Abstract
Reducing airborne microorganisms may potentially improve the environment in layer breeding houses. The effectiveness of slightly acidic electrolyzed water (SAEW; pH 5.29–6.30) in reducing airborne microorganisms was investigated in a commercial layer house in northern China. The building had a tunnel-ventilation system, with an evaporative cooling. The experimental area was divided into five zones along the length of the house, with zone 1 nearest to an evaporative cooling pad and zone 5 nearest to the fans. The air temperature, relative humidity, dust concentration, and microbial population were measured at the sampling points in the five zones during the study period. The SAEW was sprayed by workers in the whole house. A six-stage air microbial sampler was used to measure airborne microbial population. Results showed that the population of airborne bacteria and fungi were sharply reduced by 0.71 × 105 and 2.82 × 103 colony-forming units (CFU) m−3 after 30 min exposure to SAEW, respectively. Compared with the benzalkonium chloride (BC) solution and povidone-iodine (PVP-I) solution treatments, the population reductions of airborne fungi treated by SAEW were significantly (P < 0.05) more, even though the three disinfectants can decrease both the airborne bacteria and fungi significantly (P < 0.05) 30 min after spraying.
Implications:
There are no effective methods for reducing airborne microbial levels in tunnel-ventilated layer breeding houses; additionally, there is limited information available on airborne microorganism distribution. This research investigated the spatial distribution of microbial population, and the effectiveness of spraying slightly acidic electrolyzed water in reducing microbial levels. The research revealed that slightly acidic electrolyzed water spray was a potential method for reducing microbial presence in layer houses. The knowledge gained in this research about the microbial population variations in the building may assist producers in managing the bird housing environment and engineers in designing poultry houses.
Introduction
Livestock operations harbor a variety of bacterial, viral, and protozoal pathogens, and several of these pathogens pose a risk to other animals and humans (Dungan, Citation2010). Many factors such as the movement of workers and animals cause aerosolization of microorganisms (Paez-Rubio et al., Citation2007). Airborne microorganisms can lead to detrimental effects in both the health of farmers and animals, and can be responsible for infectious and noninfectious diseases (Donham et al., Citation2000; Hoopmann et al., Citation2006). The health of workers, as well as residents living near livestock operations, are threatened when pathogens are both zoonotic and airborne transmittable (Radon et al., Citation2007).
Airborne microorganisms in animal buildings may originate from several sources, including the animal body, manure, and dust (Andersson et al., Citation1999; Venglovsky et al., Citation2009). Environmental conditions such as temperature, relative humidity, and ultraviolet radiation can affect the biological survival of airborne microorganisms (Cox and Wathes, Citation1995). Many bacteria and fungi are attached to airborne particulate matter (PM) (Just et al., Citation2009), which thereby has the ability to influence the survival of microorganisms in air (Cambra-López et al., Citation2010; Adell et al., Citation2011). Bird activity is also a major cause of dust concentration changes in modern hen houses with cages (Heber et al., Citation2006).
Spraying disinfectants is used extensively in modern intensive livestock operations (Rodríguez Ferri, et al., Citation2010). Chemical disinfectants (benzalkonium chloride, formaldehyde, and glutaraldehyde) are widely used as a preventative or precautionary measure against bacterial infections in livestock animals such as cattle, swine, and poultry (Sundheim et al., Citation1998). However, chemical disinfectants are potentially toxic, corrosive, and/or volatile (Lewis and McIndoe, Citation2004). The emergence of disinfection-resistant pathogens, such as Listeria monocytogenes, which displays cross-resistance to chemical disinfectants, has also been reported (Whyte et al., Citation2002). Therefore, to improve current measures to control the transmission of pathogens in livestock environments, there is a need to develop effective disinfectants with less residues.
In the past, slightly acidic electrolyzed water (SAEW; pH 5.0–6.5) was increasingly used for the prevention and control of microorganisms (Koide et al., Citation2011). With a nearly neutral pH value, the effective form of chlorine compounds in SAEW is primarily hypochlorous acid (HOCl; 95%), which has strong antimicrobial activity (Hricova et al., Citation2008). SAEW is considered environmental friendly because it reverts to water after use (Møretrø et al., Citation2012). Electrolyzed water has been previously applied for disinfection in food processing (Huang et al., Citation2008), hospital activities (Vorobjeva et al., Citation2004), and some agricultural settings (Guentzel et al., Citation2010; Zhang et al., Citation2011).
