Abstract
Hexavalent chromium (Cr(VI)) and trivalent chromium (Cr(III)) are the primary chromium oxidation states found in ambient atmospheric particulate matter. While Cr(III) is relatively nontoxic, Cr(VI) is toxic and exposure to Cr(VI) may lead to cancer, nasal damage, asthma, bronchitis, and pneumonitis. Accurate measurement of the ambient Cr(VI) concentrations is an environmental challenge since Cr(VI) can be reduced to Cr(III) and vice versa during sampling. In the present study, a new Cr(VI) sampler (Clarkson sampler) was designed, constructed, and field tested to improve the sampling of Cr(VI) in ambient air. The new Clarkson Cr(VI) sampler was based on the concept that deliquescence during sampling leads to aqueous phase reactions. Thus, the relative humidity of the sampled air was reduced below the deliquescence relative humidity (DRH) of the ambient particles. The new sampler was operated to collect total suspended particles (TSP), and compared side-by-side with the current National Air Toxics Trends Stations (NATTS) Cr(VI) sampler that is utilized in the U.S. Environmental Protection Agency (EPA) air toxics monitoring program. Side-by-side field testing of the samplers occurred in Elizabeth, NJ, during the winter and summer of 2012. The average recovery values of Cr(VI) spikes after 24-hr sampling intervals during summer and winter sampling were 57 and 72%, respectively, for the Clarkson sampler, while the corresponding average values for NATTS samplers were 46% for both summer and winter sampling, respectively. Preventing the ambient aerosol collected on the filters from deliquescing is a key to improving the sampling of Cr(VI).
This study describes a sampler that provides cooling and drying of the particle collection filter by reducing the ambient air relative humidity to below deliquescence relative humidity of ambient particles. This Clarkson Cr(VI) sampler improved the measurement of ambient Cr(VI) concentration. It showed higher Cr(VI) recovery during field tests (71.8 ± 4.9% in winter and 57.1 ± 0.2 % in summer) compared to the current EPA Cr(VI) sampler (46.2 ± 10.8% in winter and 46.0 ± 1.6% in summer) that is employed in the National Air Toxics Trends Stations (NATTS) monitoring program.
Supplemental Materials: Supplemental materials are available for this paper. Go to the publisher's online edition of the Journal of the Air & Waste Management Association.
Introduction
Cr(III) and Cr(VI) are the two common oxidation states of chromium in the environment. Cr(VI) is toxic and exposure to Cr(VI) may lead to cancer, nasal damage, asthma, bronchitis, pneumonitis, dermatitis, and skin allergies (CitationBarceloux, 1999; CitationPark et al., 2004). In contrast, Cr(III) is a trace element essential for the proper function of living organisms (CitationIndependent Environmental Technical Group [IETEG], 2005).
CitationMeng et al. (2011) determined the soluble Cr(VI) concentration in ambient air of Paterson, NJ (0.44 U.S. 0.35 ng m−3), and Chester (0.40 ± 0.53 ng m−3), NJ, which are an industrial city and a background site, respectively. CitationSwietlik et al. (2011) performed ambient air sampling in Radom, Poland, and reported the average values for total Cr and Cr(VI) concentrations to be 25 and 6 ng m−3 respectively. They determined the Cr(VI) to total Cr ratio to be 36%. In total, 1466 Cr(VI) measurements were conducted over 22 sites from January 2005 to December 2005 in the United States (CitationEastern Research Group [ERG], 2007). The concentration of soluble Cr(VI) ranged from 0.001 to 2.97 ng m−3, and the average Cr(VI) concentration was determined to be 0.044 ng m−3. The soluble and total Cr(VI) concentrations in ambient PM10 collected from four locations in New Jersey, that is, Meadowlands, Elizabeth Trailer, and Rahway (with mixed Cr emission sources), and Piscataway (a suburb area) were determined by CitationHuang et al. (2013). In the locations with mixed Cr emission sources, the mean concentrations were 1.05–1.41 ng m−3 (winter) and 0.99–1.56 ng m−3 (summer) for total Cr(VI), and 0.11–0.19 ng m−3 (winter) and 0.18–0.37 ng m−3 (summer) for soluble Cr(VI). In Piscataway, the mean concentrations were 1.07 ng m−3 (winter) and 0.99 ng m−3 (summer) for total Cr(VI), and 0.03 ng m−3 (winter) and 0.12 ng m−3 (summer) for soluble Cr(VI). Their results indicate that the ambient PM in these sampling locations contain soluble and insoluble Cr(VI), with insoluble Cr(VI) being most prevalent.
Table 1 reviews the total Cr and Cr(VI) concentrations as well as Cr(VI) to total Cr ratios determined in previous studies. shows that Cr(III) comprises the majority of ambient chromium. Cr(VI) concentrations ranging from 0.001 (ERG, 2007) to 70 ng m−3 (CitationMandiwana et al., 2006), and the average Cr(VI) to total Cr ratios varied from 1 to 30%.
Table 1. Total Cr and Cr(VI) concentration values and Cr(VI) to total Cr ratio reported in previous studies
ERG developed a sampler (Figure S1) (ERG, 2007) for the U.S. Environmental Protection Agency National Air Toxics Trends Stations (NATTS) monitoring program to collect total suspended particles (TSP). The sampler operates for 24 hr from midnight to midnight using a carbonate-impregnated cellulose filter (see Figure S1 in the supplemental material), and the samples typically remain in the sampler after the sampling interval. ERG (2007) has shown that when filters are left in the NATTS sampler for longer than 12–24 hr, conversion of Cr(VI) occurs. When filters are spiked with Cr(VI) solution and left in the field for 33 to 105 hr, the reduction in Cr(VI) mass values ranged from 30% to 58% (ERG, 2007).
CitationMeng et al. (2011) found the average Cr(VI) recovery to be 57 ± 9% for the 24-hr sampling in Paterson, NJ, and Chester, NJ, using filters spiked prior to sampling. This recovery value was significantly lower than 67 ± 23% average Cr(VI) recovery for filters spiked after sampling.
