Abstract
Incineration is a traditional method of treating sewage sludge and the disposal of derived ash is a problem of secondary waste treatment. In this study, sewage sludge ash (SSA) was coated with ferrite through a ferrite process and then used as an adsorbent for ionic dyes (methylene blue [MB] and Procion Red MX-5B [PR]). The modified SSA possessed surface potential that provided electrostatic attraction toward MB and PR. Adsorbent FA10 (named on the basis of being produced from 10 g of SSA in the ferrite process) was used for the adsorption of MB. Ideal pH for adsorption was 9.0 and maximum adsorption capacity based on Langmuir isotherm equation was 22.03 mg/g. Adsorbent FA2.5 (named on the basis of being produced from 2.5 g of SSA in the ferrite process) was used for PR adsorption. Ideal pH for adsorption was 3.0 and the maximum adsorption capacity (calculated as above) was 28.82 mg/g. Kinetic results reveal that both MB and PR adsorption fit the pseudo-second-order kinetic model better than the pseudo-first-order model. The values of activation energy calculated from rate constants were 61.71 and 9.07 kJ/mol for MB and PR, respectively.
Implications:
Magnetic modified adsorbent could be synthesized from sewage sludge ash (SSA). In this study, the adsorption ability of SSA toward ionic dye (methylene blue [MB] and Procion Red MX-5B [PR]) was enhanced by ferrite process. The synthesized Fe3O4 can act as an active site and provide electrostatic attraction toward cationic dye and anionic dye at different pH. The application of magnetic modified adsorbent in wastewater treatment can not only recycle the SSA, but also make SSA become an environmentally friendly material.
Introduction
Incineration is a traditional method of sewage sludge disposal. It possesses several advantages, including the reduction of sludge volume, thermal destruction of toxic organic constituents, and energy recovery during sludge combustion (CitationWerther and Ogada, 1999; CitationWang et al., 2012). The major solid product of sewage sludge incineration is sewage sludge ash (SSA). Although SSA is an inorganic and stable material, it still needs final disposal. Therefore, SSA recycling techniques have been developed. One use of incinerated ash has been as a base material for the production of lightweight aggregates used in construction (CitationCheeseman and Virdi, 2005; CitationHu et al., 2012). Other uses include its use as the raw material for cement (CitationLin and Lin, 2005; CitationGarcés et al., 2008), bricks (CitationAnderson, 2002; CitationAnderson et al., 2002), and tiles (CitationChen and Lin, 2009; CitationKikuchi, 1998). On the other hand, SSA can be recycled as fertilizer to increase the nutrient level of agricultural soils (CitationWeigand et al., 2013; Franz, 2008).
Besides the above applications, SSA can be utilized as an adsorbent in wastewater treatment. It is a win-win strategy in that it uses SSA as an inexpensive, environmentally friendly material for turning waste into useful material (CitationMoghaddam et al., 2009). The adsorbates of SSA adsorbent could be heavy metal ions or organic dyes. CitationLiu et al. (2011a, Citation2011b) and Elouear et al. (2009) utilized sewage sludge incineration slag as an adsorbent for removing Cu, Ni, or Cd from wastewater; the adsorption pH was either weak acid or neutral. CitationWeng (2002) studied the adsorption characteristics of an anionic dye (New Coccine) by SSA. The experimental data correlated well with a nonlinear multilayer adsorption isotherm. The adsorption capacities ranged between 1.96 and 3.45 mg/g, with pH being the key factor affecting adsorption. Dye adsorption density increased abruptly below pH 5.0; this phenomenon was attributed to SSA having a positively charged surface when the solution pH was less than the pH zero point of charge (pHzpc). Below this pH, the surface acidity of the hydrolyzed ash and its positively charged surface provided favorable electrostatic conditions for the single anionic dye species to be adsorbed. CitationMoghaddam et al. (2012) studied the adsorption of Acid Red 119 by mixtures of dried sewage sludge (DSS) and SSA. Results revealed that a DSS mixture of 40 wt%, 5 g/L of adsorbent dose, 200 mg/L of initial dye concentration, initial pH 6, and 60 min of contact time were the optimal experimental conditions for giving a removal efficiency of higher than 95%.