Ge et al. (Citation2012) reported that electrolyzed water with a pH of 6.53 can significantly reduce the microorganisms on the skin of swine. The spray application of electrolyzed water for dust reduction was tested in layer breeding houses (Zheng et al., Citation2012). Hao et al. (Citation2013b) reported that SAEW can significantly reduce the airborne microorganisms in swine barns. However, little work has been done to assess the effect of SAEW on airborne microorganisms in layer buildings. The objectives of this work were to determine the airborne microbial population in different spatial locations in a typical tunnel-ventilated layer breeding house, and to examine the effectiveness of slightly acidic electrolyzed water spray in microbial reduction.
Materials and Methods
Experimental facilities
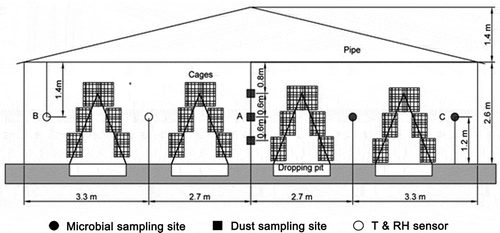
Experiments were conducted in a layer house with dimensions of 100 m × 12 m (length × width), located in the Pinggu District of Beijing, China. The study was carried out in the winter of 2011. The facility housed approximately 16,000 breeding hens of 351 days old and confined in four rows of three tiers stair-step cages, which provided an average space of 450 cm2 per hen (). The building was tunnel-ventilated. Five axial flow fans (9FJ12.7; Shanghai Zhengcheng Electrical and Mechanical Manufacturing Co., Ltd., Beijing, China), with an airflow of 41,750 m3 hr−1 per fan were installed on one end wall and an evaporative cooling pad (1.5 m × 11.2 m) at the other end (which was not operated in winter). The fans operated when the indoor ambient temperature was higher than 15 °C. Additionally, 27 adjustable air inlets distributed along the sidewalls were opened slightly due to the low ambient temperature during the experimental period. Manure was collected in four dropping pits under the cages and scraped out of the house with a mechanical scraper in each pit twice a day at approximately 8:00 a.m. and 5:00 p.m. The lights were off from 6:30 p.m. to 4:30 a.m. Feed was delivered at 8:30 a.m. and 5:30 p.m. with automatic feeding troughs, and water was supplied with drinking nipples. Artificial insemination was performed daily from 1:30 p.m. to 4:30 p.m. During the study period, the layers remained in their cages, while the researchers and workers applied strict hygiene procedures to prevent cross-infections.
Disinfectants
Slightly acidic electrolyzed water was generated with a continuous SAEW generator (CWD-A; Shenyang Dongyu Xinbor Technology Company Ltd., Shenyang, China). The generator was set to a voltage and current of 45 V and 30 A, respectively, to electrolyze NaCl (1 g L−1) containing an HCl (100 μL L−1) solution. The electrodes were two 30 × 15 cm2 plates of Ti/Pt-IrO2 with a distance of 1 cm between the anode and cathode. Produced SAEW solution had a pH of 5.29–6.30, an oxidation-reduction potential (ORP) of 974–987 mV, and an available chlorine concentration (ACC) of 250 mg L−1. All chemicals used were of analytical grade.
The physicochemical properties of SAEW were measured immediately after production. The pH and ORP values were measured using a dual scale pH/ORP meter (CON60; Trans-Wiggens, Singapore) with a pH electrode (PE02; range: 0.00–14.00) or an ORP electrode (ORP06; range: −999 to +999 mV). The ACC was determined using a digital chlorine test kit (RC-2Z; Kasahara Chemical Instruments Corp., Saitama, Japan). The resolution is 1 mg L−1 and the detection range is 0–320 mg L−1. The SAEW was used within 1 hr after preparation.
Benzalkonium chloride solution (BC; Bayer Animal Health Co., Ltd., Sichuan, China) and povidone-iodine solution (PVP-I; Beijing Realm-Zone Animal Health Co., Ltd., Beijing, China) were purchased from commercial suppliers. The product name, class, effective components, and recommended and practical concentrations of each disinfectant are shown in . Product dilutions were performed using tap water.
Table 1. Main characteristics of the three disinfectants
Experimental zones and air quality measurements
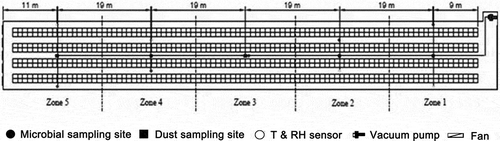
The experimental cage area was divided into five zones (named zone 1, zone 2, zone 3, zone 4, and zone 5) along the length of the building. Zone 1 was nearest to the evaporative cooling pads and zone 5 was nearest to the fans (). The spatial distribution and time courses of air temperature (T), relative humidity (RH), dust concentration, and microbial concentration were measured, respectively. All the measurements taken during the experimental period (samples taken 2× per week for 8 weeks = 16 collections, five zones) in each of the five zones were averaged to reflect the conditions of the whole building.
