Abstract
Mesoporous MCM-41 was synthesized using cetyltrimethyl ammonium bromide (CTAB) as a cationic surfactant and spent quartz sand as the silica source. Modification of the mesoporous structure to create an absorbent was then completed using 3-aminopropyltrimethoxysilane. Amine-Quartz-MCM (The A-Q-MCM) adsorbents were then characterized by N2 adsorption/desorption, elemental analysis (EA), X-ray fluorescence (XRF), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), as well as the carbon dioxide (CO2) adsorption/desorption performance. In this study, spent quartz sand was utilized to synthesize Quartz-MCM (Q-MCM) and the amine functionalized material, A-Q-MCM, which exhibited a higher uptake of CO2 at room temperature compared with the nongrafted material. The results showed that Q-MCM is similar to MCM-41 synthesized using commercial methods. The surface area, pore volume, and pore diameter were found to be as high as 1028 m2/g, 0.907 cm3/g, and 3.04 nm, respectively. Under the condition of CO2 concentration of 5000 ppm, retention time of 50 cc/min, and the dosage of 1 g/cm3, the mean adsorption capacity of CO2 onto A-Q-MCM was about 89 mg/g, and the nitrogen content of A-Q-MCM was 2.74%. The adsorption equilibrium was modeled well using a Freundlich isotherm.
Implications:
In this study, spent quartz sand was utilized to synthesize Q-MCM. The amine functionalized material exhibited a higher uptake of CO2 at room temperature compared with the nongrafted material. The results showed that Q-MCM is similar to MCM-41 synthesized using commercial methods. The adsorption equilibrium was modeled well using a Freundlich isotherm.
Introduction
Global warming has spurred demand for effective, practical energy alternatives and carbon dioxide (CO2) mitigation strategies (Hansen and Houghton, Citation1998; Hoffert et al., Citation2002). Several scenarios have been proposed to mitigate global warming, including energy savings, development of renewable energy sources, expansion of natural CO2 sinks by forestation, and sequestration of anthropogenic CO2 (Kakizawa et al., Citation2001). Existing methods for capturing CO2 from pollutants include catalytic degradation, sequestration, and physical and chemical adsorption. Adsorption is considered one of the most promising methods for separating CO2 from gas mixtures, and in the last two decades numerous studies have been conducted on the separation of CO2 by adsorption. Various adsorbents, such as activated carbons, pillared clays, metal oxides, and zeolites, have been investigated (Xu et al., Citation2002). Functionalization or impregnation of amine onto porous supports has attracted much attention (Demessence et al., Citation2009; Yue et al., Citation2008; Bacsik et al., Citation2010; Sayari et al., 2010). Ordered mesoporous silica MCM-41 (Harlick et al., 2007; Son et al., Citation2008), MCM-48 (Son et al., Citation2008), SBA-15 (Son et al., Citation2008; Belmabkhout et al., Citation2010; Zeleňák et al., Citation2008a; Chang et al., Citation2009; Sanz et al., 2009; Khatri et al., Citation2006), SBA-16 (Son et al., Citation2008), and SBA-12 (Zeleňák et al., Citation2008a, 2008b) have been investigated for this purpose owing to their high pore volume, large surface area, and ease of functionalization (Beck et al., Citation1992; Zhao et al., 2008; Ying et al., Citation1999). The used materials were mixed with other catalyst to recover CO2 to produce energy, such as fuel, as mentioned in the study of Li et al. (Citation2010). Sathitsuksanoh (Citation2007) indicated that the microfibrous entrapped K2CO3/activated carbon particulate sorbent and regenerable system were used for CO2 removal to supply CO2-free gas stream for low temperature and low CO2 concentration applications, such as alkaline fuel cells, metal-air batteries, and portable air-purifying respirators.
In this study, the adsorption of CO2 was tested with amine-modified MCM-41 and mesoporous silica materials synthesized from spent quartz sand. The amount of materials gotten in this process can be reached to 100 g, much higher than some other materials, such as mesoporous carbon and b-zeolite (Saha et al., Citation2010; Xu et al., 2010). The effect of concentration of CO2 was also studied in this paper. The results show that the concentration will affect the breakthrough time strongly, and the result can be used as references for further industry application. The prepared adsorbents were characterized by nitrogen adsorption desorption analysis, transmission electron microscopy (TEM), scanning electron microscopy (SEM), element analysis (EA), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR). The content of the material was tested by X-ray fluorescence spectrometry (XRF). The concentration of CO2 was detected using a carbon analyzer.
