Abstract
Continuous monitoring of exhaust flue gas has become a common practice in power plants in response to Federal Mercury and Air Toxics Standards (MATS) standards. Under the current rules, hydrochloric acid (HCl) is not continuously measured at most plants; however, MATS standards have been proposed for HCl, and tunable diode laser (TDL) absorption spectroscopy is one method that can be used to measure HCl continuously. The focus of this work is on the evaluation and verification of the operation performance of an HCL TDL over a range of real-world operating environments. The testing was conducted at the University of California at Riverside (UCR) spectroscopy evaluation laboratory. Laboratory tests were conducted at three separate temperatures, 25ºC, 100ºC, and 200ºC, and two distinct moisture levels for the enhanced temperatures, 0%, (2 tests) and 4%, over a concentration range from 0 ppmv to 25 ppmv-m at each of the elevated temperatures. The results showed good instrument accuracy as a function of changing temperature and moisture. Data analysis showed that the average percentage difference between the ammonia concentration and the calibration source was 3.33% for varying moisture from 0% to 4% and 2.69% for varying temperature from 25 to 100/200ºC. An HCl absorption line of 1.742 μm was selected for by the manufacturer for this instrument. The Hi Tran database indicated that CO2 is probably the only major interferent, although the CO2 absorption is very weak at that wavelength. Interference tests for NO, CO, SO2, NH3, and CO2 for a range of concentrations typical of flue gasses in coal-fired power plants did not show any interference with TDL HCl measurements at 1.742 μm. For these interference tests, CO2 was tested at a concentration of 11.9% concentration in N2 for these tests. Average precision over the entire range for all 10 tests is 3.12%.
Implications: The focus of this study was an evaluation of the operation performance of a tunable diode laser (TDL) for the measurement of hydrochloric acid (HCl) over a range of real-world operating environments. The results showed good instrument accuracy as a function of changing temperature from 25ºC to 200ºC and moisture from 0% to 4%. Such as an instrument could be used for continuous monitoring of exhaust flue gas in power plants once the Federal Mercury and Air Toxics Standards (MATS) standards have been fully implemented.
Introduction
In the United States, consent decrees, state laws, and the proposed Federal Mercury and Air Toxics Standards (MATS) standards for coal-fired plants have driven the need for robust (U.S. Environmental Protection Agency [EPA], 2013), accurate, and certifiable continuous emissions monitors (CEMS) for mercury, particulate matter (PM), ammonia (NH3), and acid gases. One of the key elements of successful control programs is the development of instrumentation that is simple to use, robust, and accurate under a range of different operating environments. The application of tunable diode laser absorption spectroscopy (TDLAS) for the measurement of a wide range of species has expanded considerably over the past decade (CitationHimes et al., 2006; CitationVon Drasek et al., 2006). TDLAS provides advantages in that the laser can be tuned to a wavelength that is very specific to a particular species of interest (CitationAhlber et al., 1994; CitationSchiff et al., 1994; CitationFried et al., 1991). TDLAS spectroscopy is now one of the main techniques utilized for monitoring and controlling NH3 emissions from power plants and other stationary source generation applications (CitationHimes et al., 2006).
As new rules have expanded the monitoring needs within a power plant, it has been important to evaluate instrument performance both in field and in the laboratory. Hydrochloric acid (HCl) is another species that can readily be measured with TDLAS. Under the current rules, hydrochloric acid (HCl) is not continuously measured at most plants. However, as MATS standards have been proposed for HCl, it is likely that many plants will elect to monitor HCl continuously as well. The typical level of HCl expected at plants with highly controlled stack emissions is expected to be much less than 2 ppm. With such low emission levels, it is important to understand the limit of detection and any potential measurement interferences for these instruments. As this application is only beginning to potentially expand, experience with instruments for this purpose is more limited. Also, the sensitivity and reproducibility requirements for the needed measurements are more stringent, as, unlike the NH3 measurements that are used for process measurements, the acid gas measurements are for CEMS applications, and ultimately would need to meet U.S. EPA requirements.