Air temperature (T) and relative humidity (RH) sensors (thermo recorder RS-12; ESPEC MIC Corp., Aichi, Japan) were positioned 1.2 m above the floor. Temperature and relative humidity were recorded at 15-min intervals. Dust concentration in the air was determined using a multipoint dust sampler, which consists of a commercially available vacuum pump (Mink MM 1252 AV; Busch Co., Ltd., Marburg, Germany), a pressure monitor, a pressure regulator, and an array of critical venturis with glass fiber filters (Wang et al., Citation1999). The filters were 37-mm glass microfiber filters (Whatman 934-AH; GE Healthcare Bio-Sciences Corp., Piscataway, NJ) housed in a holding cassette located upstream of a critical venturi facing the evaporative cooling pad. Five sampling points were placed along the length of building (19 m apart) and three along the building height (0.6 m apart). The sampling point heights were approximately at the breathing heights of birds in the three tiers. The sampling duration was 2 hr. The average flow rate of the samplers was 16.6 ± 0.99 L min−1. The concentration of airborne dust was calculated using the following equation (Wang et al., Citation2002):
The filters before sampling (filter only) and after sampling (filter with dust) were placed in a vacuum dryer for 24 hr. Then, all the filters were weighed with an analytical balance (Sartorius AG, Göttingen, Germany; accuracy: 0.1 mg) in a controlled environment. The net mass increases of dust were recorded. The average dust concentration in each zone was obtained by averaging the values measured at three vertically aligned sampling points.
Airborne microorganisms were collected with a six-stage microbial air sampler (JWL-S6; Beijing Senon Technology Development Co., Ltd., Beijing, China) in five locations 1.2 m above the floor ( and ). The sampling time was 1 min at a flow rate of 28.3 L min−1. The airborne microbes were collected on nutrient agar media (specific to either fungi or bacteria) in the plates (Jo and Kang, Citation2005). Six plates with nutrient agar media were placed inside the samplers as a group. The microbial particles were directly collected on a nutrient agar medium while sampling.
The population of microorganisms in the air was calculated using following relationship:
All of the parameters were analyzed using variance (ANOVA; SAS Institute Inc., Cary, NC, USA). Results were reported as means and standard deviations. Tukey’s honest significance test was used to determine the significant differences among the means at the 5% probability level.
Microbial reduction after spraying SAEW
Manure was removed and the house was cleaned with tap water before spraying disinfectants in the building. Slightly acidic electrolyzed water (at an ACC of 250 mg L−1) was sprayed at a rate of 120 mL m−2 with a high pressure sprayer, starting at 11:00 a.m. and lasting for 20 min, the spraying pressure was 3–4 bar, and the nozzle diameter was 0.7 mm. All the surfaces in the whole building, including cages, wall, floor, and ceiling, were sprayed with SAEW.
Microorganism samples were collected both 2.5 hr before spraying (from 8:30 a.m. to 11:00 a.m.) and 5 hr after spraying (from 11:30 a.m. to 4:30 p.m.) with SAEW at an ACC of 250 mg L−1, respectively. The microbes were tested in 1 day with no spray to observe their common variations, and the interval was 2 hr. The diurnal changes of bacteria and fungi were measured in the same way. During the tests, the ventilation in the house remained stable with the five fans in operation. The plate of dichloran glycerol 18 agar (DG-18; Beijing Land Bridge Technology Co., Ltd., Beijing, China) was applied for fungal collection with the addition of chloramphenicol to inhibit bacterial growth (Jo and Kang, Citation2005). The trypticase soy agar (TSA) was used to collect the airborne bacteria with the addition of cycloheximide to inhibit fungal growth (Jo and Kang, Citation2005). All samples were sent to the laboratory under ambient conditions on the day of collection. The DG-18 and TSA plates were incubated at room temperature for 3–5 days and 2–3 days, respectively. The microbial populations on all the plates were calculated manually (only the alive microorganisms can grow up to colonies and be calculated).
Statistical analysis was performed using the SAS software (SAS Institute). Paired-sample t test was used to analyze the significant differences before and after disinfection at the 5% probability level.
Disinfection effectiveness of spraying the three disinfectants
Manure was removed and the house was cleaned with tap water before spraying disinfectants in the building. Slightly acidic electrolyzed water at an ACC of 250 mg L−1 was sprayed as above. The BC (1:2000) and PVP-I (1:1000) solutions were used as controls. The samples were collected before (10:30 a.m.) and 30 min after (11:50 a.m.) disinfection. Data were compared using analysis of variance (ANOVA; SAS Institute). Results were reported as means and standard deviations. A significance level of less than 0.05 was used as the minimum acceptable P value. Paired-sample t test was used to analyze the significant differences before and after disinfection. The disinfection effectiveness differences among disinfectants were performed by Tukey’s honest significance test (P < 0.05).