The above studies support the potential of SSA as a dye adsorbent; however, there has not been abundant research into its adsorption application. According to other reports (Weng and Pan, 2006; CitationWeng, 2002), the adsorption capacity of SSA is 1.6 mg/g for MB and 1.96–3.45 mg/g for New Coccine dye. This study examines a process by which these results can be improved. A ferrite synthesis process is proposed and used to produce crystalline Fe3O4 on the surface of SSA particles. Ferrite is a spinel structure that crystallizes in a cubic close-packed arrangement (CitationMathew and Juang, 2007). The chemical mechanism for the formation of Fe3O4 is listed in Equationeq 1 (CitationZhu et al., 2007):
The surface potential of Fe3O4 can change with pH adjustment (CitationBarale et al., 2008); it acts as an adsorption site for cationic or anionic dye in aqueous solution. Furthermore, the magnetic adsorbent can be easily separated by magnetic field, which provides a more convenient recovery method than conventional separation techniques. The adsorbates of this experiment were methylene blue (MB) and Procion Red MX-5B (PR). Langmuir isotherm and Freundlich isotherm are adopted to evaluate the adsorption isotherm, and adsorption kinetics are studied using pseudo-first-order and pseudo-second-order kinetic models. Finally, the activation energy is obtained from the kinetic rate constant and Arrhenius equation.
Materials and Methods
Characteristics analysis of sewage sludge
The sewage sludge from a wastewater treatment plant in northern Taiwan was incinerated at 900 °C for 1 hr and the residue was named as sewage sludge ash (SSA). The SSA was ground and sieved with a 0.59-mm sieve before adsorption experiments. Chemical analysis, X-ray diffraction (XRD) analysis, and ignition-loss testing were utilized to analyze the characteristics of SSA. For chemical analysis, 0.1 g SSA powder was mixed with 16 mL of aqua regia and 8 mL of hydrofluoric acid at 180 °C for 3 hr. After cooling and filtration, the filtrate was diluted and the concentrations of Si, Al, Fe, Na, K, Ca, and Mg were analyzed by atomic absorption spectroscopy (Varian AA240; CA, USA). X-ray diffraction (XRD) analysis was conducted with an X-ray diffractometer (Rigaku D/max-2500, Tokyo, Japan). It was scanned from 10° to 80° (2θ) at a scan rate of 4° per minute with voltage of 30 KV and current of 50 mA. For ignition-loss testing, 5 g (W 1) of SSA were heated in the furnace at 800 °C 3 hr. The heating rate was fixed at 15 °C per minute. The weight (W 2) of the residue was recorded while the sample was cooled. The results of ignition loss were calculated according to Equationeq 2:
Synthesis and characteristics of modified magnetic adsorbent
Different dosages (0, 2.5, 5, 10 g) of SSA were mixed with 250 mL of solution containing 0.2 M FeSO4 (FeSO4·7H2O, 99%; Panreac, xx, xx) and 0.2 M FeCl3 (FeCl3·6H2O, 99%; Panreac). The mixture was agitated and its pH adjusted to 11 with 10 N NaOH. Then, the mixture was heated to 90 °C for 2 hr. After the ferrite process, the mixture was cooled, filtrated, washed with deionized (DI) water twice, dried, and ground to powder. The magnetic adsorbents produced from 0, 2.5, 5, and 10 g of SSA were named FA0, FA2.5, FA5, and FA10.
XRD, magnetic properties, specific surface area, and zeta potential analyses were conducted to understand the characteristics of adsorbents. The XRD analysis procedure was the same as that for SSA analysis. Magnetization testing was conducted at room temperature in a vibrating sample magnetometer (MPMS SQUID VSM; Quantum Design Inc, CA, USA). The magnetic field was set from 20,000 to −20,000 Oe. The specific surface area was measured from N2 adsorption isotherms of 77 K with adsorption apparatus (Autosorb-1; Quantachrome, FL, USA) and calculated with the Brunauer-Emmett-Teller (BET) equation. Zeta potential of the magnetic adsorbents was determined by a zeta potential analyzer (Nano Z; Malvern, Worcs, UK), and 0.1 g of adsorbent was mixed with 1000 mL of 0.001 M NaCl solution. The mixture was dispersed in an ultrasonic cleaner for 30 min and 1 N HCl or NaOH solution was used to adjust the pH within a range of 2–10 for measurement.