The corresponding values for Cr(III) conversion were reported to be 17 ± 9% and 11 ± 5% for filters spiked prior to sampling and after sampling, respectively. These results suggest that ∼10% of the Cr(VI) and ∼6% of the Cr(III) were converted during sampling. CitationHuang et al. (2013) studied chromium stability during sampling (NATTS sampler) using the method of filter-spiked prior to and after sampling such that reduction of Cr(VI) occurred during summer and winter. However, sampling had no significant influence on the Cr(III) oxidation. CitationTirez et al. (2011) determined the Cr(VI) recovery to be 75 ± 39%, and the Cr(III) conversion was 1.7 ± 1.2% during sampling in the Flemish region of Belgium. These previous results regarding chromium reduction and oxidation during sampling indicate that in general, there is conversion of Cr(VI) to Cr(III) during sampling. However, since Cr(III) is much higher in concentration than Cr(VI) in ambient PM, even a small conversion of Cr(III) to Cr(VI) can lead to a substantial positive bias in Cr(VI) measurements. The observed Cr(VI) and Cr(III) conversion in previous studies is attributed to the conversion during sampling, filter storage, extraction, and analysis. The differences in the reported Cr(VI) recovery and Cr(III) conversion among previous studies could be also due to differences in the type of filters and in the analytical and filter extraction methods.
The conversion of Cr(VI) during sampling could be the result of deliquescence of the collected ambient particulate matter that provides aqueous reaction media, and subsequent reactions with organic matter, SO2, and other reductants (CitationHuang et al., 2013; CitationAmouei Torkmahalleh et al., 2012; CitationGrohse et al. 1988). Under typical atmospheric conditions, the relative humidity (RH) varies over the 24-hr sampling period. It may exceed the deliquescence relative humidity (DRH) at least 2 times during the 24 hr sampling periods commonly employed: after sunset and early in the morning. Once the relative humidity (RH) reaches or exceeds the DRH of the ambient particulate matter (76%) (CitationAmouei Torkmahalleh et al., 2012), conversion of Cr(VI) can occur even if the relative humidity subsequently drops below the DRH. The conversion of Cr(III) during sampling could be attributed to the reaction of Cr(III) with dissolved Mn (CitationSeigneur and Constantino, 1995; CitationNico and Zasoski, 2000), or with water-soluble organic compound (WSOC) that include secondary organic aerosol (SOA) (CitationHuang et al., 2013), and also reactions with gaseous oxidants such as O3 and particle-bound reactive oxygen species (ROS) (CitationAmouei Torkmahalleh et al., 2013; Werner et al., 2006).
The reported Cr(VI) concentrations and Cr(VI) to total Cr ratios in previous studies may have been biased by the Cr(VI) reduction and Cr(III) oxidation during sampling, filter storage, filter extraction, and analysis.
The goal of the current study was to design, construct, and test a new Cr(VI) sampler (Clarkson sampler) and compare the recovery of Cr(VI) using the Clarkson sampler with the NATTS sampler. The new sampler preserves Cr(VI) by reducing the humidity of the ambient air during sample collection to avoid deliquescence and slightly cooling the ambient air during summer.
Experimental Methods
Clarkson Cr(VI) sampler
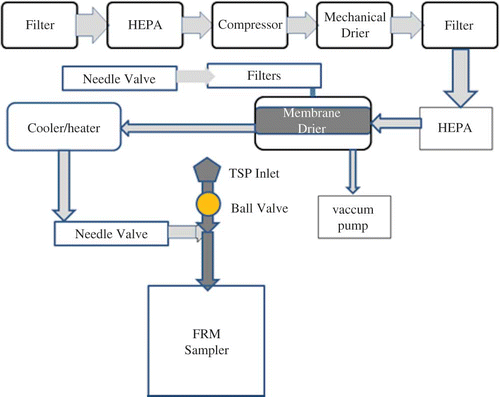
The Clarkson Cr(VI) sampler was designed such that the sampling filters remain dry (RH < 76%) during the sampling and postsampling intervals. To keep the filter dry, the RH of the air passing through the sampling filter was maintained below 76 ± 2%, the deliquescence relative humidity (DRH) of the ambient particles reported by CitationAmouei Torkmahalleh et al. (2012). The dry filter should slow the reduction reactions of Cr(VI) with ambient particles by preventing aqueous Cr(VI) chemistry so only solid–gas and solid–liquid reactions can occur (CitationAmouei Torkmahalleh et al., 2013; CitationHuang et al., 2013). Thus, a clean and dried airflow was added to sampled air in an FRM sampler (Rupprecht and Patashnick MODEL 2000H) equipped with TSP inlet (). The clean air is also cooled to 10ºC in the summer to decrease the temperature of the sampled air to slow Cr(VI) reactions on the filter. The clean air is warmed to 10ºC in the winter to decrease the relative humidity. The FRM sampler pump draws air through two paths, the clean air and the ambient air paths.
The sampling flow rate was adjusted to 15 LPM, and clean air was introduced into the sampler at 5 LPM. The TSP sampler was operated from 00:00 to 24:00. The ambient airflow was controlled by a solenoid valve installed between the sampler inlet and the clean air inlet. The valve was controlled by the sampler's controller. The solenoid valve is normally closed. The controller provides a signal to open the valve at 00:00 and close the valve at 24:00. In the subsequent period, the clean airflow rate increased to 15 LPM with the pump continuously pulling air through the filter/dryer/cooler system.
To provide dry air, filtered air was forced through a mechanical dryer (model D18IN, Ingersoll Rand, USA) to provide initial water removal. The air stream leaving the dryer was divided into four paths; each of them entered the drier enclosure (DE) of each Clarkson sampler. However, the mechanical dryer is required to operate even one Clarkson sampler because of the amount of water that needs to be removed from the air in hot and humid weather.
Each DE included two 47-mm cellulose filters, two HEPA filter capsules, a membrane dryer (Thermo Fisher Scientific, USA), a chiller/heater (TE Technology; model CP-065), two needle valves, a vacuum pump, and a vacuum pressure gage. The chiller/heater cooled the air in the summer, and heated the air during the winter. The set point of the chiller/heater was 10ºC, and the actual working temperature was 10 ± 0.5ºC. The temperature reached approximately 5 and 15ºC during winter and summer, respectively, at the end of the line, before mixing with ambient air. The needle valve was used to adjust the clean air flow rate as needed. The membrane dryer removed the water from the airflow. To prevent water saturation in the dryer, a counterflow purge air driven by 24 ± 1 inches Hg pressure drop was applied continuously across each dryer. The vacuum pump was used to provide purge airflow in the dryer. The purge air was passed through the cellulose filter and a HEPA filter capsule before entering the dryer. The needle valve was used to adjust the flow rate of the purge air to 0.5 LPM (). Abrasion-resistant gum rubber tubing (1/4 inch ID, 1/2 inch OD, 1/8 inch wall thickness) was used in the clean air path and the tubing was insulated by semiflexible polyethylene foam rubber (McMaster Carr) to minimize the temperature drop. Because of heat transfer across the clean air line, the temperature of the clean air slightly deviated from the cooler/heater set point. Figures S2 and S3 show the FRM sampler with TSP inlet and DE connected to the sampler. The relative humidity and the temperature of the ambient air and clean air were continuously monitored using a temperature and relative humidity sensor (iButton, Maxim).