Results and Discussion
Environmental conditions in the test building
During the experimental period, the air temperature in the layer house ranged from 14.1 to 16.1 °C, and the temperatures outside the building ranged from −7.5 to 1.6 °C. The relative humidity values were 58–65% inside but only 30–34% outside. Dust concentrations were 2.98–3.90 mg m−3 and the microbial populations were 5.18–5.37 log10 CFU m−3 ( and ). The temperature increased from zone 1 to zone 4 and decreased from the zone 4 to zone 5. It is indicated in the China Environmental Quality Standard for the Livestock and Poultry Farm (NY/T388-1999) that the particle matters and the microbial populations in layer house should be less than 4 mg m−3 and 2.5 × 104 CFU m−3, respectively, from the viewpoints of public health. The microorganisms in the air of the layer house were more than 2.5 × 104 CFU m−3 ( and ).
Table 2a. Environmental parameters of air in the layer breeding house by zone
Table 2b. Environmental parameters of air in the layer breeding house by Time
Cleaning and spraying in an open area beside the entrance in zone 1 might lead to the lower temperature in zone 1. The indoor/outdoor air exchange because of the fans in zone 5 contributed to the low temperature. In the middle of the building, zone 3 and zone 4, the effect of heat production by birds was the same as with other zones, but the effect of inlets and space was less, which can explain the highest temperature in zone 4. According to the hythergraph, the relative humidity decreased with increasing temperature. Therefore, the relative humidity should be higher in zone 1 and zone 5, which is in accordance with the results in .
Dust concentrations increased from zone 1 to zone 3, and decreased from zone 3 to zone 5. The layers in zone 1 were approximately the same as the other zones, which means that the dust production by birds’ movement was the same as the other zones. However, there was more space in zone 1 compared with the other zones, which might have also contributed to the lower temperature in zone 1. The cleaning and spraying in an open area beside the entrance might have led to lower dust concentrations in zone 1. Placing the exhaust fans at the end of the house may have brought air from the upstream fans, in which a portion was withdrawn from the house. The high airflow rate caused by the fans may lead to the high airflow rate of the inlets in zone 5, which means the fresh air brought into the house from these inlets was more than that from other zones. The low dust concentration in the fresh air (not shown) contributed to the lower dust concentrations in zone 5. Zone 4 might have been affected by the air inlets. As a result, the highest dust concentration was observed in zone 3.
As the data shown in , the dust concentration was higher in zone 5 compared with zone 1. This may due to the exhaust fans at the end of the house, which brought air and dusts from the upstream the fans. As a result, a portion of the bioaerosol was withdrawn from the house. But lots of them were kept in the zone 5, because the operation of the middle fan in the wall was enough to keep the indoor temperature around 15 °C most of the time in the winter, so only one of the five fans actually worked.
During the animal rearing cycle, with regards to the animal productivity parameters, the same results were generally found within the upper ranges of previous studies (Al Homidan et al., Citation1998; Feddes et al., Citation2002). It has been reported that airborne microorganisms were associated with airborne particulate matters (Zhao et al., Citation2011). The total microbial populations increased from zone 1 to zone 3, and decreased from zone 3 to zone 5 (). The atmospheric microorganism may also interfere the microbial concentration while being brought into the house randomly. The atmospheric microorganism concentration near to zone 1 was approximately 24 CFU/m3, whereas it was 5.6 × 103 CFU/m3 close to the fans. So the inside/outside (I/O) ratio ranged from 90 to 6307 during the experiment.
shows that all the parameters did not change significantly during the day time, although the concentrations of dust and microorganisms increased slightly from the morning to afternoon. The increase may have been caused by the movement of workers and animals.
Microbial reduction due to SAEW spray
The variations in populations of airborne bacteria and fungi during the daytime, as well as before and after disinfection, are illustrated in . The population of bacteria increased from 1.57 × 105 to 2.30 × 105 CFU m−3 without disinfection in the daytime, whereas the fungal population increased from 5.27 × 103 to 8.43 × 103 CFU m−3. This may be because the continuous movement of birds can lead to more microorganisms in the air.
Figure 3. Variations of airborne bacteria and fungi in a day’s time. Points represent the average means ± standard deviations in five zones. SAEW spray was employed at 11:00 a.m. for 20 min.