For the NH3 application, TDLAS has undergone extensive testing for power plant applications (CitationHimes and Pisano, 2009; CitationHimes et al., 2012). This includes both laboratory testing, as well as field testing and installation. The University of California at Riverside (UCR), in conjunction with the Electric Power Research Institute (EPRI), has constructed a laboratory facility for simulating in situ measurements for instrument evaluations (CitationHimes et al. 2006; CitationHimes and Pisano, 2007a; CitationHimes and Pisano, 2007b; CitationHimes and Pisano, 2007c; CitationHimes et al., 2009; CitationHimes and Pisano, 2009; CitationHimes et al., 2012; CitationDene et al., 2011). The role of the laboratory was to verify the response of various tunable diode laser (TDL)-based instrumentation over temperature ranges and moisture levels that are representative in typical flue gases at coal-fired power plants. For the laboratory experiments, gas mixtures with temperatures between 300 and 400°C and up to 12% moisture were typically utilized, which represents flue gas conditions in a power plant exhaust stream found past the economizer but prior to the air heater. The laboratory apparatus was constructed and finalized in 2007 and has been used to successfully evaluate eight different TDL instruments for NH3 from six manufacturers (CitationHimes et al. 2006; CitationHimes and Pisano, 2007a; CitationHimes and Pisano, 2007b; CitationHimes and Pisano, 2007c; CitationHimes et al., 2009; CitationDene et al., 2011).
The focus of this work is on the evaluation and verification of the operational performance and specifications for an HCl instrument over a range of real-world operating environments (CitationDene et al., 2011). This includes verification of detection limits and testing for potential interfering species. The testing was conducted at the UCR spectroscopy evaluation laboratory. The laboratory was modified to be able to measure acid gases, such as HCl, HF, and HBr. For these types of gases, the flue gas temperatures are normally lower at 100–200°C at the point in the stack where the HCl is to be measured. For this study, evaluations were conducted at temperatures of 25°C, 100°C, and 200°C, and for moisture levels of 0% and 4%. The 4% moisture level tests were only conducted at 100°C and 200°C.
Test Methods
Instrument
The instrument evaluated for this study was a Unisearch Associates, Inc., LasIR 410 S-Series (CitationMackay et al., 2010). This instrument was chosen because it is already being used at one coal-fired power plant site for continuous HCl measurements. A similar instrument has also performed satisfactorily during the EPRI sponsored NH3 evaluations.
Experimental setup
shows the laboratory setup, including the sample cell and the radiative heater. Measurements were made in a sample cell that was 1.0 m long with an ID of 5.0 cm. This sample cell is typical of those commonly used to facilitate in situ flue gas TDL measurements for HCl. It is designed to accommodate varying flows of a source gas and dilution air to provide different gas concentrations. The sample cell is made of electropolished 314 stainless steel and is quartz lined. It is mounted on a rigid assembly that is bolted to the structural beams of the laboratory. The sample cell has a path length of 1.00 m for single-pass optics, or 2.00 m for dual-pass optics. Each end of the sample cell is equipped with standard 4-inch flanges. This cell has a sufficient diameter for a TDL beam to traverse the path length. When multiplied by the 1-m path length, HCl can be detected in units of ppmv-m. The sample cell is maintained at atmospheric pressure.
Figure 1. Experimental setup with Unisearch Optics mounted, instrument tested, and sample cell with base path of 1.0 m, radiative heater, and white insulation.
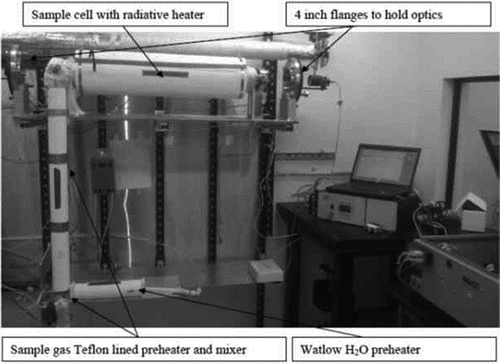
The sample cell has a radiative heater, is insulated, and is capable of a temperature range of 300–700ºK. The heater operates on 120 VAC and can deliver over 25 W/inch2 to the apparatus. Type K thermocouples (TCs) are used for all temperature measurements in the system. The temperature controllers used in the system are from Omega Engineering. An error analysis shows an overall system accuracy for temperature measurement of ± 3.15ºC up to 277ºC and ± 3.27ºC at 315ºC.