Sampling filters
Cellulose filters were used because they have low chromium background concentrations (ERG, 2007). Cellulose filters spiked with Cr(VI) solution and then frozen showed no Cr(VI) reduction for up to 11 days at –18ºC (ERG, 2007). The cellulose filters were leached overnight in 10% nitric acid solutions to remove any chromium contamination. The filters were washed with ultrapure water, and dried overnight in a clean bench. The pH of the filters was measured using pH indicator paper, and found to be between 5 and 6. To prepare basic cellulose filters, the acid-washed filters were impregnated with 500 mL of a 0.12 M sodium bicarbonate solution. The filters were dried in a clean bench producing filters with pH values between 9 and 10 (ERG, 2007).
Isotopic spiking
Solutions of isotopically enriched Cr(III) (chromium(III) nitrate, Cr(NO3)3) and Cr(VI) (potassium dichromate, Cr2K2O7) standards were purchased from Applied Isotope Technology and refrigerated at 4ºC. 53Cr(VI) and 50Cr(III) isotopes were spiked on the filters to simultaneously monitor the oxidation and reduction of Cr(III) and Cr(VI), respectively, using ion chromatography (IC) coupled with inductively coupled plasma mass spectrometry (ICPMS) (IC/ICPMS).
Cr(VI) standard was spiked on the filters to determine Cr(VI) recovery using ion chromatography-ultraviolet spectroscopy (ICUV) for a positive control study that was performed during the field (summer) sampling campaign. All unspiked filters including field and laboratory blank filters were analyzed using ICUV.
Spiked filters were placed in a laminar flow clean bench for 10 min to dry and then stored in a freezer at –20ºC. All of the frozen filters were then dried in vacuum desiccators before being used for sampling to remove any possible absorbed water.
Analytical methods
To determine Cr(VI) concentration using IC/ICPMS (Thermo X-series, MA) analysis, a CS5A ion-exchange column (Dionex IonPac, 250 × 4.0 mm, 5 µm size) was employed. The sample delivery system consisted of high-performance liquid chromatography (HPLC), a Spectrosystem peristaltic pump, and a quartz spray chamber with a Conikal concentric glass nebulizer. Collision cell technology (CCT) was used to reduce polyatomic interferences such as 52ArC+ and 53ClO+. The instrument was optimized daily using a Thermo A25 Tune solution. External calibration curves were generated using a blank and Cr(VI) standards of 0.2, 0.5, 1, 2, 5, and 10 ng/mL. The method detection limit (MDL; 40. CFR 136, Appendix B) was determined as 0.042 ng/mL. The mean of the blank filter extract was 0.004 ng/mL (n = 4), which was below the MDL. Relevant HPLC parameters and analytical conditions for HPLC-ICPMS are given in Table S1 (supplemental material).
To determine the concentration of Cr(VI) using ICUV analysis, Cr(VI) is separated by ion chromatograph (IC) using an anion-exchange analytical column (AS7, Dionex) with a supporting guard column (NG1, Dionex), and reacted using a postcolumn derivatization module with diphenylcarbohydrazide (DPC) to form a complex that can be detected at 530 nm with a UV-visible detector.
The conversion of Cr(VI) to Cr(III) cannot be directly compared from IC/ICPMS analyses and the ICUV analyses. The samples were extracted using an acidic solution when prepared for IC/ICPMS, and with a slightly basic solution when prepared for ICUV analysis.
Data analyses
The relative standard deviations (RSD) of Cr(VI) recovery and Cr(III) conversion were calculated as the ratio of the standard deviation of the group of data to average of the data. Data for samples with more than two repetitions are presented as average ± standard deviation, while data from duplicated samples are presented as average ± relative percent difference (RDP). Variability is defined as absolute (value of sample 1 – value of sample 2)/average (value of sample 1 and value of sample 2).
One-way analysis of variance (ANOVA) and two-way ANOVA were used for statistical comparisons in the campus and the field studies, respectively.
Clarkson campus study
Basic pH filters were spiked with 10 ng Cr(VI) and 20 ng Cr(III). Four FRM samplers either were used for the NATTS samplers or were assembled for the Clarkson samplers. The samplers were operated simultaneously using spiked basic cellulose filters prepared a day before the sampling day, from November 2010 to February 2011. Sampling was performed with flow rates of 15 L min−1 on the roof of an academic building on the Clarkson University campus in Potsdam, NY. After sampling, the filters were transferred to petri dishes and stored in a freezer at –20ºC until analysis.
To examine the precision of the four Clarkson samplers on Cr(VI) recovery and Cr(III) conversion, four samplers were operated simultaneously for 24 hr, and the sampling was repeated for 4 days to collect a total of 16 filters.
NATTS and Clarkson samplers were operated side by side to compare the performance of both types of the samplers to recover Cr(VI). The experiments were performed by operating two Clarkson samplers and two NATTS samplers simultaneously with 15 LPM flow rate. The comparison was made on two different days to duplicate the study. Overall, four filters were collected per sampler type.
Field study
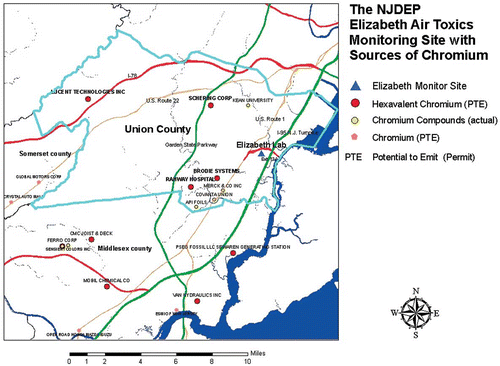
Field sampling was conducted at the New Jersey Department of Environmental Protection (NJDEP) air toxics monitoring site in Elizabeth, NJ. The site is downwind from a number of potential sources of hexavalent chromium emissions, and is located adjacent to interchange 13 on the New Jersey Turnpike (). The site has power, and is easily accessible by field sampling crews 24 hr/day, 7 days/week. In total, eight samplers (four Clarkson samplers and two dual-channel NATTS samplers) were deployed in the field for the sampling at average flow rates of 13.1 (NATTS) and 15.2 (Clarkson) LPM. To minimize potential airflow interferences, the collocated samplers were placed at least 1 m apart from each other at the monitoring site, but close enough to ensure that there were no significant differences (<20%) in the collected PM mass by the samplers. The mass precision tests were conducted for four rounds, from August 1, 2011, to August 4, 2011. The sampling was performed 14 ± 1 LPM for 24 hr on Teflon filters, with no Cr spiking. The sampled filters were kept in petri dishes in a weighing room (20 ± 2ºC and 35 ± 5%) at least 24 hr until gravimetric analysis. The mass of the filters before and after sampling was monitored using a microbalance to determine the collected PM mass.
