Abstract
Although widely used in air quality regulatory frameworks, the term “volatile organic compound” (VOC) is poorly defined. Numerous standardized tests are currently used in regulations to determine VOC content (and thus volatility), but in many cases the tests do not agree with each other, nor do they always accurately represent actual evaporation rates under ambient conditions. The parameters (time, temperature, reference material, column polarity, etc.) used in the definitions and the associated test methods were created without a significant evaluation of volatilization characteristics in real world settings. Not only do these differences lead to varying VOC content results, but occasionally they conflict with one another. An ambient evaporation study of selected compounds and a few formulated products was conducted and the results were compared to several current VOC test methodologies: SCAQMD Method 313 (M313), ASTM Standard Test Method E 1868-10 (E1868), and U.S. EPA Reference Method 24 (M24). The ambient evaporation study showed a definite distinction between nonvolatile, semivolatile, and volatile compounds. Some low vapor pressure (LVP) solvents, currently considered exempt as VOCs by some methods, volatilize at ambient conditions nearly as rapidly as the traditional high-volatility solvents they are meant to replace. Conversely, bio-based and heavy hydrocarbons did not readily volatilize, though they often are calculated as VOCs in some traditional test methods. The study suggests that regulatory standards should be reevaluated to more accurately reflect real-world emission from the use of VOC containing products.
Implications:
The definition of VOC in current test methods may lead to regulations that exclude otherwise viable alternatives or allow substitutions of chemicals that may limit the environmental benefits sought in the regulation. A study was conducted to examine volatility of several compounds and a few formulated products under several current VOC test methodologies and ambient evaporation. This paper provides ample evidence to warrant a reevaluation of regulatory standards and provides a framework for progressive developments based on reasonable and scientifically justifiable definitions of VOCs.
Introduction
Volatile organic compounds (VOCs) are among the key precursors involved in the chemical reactions that form tropospheric ozone. Accurately defining VOC and volatility is critically important because multiple metropolitan areas and states are developing low VOC (≤ 50 g/L) regulations and implementing programs that reduce VOC emissions in order to attain the federal and state ambient air quality standards for ozone.
Test methods are often incorporated into these regulations and programs that define volatility by specifying analytical procedures and parameters (time, temperature, reference material, column polarity, etc.) for determining VOC content. Consequently, the different test methods may result in inconsistent volatility classifications for identical compounds, potentially resulting in ineffective environmental regulations or exclusion of alternative compounds in formulations.
ASTM Standard Test Method D 2369 (D2369) is the original test method for determining the VOC content of coatings. D2369 initially required specimens to be heated at 110°C for 20 min in a forced-draft oven, which limited the definition of volatility to the relatively low boiling point solvents that evaporated under these conditions. Coatings at that time were predominantly formulated with high vapor pressure solvents such as methyl ethyl ketone and toluene. With the adoption of D2369, manufacturers seeking to lower VOC content altered their formulations to include lower vapor pressure solvents, water, higher solids content, and multicomponent coatings. The original test method parameters did not yield accurate or precise results for the newer waterborne and high-solids coatings; therefore, the test time was extended to 60 min (CitationBrezinski, 1993). This time extension not only resolved the accuracy and precision issues, but also redefined which organic compounds were considered volatile. In October 1980, the U.S. Environmental Protection Agency (EPA) published M24 (CitationU.S. EPA, 2000) which incorporated D2369 with the extended test time. Generally, M24 is the accepted standard for VOC testing for all surface coatings, lubricants, cleaning materials,and some inks.
M24 determines the VOC content of a coating by measuring the water, exempt compound, and nonvolatile fractions, with the remainder calculated as VOCs. For low-VOC coatings, as the water, exempt compound, and nonvolatile fractions of the coating approach 100%, the precision of M24 becomes unreliable. To address this issue, gas chromatography (GC) methods, such as ASTM Standard Test Method D 6886 (D6886) and M313, have been developed (CitationJones and Wills, 2009). GC methods reduce these uncertainties by directly measuring the VOC present in the coating. Another difference between M24 and GC methods is that GC methods classify individual compounds as only volatile or nonvolatile, with no semivolatile category. Implementation of M313 includes methyl palmitate as an endpoint, primarily to reproduce VOC results from M24 (CitationMorris, 2010).