The effect of pH on dye adsorption
MB and PR were chosen as adsorbates, and the adsorption of dyes was studied in batch operation mode and the concentration of residual dye in solution was determined by ultraviolet/visible (UV/Vis) spectrophotometry (Lambda 25; PerkinElmer, Taiwan, China) at wavelengths of 664 nm for MB and 538 nm for PR. SSA and FA5 were chosen as adsorbents and the ratio of adsorbent versus dye solution was fixed at 0.5 g/50 mL. The initial concentration of dye solution was 100 mg/L. Adsorbents and dye solution were added to the Erlenmeyer flask, which was placed into the water bath equipment (BT-150D; Yihder, Taiwan, China) and the shake speed was fixed at 100 rpm. NaOH (1 N) and HCl (1 N) was used to adjust the pH value of the dye solution, and adsorption experiments were conducted from pH 2 to 10 at 30 °C for 6 hr. After the adsorption experiments, the solutions were filtered with Advantec 5C filter paper (Tokyo, Japan) by gravitational filtration and the concentrations of MB and PR determined with UV-Vis spectrophotometry. The adsorption percentage was obtained from Equationeq 3:
Isotherm adsorption
The equilibrium time of adsorption was determined before isotherm adsorption testing. The experimental conditions were 200 mg/L of initial dye concentration, 0.5 g/50 mL of adsorbent versus dye solution at 30 °C for 0.5–16 hr. The pH value for MB adsorption was 9.0 and that for PR was 3.0. Isotherm adsorption experiments were conducted for different initial concentrations (100, 200, 400, 600, and 800 mg/L) of MB and PR. The ratio of adsorbent versus dye solution and adsorption temperature were as above. Adsorption duration was 4 hr. After adsorption equilibrium of 4 hr, the experimental results were examined using Langmuir ( Equationeq 4) and Freundlich ( Equationeq 5) isotherm models, as follows (CitationGobi et al., 2011):
Dimensionless separation factor, R L, was calculated from the Langmuir isotherm equation to determine the type of adsorption process (CitationTseng, 2007):
Adsorption kinetics
The results of the kinetic study were obtained for different temperatures (30, 45, and 60 °C) and durations (20, 40, 60, and 80 min). Other experimental conditions were the same as adsorption isotherm testing and the initial concentration of dye was 400 mg/L. The results were analyzed with two models, namely, the pseudo-first-order ( Equationeq 7) and pseudo-second-order kinetic models ( Equationeq 8) (CitationLin et al., 2011):
The temperature dependence of rate constants can be described by the Arrhenius equation:
Desorption experiment
Desorption experiments were conducted to illustrate the adsorption process. 0.1 N of HCl and NaOH were used as desorption reagent for FA10 and FA2.5, respectively. The adsorption experiments were conducted using 200 mg/L of initial dye concentration, 0.5 g/50 mL of adsorbent dosage, pH 3 for PR, pH 9 for MB, and a shake speed of 100 rpm at 30 °C for 4 hr. After adsorption, the dye-loaded adsorbent was dried and mixed with 0.1 N HCl or NaOH solution and the desorption conditions were 0.5 g/50 mL of adsorbent and 100 rpm shake speed at 30 °C for 10 min to 4 hr. After desorption, the concentrations of MB and PR in the filtrate were determined with UV-Vis spectrophotometry. The desorption efficiency (DE) was calculated from Equationeq 10:
Results and Discussion
Characteristics analysis of sewage sludge ash
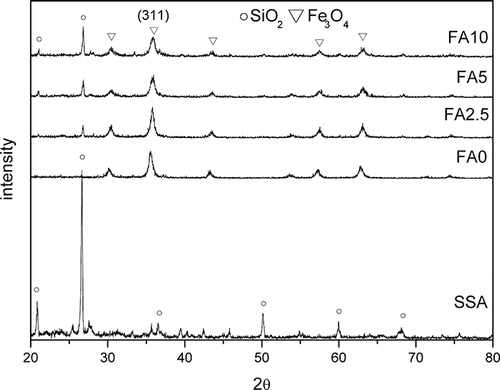
SSA is a residue of sewage sludge incineration. Its major components were inorganic compounds. The results of chemical analysis and ignition-loss testing are shown in . The value of ignition loss was 0.1%, which indicates organic compounds having been burnt off during initial incineration. SiO2 constituted 44.6% of SSA. XRD analysis is shown in . It shows that the major crystal phase of SSA was SiO2, which corresponds to the results of chemical analysis.