The output signals from the mass flow controllers (MFCs) for the source gas flow and the dilution air flow and five thermocouples are recorded on a Campbell CR10 data logger using seven defined channels. This system provided instantaneous readouts, plus a 1-min average from each channel. The collected data from each test run were then converted to Excel files for data validation and interpretation. The HCl concentration is calculated from the cylinder concentration, the span gas flow rate, and the dilution air flow rate and is checked using a Unisearch LasIR Two-Tone FM (TTFM) TDL as a reference.
Calibration and gas and water delivery systems
The calibration system provides precise concentrations of the HCl target gas by utilizing precision gas dilution. The TECO 146C Calibrator employs a central processor unit (CPU) and controlled precision MFCs. A Linde certified gas cylinder containing 100 ppmv of HCl gas in an inert (nitrogen) carrier provided the source gas through a precision, low-flow MFC. The source gas is mixed with zero air in predetermined flow rates using MFCs to achieve the desired concentrations. The dilution air and source gas MFCs have nominal ranges of 0–10,000 and 0–500 cm3/min, respectively. The MFC used to control the diluent air had a higher volume capability (approximately 5 standard liters per minute [slpm]) than the MFC used to control the source gas (approximately 100 standard cubic centimeters per minute [sccm], or 0.1 slpm). After the HCl is mixed with dilution air, the effluent is heated to the desired temperature.
Gaseous water is introduced into the blended HCl sample gas stream to provide the desired water content. The water generation system consists of a water reservoir, a graduated burette, a positive displacement pump, and a heating system. The pump speed can be adjusted to the desired flow rate, as measured by noting the change in volume in the burette over time. The water is drawn through the pump into a 1/8-inch stainless-steel line, which is routed through a heated aluminum block with a particulate filter to gasify the water. The gaseous water effluent from the block then enters the diluted HCl gas stream.
Installation
The TDL was installed according to the manufacturer’s specifications. The laser output was checked to ensure that the output power from the TDL was 8.2 mW, as specified for this instrument. The optics were mounted and the optical alignment was verified to ensure that there were no optical artifacts, such as etalons or optical fringes, that could have degraded or altered the optical signal. This was a very important and critical step, as minimization of optical effects is an iterative procedure. For this, windows installed at seven degrees from parallel at each entrance/exit of the sample cell were required. These windows have an antireflective coating specific for the infrared (IR) wavelength range measured. The windows were then rotated to generate as flat a background signal as possible. Prior to making measurements, it was important to verify the stability of the alignment by recording the signal power for a 30-min period. The alignment was considered to be stable if the standard deviation of the signal power was less than 10% of the nominal power level.
Test matrix
The source gas levels for the evaluation were chosen to cover HCl levels that can be found over a wide range of process conditions, which was determined to be in the range of 0.5 to 5 ppmv from field tests. Since most exhaust stacks are around 5 m in diameter, and a cross-duct optical setup is required for in situ monitoring, the levels chosen were from 2.5 to 25 ppmv-m, as TDL sensitivity is nominally reported in ppmv-m, where the levels are multiplied by the path length. Since our sampling cell is only 1 m long, the values chosen for the evaluation were 0, 2.5, 5, 10, 15 and 25 ppmv, which yielded levels of 0, 2.5, 5, 10, 15 and 25 ppmv-m, similar to the levels found in coal-fired power plant flue gases.