The South Coast Air Quality Management District (SCAQMD) found that for semivolatile metalworking fluids, M24 was unable to yield reproducible VOC content results and GC methods were difficult to implement (CitationMorris, 2010). In an effort to more accurately and precisely measure VOC, SCAQMD evaluated alternative approaches, including thermogravimetric analysis (TGA), to measure VOCs in semivolatile fluids. The time and temperature parameters for the TGA approach were selected to simulate the results of W. S. Dodge Oil’s (Maywood, CA) 6-month evaporation study conducted on naphthenic base oils (CitationBurke et al., 2010). Subsequently, these TGA parameters were incorporated into ASTM Standard Test Method E 1868-10 (E1868) and approved by SCAQMD for VOC determination of metalworking fluids and direct-contact lubricants. While TGA was an innovative approach to measuring the VOC content of non-film-forming metalworking fluids, it may have limited applicability for paint and coatings due to the presence of water and exempt compounds.
Many regulatory agencies and third-party certification agencies have attempted to define VOC by establishing endpoints based on physical properties to exclude chemicals that do not evaporate or volatilize in a reasonable time under ambient conditions. The U.S. EPA exempts solvents in consumer products with a vapor pressure of 0.1 mm Hg or less at 20°C, concluding that those compounds have little or no volatility and that an exemption will not result in significant VOC emissions and contribute to ozone formation (CitationU.S. EPA, 1998). However, the measurement for vapor pressure becomes difficult at values below 7.5 mm Hg (CitationRuzicka, Fulem, and Ruzicka, 2008), and most vapor pressure endpoints are 10 to 100 times lower. In efforts to develop a more easily measured surrogate for vapor pressure, boiling point and number of carbon atoms have been substituted. The California Air Resources Board (CARB) exempts low vapor pressure (LVP) solvents in consumer products with a vapor pressure less than 0.1 mm Hg, a boiling point greater than 216°C, or more than 12 carbon atoms (CitationCARB, 2011). The U.S. EPA and Ozone Transport Commission (OTC) exempt LVP solvents with a vapor pressure less than 0.1 mm Hg, those with more than 12 carbon atoms, or those that have a melting point higher than 20°C and do not sublime (CitationU.S. EPA, 1998; CitationOTC, 2006). The European Union (EU) and Canada exempt solvents with a boiling point greater than 250°C (European Union, 2004; CitationHealth Canada, 1995). Green Seal exempts solvents with a boiling point greater than 280°C (CitationGreen Seal, 2010). Some regulatory agencies utilize maximum incremental reactivity (MIR) values to determine a compound’s contribution to ozone formation, as well as a parameter to define what is considered an exempt solvent. MIR measures the relative photochemical reactivity of chemicals on a common, continuous scale. MIR values are typically expressed in mass of additional ozone formed per mass of VOC added to the emissions (CitationCarter, 2010).
A study was conducted to examine the volatility of several compounds and a few formulated products using several commonly applied VOC test methodologies—M313, E1868, and M24—and ambient evaporation. The term “compound” as used in this work is intended to include formulated products. The compounds tested are often used in paints, coatings, metalworking fluids, and consumer products. For example, propylene glycol, ethylene glycol, and 2,2,4-trimethylpentanediol diisobutyrate are common solvents in architectural coatings. Light distillates are used in metalworking fluids and consumer multipurpose solvents. Benzyl alcohol and n-methyl-2-pyrrolidone (NMP) are used in consumer paint stripping products. Other compounds are included as marker compounds. The results of the test methods were compared to the ambient evaporation results. Ambient evaporation results were also compared to VOC endpoints used by various regulatory agencies and third-party certifiers. Ambient conditions were chosen as the comparative standard because these are the conditions in which these products are most commonly used. This study provides a comparative review of compounds and may not reflect their behavior in other complex blends or mixtures. This paper discusses the strengths and weaknesses of each test method and regulation. The results from this work may be useful in refining each test method and regulation to more accurately reflect real-world emissions of VOC-containing products.