During the sampling campaign, field blank samples were placed in all samplers for 24 hr and then were collected with field samples to store in the freezer until analyses. After sample collection, the field and field blank samples were shipped overnight to the designated laboratories for analysis by mail on dry ice (without weekend delivery). Accordingly, laboratory blank samples were shipped from either ERG or Rutgers University laboratories to the designated laboratories. Samples were kept at –20ºC in freezers at each laboratory until analysis. Samples were analyzed by each laboratory soon after receipt.
Tables S2 and S3 (supplemental material) present the details of field samples during winter and summer 2012, respectively. Tables S4 and S5 (supplemental material) show the sampling information for field and laboratory blank samples during winter and summer sampling campaigns, respectively. Rounds 5 and 6 in Table S3 are considered as positive control study.
Winter and summer sampling were performed in January 2012 (eight rounds) and June 2012 (six rounds), respectively. During winter sampling, in each round, eight samplers (four Clarkson and four NATTS samplers) were operated side by side except during round 1. During round 1, six samplers including four Clarkson samplers and two NATTS samplers were operated. Day 1 pickup represents 24-hr sampling plus the subsequent overnight field storage period, and Day 3 pickup refers to 24-hr sampling plus subsequent 48 hr and an overnight field storage period. During the winter sampling, sample analysis was performed by ICUV at Clarkson University, Rutgers University, and ERG, and also by IC/ICPMS at Clarkson University and Rutgers University, under the same sampling and analysis conditions per analytical method. All recovery and conversion calculations were made using the determined concentrations by IC/ICPMS.
During summer sampling, four NATTS and three Clarkson samplers (one sampler had failed) were operated side by side. Rounds 3 and 4 were designed to monitor Cr(VI) recovery and Cr(III) conversion using IC/ICPMS, while rounds 5 and 6 (positive control study) were performed to monitor only the Cr(VI) recovery using ICUV. During the summer campaign, sample analysis was performed by ICUV at ERG and IC/ICPMS at Rutgers University.
Table S6 (supplemental material) shows the method detection limit (MDL) for each analytical method at each laboratory.
Ambient Cr(VI) concentration was defined as the mass of the collected Cr(VI) after 24-hr sampling divided by the sampling volume. For laboratory and field blank samples, the Cr(VI) concentration was estimated by the mass of the determined Cr(VI) divided by the average sampling volume (20.2 m3 for winter sampling and 20.5 m3 for summer sampling) during the sampling period. The precision in sampling flow rate was higher for Clarkson samplers compared to NATTS samplers.
Results and Discussion
All of the laboratories analyzed quality assurance/quality control (QA/QC) samples before field sampling as well as during field campaign to ensure the accuracy for each method at each laboratory. All of the laboratories showed acceptable results for Cr(VI) and Cr(III) audit samples during winter sampling. During summer sampling, Clarkson laboratory and Rutgers University laboratory (for ICUV method) did not show acceptable results and thus were not included for analyses. Audit samples were prepared and distributed by an independent third party (Wibby Environmental, Golden, CO), as well as by the three laboratories.
The ratio of the measured 53Cr(VI) mass in the sample extract subtracted from the portion of 53Cr(VI) in the ambient air divided by the spiked 53Cr(VI) mass was defined as “recovery” of 53Cr(VI) when IC/ICPMS was used for analysis. Likewise, the ratio of the measured natural Cr(VI) mass in the sample extract subtracted from the portion of natural Cr(VI) in the ambient air divided by the spiked Cr(VI) standard mass was defined as “recovery” of Cr(VI) when ICUV was used for analysis. The conversion of 50Cr(III) to 50Cr(VI) was defined as the measured mass of 50Cr(VI) in the sample extract subtracted by the portion of 50Cr(VI) in the ambient air divided by the spiked 50Cr(III) mass. To estimate the mass of 50Cr(VI) and 53Cr(VI) in the ambient air, the determined mass of 52Cr(VI) using IC/ICPMS was corrected by the abundance isotopic ratio of each chromium isotope.
Campus study
Evaluation of the membrane dryer
This section examines the performance of the DE in drying the clean air, as all of the data presented in this section were obtained when the mechanical dryer was off. The pressure of the purge air strongly affects the intensity of the drying by the membrane dryer. Increasing the pressure drop of the purge air decreases the relative humidity of the clean air. Therefore, the pressure of the purge air was adjusted at the lowest possible pressure (24 inches Hg vacuum), maintained by the vacuum pump.
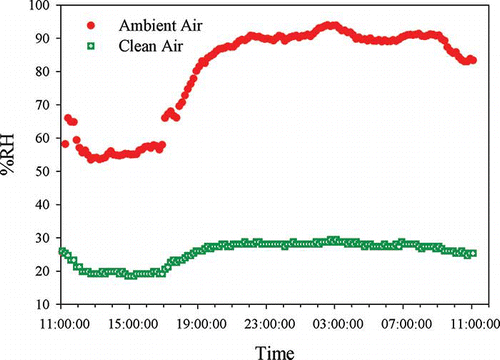
The Clarkson sampler efficiently reduced the RH of the clean air below 30% during 24-hr sampling (), even during the early morning around 3 a.m. when the ambient RH reached 94%. The average temperature for the ambient air and the clean air were 5.1 ± 1.2ºC and 5.0 ± 1.3ºC, respectively.
Figure 3. RH variations for the ambient air and the clean air during 24 hr, purge air pressure = 24 inches Hg vacuum, purge air flow rate = 0.5 LPM, and the clean air flow rate = 5 LPM.