Materials and Methods
Chemicals and starting materials
Hexadecyl trimethyl ammonium bromide (C16H33(CH3)3N+Br−; CTABr; 99%), tetraethyl orthosilicate (TEOS; 98%), ammonium hydroxide (NH4OH; 28%), sulfuric acid (H2SO4; 98%), toluene (C7H8; 99.9%), 3-aminopropyltrimethoxysilane (C6H17NO3Si; 99%), carbon dioxide (CO2; 99%), and sodium hydroxide (NaOH; 98%) were all purchased from Aldrich Co. (St. Louis, MO) and spent quartz sand was obtained from the Taiwan Semiconductor Manufacturing Company (TSMC) of Taiwan.
Synthesis of MCM-41, Q-MCM, and A-Q-MCM
A method proposed by Cheng et al. (Citation2010) and Hu et al. (2007) was utilized to synthesize MCM-41, which was prepared for comparison. Beginning with pure chemical sources, mesoporous MCM-41 was prepared by dissolving 2.5 g of hexadecyl trimethylammonium bromide (CTABr), which is widely used in the preparation of MCM-41, as described by Bouhadjar et al. (Citation2013), Adam et al. (Citation2014), and Wu et al. (Citation2013), in 125 mL of distilled water. A homogeneous, clear solution was made by continuously stirring with a laboratory stirrer, after which 20 mL of 28 wt% aqueous ammonia solution (NH4OH) was added to the clear solution and the mixture was stirred for 15 min. Then, 5.2 mL of tetraethyl orthosilicate (TEOS) was added dropwise. The mixture was stirred at room temperature for 15 min and then the white precipitate was filtered and washed consecutively with distilled water and dried at 383 K for 8 hr. The as-synthesized material was calcined at 823 K for more than 5 hr to remove the template and obtain a surfactant-free sample.
In order to extract SiO2, which is needed when synthesizing mesoporous silica materials from spent quartz sand, 20 g of spent quartz sand had been previously grinded and sifted through a 200 mesh, was predispersed in 100 mL of sodium hydroxide solution and stirred for 24 hr at 423 K. Then, the supernatant was obtained after filtration of the suspension, whereas the surfactant mixture was prepared by dissolving NH4OH and CTABr in distilled water at room temperature. The surfactant solution and sodium silicate solution were mixed and stirred vigorously for 8 hr. The pH of the solution was adjusted to 11 with sulfuric acid (H2SO4; 98%). The precipitation was filtered and washed several times with distilled water and then dried at 383 K for 8 hr. The obtained materials were then calcined at a heating rate of approximately 278 K/min to 823 K for 5 hr to remove the organic template. The mesoporous silica materials synthesized from spent quartz sand was designated as Q-MCM.
Dried Q-MCM or MCM-41 (1.5 g) was added to 15 mL of toluene with continuously stirring, and 3-aminopropyltrimethoxysilane (0.92 g) was added to the solution and stirred for 6 hr at room temperature, and then refluxed with Soxhlet extractor for 4 hr at 383 K. The obtained material was filtered and washed by ethanol and then dried at 343 K under vacuum conditions. The products were designated as A-Q-MCM.
Characterization of the adsorbents
The formation of a mesoporous structure was confirmed by X-ray diffraction (XRD). X-ray powder diffraction patterns were recorded using a Rigaku Miniflex diffractometer with a Cu Kα radiation wavelength of 0.15405 nm. The surface area and pore size were determined with a nitrogen adsorption/desorption isotherm analyzer. Nitrogen adsorption/desorption isotherms of adsorbent samples were obtained at 77 K using a Micromeritics ASAP 2020 N volumetric adsorption analyzer. Fourier transform infrared (FTIR) spectra of the samples were recorded at room temperature on a PerkinElmer spectrometer. Each sample was scanned 20 times at 4 cm−1 resolution over the range 4000–400 cm−1. The morphologies of the samples were studied by SEM after gold coating using a Hitachi S-4700 instrument. The structure, size, and morphology of the mesoporous silica materials were examined by analytical scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS). The abundance of amine modified on the Q-MCM was estimated using element analysis (EA). Element analysis was also used to determine the amine content (wt%) in the A-Q-MCM-41. The amine content was defined as the number of nitrogen atoms per gram of the impregnated mesoporous structure.