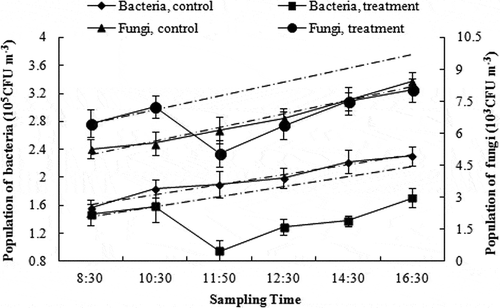
A large reduction in microbial population occurred after the SAEW was sprayed. The population reductions were 0.64 ×105 and 2.18 × 103 CFU m−3 for bacteria and fungi from 10:30 a.m. to 11:50 a.m., respectively. In , the population of bacteria and fungi should be appropriately 1.65 × 105 and 7.89 × 103 CFU m−3 at 11:50 a.m. with no spray disinfection, as shown by the dotted lines. The spraying significantly (P < 0.05) reduced 0.71× 105 CFU m−3 bacteria and 2.82 × 103 CFU m−3 fungi after 20 min spraying and 30 min exposure to SAEW. This reduction might be attributed to (a) the antimicrobial effect of SAEW (Landa-Solis et al., Citation2005; Guentzel et al., Citation2010; Nan et al., Citation2010); it has been found that the effective chlorine in SAEW, primarily hypochlorous acid (HOCl; 95%), has strong antimicrobial activity (Hricova et al., Citation2008); and (b) reduction of airborne dust level, which might subsequently lead to the reduction of microorganisms that were attached to airborne dust particles. Zheng et al. (Citation2012) reported that SAEW can significantly reduce the airborne dust level in layer breeding house. It has been reported that the airborne microbes in swine barns decreased by 59% and 12% after 30 min exposure to SAEW and tap water (Hao et al., Citation2013b). The result indicates that biochemical disinfection made the most contribution to the reduction of airborne dust and microbes.
Nan (Citation2011) reported that the mechanical spraying process can cause 21–55% loss of ACC in SAEW, whereas 47–69% ACC was lost in strong acid electrolyzed water. The result is in accordance with Hsu’s study (Citation2004), which indicated that spraying reduced the chlorine in electrolyzed oxidizing water by 20–97%. Park et al. (Citation2007) also demonstrated that fogging reduced the ACC by approximately 70% in hypochlorous acid (HOCl) solution, as well as the inactivation efficacy of disinfectant.
Microbial reduction after treating with different disinfectants
As shown in , both the population of airborne bacteria and fungi were reduced significantly (P < 0.05) by spraying with all three disinfectants (SAEW, BC, and PVP-I). These results agree with Hao et al. (Citation2013b), which revealed that SAEW can significantly reduce the airborne microorganisms in swine barns. Additionally, there were no significant differences (P > 0.05) between the surviving counts of airborne bacteria after spraying with SAEW (250 mg L−1), BC (1:2000, v v−1), and PVP-I (1:1000, v v−1). However, the surviving count of airborne fungi in the layer house after spraying SAEW was significantly lower (P < 0.05) than that after spraying with the other two disinfectants. This indicates that SAEW was more effective than either BC or PVP-I.
Table 3. Spraying disinfection effect of the three disinfectants for reducing airborne bacteria and fungi in the layer house
Hao et al. (Citation2013a, Citation2013b) used SAEW, BC, and PVP-I to reduce microorganisms in animal buildings, the results were that the disinfection effectiveness of SAEW is much better than those of BC and PVP-I, which is in accordance with the results given in . As widely used disinfectants, the disinfection mechanisms of BC and PVP-I were reported for decades. The permeability of the cell membrane in microbes can be changed by benzalkonium chloride. The extravasation of intracellular materials can lead to death of pathogens. The free iodine in the PVP-I is the main effective component, which can cause the denaturation of protein, thereby causing microbes to die. The three chlorine forms of Cl2, HOCl, and OCl− in electrolyzed water contribute to available chlorine or free chlorine. The relative amount of each chlorine compound is dependent on the pH value and temperature (Koide et al., Citation2009). The chlorine form in SAEW at a pH value of 5.0–6.5 is hypochlorous acid (HOCl; approximately 95%), which has strong antimicrobial activity. The antimicrobial activity of hypochlorous acid (for inactivating Echerichia coli) is 80 times greater than that of the hypochlorite ion (ClO−) at the same chlorine concentration and treatment time (Honda, Citation2003). Additionally, the pH value and oxidation-reduction potential (ORP) also contribute to the disinfection effect of electrolyzed water (Kiura et al., Citation2002; Liao et al., Citation2007). Liao et al. (Citation2007) indicated that electrolyzed water influenced the shape of the cells because of the ORP. Nakajima et al. (2004) illustrated that electrolyzed water can kill cells by affecting genetic materials such as DNA. The disinfection mechanism of SAEW is still not completely clear. Further work need to be undertaken.