Table 1. Results of chemical analysis and ignition loss of SSA
Formation and characteristics of modified magnetic adsorbent
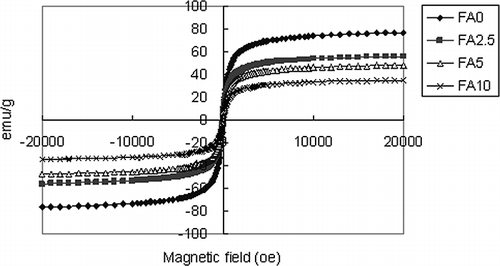
Modified SSA was synthesized in the ferrite process. The ferric and ferrous ions were reacted at pH 11 for 2 hr at 90 °C. Fe3O4 coated the surface of SSA particulates. XRD analysis of magnetically modified SSA is shown in . It is obvious from the diffraction patterns that Fe3O4 and SiO2 are the major phases of these samples. Magnetization of magnetically modified adsorbent is shown in . The results show that the value of saturated magnetizations decreased with the increased SSA dosage. The magnetization values of FA0, FA2.5, FA5, and FA10 were 73.7, 53.6, 45.8, and 33.0 electromagnetic units (EMU)/g, respectively. It is thought that the higher SSA dosages led to lower relative coverage by Fe3O4 on the surface of the adsorbent, leading to a decrease in saturated magnetization.
Effect of pH on dye adsorption
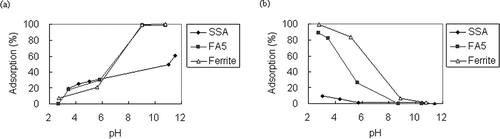
pH is an important factor in ionic dye adsorption because the surface charge of adsorbents varies with pH value. The adsorption results of SSA, FA5, and ferrite from pH 2 to pH 12 are shown in and b.The experimental conditions were 100 mg/L of initial dye concentration and 0.5 g/50 mL of adsorbent at 30 °C for 6 hr. For MB, adsorption percentages by SSA and FA5 increased with increased pH. CitationPan et al. (2003) studied copper ion adsorption by SSA and mentioned that silicon or aluminum oxide may be hydrolyzed. The chemical reaction listed below may occur under different pH:
Figure 3. Adsorption of ionic dyes at different pH values for different adsorbents: (a) MB and (b) PR. (Initial dye concentration: 100 mg/L.)
Effect of SSA dosage on dye adsorption
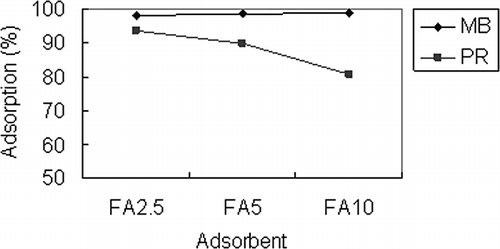
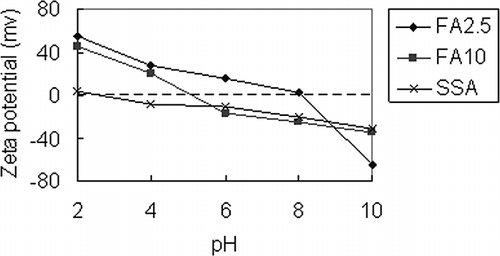
Fe3O4 acts as an active site for dye adsorption; therefore, SSA dosing may affect the adsorption ability of the adsorbent because it affects Fe3O4 content. The adsorption abilities of FA2.5, FA5, and FA10 toward MB and PR are shown in . The experimental conditions were 100 mg/L, initial dye concentration, and 0.5 g/50 mL adsorbent at 30 °C for 6 hr. The pH value of MB adsorption was 9.0 and that of PR was 3.0. For MB, adsorption percentages were close among the adsorbents (98.2% for FA2.5, 98.6% for FA5, and 99.0% for FA10). For PR, adsorption percentage increased with decreased SSA dosing. The respective adsorption percentages were 93.7%, 89.8%, and 80.6% for FA2.5, FA5, and FA10, respectively. Due to electrostatic attraction being an important driving force for ionic dye adsorption, the zeta potentials for FA2.5 and FA10 from pH 2 to pH 10 are shown in .The zeta potentials of FA2.5 and FA10 are negative at pH 9 and the adsorption percentages of MB for the two adsorbents are higher than 98%. Under acidic conditions, the zeta potential of FA2.5 and FA10 are positive. The value for FA2.5 is about +40 mV and that for FA10 is about +30 mV at pH 3. It is thought that FA2.5 can provide higher electrostatic attraction toward PR and obtain a higher adsorption percentage than FA10. According to , the value of the zeta potential decreased with increased SSA dosing under acidic conditions. Therefore, the adsorbent's PR adsorption ability is enhanced by increasing Fe3O4 content (or decreasing SSA content). As a result, FA2.5 and FA10 were chosen as adsorbents for PR and MB, respectively, as they provided the highest adsorption abilities (FA2.5 adsorbed 93.7% of PR and FA10 adsorbed 99.0% of MB). These two adsorbents were studied for their adsorption isotherm and kinetics.