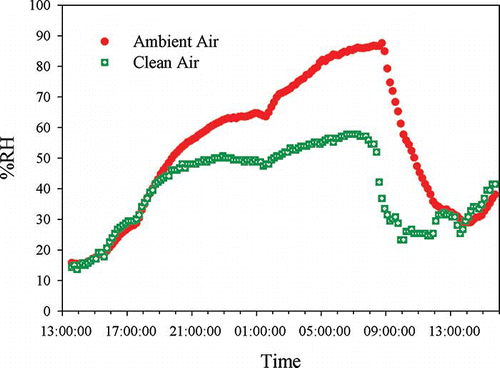
The RH of the clean air increased as its flow rate increased to 15 LPM (). During the postsampling period, the increase in the clean air flow rate decreased its residence time inside the membrane, and thus at constant ambient RH, purge airflow rate, and membrane pressure drop, the RH of the clean air increased compared to the sampling period (). The RH of the clean air was below 60% during the most humid condition when the RH of the ambient air exceeded 85%. During the post-sampling time, the sampling filter faced only the clean stream with a RH value below 76%, indicating that the Clarkson sampler will keep the filter dry during the postsampling period.
Figure 4. RH variations for the ambient air and the clean air during 24 hr, purge air pressure = 24 inches Hg vacuum, purge air flow rate = 0.5 LPM, and the clean air flow rate =15 LPM.
Evaluation of final RH
To examine the RH of ambient air after mixing with the clean air produced by the mechanical and membrane dryers, an experimental study was conducted with the Clarkson sampler during summer. In this study, the RH of the clean air, ambient air and the ambient air passing through the filter were monitored simultaneously for 24 hr (). The final RH after mixing was below the DRH of ambient particles indicating that the Clarkson sampler design works to reduce RH.
Figure 5. RH variations for the ambient air, the clean air, and the ambient air mixed with the clean air.
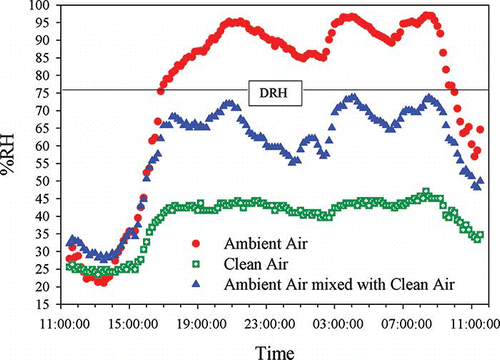
During winter sampling, the clean air was slightly heated by the heater to reduce its RH. However, heating the clean air increases the ambient temperature by 1ºC after mixing. This change in temperature is negligible and should not affect the collection of the particulate matter on the filter.
Campus sampling
The samplers showed good precision (<20%), in particular for Cr(III) conversion (). Sampling during a windy day (round 2) resulted in higher Cr(VI) recovery (over 95%) compared to the rainy day (round 3). This result is in agreement with our recent finding showing that relative humidity plays an important role in the reduction of Cr(VI) during sampling (CitationAmouei Torkmahalleh et al., 2012). During a rainy day, the average and maximum humidity values were found to be higher compared to a windy day. During round 1, the ambient RH exceeded the DRH for only 6 hr, and during round 2, the ambient RH never exceeded the DRH (data not shown). During round 3, the ambient RH exceeded the DRH of ambient particles for approximately 16 hr (data not shown). The difference in the Cr(VI) recovery between rounds 2 and 3 was determined to be statistically significant (P = 0.014).
Table 2. The 53Cr(VI) recovery and 50Cr(III) conversion determined by four Clarkson samplers (precision study), campus study
The average and maximum temperature values were lower in round 3 compared to round 2 (). Round 3 with the lower temperature that slows Cr(VI) conversion resulted in lower recovery compared to round 2. This result suggests that the role of humidity might be more important than the role of temperature for the temperature range of this study. It is assumed that the difference in collected PM mass among different sampling events is low such that the Cr conversion is not biased by this difference. The data presented by CitationSwietlik et al. (2011) show the importance of humidity in Cr(VI) to total Cr ratio in the atmosphere. For example, sampling at the same location in Radom, Poland but different days showed Cr(VI) to total Cr ratio to be 27.6 ± 15.5% (n = 3) at cold and humid atmospheric conditions (average T = 3–6ºC, average RH = 90%, PM concentration = 116.5 μg.m−3, and Cr total concentration = 14.3 ± 2.5 ng m−3) and 38.3 ± 5.7% (n = 3) for moderate temperature and humidity conditions (average T = 22–25ºC, average RH = 57%, PM concentration = 100.0 μg m−3 and Cr total concentration = 15.3 ± 2.3 ng m−3).
Cr(III) conversion was found to be consistent with higher precision independent of meteorological conditions. Oxidants such as ozone and reactive oxygen species (CitationAmouei Torkmahalleh et al., 2013; CitationHuang et al., 2013) can oxidize Cr(III) to Cr(VI).
Side-by-side comparison between NATTS and Clarkson samplers showed no statistical difference in the Cr(VI) recovery. The average Cr(VI) recovery values after 24-hr sampling were determined to be 87 ± 2 (n = 4) and 89 ± 13 (n = 4) for Clarkson and NATTS samplers, respectively. The Clarkson samplers had better precision compared to NATTS samplers. Potsdam, NY, is a clean rural area where PM mass concentrations are low providing little to react with spiked Cr(VI) during campus sampling. Therefore, it was expected that there would be no difference in the Cr(VI) recovery between the two sampling trains.
Field study
Overall, both types of the samplers showed a good precision individually, on each sampling day (Table S7). Also, the precision among all eight samplers on each sampling day was found to be 14 ± 5%, which is better than the acceptable range (<20%).
Cr(VI) concentration
The average (n = 61) Cr(VI) concentrations during the laboratory and field blank study for winter and summer sampling campaigns were below the analytical MDL (Table S6). The very low Cr(VI) concentrations suggest that no chromium contamination was introduced by the samplers and sampling filters and during the analyses. However, for some samples, IC/ICPMS analysis showed chromium background contamination that could cause the results to be biased.