Materials and Methods
Manufacturer, purity, number of carbon atoms, boiling point, vapor pressure, and MIR values (CitationCarter, 2010) of compounds used in this study are listed according to ambient evaporation rate in .
Table 1. Compound information listed by evaporation rate
Ambient evaporation (ambient evap)
Ambient evaporation experiments were carried out in duplicate in a non-climate-controlled interior storage space with no discernible air flow. Approximately 1 g of neat compounds was added to 9-mm-diameter petri dishes (Gelman Sciences, Ann Arbor, MI). For compounds that reacted with the petri dish, the experiment was repeated using 64-mm-diameter aluminum weighing dishes (Fisher Scientific, Hampton, NH) that had been preconditioned at 110°C for 24 hr. Average compound weights, time, temperature, and humidity were recorded daily for the first month and weekly thereafter. The number of days required for complete evaporation of the compound or the weight percent remaining at 6 months is reported in order of evaporation from fastest to slowest. The average ambient temperature was 24°C with a high of 30°C and a low of 20°C. The humidity ranged between 16% and 54% with an average humidity of 41%.
SCAQMD method 313 (M313)
M313 experiments were carried out on a Finnigan Trace GC with a Finnigan Trace mass spectrometer (MS) equipped with a Thermo Finnigan AS 2000 liquid autosampler (Thermo Fisher Scientific, Inc., Waltham, MA) and an Agilent DB-624, 30 m × 0.32 mm × 0.25 μm column (Agilent Technologies, Inc., Santa Clara, CA). The initial oven program was held at 40°C for 5.5 min, then ramped to 95°C at 3°C/min, then ramped to 225°C at 25°C/min and held for 10.97 min. Inlet temperature was set at 190°C with helium carrier gas flow set at 4.5 mL/min operated in constant flow mode. A 1-μL split injection with a split flow of 36 mL/min was made. The transfer line temperature was set at 250°C. The detector temperature was set at 200°C. Compounds were qualitatively diluted in tetrahydrofuran and analyzed by GC/MS/flame ionization detection (FID) for their retention times. M313 uses methyl palmitate as a retention time standard. All compounds that elute before methyl palmitate are calculated as volatile and those that elute with or after are calculated as nonvolatile.
ASTM Standard Test Method E 1868-10 (E1868)
E1868 experiments were carried out according to SCAQMD Rule 1144 specifications on a STA 449 F1-Jupiter (NETZSCH Instruments, Inc., Burlington, MA) equipped with: a silicon carbide furnace with a type S thermocouple and a thermogravimetric (TG) sample carrier with a radiation shield, a 10-mm aluminum oxide slip-on plate, a Q5000 100-μL platinum pan (TA Instruments, New Castle DE), and a type S thermocouple. Neat compounds were analyzed and volatilization was reported as weight percent nonvolatile. E1868 calculates volatility by converting weight percent loss at the end of 110 min at 81°C in a TGA into VOC content.
U.S. EPA Reference Method 24 (M24)
M24 experiments were carried out according to D2369 in a Thermolyne mechanical oven (Thermo Fisher Scientific, Inc., Waltham, MA). Neat compounds were analyzed and volatilization was reported as weight percent nonvolatile.
