high-pressure nozzle sprayer (model LF-80; Chengdu Lifeng Cleaning Machine Co., Ltd., Chengdu, China; pressure: 3.5 MPa, maximum flow rate: 36 L min−1) was used for spraying disinfectants in the experiment. The sprayer was operated to consistently and evenly spray disinfectants into the air above the top ties by workers from one end of the building to the other. Spraying was administrated starting at 11:00 a.m. and continued for approximate 5 min in the amount of 120 or 90 mL m−2 every day.
Disinfectants in the experiment
As shown in , SAEW and diluted didecyl dimethyl ammonium bromide (DDAB) (10%; Bestar Biochemical Co., Ltd., Shanghai, China) were sprayed in this study. SAEW with an available chlorine concentration (ACC) of 150–250 mg L−1 and pH of 5.8–6.2 was used in this study. It was generated by electrolysis of a 1% sodium chloride solution in a generator that consisted of an electrolytic cell without a separating membrane. Hydrochloric acid was added to the sodium chloride solution before electrolysis to reach the desired pH of 5.9–6.1 for SAEW. The ACC was determined by a colorimetric method using a digital chlorine test kit (RC-3F; Kasahara Chemical Instruments Corporation, Saitama, Japan), and the pH was measured using a dual-scale pH/oxidation-reduction potential (ORP) meter (HM-30 R; DKKTOA Corporation, Tokyo, Japan) with a pH electrode (GST-5741C) measuring from 0.0 to 14.0. The pH meter was calibrated using commercial standard buffers with pH of 4.01 and 6.86 supplied by the manufacturer. DDAB (10%; Bestar Biochemical) is a common commercial disinfectant in China. DDAB diluted with tap water was used in the experiment and active compound (DDAB) concentrations of diluted DDAB (167–500 mg L−1) were calculated from the active compound concentration given by the manufacturer and different dilution factors.
Table 1. Disinfectants used in the experiment
Air temperature, relative humidity, and airborne bacterial measurement
An air temperature (T) and relative humidity (RH) sensor (Thermo recorder RS-11; ESPEC MIC Corp., Aichi, Japan) were positioned at the B test point (), and an identical sensor was used to monitor the air temperature and relative humidity outdoors. The accuracy for air temperature and relative humidity is 0.5 °C and 5%, respectively. The two sensors were calibrated using an aspirated dry-wet bulb hygrometer before the experiment.
A six-stage viable Andersen cascade impactor (model S6; Sennon Technology Development Co., Ltd., Beijing, China) was used for airborne bacterial measurement in the experiment. Airborne bacterial measurement was performed at sampling points A, B, and C, which were at the height of 1.5 m (approximately breathing height of humans) in the laying-hen house (as shown in and ). Airborne bacterial sampling was administrated at 10:45–11:00 a.m., 11:30–11:45 a.m., 12:15–12:30 p.m., 1:15–1:30 p.m. and 3:15–3:30 p.m., 15 min for each sampling. During the 15-min sampling period, airborne bacteria were measured from points A to C (), min for each point. During the 5-min sampling period for each point, the sampling duration of the impactor was 1 min and the remaining 4 min was for sampling preparation. The impactor consists of an air pump and six orifice stages along with Petri dishes at each stage level. The airflow rate through the impactor with Petri dishes was calculated to be 28.3 L min−1 using a calibrated rotameter (Dwyer RMC-123-SSV Rate-Master flow meter; Michigan City, IN, USA) before the experiment. To each Petri dish, 27 mL of Trypticase Soy Agar-Yeast Extract (TSA-YE; Qingdao Hope Bio-Technology Co., Ltd, Qingdao, China) was transferred using sterile pipette tips on a clean bench. Each Petri dish with TSA-YE was located on every stage to collect airborne microbial particles. After sampling, each Petri dish was incubated at 37 °C for 24 hr. The number of colony-forming units (CFU) on each Petri dish was counted to calculate the airborne bacterial concentration. The airborne bacterial concentration was calculated using the following equation:
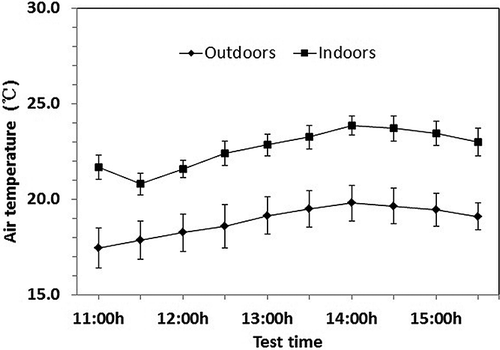
Figure 3. Air temperature outdoors and indoors during the test period. Vertical bars represent means ± standard errors.