Conclusion
It has been found that the air temperature, dust concentration, and microbial population increased from zone 1 to zone 3 or 4 along the length of the house from the evaporative cooling pad to fans in the tunnel-ventilated layer house, and decreased from zone 3 or 4 to zone 5. The population of airborne bacteria and fungi sharply (P < 0.05) decreased by 0.71 × 105 and 2.82 × 103 CFU m−3 after 20 min spraying and 30 min exposure to SAEW, respectively. Additionally, compared with the BC and PVP-I treatments, the effectiveness of disinfection using SAEW to airborne fungi were significantly (P < 0.05) higher in the 30 min after spraying in the layer house. Slightly acidic electrolyzed water is shown to be a potential agent to reduce airborne microbes in layer houses.
Funding
This work was supported by the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-41) and the National Natural Science Foundation of China (grant number: 21106179 and 31372350).
Acknowledgment
The authors would like to thank Beijing Huadu Yukou Fowl Industry Co., Ltd., Beijing, China.
Additional information
Notes on contributors
Xiaoxia Hao
Xiaoxia Hao is a Ph.D. candidate and Wei Cao, Baoming Li, and Chaoyuan Wang are professors in the College of Water Resources and Civil Engineering, China Agricultural University, Beijing, People’s Republic of China.
Wei Cao
Xiaoxia Hao is a Ph.D. candidate and Wei Cao, Baoming Li, and Chaoyuan Wang are professors in the College of Water Resources and Civil Engineering, China Agricultural University, Beijing, People’s Republic of China.
Baoming Li
Xiaoxia Hao is a Ph.D. candidate and Wei Cao, Baoming Li, and Chaoyuan Wang are professors in the College of Water Resources and Civil Engineering, China Agricultural University, Beijing, People’s Republic of China.
Qiang Zhang
Qiang Zhang is professor in the Department of Biosystems Engineering, University of Manitoba, Winnipeg, Manitoba, Canada.
Chaoyuan Wang
Xiaoxia Hao is a Ph.D. candidate and Wei Cao, Baoming Li, and Chaoyuan Wang are professors in the College of Water Resources and Civil Engineering, China Agricultural University, Beijing, People’s Republic of China.
Liangpeng Ge
Liangpeng Ge, Ph.D., is a researcher in Chongqing Academy of Animal Sciences, Chongqing, People’s Republic of China.
References
- Adell, E., V. Moset, Y. Zhao, A. Cerisuelo, and M. Cambra-López. 2011. Concentración, distribución espacial y por tamaño de bacterias aerobias mesófilas en elaire de granjas de broilers. ITEA Inf. Téc. Econ. Agrar. 107:77–93.
- Al Homidan, A., J.F. Robertson, and A.M. Petchey. Comp:Ref au lists. Close up initials pls. 1998. Effect of environmental factors on ammonia and dust production and broiler performance. Br. Poult. Sci. 39: S9–S10. doi:10.1080/00071669888052
- Andersson, A.M., N. Weis, F. Rainey, and M.S. Salkinoja-Salonen. 1999. Dust-borne bacteria in animal sheds, schools and children’s day care centres. J.Appl. Microbiol. 86:622–634. doi:10.1046/j.1365-2672.1999.00706.x
- Cambra-López, M., A.J.A. Aarnink, Y. Zhao, S. Calvet, and A.G. Torres. 2010. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 58:1–17. doi:10.1016/j.envpol.2009.07.011
- Cox, C.S., and C. Wathes. 1995. Bioaerosols Handbook. Boca Raton, FL: CRC Press.
- Donham, K.J., D. Cumro, S.J. Reynolds, and J.A. Merchant. 2000. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: Recommendations for exposure limits. J.Occup. Environ. Med. 42:260–269. doi:10.1097/00043764-200003000-00006
- Feddes, J.J., E.J. Emmanuel, and M.J. Zuidhoft. 2002. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 81:774–779.