Adsorption experiments
The CO2 adsorption experiment used MCM-41, Q-MCM-41, and A-Q-MCM as adsorbent materials. The adsorption performance test was carried out in a fixed-bed reactor, with the initial concentration of CO2 adjusted to 500, 1000, 1500, and 2000 ppm. An adsorbent weight of 1000 mg was loaded into a glass tube (length: 70 cm, inside diameter: 2 cm, outside diameter: 2.7 cm) at room temperature for the experiment. The adsorption was carried out using different CO2 concentration prepared by using a high-purity CO2 (99%) gas cylinder and an air compressor. In addition, the flow rate was controlled at 50 mL min−1 using a massflow controller connected to the fixed-bed reactor in the experimental setup. The breakthrough curve of CO2 adsorption was obtained from data measured from the CO2 analysis.
Equilibrium adsorption models
The amount of CO2 uptake per unit of adsorbent (Q) was calculated by the following equations:
Langmuir and Freundlich adsorption models were used to perfrom adsorption capacity analyses. The pattern obtained form the experimental data was fitted to the models and the most appropriate model was determined and then used to count the maximum adsorption capacity of MCM-41, Q-MCM, and A-Q-MCM separately. The Langmuir and Freundlich models are defined as follows.
Freundlich adsorption isotherm
The Freundlich isotherm (Cheng et al., Citation2010), which is based on sorption on a heterogeneous surface, is the earliest known relationship that describes sorption equilibrium. Its linearized form is given as
Langmuir adsorption isotherm
The Langmuir isotherm, which is regarded as the most applicable isotherm, is commonly applied in solid/liquid systems to describe saturated monolayer sorption (Hu et al., 2007). The linearized form of the Langmuir equation is the following:
Results and Discussion
Characterization analysis
An X-ray fluorescence spectrometer was used to analyze the spent quartz sand and Q-MCM. Results are shown in . Both the spent quartz sand and Q-MCM showed a high-purity concentration of silica. We used sodium hydroxide solution to extract the SiO2 from spent waste quartz to obtain a high concentration of silica in the Q-MCM, as demonstrated in Kakizawa et al. (Citation2001). From the result of XRF, we can know that 99.9% of the waste quartz is silicate. So the heavy metal contents are very limited. On the other hand, the heavy metal is hard to be leached by caustic sodium.
Table 1. XRF analysis
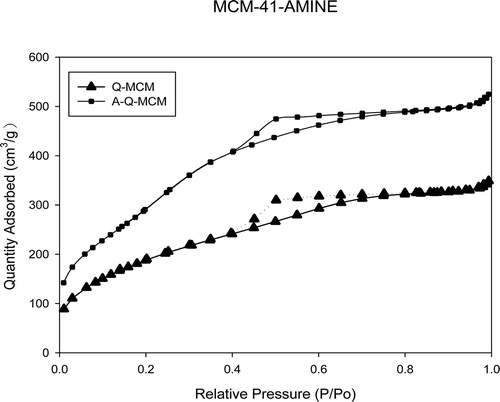
N2 adsorption/desorption isotherms of MCM-41, Q-MCM, and A-Q-MCM at −196 °C correspond to a typical type IV isotherm characteristic of a mesoporous material (). Physical properties of the samples were characterized by XRF and BET analyses. Surface areas, total pore volumes, and nitrogen contents were calculated and are listed in . The surface area and pore volume of Q-MCM are smaller than those of MCM-41. It can be explained by that the pores were blocked by the impurities in quartz sand. The nitrogen content of A-Q-MCM is much higher than MCM-41 and Q-MCM-41. It means that the ammonia was successfully loaded on Q-MCM.
Table 2. Physical properties characterized by BET analysis
A-Q-MCM shows much lower surface area and pore volume than MCM-41 and Q-MCM, which most likely resulted from pores being blocked by the animes impregnated on Q-MCM. Similar results can be seen in previous studies (Sayari et al., 2010; Jadhav et al., Citation2007; Plaza et al., Citation2007). The results of Lee et al. (Citation2007) show that the surface area, pore volume, and pore diameter of MCM-41 are 1003.5 m2/g, 0.76 cm3/g, and 3.95 nm, respectively. For comparing with the result mentioned above, self-prepared MCM-41 in our study has smaller pore diameter, higher surface area, and pore volume.
Elemental analyses of Q-MCM, A-Q-MCM, and the absorbent that has captured the CO2 (C-MCM) are shown in . Elemental analysis revealed different nitrogen contents associated with the Q-MCM and A-Q-MCM samples. The observed increase of nitrogen content demonstrated that the anime had been impregnated onto the Q-MCM and the enhanced carbon content of the A-Q-MCM after absorbing CO2 also showed that the CO2 has been captured by A-Q-MCM. From the C/N ratio, the amine concentration is estimated to be 2.39%.