Experimental design and data analysis
Airborne bacterial reduction by spraying SAEW and thermal environmental changes during spraying
As shown in , spraying diluted DDAB (active compound concentration, about 167 mg L−1) (treatment 1) and spraying SAEW (ACC = 156 ± 5 mg L−1) (treatment 5) were administrated starting at 11:00 a.m. and continuing approximately 5 min in the amount of 120 mL m−2. Spraying diluted DDAB was performed in 2 weeks, twice for each week (4 days total). Spraying SAEW was carried out in the following 2 weeks, twice for each week (4 days total). The control (no spraying) was performed once a week for the 4 weeks (4 days total). Airborne bacterial samplings were performed at 10:45–11:00 a.m., 11:30–11:45 a.m., 12:15–12:30 p.m., 1:15–1:30 p.m. and 3:15–3:30 p.m. in each sampling day. Airborne bacterial concentrations at the three sampling points during each sampling period were collected. Air temperature and relative humidity were recorded at 11:00 a.m., 11:30 a.m., 12:00 p.m., 12:30 p.m., 1:00 p.m., 1:30 p.m., 2:00 p.m., 2:30 p.m., 3:00 p.m., and 3:30 p.m. in all the sampling days (12 days total) and averaged. During the tests, the ventilation of the house was kept stable at about 2.5 m3 hr−1 per bird, with four fans in operation.
Table 2. Experimental treatments in the laying-hen house
Statistical analysis was performed using the SAS9.2 software (SAS Institute Inc., Cary, NC, USA). Tukey's studentized range test was used to determine the significant differences among the airborne bacterial concentrations at 10:45 a.m. (before spraying) of the control, treatment 1, and treatment 5 (5% probability level). It was also used to determine the significant differences among the airborne bacterial concentrations at 11:15 a.m., 12:15 p.m., 1:15 p.m., and 3:15 p.m. (after spraying) of the control, treatment 1, and treatment 5 (5% probability level).
Effects of active compound concentration and spraying volume on airborne bacterial reduction by spraying SAEW
As shown in , eight treatments were performed and each treatment was carried out in four different sampling days during the experimental period. In each day, airborne bacteria were measured at the three test points starting at 10:45 a.m., 11:15 a.m., 12:15 p.m., 1:15 p.m., and 3:15 p.m. and the sampling duration for each point was 1 min. Two factors on the airborne bacterial reduction effects by spraying SAEW and diluted DDAB were investigated. The active compound concentrations of diluted DDAB and ACCs of SAEW were studied at three levels (167, 333, and 500 mg L−1 for diluted DDAB; 156, 206, and 262 mg L−1 for SAEW) at the spraying volume of 120 mL m−2, respectively. The spraying volume of SAEW (ACC, 262 ± 12 mg L−1) and diluted DDAB (active compound concentration, 333 mg L−1) were studied at two levels (120 and 90 mL m−2, respectively).
Statistical analysis was performed using the SAS9.2 software (SAS Institute). Tukey's studentized range test was used to determine the significant differences among the airborne bacterial concentrations at 10:45 a.m. (before spraying) of treatment 1 to treatment 8 () at the 5% probability level. It was also used to determine the significant differences among the airborne bacterial concentrations at 11:15 a.m., 12:15 p.m., 1:15 p.m., and 3:15 p.m. (after spraying) of treatment 1 to treatment 8 () at the 5% probability level.
Results and Discussion
Air temperature and relative humidity before and after spraying
The air temperature and relative humidity outdoors and indoors before and after spraying were shown in and . Spraying caused an air temperature drop and relative humidity rise. Spraying was administrated starting at 11:00 a.m. and continued for approximately 5 min in the amount of 120 mL m−2 without dampening the floor and chicken feather. The average air temperature indoors at 11:30 a.m. (21.7 ± 1.8 °C, ± SD) was 1 °C lower than that at 11:00 a.m. (before spraying) (20.8 ± 1.6 °C, ± SD) for a stable ventilation rate (about 2.5 m3 hr−1 per bird) in the building and increased outdoor air temperature (from 17.5 ± 3.0 to 17.9 ± 2.8 °C). The average relative humidity indoors at 11:30 a.m. (53 ± 3%, ± SD) was more than 3% of that at 11:00 a.m. (before spraying) (50 ± 3%, ± SD), whereas the relative humidity outdoors decreased by 3% (from 51 ± 5% to 48 ± 4%) and the ventilation was stable at about 2.5 m3 hr−1 per bird. Spraying increased the moisture in the air and caused the relative humidity to increase. Heat absorption by water evaporation due to the high ventilation possibly reduced the air temperature.