Tests were conducted at three separate temperatures, 25ºC, 100ºC, and 200ºC, and 2 moisture levels for the higher temperatures, 0% (2 tests) and 4%. The 4% moisture level was selected since this was the highest moisture level that could be set up with the system at the time of the testing. The two tests at the 0% moisture level were done to provide a replicate for one of the test conditions. This resulted in 3 different measurements for each of the target concentrations of 0 ppmv, 2.5 ppmv-m, 5.0 ppmv-m, 10 ppmv-m, 15 ppmv-m, and 25 ppmv-m at each of the elevated temperatures. The initial tests were done at ambient temperatures with no moisture over the defined range. The 25ºC sample was used as a control only. Each test was conducted over a 15-min interval. The instrument was configured to provide 1-min measurements with a 15-min integrated response. Note that the test matrix was configured to complete all the tests at a specific temperature, varying the concentration of the target gas at each of the moistures investigated.
Additional tests were also run with other gases present in the exhaust stream that could be potential interferences or gases that could bias the TDL measurements. The possible interfering species were identified from typical known constituents of gases in coal-fired power plant exhaust streams. These were H2O, CO, CO2, NO, and SO2. Also, with the prevalence of selective catalyst reduction (SCR) and/or nonselective catalyst reduction (NSCR) technologies being employed for NOx control, possible interference from NH3 was evaluated as well.
Results
Standard and temperature corrections
The agreement of the Unisearch S Series LasIR SM410 with the HCl gas standards was assessed by taking fifteen 1-min averaged samples at each of the test matrix configurations. The values and associated standard deviations presented below are for the fifteen 1-min averages of the sample gas measurement.
A temperature correction algorithm must also be applied to the data to compensate for two other factors. All spectroscopic instruments measure a mixing ratio, which is a ratio of the number of molecules of the measured species to the total number of molecules in a specific volume. As temperature changes, the number of total molecules for a given volume changes. For example, the number of total molecules at 25ºC in 1 L of sample gas is different from the number of total molecules at 200ºC in 1 L of sample gas. An ideal gas law correction is needed to compensate the sample back to the condition of the instrument calibration. Also, the ability for any unsymmetrical polyatomic molecule to absorb energy changes with temperature, so the temperature correction algorithm must also deal with how the molecular absorption of the target molecule behaves with changes in temperature. This effect is called the line strength ratio.
The instrument is configured to read temperatures using a Type K thermocouple and automatically corrects the values for ideal gas law as well as the line strength temperature effects. For field installations, the instrument is capable of correcting for temperature based on an empirically derived algorithm. For the current study, the temperature values were simply input into the software, since only 3 discrete levels were used, 25°C, 100ºC, and 200ºC. For this study, Unisearch calibrated its instrument at 25ºC.
The results of the tests over the full range of concentration levels are presented in Table 1 and Figure 2, at 25ºC, 100ºC, and 200ºC at two moisture levels. The data were analyzed for both moisture effects and temperature effects to determine the average percentage differences. All data was used, including the interference tests discussed later, since they were part of the total data set.
Table 1. Results of Unisearch S Series LasIR SM410 for 1.0-m sampling system at 25ºC, 100ºC, and 200ºC, where the uncertainties represent one standard deviation of the measurement average
For moisture effects, the average percent difference for each matrix point was determined by the following equation:
For temperature effects, the average percent difference for each matrix point was determined by the following equation:
The average percent differences of the measured results obtained with respect to the standard gas values for the 1 meter single pass cell configuration are presented in and for the following groupings: Group 1, 1.0 m sampling cell at 100ºC; Group 2, 1.0 m sampling cell at 200ºC; and Group 3, 1.0 m sampling cell at 200ºC with interference testing.
Figure 2. Graphical representations of system response over the concentration range at 100°C and 200°C.
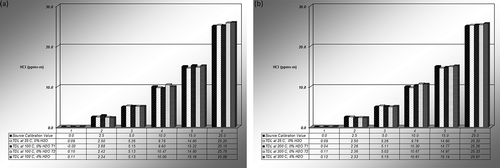
Figure 3. Graphical representation of the percent difference over the three groupings at each of the sampling levels, Group 1: 100 °C, Group 2: 200 °C, Group 3: 200 °C interference tests.