Results and Discussion
Ambient evaporation
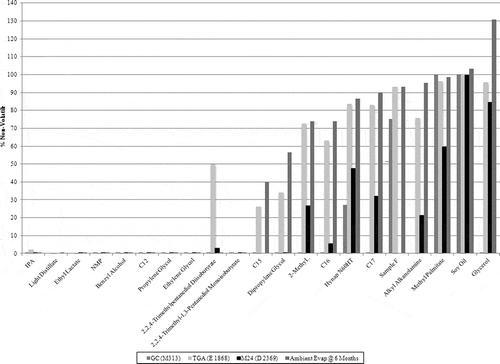
For the purpose of this study, the compounds are evaluated at 6 months. Using this as the reference point, the results from the ambient evaporation study delineated the compounds into three categories: volatile, nonvolatile, and semivolatile. A comparison of the test results are depicted in .
Volatile compounds
A volatile compound is defined as a compound that evaporates more than 95% by weight within 6 months under ambient evaporation testing conditions. Isopropyl alcohol (IPA), light distillate, and ethyl lactate all evaporate completely within 2 days. It takes 10 days for complete evaporation of NMP, 2 weeks for benzyl alcohol to fully evaporate, and C12 takes 16 days to completely evaporate. The remainder of the volatile compounds—propylene glycol, ethylene glycol, 2,2,4-trimethylpentanediol diisobutyrate, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate—are fully evaporated by 6 months.
Nonvolatile compounds
A nonvolatile compound is defined here as a compound that evaporates less than 5% by weight in 6 months under ambient evaporation testing conditions. Alkyl alkanolamine, methyl palmitate, soy oil, and glycerol all fall under this category. Several compounds had minor weight gain during the study. In the case of glycerol, significant weight gain was noticed, particularly during periods of higher humidity. This weight gain can probably be attributed to glycerol’s hygroscopic properties (CitationReAgent, 2010). Further evaluation of this issue is planned in future studies, including testing the compound in a moisture-free environment.
Semivolatile compounds
A semivolatile compound is defined here as a compound that evaporates between 5 and 95% by weight during the 6 months under ambient evaporation testing conditions. The following compounds exhibit semivolatile behavior: C15, dipropylene glycol, 2-methyl hexadecane, C16, Hynap N60HT, C17, and a formulated naphthenic-based metalworking fluid (MWF).
Comparing ambient evaporation results to various test methods
compares ambient evaporation results to the VOC test methods results. Several observations can be made about current test methods when comparing them to the results of the ambient evaporation study. For the purpose of this discussion, E1868 and M24 will have the same definition for volatile (evaporates more than 95% by weight), nonvolatile (evaporates less than 5% by weight), and semivolatile (evaporates between 5 and 95% by weight) as used for ambient evaporation.
Table 2. Combined results: ambient evaporation, GC, TGA, and M24
All methods agree on most of the volatile compounds—IPA, light distillate, ethyl lactate, NMP, benzyl alcohol, C12, propylene glycol, ethylene glycol, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate.
Soy oil is nonvolatile by every test method.
When compared to ambient evaporation results, M24 shows higher volatility. Of the 18 compounds where M24 and ambient evaporation data are available, at 6 months, the average difference is 24%, with the largest discrepancy being 74% for alkyl alkanolamine.
Of the three test methods, M313 differs the most with ambient evaporation results in the semivolatiles region.
M313 is the only test method that classifies glycerol as a volatile. Ambient evaporation and TGA show glycerol as a nonvolatile. Glycerol is a semivolatile by M24.
Of the three test methods, the results from E1868 agree most closely with the ambient evaporation results, particularly for the semivolatiles. Of the 21 compounds where E1868 and ambient evaporation data are both available, the average difference is 6.6% at 6 months, with the largest discrepancy 50% for 2,2,4-trimethylpentanediol diisobutyrate.