Figure 4. Relative humidity outdoors and indoors during the test period. Vertical bars represent means ± standard errors.
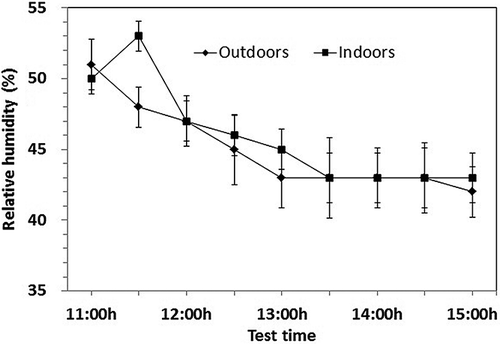
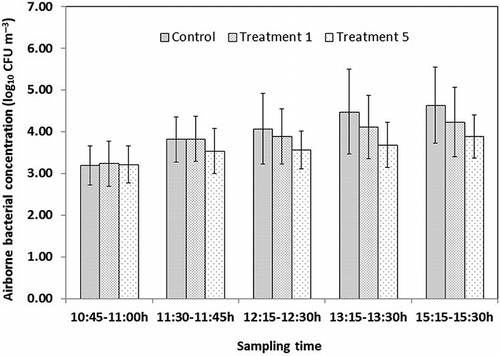
Figure 5. Airborne bacterial concentration variations in the control, treatment 1, and treatment 5. Spraying diluted DDAB (167 mg L−1) and SAEW (156 mg L−1) were administrated starting at 11:00 a.m. in the amount of 120 mL m−2 in treatment 1 and treatment 5, respectively. No spraying was carried out in the control. Vertical bars respect means ± standard deviations with n = 12 (3 sampling points each day, 4 sampling days).
Airborne bacterial reduction by spraying slightly acidic electrolyzed water
Airborne bacterial concentrations at the different sampling time for the control, treatment 1, and treatment 5 are shown in . The trends of airborne bacterial concentrations after spraying varied between the control, spraying 167 mg L−1 diluted DDAB in the amount of 120 mL m−2 (treatment 1), and spraying 156 mg L−1 SAEW in the amount of 120 mL m−2 (treatment 5). Treatment 1 tended to be higher than the control and lower than treatment 5. The statistical analysis showed that airborne bacterial concentrations at 10:45 a.m. (before spraying) had no significant differences among the control, treatment 1, and treatment 5 (P > 0.05). Airborne bacterial concentrations after spraying 167 mg L−1 diluted DDAB in the amount of 120 mL m−2 (treatment 1) did not differ significantly (P = 0.153) from the control. However, spraying 156 mg L−1 SAEW in the amount of 120 mL m−2 (treatment 5) significantly reduced the airborne bacterial concentrations (P = 0.023) compared with the control. The finding showed that spraying SAEW (ACC, 156 mg L−1) in the amount of 120 mL m−2 has a great effect on airborne bacterial reduction in the laying-hen house. Compared with the control without spaying, the airborne bacterial concentration was reduced by 0.70 and 0.37 log10 CFU m−3 in the 4 hr after spraying 120 mL m−2 SAEW (ACC, 156 mg L−1) and diluted DDAB (active compound concentration, 167 mg L−1), respectively. The effect of airborne bacterial reduction by spraying SAEW (ACC, 156 mg L−1) was greater than spraying diluted DDAB with the equivalent active compound concentration (167 mg L−1) in the amount of 120 mL m−2.
The efficiency of weak acid hypochlorous water (WAHW) on disinfecting indoor microbes in a child daycare room was investigated, and indoor microbial concentrations were reduced significantly with WAHW intervention (CitationChen et al., 2012). SAEW improves the bactericidal activity by maximizing the use of hypochlorous acid, which is primarily responsible for inactivation of Escherichia coli O157:H7 and Salmonella enteritidis in SAEW (Zheng et al., 2012a). CitationBailey et al. (1986) showed that with the same spraying conditions, a 20-ppm chlorine solution could reduce Salmonella contamination by 50%, and a 40-ppm chlorine solution could reduce Salmonella contamination by 96%. Therefore, spraying SAEW for disinfecting airborne bacteria has a good potential and is a highly efficient method for airborne bacterial reduction in laying-hen houses.