Isotherm adsorption
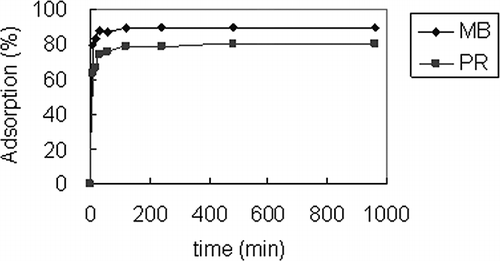
FA2.5 is chosen as the PR adsorbent and FA10 for MB. The specific surface areas calculated from BET are 61.55 and 44.94 m2/g, respectively. The adsorption percentages at different time are shown in .The experiments were conducted at 30 °C, 0.5 g/50 mL of adsorbent, and 200 mg/L of initial dye concentration. The pH of MB adsorption was controlled at 9.0 and that of PR at 3.0. According to , it took more than 2 hr to reach adsorption equilibrium. Therefore, the equilibrium time for both MB and PR was chosen to be 4 hr. This time was adopted for isotherm adsorption.
The results of isotherm adsorption are shown in and . Based on Equationeqs 5 and Equation6, the values of Q m and K L in are derived from the slope and intercept of the linear plots of C e/Q e versus C e, whereas 1/n and K F are from the slope and intercept of the linear plots of log Q e versus log C e. The r 2 value of the Langmuir equation is higher than that of Freundlich, which implies the adsorption of MB and PR suitably fit the Langmuir isothermal equation. The dimensionless separation factor R L of MB and PR in this study is between 0 and 1 when C i was between 100 and 800 ppm. The R L value indicates that the adsorption types for MB and PR for FA10 and FA2.5 are favorable.
Figure 7. Results of isotherm adsorption. (a) Fitting of Langmuir equation; (b) fitting of Freundlich equation.
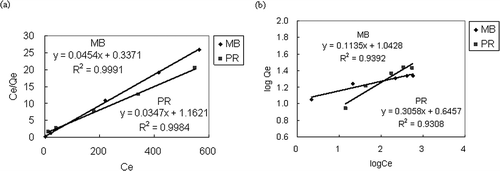
Table 2. Parameters of adsorption isotherm model fitting for MB and PR
Adsorption kinetics
Adsorption kinetics can be analyzed using pseudo-first-order and pseudo-second-order kinetic models. The equations for pseudo-first-order and pseudo-second-order kinetics are given by Equationeqs 7 and Equation8, respectively. The experimental conditions were 30, 45, and 60 °C for adsorption temperature, 400 mg/L for initial dye concentration, and 0.5g/50 mL for adsorbent concentration. The pH value for MB adsorption was 9.0 and that of PR was 3.0. The results are shown in and the r 2 values of pseudo-first-order and pseudo-second-order models are both higher than 95%. Besides the r 2 value, the applicability of both kinetic models can be verified through the sum of error squares, SSE(%) (CitationHameed et al., 2007; CitationAnirudhan and Tharun, 2012). SSE(%) is given by
Table 3. Results of kinetic model fitting for MB and PR
Activation energy E a can be obtained by the slope of plotting ln k 2 against 1/T in Arrhenius equation and the values of E a for MB and PR adsorption are 61.71 and 9.07 kJ/mol. The magnitude of E a can be considered a parameter for the differentiation between chemical or physical adsorption (CitationHan et al., 2009). The E a value of physical adsorption is usually less than 4.2 kJ/mol, because the forces involved in physical adsorption are weak. Chemical adsorption involves forces much stronger than those in physical adsorption. The E a of activated chemical adsorption is between 8.4 and 83.7 kJ/mol but that of nonactivated chemical adsorption is near zero (CitationAksu, 2002). In this study, the E a values of PR and MB adsorption range between 8.4 and 83.7 kJ/mol, indicating that the adsorption of PR and MB on magnetically modified SSA adsorbent is possibly a process of chemical adsorption.
Other adsorption studies on MB and PR were gathered to compare the data of isotherm adsorption and kinetic data (see ). The adsorption capacity of FA10 is higher than some inorganic adsorbents but lower than sewage sludge and bamboo-based activated carbon due to diverse force or other interaction between organic functional group. On the other hand, small differences in adsorption capacity are observed between FA2.5 and other adsorbents. In terms of kinetic modeling, most dye adsorption best fits the pseudo-second-order model, with only PR adsorbed on Bokbunja wine industry waste (BWIW) fitting pseudo-first-order kinetics well.