Comparing ambient evaporation results to physical properties
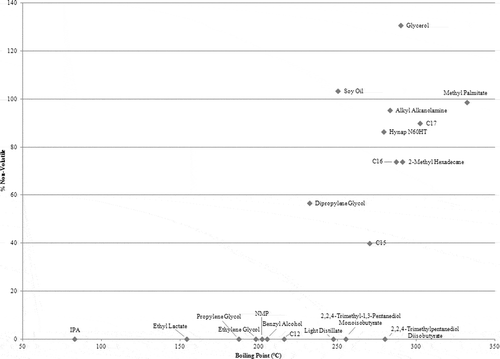
Boiling point
shows the correlation between a compound’s ambient evaporation and its boiling point. Compounds with a boiling point of less than 220°C—IPA, ethyl lactate, propylene glycol, ethylene glycol, NMP, benzyl alcohol, and C12—are volatile. The four nonvolatile compounds—soy oil, alkyl alkanolamine, glycerol, and methyl palmitate—have boiling points of 250°C, 283°C, 290°C, and 332°C, respectively. Compounds with boiling points between 220°C and 280°C fall under all three volatility categories: nonvolatile (soy oil), volatile (light distillate, 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate, and 2,2,4-trimethylpentanediol diisobutyrate), and semivolatile (dipropylene glycol, C15, and Hynap N60HT). There are no volatile compounds with a boiling point greater than 280°C, only semivolatile (C16, 2-methyl hexadecane, C17, and MWF) and nonvolatile (alkyl alkanolamine, glycerol, and methyl palmitate) compounds.
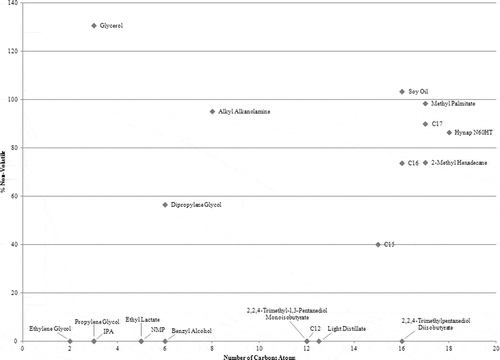
Number of carbon atoms
The correlation between a compound’s ambient evaporation with its number of carbon atoms is shown in . With the exception of glycerol, dipropylene glycol, and alkyl alkanolamine, all compounds with 12 carbon atoms or fewer are volatile. Compounds with more than 12 carbon atoms fall under all three volatility categories: nonvolatile (soy oil and methyl palmitate), volatile (light distillate and 2,2,4-trimethylpentanediol diisobutyrate), and semivolatile (C15, C16, 2-methyl hexadecane, C17, and Hynap N60HT).
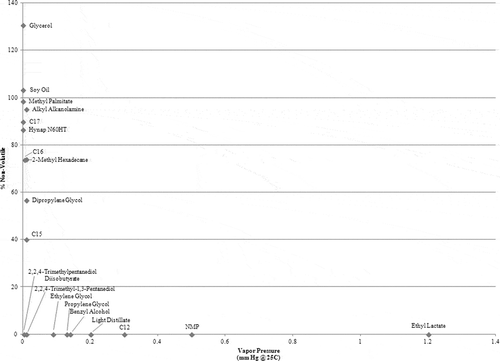
Vapor pressure
(excluding IPA) shows the correlation between a compound’s ambient evaporation with its vapor pressure. Nearly all of the compounds tested have vapor pressures below 7.5 mm Hg at 25°C, at which point the vapor pressure becomes difficult to measure. Compounds with a vapor pressure greater than 0.1 mm Hg at 25°C—propylene glycol, benzyl alcohol, light distillate, C12, NMP, ethyl lactate, and IPA are volatile. Compounds with a vapor pressure less than 0.1 mm Hg at 25°C fall under all three categories: nonvolatile (methyl palmitate, soy oil, glycerol, and alkyl alkanolamine), volatile (ethylene glycol, 2,2,4-trimethylpentanediol diisobutyrate, and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate), and semivolatile (C15, dipropylene glycol, 2-methyl hexadecane, C16, Hynap N60HT, C17, and MWF).
Summary of results
summarizes each compound’s volatility by ambient evaporation, test method, and regulatory standard.