Effects of spraying SAEW and diluted DDAB with different active component concentrations and spraying volumes
Airborne bacterial concentrations plotted in show different trends in treatment 1, treatment 2, treatment 3, and treatment 4. The statistical analysis showed that airborne bacterial concentrations at 10:45 a.m. (before spraying) had no significant differences among treatment 1, treatment 2, treatment 3, and treatment 4 (P > 0.05). Spraying 167 mg L−1 diluted DDAB (treatment 1) was significantly different (P = 0.009) from spraying 333 mg L−1 diluted DDAB in the amount of 120 mL m−2 (treatment 2) on the airborne bacterial concentrations. Airborne bacterial concentrations after spraying 333 mg L−1 diluted DDAB (treatment 2) were statistically higher (P = 0.027) than those after spraying 500 mg L−1 diluted DDAB in the amount of 120 mL m−2 (treatment 3). Similarly, airborne bacterial concentrations at different sampling time for treatment 5, treatment 6, treatment 7, and treatment 8 are plotted in . There was also no significant difference in airborne bacterial concentrations at 10:45 a.m. (before spraying) for treatment 5, treatment 6, treatment 7, and treatment 8 (P > 0.05). Spraying 156 mg L−1 of SAEW in the amount of 120 mL m−2 (treatment 5) was significant different (P = 0.031) from spraying 206 mg L−1 SAEW in the amount of 120 mL m−2 (treatment 6) on the airborne bacterial concentrations after spraying. Airborne bacterial concentrations after spraying 206 mg L−1 SAEW (treatment 6) were statistically higher (P = 0.033) than those of spraying 262 mg L−1 SAEW in the amount of 120 mL m−2 (treatment 7). These results indicated that the effects of spraying diluted DDAB and SAEW on airborne bacterial reduction were more effective with higher active compound concentrations.
Figure 6. Airborne bacterial concentration variations in treatment 1, treatment 2, treatment 3, and treatment 4. Spraying diluted DDAB (167, 333, and 500 mg L−1) in the amount of 120 mL m−2 and spraying diluted DDAB (333 mg L−1) in the amount of 90 mL m−2 were administrated starting at 11:00 a.m. in treatment 1, treatment 2, treatment 3, and treatment 4, respectively. Vertical bars respect means ± standard deviations with n = 12 (3 sampling points each day, 4 sampling days).
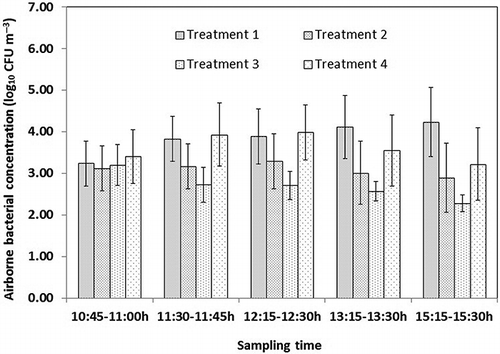
Figure 7. Airborne bacterial concentration variations in treatment 5, treatment 6, treatment 7, and treatment 8. Spraying SAEW (156, 206, and 262 mg L−1) in the amount of 120 mL m−2 and spraying SAEW (262 mg L−1) in the amount of 90 mL m−2 were administrated starting at 11:00 a.m. in treatment 5, treatment 6, treatment 7, and treatment 8, respectively. Vertical bars respect means ± standard deviations with n = 12 (3 sampling points each day, 4 sampling days).
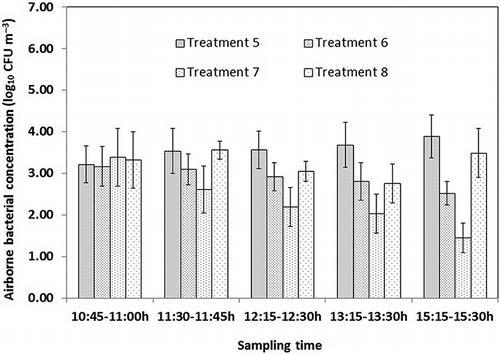
Statistical analysis showed that airborne bacterial concentrations after spraying 333 mg L−1 diluted DDAB in the amount of 120 mL m−2 (treatment 2) were significantly lower (P = 0.043) than those of spraying 333 mg L−1 diluted DDAB in the amount of 90 mL m−2 (treatment 4). Similarly, airborne bacterial concentrations after spraying 262 mg L−1 SAEW in the amount of 120 mL m−2 (treatment 7) were significantly lower (P = 0.017) than those after spraying 262 mg L−1 SAEW in the amount of 90 mL m−2 (treatment 4). The results indicated that the effects on airborne bacterial reduction by spraying diluted DDAB and SAEW both increased with a higher spraying volume in the laying-hen house.