- Dungan, R.S. 2010. Board-invited review: Fate and transport of bioaerosols associated with livestock operations and manures. J.Anim. Sci. 88:3693–3706. doi:10.2527/jas.2010-3094
- Ge, L.P., X.Ch. Zhang, Ch. Cao, Zh.B. Gu, Z.H. Liu, L.B. Liu, and B.Zh. Lin. 2012. Feasibility study of the sterilization of pigskin used as wound dressings by neutral electrolyzed water. J.Trauma Acute Care Surg. 72: 1584–1587. doi:10.1097/TA.0b013e318243a1dc
- Guentzel, J.L., K.L. Lam, M.A. Callan, S.A. Emmons, and V.L. Dunham. 2010. Postharvest management of gray mold and brown rot on surfaces of peaches and grapes using electrolyzed oxidizing water. Int. J.Food Microbiol. 143:54–60. doi:10.1016/j.ijfoodmicro.2010.07.028
- Hao, X.X., B.M. Li, C.Y. Wang, Q. Zhang, and W. Cao. 2013a. Application of slightly acidic electrolyzed water for inactivating microbes in a layer breeding house. Poult. Sci. 92:2560–2566. doi:10.3382/ps.2013-03117
- Hao, X.X., B.M. Li Q., Zhang B., Zh. Lin, L.P., Ge C.Y. Wang, and W. Cao. 2013b. Disinfection effectiveness of slightly acidic electrolyzed water in swine barns. J.Appl. Microbiol. 115: 703–710. doi:10.1111/jam.12274
- Heber, A.J., T.T. Lim, J.Z. Gallien, J.Q. Ni, P.C. Tao, A.M. Schmidt, J.A. Koziel, S.J. Hoff, L.D. Jacobson, Y. Zhang, and G.B. Baughman. 2006. Quality assured measurement of animal building emission: Particulate matter concentrations. J.Air Waste Manage. Assoc. 56:1642–1648. doi:10.1080/10473289.2006.10464569
- Honda, Y. 2003. Improvement of the electrolysis equipment and application of slightly acidic electrolyzed water for dairy farming. J.Japn. Soc. Agric. Machinery 65:27–29.
- Hoopmann, M., O. Hehl, F. Neisel, and T. Werfel. 2006. Associations between bioaerosols coming from livestock facilities and asthmatic symptoms in children. Gesundheitswesen 68:575–584. doi:10.1055/s-2006-926987
- Hricova, D., R. Stephan, and C. Zweifel. 2008. Electrolyzed water and its application in the food industry. J.Food Protect. 71:1934–1947.
- Hsu, S.Y., C. Kim, Y.C. Hung, and S.E. Prussia. 2004. Effect of spraying on chemical properties and bactericidal efficacy of electrolyzed oxidizing water. Int. J.Food Sci. Technol. 39:157–165. doi:10.1046/j.0950-5423.2003.00762.x
- Huang, Y.R., Y.C. Hung, S.Y. Hsu, Y.W. Huang, and D.F. Hwang. 2008. Application of Electrolyzed water in the food industry. Food Control. 19:329–345. doi:10.1016/j.foodcont.2007.08.012
- Jo, W.-K., and J.-H. Kang. 2005. Exposure levels of airborne bacteria and fungi in Korean swine and poultry sheds. Arch. Environ. Occup. Health 60:140–146. doi:10.3200/AEOH.60.3.140-146
- Just, N., C. Duchaine, and S. Baljit. 2009. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J.Occup. Med. Toxicol. 4:13. doi:10.1186/1745-6673-4-13
- Kiura, H., K. Sano, S. Morimatsu, T. Nakano, C. Morita, M. Yamaguchi, T. Maeda, and Y. Katsuoka. 2002. Bactericidal activity of electrolyzed acid water from solution containing sodium chloride at low concentration, in comparison with that at high concentration. J.Microbiol. Methods 49:285–293. doi:10.1016/S0167-7012(01)00385-2
- Koide, S., D. Shitanda, M. Note, and W. Cao. 2011. Effects of mildly heated, slightly acidic electrolyzed water on the disinfection and physicochemical properties of sliced carrot. Food Control 22:452–456. doi:10.1016/j.foodcont.2010.09.025
- Koide, S., J. Takeda, J. Shi, H. Shono, and G.G. Atungulu. 2009. Disinfection efficacy of slightly acidic electrolyzed water on fresh cut cabbage. Food Control 20:294–297. doi:10.1016/j.foodcont.2008.05.019
- Landa-Solis, C., D. González-Espinosa, B. Guzmán-Soriano, M. Snyder, G. Reyes-Terán, K. Torres, and A.A. Gutierrez. 2005. Microcyn™: A novel super-oxidized water with neutral pH and disinfectant activity. J.Hosp. Infect. 61:291– 299. doi:10.1016/j.jhin.2005.04.021
- Lewis, S., and A.K. McIndoe. 2004. Cleaning, disinfection and sterilization of equipment. Anaesth Intensive Care 11:360–363.
- Liao, L.B., W.M. Chen, and X.M. Xiao. 2007. The generation and inactivation mechanism of oxidation-reduction potential of electrolyzed oxidizing water. J.Food Eng. 78:1326–1332. doi:10.1016/j.jfoodeng.2006.01.004
- Møretrø, T., E. Heir, L.L. Nesse, L.K. Vestby, and S. Langsrud. 2012. Control of Salmonella in food related environments by chemical disinfection. Food Res. Int. 45:532–544. doi:10.1016/j.foodres.2011.02.002
- Nan, S.J. 2011. Disinfection effect of slightly acidic electrolyzed water in the dairy farm and application on the control of dairy mastitis. Doctoral dissertation, China Agricultural University, Beijing, China. Document ID 20110617111454.