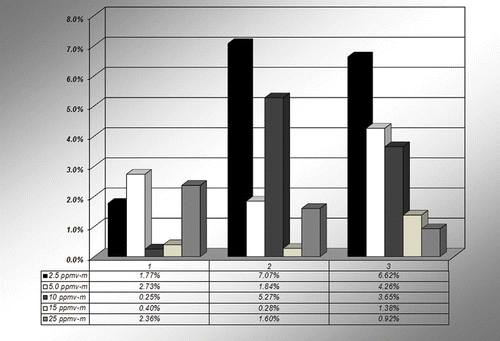
Table 2. Average percent difference of the measured results obtained with respect to the standard gas values obtained with the following grouping: Group 1: 100ºC, Group 2: 200ºC, Group 3: 200ºC interference testing
The average percentage differences are within the typical precision limits of the system itself, which are approximately 3–5%. Larger percentage differences were seen in some cases, for example, at the 2.5 ppmv-m concentration in Table 2 for 100ºC and 200ºC, and in comparing the repeat tests at the 100ºC/0% moisture tests at 2.5 ppmv-m, as shown in Table 1. These could be due to slight differences in the delivery system for specific tests, but do not appear to be distinct trends with respect to either temperature or moisture content. The overall system precision is further evaluated in the following.
IR spectroscopy of species typical of coal combustion applications
This section explores the IR spectra of possible interfering species from combustion applications and uses this to first determine theoretically whether there are any possible interferences with the HCl measurements that may result from other combustion gases. Combustion applications generate high amounts of water vapor and CO2. Typically, their absorption spectra strongly overlap with other species of interest for the combustion analyses. The difference in the concentration levels of water vapor and CO2 with respect to other combustion products can range from 1 to 5 orders of magnitude. The high-intensity spectral features of gaseous water and CO2 at high concentrations work like narrow-band-pass filters, which block significant amounts of the available light in the spectral regions they occupy. The less intense features of water and CO2 that overlap with other combustion species will normally show a nonlinear behavior in their absorption spectrum. The best wavelength window for a target species is to find suitable lines that are not perturbed by either the high concentrations of water and CO2 or other gases, such as CO, NO, NH3, and SO2.
shows typical gaseous infrared spectra of major flue gas species observed from coal-fired combustion processes. Water was tested for as part of the main test matrix, so it is not discussed further here. Temperatures of these spectra are around 200°C (392°F). Most of the reported HCl absorption lines are between 5500 and 6000 cm−1. CO2 could interfere, but the other gases of interest have much weaker absorptions past 4000 cm−1. Most of the CO2 lines weaken considerably at these wavelengths.
Figure 4. Typical gaseous infrared spectra of major flue gas species observed from coal-fired combustion processes.
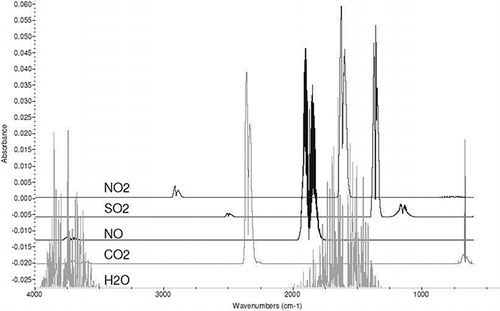
To further investigate potential interferences and which HCl lines are less likely to be interfered with from combustion gas processes, high-resolution transmission molecular absorption database (Hi Tran) database spectra were generated for the each of the prominent combustion species, as well as HCl, over the 5500 and 6000 cm−1 range (CitationGoldman et al., 2000; CitationHarvard-Smithsonian Center for Astrophysics, 2013). From the Hi Tran database evaluation, only NO and CO2 were identified as possible interfering species, with spectral features in the same region where HCl spectral features were found. For the manufacturer-selected HCl aborption line for this instrument (1.742 μm), the Hi Tran database indicated that CO2 is probably the only major interferent, although the CO2 absorption is very weak at that wavelength.