The efficiency of SAEW for inactivation of pure cultures increased with increasing available chlorine concentration and treatment time (CitationCao et al., 2009; CitationZhang et al., 2011). Available chlorine is responsible for the bactericidal effects of SAEW. Higher available chlorine concentrations in SAEW result in more effective bactericidal activity on airborne bacteria. Spraying SAEW with a higher volume results in more airborne bacteria exposed to available chlorine. The effect on airborne bacterial reduction by spraying SAEW with a volume of 120 mL m−2 is significantly higher than that of 90 mL m−2 (P < 0.05).
Conclusion
Spraying diluted DDAB and SAEW were carried out during production in a laying-hen house. The results provide findings on the effects of spraying diluted DDAB and SAEW on airborne bacterial levels in the laying-hen house.
| • | The results suggested that spraying SAEW is an efficient approach to reduce airborne bacterial contamination in laying-hen houses. Furthermore, spraying SAEW was more effective than spraying diluted DDAB at the same active compound concentration and spraying volume in a laying-hen house. | ||||
| • | The ability of spraying SAEW and diluted DDAB to reduce airborne bacterial contamination in a laying-hen house showed a dose-dependent relationship with the concentration of active compounds and showed significant differences (P < 0.05) between spraying volumes of 120 and 90 mL m−2. | ||||
Acknowledgment
This study was funded by the China Agricultural Research System (CARS-41), National Department Public Benefit Research Foundation of China (200903009), and National Fund of Natural Science of China (30871957). The authors would also like to thank Sun Daily Farm, Mianyang, Sichuan Province, China.
References
- Abdulsudi , I.Z. , Yoshinori , K. , Nami , M. , Happiness , M. and Koichi , I. 2011 . Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts . Food Control , 22 : 601 – 607 . doi: 10.1016/j.foodcont.2010.10.011
- Bailey , J.S. , Thomson , J.E. , Cox , N.A. and Shackelford. , A.D. 1986 . Chlorine spray washing to reduce bacterial contamination of poultry processing equipment . Poultry Sci , 65 : 1120 – 1123 . doi: 10.3382/ps.0651120
- Biihm , R. 1998 . Disinfection and hygiene in the veterinary field and disinfection of animal houses and transport vehicles . Int. Biodeter. Biodegrad , 41 : 217 – 224 . doi: 10.1016/S0964-8305(98)00030-4
- Cao , W. , Zhu , Z. , Shi , Z. , Wang , C. and Li. , B. 2009 . Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs . Int. J. Food Microbiol. , 130 : 88 – 93 . doi: 10.1016/j.ijfoodmicro.2008.12.021
- Chen , N.T. , Su , Y.M. , Hsu , N.Y. , Wu , P.C. and Su. , H.J. 2012 . Airborne fungi and bacteria in child daycare centers and the effectiveness of weak acid hypochlorous water on controlling microbes . J. Environ. Monit , 14 : 2692 – 2697 . doi: 10.1039/c2em30113j
- Cui , X. , Shang , Y. , Shi , Z. , Xin , H. and Cao. , W. 2009 . Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions . J. Food Eng. , 91 : 582 – 586 . doi: 10.1016/j.jfoodeng.2008.10.006
- Fabrizio , K.A. and Cutter. , C.N. 2005 . Application of electrolyzed oxidizing water to reduce Listeria monocytogenes on ready-to-eat meats . Meat Sci , 71 : 327 – 333 . doi: 10.1016/j.meatsci.2005.04.012
- Guentzel , J.L. , Lam , K.L. , Callan , M.A. , Emmons , S.A. and Dunham. , V.L. 2008 . Reduction of bacteria on spinach, lettuce, and surfaces in food service areas using neutral electrolyzed oxidizing water . Food Microbiol , 25 : 36 – 41 . doi: 10.1016/j.fm.2007.08.003
- Hajmeer , M. , Ceylan , E. , Marsden , J.L. and Fung. , D.Y.C . 2006 . Impact of sodium chloride on Escherichia coli O157: H7 and Staphylococcus aureus analysed using transmission electron microscopy . Food Microbiol , 23 : 446 – 452 . doi: 10.1016/j.fm.2005.06.005
- Kim , C. , Hung , Y.C. and Russell. , S.M. 2005 . Efficacy of electrolyzed water in the prevention and removal of fecal material attachment and its microbicidal effectiveness during simulated industrial poultry processing . Poultry Sci , 84 : 1778 – 1784 .