Table 4. Comparison of MB and PR adsorption on different adsorbents
Desorption experiment
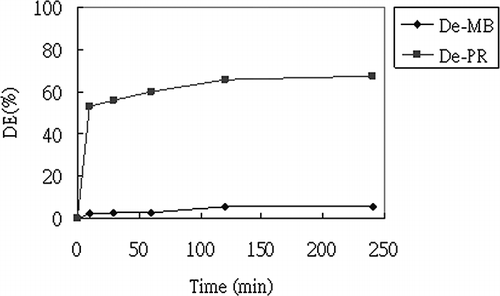
Desorption results are shown in . It is found that the DE value of PR is higher than MB (67.7% for PR and 6.0% for MB at 4 hr). The results correspond to the E a value calculated from kinetic modeling whereby the higher E a of MB adsorption (61.71 kJ/mol) reveals strong interaction between MB and FA10. Besides this, it was observed that the color of the solution changed to purple during MB desorption. After UV-Vis spectral scanning from 300 to 900 nm, it was found that the maximum adsorption wavelength changed from 663 to 598 nm. The color change implies a new compound being produced during desorption. It is thought that desorption occurred at a different position to the adsorption site, resulting in the desorbed compound having a different chemical structure, and, therefore, an altered maximum adsorption wavelength. This variation between the desorption site and adsorption site reveals that the interaction between MB and FA10 is strong and adsorption can be classified as chemical adsorption. On the other hand, the high value of DE (%) for PR desorption implies that the interaction between PR and FA2.5 is not strong and this weak interaction may be seen by the lower E a value.
Conclusion
SSA was modified by ferrite process. The synthetic Fe3O4 crystal on SSA not only provides electrostatic attraction toward ionic dyes, but also acts as active adsorption sites, leading to increased adsorption ability. Furthermore, magnetized modified SSA can be recovered using a magnetic field, which provides a more convenient recovery method than conventional separation techniques. In this study, Fe3O4 was synthesized on SSA particle by mixing SSA with a solution containing ferric and ferrous ions, adjusting the pH to 11 and reacting the mixture at 90 °C for 2 hr. Through the Fe3O4 process, magnetically modified SSA was produced. The products were termed FA2.5, FA5, and FA10 according to their respective dosing with SSA (i.e., 2.5, 5.0, and 10.0 g, respectively). The adsorption ability of modified SSA toward MB and PR was based on electrostatic attraction. Adsorbents had negative surface charge under alkaline condition, allowing them to adsorb a cationic dye (MB). On the other hand, a positive surface charge existed on the surface of adsorbents under acid conditions, allowing for anionic dye (PR) adsorption. SSA not subject to the ferrite process had weak adsorption ability, with the adsorption percentage for MB and PR being lower than 65% and 10%, respectively (100 mg/L of initial dye concentration). By comparison, the adsorption ability among FA2.5, FA5, and FA10 showed that FA10 had a very high adsorption ability toward MB (99% of adsorption percentage) at pH 9.0. For PR adsorption at pH 3.0, FA2.5 had the highest adsorption percentage (93.7%). As a result, FA2.5 and FA10 were chosen as PR and MB adsorbents and were utilized in the study of adsorption isotherms and kinetic models. For adsorption isotherms, the experimental data indicate that MB and PR adsorption best fit the Langmuir isotherm, with the adsorption capacities for MB and PR being 22.03 and 28.82 mg/g, respectively. The kinetic study revealed that MB and PR adsorption fit well the pseudo-second-order kinetic model, and the respective values of activation energy calculated from rate constants were 61.71 and 9.07 kJ/mol for MB and PR. According to the activation energy, it is thought that MB and PR adsorption can be described as chemical adsorption.
Funding
The authors would like to thank the National Science Council of the Republic of China for financially supporting this research under contract no. NSC 99-2221-E-122 -001-MY2 and NSC 102-2221-E-122-001.