Interference tests
Laboratory tests were also conducted using gas cylinders with interfering species that had concentrations similar to those found in coal-fired power plant flue gases. The interference gases and concentrations are: NO 1510 ppmv, CO 1220 ppmv, CO2 11.9%, SO2 2050 ppmv, and NH3 100 ppmv. To simplify matters, the gas in the cylinders with the interfering species was used as the carrier gas for the HCl into the sample cell instead of zero air, which was used for the earlier tests. The results of the interferences tests are listed in and shown graphically in for a 1.0-m single-pass cell configuration. All tests were done at 200°C, but without moisture, as those tests were conducted in the earlier investigations. Tests were conducted only once at each level. Note that the system was calibrated at 25ºC, and the response at the ambient temperature was left on each plot as the control.
Figure 5. Graphical representation of system response over the concentration range at 0% moisture at 200°C during the interference tests.
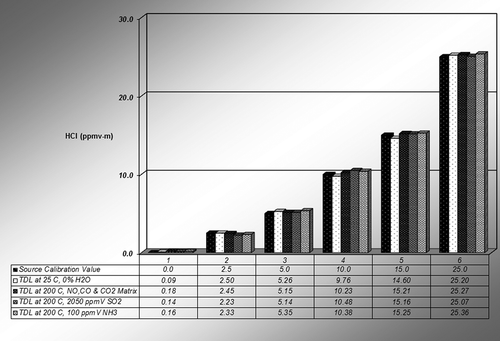
Table 3. Interfering gas test results of Unisearch S Series LasIR SM410 for 1.0-m sampling for NO, CO, CO2, SO2, and NH3 at 0% moisture and 200ºC, with the uncertainties representing one standard deviation of the measurement average
It is clear from these tests that the other gases at concentrations typical of flue gases in coal-fired power plants do not interfere with TDL HCl measurements at 1.742 μm, as there was no observed effect in the system response. The only gas that may have been of concern from the Hi Tran evaluation, CO2, also did not affect the TDL HCl response at a concentration of 11.9% CO2 in N2.
Linearity, precision, and minimum detection limits
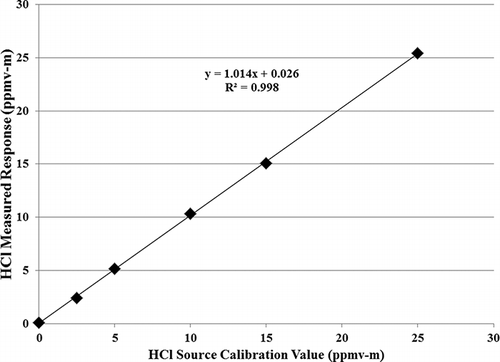
Linearity was assessed by a linear regression analysis of the 15-min averaged values for all 10 tests that were done at the same concentration over the range of moisture, temperature, and interference variables. The 10 tests include 1 test at 25ºC, 2 tests at 100ºC at 0% moisture, 1 test at 100ºC at 4% moisture, 2 tests at 200ºC at 0% moisture, 1 test at 200ºC at 4% moisture, and 3 interference tests at 200ºC and 0% moisture. Linearity is expressed in terms of slope, intercept, and coefficient of determination (R2), and is shown in .
Figure 6. Linear regression of the measured response from the Unisearch S Series LasIR SM410 as compared to the source calibration value. The measured responses are averaged from all 10 tests at each source calibration value that encompassed the range of moistures and temperatures used in this evaluation.
Precision was calculated in terms of the average percent relative standard deviation (RSD) at each concentration of the Unisearch S Series LasIR SM410 readings over the duration of each of the 10 sets included in the overall test matrix. This included 10 zero samples during each individual test. For each 15-min period, all 1-min readings from the SM410 were recorded, and the mean and standard deviation of those readings were calculated.