The average 53Cr(VI) recovery and 50Cr(III) conversion for the laboratory blank samples (n = 8) were determined to be 102.8 ± 9.1% and 3.0 ± 2.4% . The average 53Cr(VI) recovery and 50Cr(III) conversion for the winter field blank samples (n = 4) were 113.5 ± 27.2% and 14.0 ± 15.0%, respectively, on day 1 pickup. One sample with 153% Cr(VI) recovery and 36% Cr(III) conversion among those field blank samples markedly increased the standard deviation values. The average 53Cr(VI) recovery and 50Cr(III) conversion for the winter field blank samples (n = 6) were determined to be 88.2 ± 8.7% and 8.5 ± 3.5%, respectively, on day 3 pickup. The average 53Cr(VI) recovery and 50Cr(III) conversion for the summer field blank samples (n = 2) were 77.0 ± 0.1% and 6.0 ± 0.0%, respectively, on day 1 pickup. The average 53Cr(VI) recovery and 50Cr(III) conversion for the summer field blank samples (n = 2) were found to be 67.0 ± 0.04% and 11.5 ± 0.08%, respectively, on day 3 pickup. Decreases in Cr(VI) recovery values were observed for summer and winter field blank samples when the blank filters were left in the field for 3 days compared to 1 day field blank samples. Also, spiked field blank filters showed lower Cr(VI) during summer compared to winter demonstrating the negative effect of temperature on Cr(VI) recovery.
CitationUnceta et al. (2010) stated that reduction of Cr(VI) can occur by reaction of Cr(VI) with the polymeric materials used in sampling filters such as cellulose and glass during Cr(VI) sampling. In addition, filter holders may add to Cr(VI) conversion as a results of the presence of plasticizers materials. However, the high recovery of Cr(VI) in the laboratory blank samples as well as winter field blank samples at day 1 pickup implies very low impact of cellulose filter and filter holder on Cr(VI) conversion. This observation is in agreement with findings of CitationHuang et al. (2013).
Twenty-four-hour samples showed no statistically significant differences between Cr(VI) concentrations determined by NATTS and Clarkson samplers for each campaign (). The observed ambient Cr(VI) concentration during our winter and summer sampling campaigns in Elizabeth, NJ, was much less than previous findings (). The average ambient Cr(VI) concentrations, 0.03 ± 0.01 ng m−3 (summer) and 0.02 ± 0.01 ng m−3 (winter) determined in this study are lower than the average Cr(VI) concentration of 0.044 ng m−3 determined over 22 locations in the United States (ERG, 2007). The difference in the total Cr(VI) concentrations between the current study and previous investigations could also be due to difference in analytical methods. The results of this study show statistically higher levels of Cr(VI) concentration during the summer sampling compared to the winter sampling (P = 0.0003) in Elizabeth, NJ.
Table 3. Ambient Cr(VI) concentration determined in this study, field study
Cr(VI) recovery and Cr(III) conversion
All of the data reported in this section were obtained using IC/ICPMS analysis unless otherwise stated.
(1) Cr(VI) recovery
The average Cr(VI) recovery on day 1 for Clarkson sampler during summer and winter sampling periods was found to be 57.1 ± 0.2 and 71.8 ± 4.9%, respectively, while the corresponding average values for the NATTS samplers were 46.0 ± 1.6 and 46.2 ± 10.8% for the summer and winter sampling campaigns, respectively (). The average Cr(VI) recovery on day 3 for the Clarkson sampler during summer and winter sampling were found to be 43.9 ±17.6 and 72.3 ± 4.3%, while the corresponding average values for the NATTS samplers were 32.4 ± 2.1 and 33.9 ± 7.6% for the summer and winter sampling programs, respectively. No statistically significant difference was observed between day 1 and day 3 for both samplers during the winter sampling campaign. This result could be due to the low ambient temperature during winter. During summer sampling, a statistically significant difference (P = 0.038) was observed between day 1 and day 3 Cr(VI) recovery for the both samplers. The reduction in Cr(VI) recovery between day 1 to day 3 was found to be 23 and 30% for Clarkson and NATTS samplers during summer, respectively. The statistical analyses of the results revealed that Cr(VI) recovery by Clarkson sampler was greater compared to NATTS sampler during winter sampling for both day 1 and day 3 pickup (P ≤ 0.001), while no statistical difference was observed during summer. Only two filters were sampled during summer by the Clarkson sampler, so these results may not truly reflect its performance. The results from positive control study that were obtained using ICUV analysis showed that the average Cr(VI) recovery values by Clarkson and NATTS samplers were found to be 75.5 ± 0.3 and 17.8 ± 15.8%, respectively.
Table 4. Average Cr(VI) recovery and Cr(III) conversion determined by Clarkson and NATTS samplers, field study, with all data presented as average ± standard deviation except data for Clarkson sampler during summer and positive control, which are presented as average ± variability
The improvement in Cr(VI) recovery by Clarkson samplers indicates that drying was influential in preserving Cr(VI) during sampling. Clarkson samplers keep the filters dry until the end of the postsampling period to prevent deliquescence of ambient particles, while NATTS samplers may experience deliquescence of ambient particles resulting in the conversion of Cr(VI).
(2) Cr(III) conversion
The average Cr(III) conversion on day 1 for the Clarkson sampler during the summer and winter sampling periods was determined to be 8.6 ± 0.2 and 12.7 ± 2.1%, respectively, while the corresponding average values for NATTS samplers were 5.1 ± 0.8 and 8.4 ± 5.9% for the summer and winter sampling, respectively. However, only duplicate samples were taken for Clarkson samplers during the summer campaign to determine the Cr(III) conversion on day 1.
The average Cr(III) conversion by day 3 for the Clarkson sampler during summer and winter sampling campaigns was found to be 10.6 ± 2.0 and 9.2 ± 2.0%, respectively, while the corresponding average values for NATTS samplers were 9.8 ± 1.4 and 2.8 ± 1.8%, respectively. Statistically significant differences were observed between day 1 and day 3 for the two sampler types during winter sampling (P = 0.008). The observed lower Cr(III) conversion by day 3 compared to day 1 during winter sampling could be due to reconversion of 50Cr(VI) during the postsampling period. The Cr(III) conversion during summer at day 3 was statistically higher compared to day 1 for both samplers (P = 0.004). The statistical analyses of the results of both sampling seasons showed statistically significant differences between the two samplers (P = 0.017 for summer and P = 0.003 for winter). The observed conversion of Cr(III) to Cr(VI) during field sampling could be the results of the either of following cases:
| 1. | Limited deliquescence of soluble Cr(III) salts such as Cr(NO3)3 that deliquesce at 52% (CitationAmouei Torkmahalleh et al., 2012), as the Clarkson samplers keeps the ambient RH below 76% and not necessarily below 52%. | ||||
| 2. | Solid–gas and solid–liquid reactions of soluble Cr(III) with potential oxidants in the atmosphere (CitationAmouei Torkmahalleh et al., 2013) available during field sampling that result in the formation of soluble Cr(VI). | ||||
This study investigated the concentration and conversion of soluble Cr(VI) since the methods for the filter extraction and conversion monitoring are applicable only for soluble Cr(V). CitationHuang et al. (2013) showed that insoluble Cr(VI) constitutes the majority of the total ambient Cr(VI). No conclusions can be obtained regarding the concentration and conversion of insoluble Cr(VI) during sampling in the present study.