Table 3. Compound volatility by test method and regulatory standard
Ambient evaporation versus M313 (GC)
Ambient evaporation and M313 both agree on the volatile compounds: IPA, light distillate, ethyl lactate, NMP, benzyl alcohol, C12, propylene glycol, ethylene glycol, 2,2,4-trimethylpentanediol diisobutyrate, and 2,2,4-trimethyl- 1,3-pentanediol monoisobutyrate. Of the four compounds listed as nonvolatile—alkyl alkanolamine, methyl palmitate, soy oil, and glycerol—M313 calculates alkyl alkanolamine and glycerol as volatiles because they elute before methyl palmitate. All but one of the semivolatile compounds—C15, dipropylene glycol, 2-methyl hexadecane, C16, and C17—are the compounds for which ambient evaporation and M313 are not completely in agreement; these semivolatile compounds by ambient evaporation are considered volatile by M313. The reason for this discrepancy is that M313 does not have a semivolatile cutpoint for individual compounds and can classify them as volatile or nonvolatile. The formulated products HyNap N60HT and MWF are classified as semivolatile by M313 because they are formulated with a mixture of volatile and nonvolatile compounds.
Ambient evaporation versus E1868 (TGA)
Two compounds are categorized differently between ambient evaporation and E1868: 2,2,4-trimethylpentanediol diisobutyrate and alkyl alkanolamine. 2,2,4-Trimethylpentanediol diisobutyrate is a volatile compound according to ambient evaporation but is a semivolatile according to E1868. Alkyl alkanolamine is nonvolatile under ambient evaporation but semivolatile according to E1868.
Ambient evaporation versus M24
M24 calculates volatility by converting weight percent loss at the end of 60 min at 110°C in a forced draft oven into VOC content. M24 categorizes C15 and dipropylene glycol as volatiles, whereas they are semivolatiles by ambient evaporation. Alkyl alkanolamine, methyl palmitate, and glycerol are considered semivolatiles by M24 and nonvolatile by ambient evaporation.
Ambient evaporation versus U.S. EPA, CARB, and OTC
The U.S. EPA, CARB, and OTC exempt LVP solvents with a vapor pressure less than 0.1 mm Hg, a boiling point of 216°C, or 12 or more carbon atoms. Only IPA, ethyl lactate, NMP, and C12 are volatiles according to the U.S. EPA, CARB, and OTC; other compounds tested are treated as nonvolatile, whereas ambient evaporation testing showed otherwise.
Ambient evaporation versus EU and Canada
The EU and Canada exempt solvents with a boiling point greater than 250°C. Two compounds—2,2,4-trimethylpentanediol diisobutyrate and 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate—are treated as nonvolatile by the EU and Canada, but they are volatile by ambient evaporation. Dipropylene glycol is considered a volatile by the EU and Canada, but is a semivolatile by ambient evaporation. C15, 2-methyl hexadecane, C16, Hynap N60HT, C17, and MWF are semivolatile by ambient evaporation but considered nonvolatile by the EU and Canada.
Ambient evaporation versus Green Seal
Green Seal exempts solvents with a boiling point greater than 280°C. Green Seal treats 2,2,4-trimethylpentanediol diisobutyrate as nonvolatile, while it is a volatile by ambient evaporation. Three compounds—C15, dipropylene glycol, and Hynap N60HT—are volatile by Green Seal but semivolatile by ambient evaporation. Four compounds—2-methyl hexadecane, C16, C17, and MWF—are nonvolatile by Green Seal but semivolatile by ambient evaporation. Soy oil is considered a volatile by Green Seal but is nonvolatile by ambient evaporation.
Conclusion
The volatility of several compounds and a few formulated products is studied by M313, E1868, M24, and ambient evaporation. The study shows a definite distinction between nonvolatile, semivolatile, and volatile compounds.