References
- Aksu , Z. 2002 . Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto . Chlorella vularis. Process Biochem , 38 : 89 – 99 . doi: 10.1016/S0032-9592(02)00051-1
- Anderson , M. 2002 . Encouraging prospects for recycling incinerated sewage sludge ash (ISSA) into clay-based building products . J. Chem. Technol. Biotechnol , 77 : 352 – 360 . doi: 10.1002/jctb.586
- Anderson , M. , Elliott , M. and Hickson , C. 2002 . Factory-scale proving trials using combined mixtures of three by-product wastes (including incinerated sewage sludge ash) in clay building bricks . J. Chem. Technol. Biotechnol , 77 : 345 – 351 . doi: 10.1002/jctb.593
- Anirudhan , T.S. and Tharun , A.R. 2012 . Preparation and adsorption properties of a novel interpenetrating polymer network (IPN) containing carboxyl groups for basic dye from aqueous media . Chem. Eng. J , : 761 – 769 . 181– 182 doi: 10.1016/j.cej.2011.11.077
- Barale , M. , Lefévre , G. , Carrette , F. , Catalette , H. , Fédoroff , M. and Cote , G. 2008 . Effect of the adsorption of lithium and borate species on the zeta potential of particles of cobalt ferrite, nickel ferrite, and magnetite . J. Colloid Interface Sci , 328 : 34 – 40 . doi: 10.1016/j.jcis.2008.09.007
- Binupriya , A.R. , Sathishkumar , M. , Jung , S.H. , Song , S.H. and Yun , S.I. 2008 . Bokbunja wine industry waste as precursor material for carbonization and its utilization for the removal of Procion Red MX-5B from aqueous solutions . Clean , 36 : 879 – 886 . doi: 10.1002/clen.200700202
- Cheeseman , C.R. and Virdi , G.S. 2005 . Properties and microstructure of lightweight aggregate produced from sintered sewage sludge ash . Resour. Conserv. Recycl , 45 : 18 – 30 . doi: 10.1016/j.resconrec.2004.12.006
- Chen , L. and Lin , D.F. 2009 . Applications of sewage sludge ash and nano-SiO2 to manufacture tile as construction material . Construct. Build. Mater , 23 : 3312 – 3320 . doi: 10.1016/j.conbuildmat.2009.06.049
- Elouear , Z. , Bouzid , J. and Boujelben , N. 2009 . Removal of nickel and cadmium from aqueous solutions by sewage sludge ash: Study in single and binary systems . Environ. Technol , 30 : 561 – 570 . doi: 10.1080/09593330902824940
- Franz , M. 2012 . Phosphate fertilizer from sewage sludge ash (SSA) . Waste Manage , 28 : 1809 – 1818 . doi: 10.1016/j.wasman.2007.08.011
- Garcés , P. , Carrión , M.P. , García-Alcocel , E. , Payá , J. , Monzó , J. and Borrachero , M.V. 2008 . Mechanical and physical properties of cement blended with sewage sludge ash . Waste Manage , 28 : 2495 – 2502 . doi: 10.1016/j.wasman.2008.02.019
- Gobi , K. , Mashitah , M.D. and Vadivelu , V.M. 2011 . Adsorptive removal of Methylene Blue using novel adsorbent from palm oil mill effluent waste activated sludge: Equilibrium, thermodynamics and kinetic studies . Chem. Eng. J , 171 : 1246 – 1252 . doi: 10.1016/j.cej.2011.05.036
- Han , R. , Zhang , J. , Han , P. , Wang , Y. , Zhao , Z. and Tang , M. 2009 . Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite . Chem. Eng. J , 145 : 496 – 504 . doi: 10.1016/j.cej.2008.05.003
- Hameed , B.H. , Din , A.T.M. and Ahmad , A.L. 2007 . Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies . J. Hazard. Mater , 141 : 819 – 825 . doi: 10.1016/j.jhazmat.2006.07.049
- Hu , S.H. , Hu , S.C. and Fu , Y.P. 2012 . Resource recycling through artificial lightweight aggregates from sewage sludge and derived ash using boric acid flux to lower co-melting temperature . J. Air Waste Manage. Assoc , 62 : 262 – 269 . doi: 10.1080/10473289.2001.646051
- Kikuchi , R. 1998 . Vitrification process for treatment of sewage sludge and incineration ash . J. Air Waste Manage. Assoc , 48 : 1112 – 1115 . doi: 10.1080/10473289.1998.10463766
- Lin , J.X. , Zhan , S.L. , Fang , M.H. , Qian , X.Q. and Yahg , H. 2008 . Adsorption of basic dye from aqueous solution onto fly ash . J. Environ. Manage , 87 : 193 – 200 . doi: 10.1016/J.Jenvman.2007.01.001