CitationSee and Balasubramanian (2008) found insoluble Cr in indoor PM. Chromium speciation in indoor environments would be an interesting topic for future study. In particular, outdoor origin particles in indoor environment could be important sources of Cr(VI) since they account for a major part of indoor particles.
The availability of the soluble and insoluble Cr(III) in the ambient PM samples depends on the source of the chromium. However, there are studies that showed that insoluble Cr(III) dominates the ambient Cr(III) in PM. A field test conducted by CitationHuang et al. (2013) showed that soluble Cr(III) in ambient PM of the sampling locations was negligible, and thus the Cr(III) conversion is expected to be insignificant for these soluble Cr species. This conclusion was also reached by CitationTirez et al. (2011) and Werner et al. (2007). Insoluble Cr(III) was reported to be inert under the temperature and pH of the filter extraction process in this study (CitationHuang et al., 2013). Thus, the observed conversion of Cr(III) during the field study that was obtained from the conversion of soluble spiked Cr(III) does not reflect the conversion of Cr(III) in ambient PM during sampling. This conclusion suggests that the conversion of ambient Cr(III), which is one of the major challenges for the measurement of Cr(VI) using the current filter extraction procedure, may be a concern only if the insoluble Cr(III) such as Cr(OH)3(s) converts to soluble Cr(VI) such as CrO4 −2 (aq) in the presence of gaseous and particle oxidants.
Conclusion
Drying the filter during sampling of Cr(VI) below DRH of ambient particles is a key to improving the sampling of Cr(VI). Drying slows the chemistry of chromium by preventing the system from forming an aqueous phase through deliquescence. The Clarkson Cr(VI) samplers were designed and constructed based on this principle. Even though only a small amount of samples was collected, the Clarkson Cr(VI) samplers showed better Cr(VI) recovery compared to the current NATTS samplers during sampling at Elizabeth, NJ. Because of the low chromium concentrations found during the field sampling in New Jersey, a study performed where Cr(VI) concentrations may be higher would allow a better evaluation of Clarkson samplers. However, based on the limited results presented here, it appears likely that the current NATTS sampling system is systematically underestimating the ambient concentrations of Cr(VI) in ambient PM. The extent of this underestimation depends on the fraction of soluble Cr(VI) as well as on the handling practices for the collection of the samples from the samplers following the sampling period. There is thus a need for serious consideration of upgrading of the current samplers to incorporate the drying and cooling that were included in the Clarkson samplers to provide better sampling and preservation of the collected PM to produce more accurate estimates of the population exposure to Cr(VI).
Supplementary Materials
Download PDF (761.8 KB)Acknowledgment
This work has been supported by the U.S. Environmental Protection Agency under grant XA-97247301. Although the research described in this work has been partly funded by the EPA, it has not been subjected to the agency's required peer and policy review and therefore does not necessarily reflect the views of the agency and no official endorsement should be inferred. The views expressed herein are also not necessarily those of NJ Department of Environmental Protection. Dr. Fan is supported in part by the NIEHS sponsored Rutgers University Center for Environmental Exposures and Disease, Grant # NIEHS P30ES005022.
References
- Amouei Torkmahalleh , M. , Lin , L. , Holsen , T.M. , Rasmussen , D.H. and Hopke , P.K. 2012 . The impact of deliquescence and pH on chromium speciation in ambient PM . Aerosol Sci. Technol. , 46 ( 6 ) : 690 – 96 . doi: 10.1080/02786826.2011.654285
- Amouei Torkmahalleh , M. , Lin , L. , Holsen , T.M. , Rasmussen , D.H. and Hopke , P.K. 2013 . Cr speciation changes in the presence of ozone and reactive oxygen species at low relative humidity . Atmos. Environ. , 71 : 92 – 94 . doi: 10.1016/j.atmosenv.2013.02.005
- Barceloux , D.G. 1999 . Chromium . Journal of Toxicol. Clin. Toxicol. , 37 : 173 – 94 . doi: 10.1081/CLT-100102418
- Bell , R.W. and Hipfner , J.C. 1997 . Airborne hexavalent chromium in southwestern Ontario . J. Air Waste Manage. Assoc. , 47 : 905 – 10 . doi: 10.1080/10473289.1997.10464454
- Bem , H. , Gallorini , M. , Rizzio , E. and Krzeminska , M. 2003 . Comparative studies on the concentrations of some elements in the urban air particulate matter in Lodz City of Poland and Milan, Italy . Environ Int. , 29 : 423 – 28 . doi: 10.1016/S0160-4120(02)00190-3
- Borai , E.H. , El-Sofany , E.A. and Abdel-Halim , A.S. 2002 . Speciation of hexavalent chromium in atmospheric particulate samples by selective extraction and ion chromatographic determination . Trends Anal. Chem. , 21 : 741 – 45 . doi: 10.1016/S0165-9936(02) 01102-0
- Eastern Research Group . 2007 . Collection and Analysis of Hexavalent Chromium in Ambient Air , Morrisville , NC : Eastern Research Group, Inc .
- Fernández , A.J. , Ternero , M. , Barragán , F.J. and Jiménez , J.C. 2000 . An approach to characterization of sources of urban airborne particles through heavy metal speciation . Chemosphere Global Change Sci. , 2 : 123 – 36 . doi: 10.1016/S1465-9972(00)00002-7
- Grohse , P. M. , Hodson , W.F.L. and Wilson , B. M. 1988 . The Fate of Hexavalent Chromium in the Atmosphere. California Air Resources Board Sacramento, CARB contract no. A6-096-32 , Research Traingle , NC : Research Triangle Institute .
- Hagendorfer , H. and Uhl , M. 2007 . Speciation of Chromium in Particulate Matter (PM10): Development of a Routine Procedure, Impact of Transport and Toxicological Relevance , Vienna , , Austria : Umwelbundesamt, REP-0111 .