Volatile compounds, those that show more than 95% by weight evaporation under ambient conditions at 6 months, are completely available to form ozone commensurate to their individual reactivity rate. The study demonstrates that some LVP solvents being categorized as nonvolatile clearly volatilized at ambient conditions, nearly as rapidly as the traditional high-volatility solvents they are meant to replace. Light distillate evaporates at a rate nearly identical to that of IPA. Benzyl alcohol evaporates at nearly a rate nearly identical to that of NMP. IPA and NMP are both commonly accepted as volatile solvents, while light distillate and benzyl alcohol would be likely respective LVP replacements. Assuming similar use and reactivity rates, on a pound-for-pound conversion from commonly accepted volatile solvents to LVP solvents, there potentially may be no reduction in emissions or ozone formation.
Nonvolatile compounds, those that show less than 5% by weight evaporation under ambient conditions at 6 months, are generally not available to contribute to ozone formation. Methyl palmitate and glycerol are clearly nonvolatile, though they are often calculated as VOCs in traditional test methods. Due to its negligible evaporation rate, methyl palmitate is a suitable endpoint for GC VOC test methods; it elutes just after volatile and semivolatile compounds. Alkyl alkanolamine, bio-based oils (i.e., soy oil), and glycerol are sometimes considered volatile despite their extremely low evaporation rates and thus may be unnecessarily excluded as possible alternative solvents.
Semivolatile compounds, those that show between 5 and 95% by weight evaporation under ambient conditions at 6 months, are the most difficult category of compounds to classify. Regulatory exemptions and GC test methods define a compound as either volatile or nonvolatile. Semivolatile compounds challenge the pass or fail nature of endpoints in regulatory exemptions and GC VOC methods. In these cases, the choice of an endpoint may exempt one compound, allowing unrestricted use, while prohibiting another, even though they have nearly identical evaporation profiles. As an example, 2,2,4-trimethylpentanediol diisobutyrate has a boiling point of 280°C, completely evaporates in 147 days in the ambient evaporation study, and is not considered a VOC solvent by Green Seal. On the other hand, 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate has a boiling point of 255°C, is considered a VOC by Green Seal, but evaporates more slowly than 2,2,4-trimethylpentanediol diisobutyrate (154 days). A more equitable solution may be to assign partial VOC content values to semivolatile materials in GC methods and to avoid pass-or-fail exemption criteria.
This study provides ample evidence and findings to warrant a reevaluation of regulatory standards. Current definitions may lead to regulations that exclude otherwise viable alternatives or allow substitutions of chemicals that may limit the environmental benefits sought in the regulation. Boiling point and number of carbon atoms do not appear to be a reliable indicator of volatility. Vapor pressure may correlate well with volatility but at a value much lower than 0.1 mm Hg at 20°C, which is problematic to accurately measure. M24 results show higher volatility than any of the other test methods. E1868 results most closely replicated ambient evaporation results. However, more work is necessary to refine TGA parameters that better align with ambient evaporation studies. GC methods for determining VOC should incorporate an endpoint, such as methyl palmitate, to avoid overestimating VOC content from clearly nonvolatile compounds. Additionally, GC VOC methods should consider excluding compounds that elute early but do not evaporate in a reasonable time under ambient conditions, particularly glycerol. Further work is necessary to address semivolatile materials in GC VOC methods to reflect their contributions to ozone formation. Finally, efforts to establish physical property endpoints, such as vapor pressure, carbon number, or boiling point, should be carefully assessed to ensure that the exemption of LVP solvents is truly based on their lack of availability to participate in atmospheric photochemical reactions.
Acknowledgment
The authors thank and recognize the analytical expertise of the SCAQMD laboratory staff, Joan Niertit and Rudy Eden. Gratitude and appreciation is extended to Heather Farr for her organizational and planning expertise.
Additional information
Notes on contributors
Uyên-Uyên T. Võ
Uyên-Uyên T. Võ is an air quality chemist in the Science and Technology Advancement division at the South Coast Air Quality Management District in Diamond Bar, CA.
Michael P. Morris
Michael P. Morris is an air quality specialist in the Planning, Rule Development, and Area Sources division at the South Coast Air Quality Management District in Diamond Bar, CA.