The average precision <P>was then determined as
Table 4. Average precision is reported for each of the ppmv-m source calibration values
The minimum detection limit is reported as three times the average standard deviation of all the 1-min zero measurements made at each of the temperatures used in the evaluation when the instrument was evaluated over the entire range of tests. The three temperatures were 25ºC (1 set of measurements, 15 points), 100ºC (3 sets of measurements, 45 points), and 200ºC (6 sets of measurements, 90 points). The minimum detection limits for the instrument at the different temperature settings were 0.35 ppmV/m at 25°C, 0.66 ppmV/m at 100°C, and 1.10 ppmV/m at 200°C. At the 5 meter path, typical to power plant installations, detection limits improved to 0.07 ppmV/m at 25°C, 0.13 ppmV/m at 100°C, and 0.22 ppmV/m at 200°C, as detection limits improve with longer path lengths. Zero drift is reported in terms of the RSD of the stable readings obtained from the Unisearch S Series LasIR SM410 in daily sampling of the same HCl standard gas and zero gas supplied to the sample cell. In total, 10 zero samples each containing fifteen 1-min samples were taken during the evaluation. Zero drift was found to be 1.78% over the 10 tests.
Conclusion
The focus of this study was on the evaluation and verification of the operational performance of an HCL TDL over a range of real-world operating environments. The testing was conducted in the UCR spectroscopy evaluation laboratory. Tests were conducted at 3 separate temperatures, 25ºC, 100ºC, and 200ºC, and 2 distinct moisture levels for the enhanced temperatures, 0% (2 tests) and 4%. Tests were conducted at target concentrations of 0 ppmv, 2.5 ppmv-m, 5.0 ppmv-m, 10 ppmv-m, 15 ppmv-m, and 25 ppmv-m at each of the elevated temperatures. A summary of the major findings and conclusions of this study is as follows:
The results showed good instrument accuracy as a function of changing temperature and moisture. Data analysis showed that the average percentage differences between the ammonia concentration and the calibration source were 3.33% for varying moisture from 0% to 4%, and 2.69% for varying temperature from 25 to 100/200ºC. These differences are within the typical precision limits for the laboratory system.
An HCl absorption line of 1.742 μm was selected by the manufacturer for this instrument. Analysis of the Hi Tran database indicated that CO2 is probably the only possible major interferent, although the CO2 absorption is very weak at that wavelength.
Interference tests for NO, CO, SO2, NH3, and CO2 for a range of concentrations typical to flue gases in coal-fired power plants did not show any interference with TDL HCl measurements at 1.742 μm, as there was no observed effect in the system response. For these interference tests, CO2 was tested at a concentration of 11.9% concentration in N2 for these tests.
Average precision over the entire range for all 10 tests is 3.12%.
The minimum detection limits were 0.35 ppmV/m at 25°C, 0.66 ppmV/m at 100°C, and 1.10 ppmV/m at 100°C for a 1 meter path and 0.07 ppmV/m at 25°C, 0.13 ppmV/m at 100°C, and 0.22 ppmV/m at 100°C for a 5 meter path.
Zero drift was found to be 1.78% over the 10 tests.
Additional information
Notes on contributors
Chuck Dene
Chuck Dene is a senior program manager at the Electric Power Research Institute, Palo Alto, CA.
John T. Pisano
John T. Pisano and Kurt Bumiller are senior development engineers, and Thomas D. Durbin is a research engineer at the Bourns College of Engineering–Center for Environmental Research and Technology at the University of California at Riverside, CA.
Thomas D. Durbin
John T. Pisano and Kurt Bumiller are senior development engineers, and Thomas D. Durbin is a research engineer at the Bourns College of Engineering–Center for Environmental Research and Technology at the University of California at Riverside, CA.
Kurt Bumiller
John T. Pisano and Kurt Bumiller are senior development engineers, and Thomas D. Durbin is a research engineer at the Bourns College of Engineering–Center for Environmental Research and Technology at the University of California at Riverside, CA.
Keith Crabbe
Keith Crabbe is a vice-president of CEMTEK Environmental, Santa Ana, CA.
Lawrence J. Muzio
Lawrence J. Muzio is a vice-president at the Fossil Energy Research Corp., Laguna Hills, CA.
References
- Ahlberg H., S. Lundqvist, R. Tell, and T. Andersson. 1994. Industrialized high sensitivity fiber-optic near-IR diode laser based gas analysis system. In Tunable Diode Laser Spectroscopy, Lidar, and DIAL Techniques for Environmental and Industrial Measurements, ed. H. I. Schiff, A. Fried, and D. K. Killinger, Proc. SPIE 2112, 118–29, Bellingham, WA: International Society for Optics and Photonics.