- Huang , L. , Fan , X. , Yu , C.-H. , Hopke , P.K. , Lioy , P. , Buckley , B. , Lin , L. and Ma , Y. 2013 . Inter-conversion of chromium species during air sampling: Effects of O3, NO2, SO2, particle matrices, temperature and humidity . Environ. Sci. Technol. , 47 : 4408 – 15 . doi: 10.1021/es3046247
- Independent Environmental Technical Group . 2005 . Chromium (VI) Handbook , Edited by: Guertin , J. , Jacobs , J.A. and Avakian , C.P. Boca Raton , FL : CRC Press .
- Khlystov , A. and Ma , Y. 2006 . An on-line instrument for mobile measurements of the spatial variability of hexavalent and trivalent chromium in urban air . Atmos. Environ. , 40 : 8088 – 93 . doi: 10.1016/j.atmosenv.2006.09.030
- Krystek , P. and Ritsema , R. 2007 . Monitoring of chromium species and 11 selected metals in emission and immission of airborne environment . Int. J. Mass Spectrom. , 265 : 23 – 29 . doi: 10.1016/j.ijms.2007.05.003
- Li , Y. , Pradhan , N.K. , Foley , R. and Low , G.K.C. 2002 . Selective determination of airborne hexavalent chromium using inductively coupled plasma mass spectrometry . Talanta. , 57 : 1143 – 53 . doi: 10.1016/S0039-9140(02)00196-0
- Mandiwana , K.L. , Panichev , N. and Resane , T. 2006 . Electrothermal atomic absorption spectrometric determination of total and hexavalent chromium in atmospheric aerosols . Hazard Mater. , B136 : 379 – 82 . doi: 10.1016/j.jhazmat.2005.12.030
- Meng , Q. , Fan , Z. , Buckley , B. , Lin , L. , Huang , L. , Yu , C. , Stiles , H. and Bonanno , R.L. 2011 . Development and evaluation of a method for hexavalent chromium in ambient air using IC ICP-MS . Atmos. Environ. , 45 : 2021 – 27 . doi: 10.1016/j.atmosenv.2011.02.009
- Metze , D. , Herzog , H. , Gosciniak , B. , Gladtke , D. and Jakubowski , N. 2004 . Determination of Cr(VI) in ambient airborne particulate matter by a species-preserving scrubber-sampling technique . Anal. Bioanal. Chem. , 378 : 123 – 28 . doi: 10.1007/s00216-003-2250-1
- Nico , P.S. and Zasoski , R.J. 2000 . Importance of Mn(II) availability of Cr(III) oxidation on birnessite . Environ. Sci. Tecnol. , 38 : 5253 – 60 . doi: 10.1080/01490450590945988
- Nowak , B. and Kwapulinski , J. 1991 . Occurrence of selected metals in dust suspended around the “Qagisza” power plant . Ochrona Powietrza i Problemy Odpadów , 21 : 38 – 42 . in Polish
- Park , R.M. , Bena , J.F. , Stayner , L.T. , Smith , R.J. , Gibb , H.J. and Lees , P.S. 2004 . Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment . Risk Anal. , 24 : 1099 – 108 . doi: 10.1111/j.0272-4332.2004.00512.x
- Nusko , R. and Heumann , K.G. 1997 . Cr(III)/Cr(VI) speciation in aerosol particles by extractive separation and thermal ionization isotope dilution mass spectrometry . Fresenius J. Anal. Chem. , 357 : 1050 – 55 . doi: 10.1007/s002160050303
- Puxbaum , H. 1991 . “ Metal compounds in the atmosphere ” . In Metals and Their Compounds in the Environment , Edited by: Merian , E. 257 – 86 . Weinheim , , Germany : VCH .
- Richter , P. , Grino , P. , Ahumada , I. and Giordano , A. 2007 . Total element concentration and chemical fractionation in airborne particulate matter from Santiago, Chile . Atmos. Environ. , 41 : 6729 – 38 . doi: 10.1016/j.atmosenv.2007.04.053
- See , S.W. and Balasubramanian , R. 2008 . Chemical characteristics of fine particles emitted from different gas cooking methods . Atmos. Environ. , 42 : 8852 – 62 . doi: 10.1016/j.atmosenv.2008.09.011
- Seigneur , C. and Constantino , E. 1995 . Chemical kinetic mechanism for atmospheric chromium . Environ. Sci. Technol. , 29 : 222 – 31 . doi: 10.1021/es00001a029
- Swietlik , R. , Molik , A. , Molenda , M. , Trojanowska , M. and Siwiec , J. 2011 . Chromium(III/VI) speciation in urban aerosol . Atmos. Environ. , 44 : 1364 – 68 . doi: 10.1016/j.atmosenv.2010.12.001
- Talebi , S.M. 2003 . Determination of total and hexavalent chromium concentrations in the atmosphere of the city of Isfahan . Environ. Res. , 92 : 54 – 56 . doi: 10.1016/S0013-9351(02)00036-1
- Tirez , K. , Silversmit , G. , Bleux , N. , Adriaensens , E. , Roekens , E. , Servaes , K. , Vanhoof , C. , Vincze , L. and Berghmans , P. 2011 . Determination of hexavalent chromium in ambient air: A story of method induced Cr(III) oxidation . Atmos. Environ. , 45 : 5332 – 41 . doi: 10.1016/j.atmosenv.2011.06.043
- Yang , M. 2007 . Sampling and Analysis of Hexavalent Chromium in Airborne Ambient Particular Matter , Potsdam , NY : Master's thesis, Clarkson University .
- Unceta , N. , Seby , F. , Malherbe , J. and Donard , O.F.X. 2010 . Chromium speciation in solid matrices and regulation: A review . Anal. Bioanal. Chem. , 397 : 1097 – 111 . doi: 10.1007/s00216-009-3417-1
- Utsunomiya , S. , Jensen , K.A. , Keeler , G.J. and Ewing , R.C. 2004 . Direct identification of trace metals in fine and ultrafine particles in the Detroit urban atmosphere . Environ. Sci. Technol. , 38 ( 8 ) : 2289 – 97 . doi: 10.1021/es035010p
- Zereini , F. , Alt , F. , Messerschmidt , J. , Wiseman , C. , Feldmann , I. , Bohlen , A. , üller , J. M , Liebl , K. and Püttmann , W. 2005 . Concentration and distribution of heavy metals in urban airborne particulate matter in Frankfurt am Main, Germany . Envir. Sci. Technol. , 39 : 2983 – 89 . doi: 10.1021/es040040t