- Lin , K.L. and Lin , C.Y. 2005 . Hydration characteristics of waste sludge ash utilized as raw cement material . Cement Concrete Res , 35 : 1999 – 2007 . doi: 10.1016/j.cemconres.2005.06.008
- Lin , Y.F. , Chen , H.W. , Chien , P.S. , Chiou , C.S. and Liu , C.C. 2011 . Application of bifunctional magnetic adsorbent to adsorb metal cations and anionic dyes in aqueous solution . J. Hazard. Mater , 185 : 1124 – 1130 . doi: 10.1016/j.jhazmat.2010.10.002
- Liu , J. , Sun , S. , Zhang , R. , Zhong , S. and Chen , M. 2011a . Removal of Cu2+ in aqueous solutions using sludge incineration slag as an adsorbent . Appl. Mech. Mater , : 55 – 57 . 308–311 doi: 10.4028/www.scientific.net/AMM.55-57.308
- Liu , J.Y. , Ning , X.A. and Yang , Z.Y. 2011b . Study of adsorbent derived from sewage sludge incineration residue for the removal of Ni2+ in wastewater . Key Eng. Mater , : 474 – 476 . 158–161 doi: 10.4028/www.scientific.net/KEM.474-476.158
- Mathew , D.S. and Juang , R.S. 2007 . An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions . Chem. Eng. J , 129 : 51 – 65 . doi: 10.1016/j.cej.2006.11.001
- Moghaddam , S.S. , Alavi Moghaddam , M.M.R. and Arami . 2012 . Response surface optimization of acid red 119 dye adsorption by mixtures of dried sewage sludge and sewage sludge ash . Clean Soil Air Water , 40 : 652 – 660 . doi: 10.1002/clen.201000608
- Otero , M. , Rozada , F. , Calvo , L.F. , Garcia , A.I. and Morán , A. 2003 . Kinetic and equilibrium modeling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges . Biochem. Eng. J , 15 : 59 – 68 . doi: 10.1016/S1369-703X(02)00177-8
- Pan , S.C. , Lin , C.C. and Tseng , D.H. 2003 . Reusing sewage sludge ash as adsorbent for copper removal from wastewater . Resour. Conserv. Recycl , 39 : 79 – 90 . doi: 10.1016/S0921-3449(02)00122-2
- Tseng , R.L. 2007 . Physical and chemical properties and adsorption type of activated carbon prepared from plum kernels by NaOH activation . J. Hazard. Mater , 147 : 1020 – 1027 . doi: 10.1016/j.jhazmat.2007.01.140
- Wang , J. , Zheng , S. , Shao , Y. , Liu , J. , Xu , Z. and Zhu , D. 2010 . Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal . J. Colloid Interface Sci , 349 : 293 – 299 . doi: 10.1016/j.jcis.2010.05.010
- Wang , L. , Skjevrak , G. , Hustad , J.E. and Grønli , M.G. 2012 . Sintering characteristics of sewage sludge ashes at elevated temperatures . Fuel Process. Technol , 96 : 88 – 97 . doi: 10.1016/j.fuproc.2011.12.022
- Wang , S. , Li , L. , Wu , H. and Zhu , Z.H. 2005 . Unburned carbon as a low-cost adsorbent for treatment of methylene blue-containing wastewater . J. Colloid Interface Sci , 292 : 336 – 343 . doi: 10.1016/j.jcis.2005.06.014
- Weigand , H. , Bertau , M. , Hübner , W. , Bohndick , F. and Bruckert , A. 2013 . RecoPhos: Full-scale fertilizer production from sewage sludge ash . Waste Manage , 33 : 540 – 544 . doi: 10.1016/j.wasman.2012.07.009
- Weng , C.H. 2002 . Adsorption Characteristics of new coccine dye on the sludge ash . Adsorpt. Sci. Technol , 20 : 669 – 681 . doi: 10.1260/02636170260504350
- Weng , C.H. and Y.F. , Pan . 2006 . Adsorption characteristics of methylene blue from aqueous solution by sludge ash . Colloid Surf. A- Physicochem. Eng. Asp , 274 : 154 – 162 . doi: 10.1016/j.colsurfa.2005.08.044
- Werther , J. and Ogada , T. 1999 . Sewage sludge combustion . Prog. Energy Combust. Sci , 25 : 55 – 116 . doi: 10.1016/S0360-1285(98)00020-3
- Wu , C.H. 2007 . Adsorption of reactive dye onto carbon nanotubes: Equilibrium, kinetics and thermodynamics . J. Hazard. Mater , 144 : 93 – 100 . doi: 10.1016/j.jhazmat.2006.09.083
- Zhu , H. , Yang , D. and Zhu , L. 2007 . Hydrothermal growth and characterization of magnetite (Fe3O4) thin films . Surface Coat. Technol , 201 : 5870 – 5874 . doi: 10.1016/j.surfcoat.2006.10.037