- Dene, C., J.T. Pisano, T.D. Durbin, and K. Bumiller. 2011. Continuous Emission Monitor for Hydrochloric Acid. Publication no. 1022083. Palo Alto, CA: EPRI.
- Fried, A., B. Henry, D.D. Parrish, J.R. Carpenter, and M.P. Buhr. 1991. Intercomparison of tunable diode laser and gas filter correlation measurements of ambient carbon monoxide. Atmos. Environ. Part A 25:2277–84. doi:10.1016/0960-1686(91)90103-E
- Goldman, A., R.R. Gamache, A. Perrin, J.-M. Flaud, C.P. Rinsland, and L.S. Rothman. 2000. HITRAN partition functions and weighted transition-moments squared. J. Quant. Spectrosc. Radiat. Transfer. 66:455–86. doi:10.1016/S0022-4073(99)00176-4
- Harvard-Smithsonian Center for Astrophysics. 2013. http://www.cfa.harvard.edu/hitran.
- Himes R., J. Pisano, and T. Durbin. 2006. Ammonia Lab Test Verification—Unisearch LasIR SM410. Publication no. 1014664. Palo Alto, CA: EPRI.
- Himes, R., and J.T. Pisano. 2007a. Ammonia Monitor Lab Test Verification—Norsk Electo Optik’s (NEO) LaserGas II TDL Monitor. Publication no. 1015315. Palo Alto, CA: EPRI.
- Himes, R., and J.T. Pisano. 2007b. Ammonia Monitor Lab Test Verification—Opsis LD500 TDL Monitor. Publication no. 1016116. Palo Alto, CA: EPRI.
- Himes, R., and J.T. Pisano. 2007c. Ammonia Monitor Lab Test Verification—Siemens LDS 6 TDL Monitor. Publication no. 1014745. Palo Alto, CA: EPRI.
- Himes, R., J.T. Pisano, and T.D. Durbin. 2009. Ammonia Monitor Lab Test Verification—LTG LIGHTWISE TDL Monitor. Publication no. 1013972. Palo Alto, CA: EPRI.
- Himes, R., and J.T. Pisano. 2009. Survey of Ammonia Monitor Applications and Experience. Publication no. 1017560. Palo Alto, CA: EPRI.
- Himes R., L. Muzio, J. Pisano, and T. Durbin. 2012. Application Guideline for Monitoring Ammonia with Tunable Diode Lasers from Coal-Fired Power Plants. Publication no. 1025347. Palo Alto, CA: EPRI.
- Mackay G., A. Chanda, and K. Mackay. 2010. Unisearch Associates Inc. LasIR System Manual. Concord, Ontario, Canada: Unisearch Associates, Inc.
- Schiff, H.I., G.I. Mackay, and J. Bechara. 1994. The Use of Tunable Diode Laser Absorption Spectroscopy for Atmospheric Measurements. Chemical Analysis Series, vol. 17. New York, NY: Wiley.
- U.S. Environmental Protection Agency. 2013. Code of Federal Regulations 40 CFR Parts 60 and 63, Reconsideration of Certain New Source Issues: National Emission Standards for Hazardous Air Pollutants from Coal- and Oil-fired Electric Utility Steam Generating Units and Standards of Performance for Fossil-Fuel-Fired Electric Utility, Industrial-Commercial-Institutional, and Small Industrial-Commercial-Institutional Steam Generating Units. http://federal.eregulations.us/rulemaking/document/EPA_FRDOC_0001-13637
- Von Drasek, W., A. Pubill-Melsio, E. Fauve, K. Muldernik, et al. 2006. Tunable Diode Sensors for Monitoring and Control of Harsh Combustion Environments. Report from American Air Liquide and Physical Sciences Inc. Under Cooperative Agreement No. DE-FC36-00CH11030. http://www.osti.gov/scitech/biblio/882872