- Nan, S.J., Y.Y. Li, B.M. Li, Ch.Y. Wang, X.D.Cui, and W. Cao. 2010. Effect of slightly acidic electrolyzed water for inactivating Escherichia coli O157: H7and Staphylococcus aureus analyzed by transmission electron microscopy. J.Food Protect. 73:2211–2216.
- Norihito, N., T. Nakanoa, and F. Harada. 2004. Evaluation of disinfective potential of reactivated free chlorine in pooled tap water by electrolysis. J.Microbiol. Methods 57:163–173. doi:10.1016/j.mimet.2003.12.011
- Paez-Rubio, T., A. Ramarui, J. Sommer, H. Xin, J. Anderson, and J. Peccia. 2007. Emission rates and characterization of aerosols produced during the spreading of dewatered class B biosolids. Environ. Sci. Technol. 41:3537–3544. doi:10.1021/es061786p
- Park, G.W., D.M. Boston, J.A. Kase, M.N. Sampson, and M.D. Sobsey. 2007. Evaluation of liquid and fog application of Sterilox® hypochlorous acid solution (HOCl) for surface inactivation of human norovirus. Appl. Environ. Microbiol. 73:4463–4468. doi:10.1128/AEM.02839-06
- Radon, K., A. Schulze, V. Ehrenstein, R.T. van Strien, G. Praml, and D. Nowak. 2007. Environmental exposure to confined animal feeding operations and respiratory health of neighbouring residents. Epidemiology 18:300–308. doi:10.1097/01.ede.0000259966.62137.84
- Rodríguez Ferri, E.F., S. Martínez, R. Frandoloso, S. Yubero, and C.B. Gutiérrez Martín. 2010. Comparative efficacy of several disinfectants in suspension and carrier tests against Haemophilus parasuis serovars 1 and 5. Res. Vet. Sci. 88:385–389. doi:10.1016/j.rvsc.2009.12.001
- Sundheim, G., S. Langsrud, E. Heir, and A.L. Holck. 1998. Bacterial resistance to disinfectants containing quaternary ammonium compounds. Int. Biodeter. Biodegrad. 41:235–239. doi:10.1016/S0964-8305(98)00027-4
- Venglovsky, J., N. Sasakova, and I. Placha. 2009. Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresour. Technol. 100:5386–5391 doi:10.1016/j.biortech.2009.03.068
- Vorobjeva, N.V., L.I. Vorobjeva, and E.Y. Khodjaev. 2004. The bactericidal effects of electrolyzed oxidizing water on bacterial strains involved in hospital infections. Artif. Organs 28:590–592. doi:10.1111/j.1525-1594.2004.07293.x
- Wang, X., Y. Zhang, L. Zhao, and G.L. Riskowski. 1999. Development of a multi-point aerosol sampler using critical flow control devices. ASHRAE Trans. 105:1108–1113.
- Wang, X., Y. Zhang, L. Zhao, G.L. Riskowski, and M. Ellis. 2002. Measurement and analysis of dust spatial distribution in a mechanically ventilated pig building. Biosyst. Eng. 81:225–236. doi:10.1006/bioe.2001.0014
- Whyte, P., J.D. Collins, K. McGill, and C. Monahan. 2002. Assessment of sodium dichloroisocyanurate in the control of microbiological cross-contamination in broiler carcass immersion chilling systems. J.Food Saf. 22:55–65. doi:10.1111/j.1745-4565.2002.tb00330.x
- Zhang, C.L., Zh.H. Lu, Y.Y.Li, Y.Ch. Shang, G. Zhang, and W. Cao. 2011. Reduction of Escherichia coli O157: H7and Salmonella enteritidis on mung bean seeds and sprouts by slightly acidic electrolyzed water. Food Control 22: 792–796. doi:10.1016/j.foodcont.2010.11.018
- Zhao, Y., A.J.A. Aarnink, M.C.M. De Jong, N.W.M. Ogink, and P.W.G.G. Koerkamp. 2011. Effectiveness of multi-stage scrubbers in reducing emissions of air pollutants from pig houses. Trans. ASABE 54:285–293. doi:10.13031/2013.36256
- Zheng, W.C., B.M. Li, W. Cao, G.Q. Zhang, and Z.Y. Yang. 2012. Application of neutral electrolyzed water spray for reducing dust levels in a layer breeding house. J.Air Waste Manage Assoc. 62:1329–1334. doi:10.1080/10962247.2012.710553