- Koide , S. , Takeda , J. , Shi , J. , Shono , H. and Atungulu. , G.G. 2009 . Disinfection efficacy of slightly acidic electrolyzed water on fresh cut cabbage . Food Control , 20 : 294 – 97 . doi: 10.1016/j.foodcont.2008.05.019
- Koseki , S. , Yoshida , K. , Isobe , S. and Itoh. , K. 2004 . Efficacy of acidic electrolyzed water for microbial decontamination of cucumbers and strawberries . J. Food Protect. , 67 : 1247 – 1251 . doi: 10.1016/j.fm.2003.11.004
- Leach , S.A. , Williams , A. , Davies , A.C. , Wilson , J. , Marsh , P.D. and Humphrey. , T.J. 1999 . Aerosol route enhances the contamination of intact eggs and muscle of experimentally infected laying hens by Salmonella typhimurium DT 104 . FEMS Microbiol. Lett , 171 : 203 – 207 . doi: 10.1111/j.1574-6968.1999.tb13433.x
- Len , S.V. , Hung , Y.C. and Chung. , D. 2002 . Effects of storage conditions and pH on chlorine loss on electrolyzed oxidizing (EO) water . J. Agric. Food Chem. , 50 : 209 – 212 . doi: 10.1021/jf010822v
- Mitchell , B.W. , Richardson , L.J. , Wilson , J.L. and Hofacre. , C.L. 2004 . Application of an electrostatic space charge system for dust, ammonia, and pathogen reduction in a broiler breeder house . Appl. Eng. Agric , 20 : 87 – 93 .
- Park , H. , Hung , Y.C. and Brackett. , R.E. 2002 . Antimicrobial effect of electrolyzed water for inactivating Campylobacter jejuni during poultry washing . Int. J. Food Microbiol. , 72 : 77 – 83 . doi: 10.1016/S0168-1605(01)00622-5
- Quan , Y. , Choi , K.D. , Chung , D. and Shin. , I.S. 2010 . Evaluation of bactericidal activity of weakly acidic electrolyzed water (WAEW) against Vibrio vulnificus and Vibrio parahaemolyticus . Int. J. Food Microbiol. , 136 : 255 – 260 . doi: 10.1016/j.ijfoodmicro.2009.11.005
- Russell , S.M. 2003 . The effect of electrolyzed oxidative water applied using electrostatic spraying on pathogenic and indicator bacteria on the surface of eggs . Poultry Sci , 82 : 158 – 162 .
- Whyte , R.T. 2002 . Occupational exposure of poultry stockmen in current barn systems for egg production in the United Kingdom . Br. Poultry Sci. , 43 : 364 – 373 . doi: 10.1080/00071660120103639
- Zhang , C. , Lu , Z. , Li , Y. , Shang , Y. , Zhang , G. and Cao. , W. 2011 . Reduction of Escherichia coli O157: H7 and Salmonella enteritidis on mung bean seeds and sprouts by slightly acidic electrolyzed water . Food Control , 22 : 792 – 796 . doi: 10.1016/j.foodcont.2010.11.018
- Zheng , W. , Cao , W. , Li , B. , Hao , X. , Ni , L. and Wang. , C. 2012a . Bactericidal activity of slightly acidic electrolyzed water produced by different methods analyzed with ultraviolet spectrophotometric . Int. J. Food Eng , 8 : 1556 – 3758 . doi: 10.1515/1556-3758.2827
- Zheng , W. , Li , B. , Cao , W. , Zhang , G. and Yang. , Z. 2012b . Application of neutral electrolyzed water spray for reducing dust levels in a layer breeding house . J. Air Waste Manage. Assoc , 62 : 1329 – 1334 . doi: 10.1080/10962247.2012.710553
- Zheng , W. , Li , B. , Shang , Y. , Wang , C. , Yang , Z. and Cao. , W. 2010 . Experimental study on spraying disinfection with neutral electrolyzed water in a layer breeding farm . Trans. Chin. Soc. Agric. Eng , 26 : 270 – 273 .