References
- Brezinski, J. 1993. Manual on Determination of Volatile Organic Compound (VOC) Content in Paints, Inks, and Related Coating Products, 2nd ed. Philadelphia, PA: American Society for Testing and Materials.
- Burke, J., Pearce, M., Blithe, B., and Howell, J. 2010. A new method for the determination of volatile organic compounds in metalworking fluids, vanishing oils and rust inhibitors. Society of Tribologists and Lubricant Engineers. http://www.stle.org/assets/document/New_method_for_VOCs_in_MWFs_and_rust_inhibitors.pdf (accessed June 12, 2012).
- California Air Resources Board. 2011. Regulation for reducing emissions from consumer products. (Online). Sacramento, CA, December. http://www.arb.ca.gov/consprod/regs/fro%20consumer%20products%20regulation.pdf (accessed December 18, 2011).
- Carter, W. 2010. Updated Maximum Incremental Reactivity Scale and Hydrocarbon Bin Reactivities for Regulatory Applications. California Air Resources Board, Sacramento, CA, January. http://www.arb.ca.gov/research/reactivity/mir09.pdf (accessed June 12, 2012).
- Green Seal. GS-11 Green Seal Standards for Paints and Coatings. (Online). Washington, January 2010. http://www.greenseal.org/Portals/0/Documents/Standards/GS-13/GS-11_Paints_and_Coatings_Standard_Third_Edition.pdf (accessed February 7, 2011).
- Health Canada. 1995. Indoor Air Quality in Office Buildings: A Technical Guide. Ontario, Canada. http://www.publications.gc.ca/collections/Collection/H46-2-93-166Erev.pdf (accessed May 7, 2013).
- Jones, D., and M. Wills. 2009. Development of an Improved VOC Analysis Method for Architectural Coatings, California Air Resources Board, Sacramento, CA, February. http://www.arb.ca.gov/research/seminars/jones2/jones.pdf (accessed February 7, 2012).
- Morris, M. 2010. Final Staff Report for Proposed Rule 1144—Metalworking Fluids and Direct Contact Lubricants. South Coast Air Quality Management District. Diamond Bar, CA, July.
- Office Journal of the European Union. 2004. Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain paints and varnishes and vehicle refinishing products and amending Directive 1999/13/EC. O J L 143. Brussels, April. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:143:0087:0096:EN:PDF (accessed June 12, 2012).
- Ozone Transport Commission. 2006. OTC Model Rule for Consumer Products. Washington, DC, September. http://www.otcair.org/upload/Documents/Model%20Rules/OTC%20CP%20Model%20Rule%202012%20CLEAN_vs2010.2012%2005%2010.pdf (accessed June 12, 2012).
- ReAgent, Cheshire, 2010. Glycerol Tech MSDS. http://www.reagent.co.uk/uploads/documents/GLYCEROL-TECH-MSDS.pdf (accessed June 12, 2012).
- Ruzicka, K., M. Fulem, and V. Ruzicka. 2008. Vapor Pressure of organic compounds. Measurement and correlation. Department of Physical Chemistry, Institute of Chemical Technology, Prague. http://www.vscht.cz/fch/Kvetoslav.Ruzicka/ICTP_VaporPressureGroup.pdf (accessed June 12, 2012).
- U.S. Environmental Protection Agency. 1998. National Volatile Organic Compound Emission Standards For Consumer Products—Background for Promulgated Standards. Washington, DC, August. Available: http://www.epa.gov/ttn/oarpg/t1/reports/cpbid.pdf (accessed June 12, 2012).
- U.S. Environmental Protection Agency. 2000. Method 24—Determination of Volatile Matter Content, Water Content, Density, Volume Solids, and Weight Solids of Surface Coatings. (Online). Washington, DC, February. http://www.epa.gov/ttn/emc/promgate/m-24.pdf (accessed December 1, 2011).