Table 3. Elemental analysis of Q-MCM, A-Q-MCM, and C-MCM
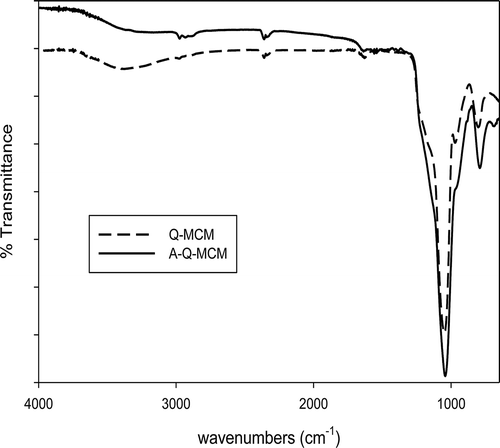
shows the FT-IR spectra of Q-MCM and A-Q-MCM over the range of 4000–550 cm−1. The main difference between the spectra of Q-MCM and A-Q-MCM were due to bands attributed to m(C–H) stretches, clearly identified at 2927 and 2852 cm−1, and weak bands due to m(N–H) stretches at 3369 and 3304 cm−1, associated with the presence of the amines impregnated on A-Q-MCM. Moreover, the absorption bands at 1620–1650 cm−1 were caused by deformation vibrations of adsorbed water molecules (Hassan et al., 2011), and several absorption bands between 1030 and 1240 cm−1 could be assigned to Si–O–Si stretching (Evangelista et al., Citation2007; Grigoropoulou et al., Citation2008).
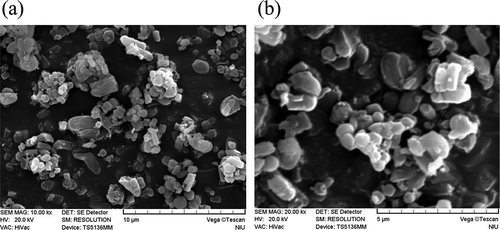
SEM micrographs show particle morphology of A-Q-MCM () and Q-MCM () made from spent waste quartz. The results of our study clearly show the spherical morphology with uniform particle size. The average size of the materials can reach 5–8 μm.
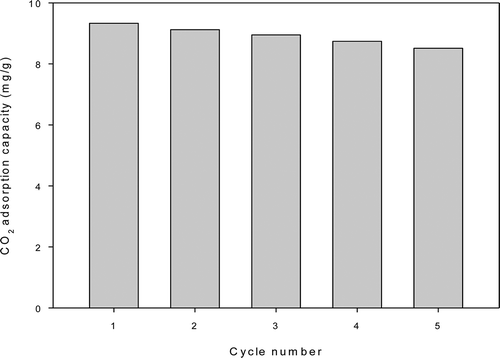
The cyclic performance of A-Q-MCM is the most important criterion to determine whether it can be generalized into practical industrial application. In this study, the performance of the A-Q-MCM in five continuously CO2 sorption-desorption runs shows that the capacity of A-Q-MCM decreases a little in each subsequent run, as shown in .
Adsorption behavior study
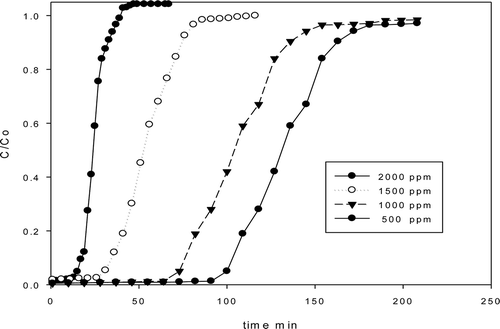
Breakthrough experiments were carried out in a fixed-bed reactor in order to calculate the CO2 adsorption capacity of Q-MCM and A-Q-MCM materials. The breakthrough curves are shown in . The initial concentration of CO2 was set at about 500, 1000, 1500, and 2000 ppm, respectively. The CO2 capacity of A-Q-MCM at different concentration was calculated to be about 1.34, 2.79, 6.53, and 9.34 mg g−1, respectively. The adsorption capacity of CO2 by Q-MCM was much lower than that of A-Q-MCM, because the impregnated anime enhanced the activity of materials. In comparison with other materials (Saha et al., Citation2010; Xu et al., 2010), the capacity of A-Q-MCM was higher than most of others under the same experiment condition. Data are showed in .
Table 4. CO2 adsorption properties of various microporous and mesoporous materials
Equilibrium adsorption models
A number of previous studies have shown that the Langmuir and Freundlich isotherms can be used to simulate the adsorption capacity of materials. From experimental results obtained in this study, the Freundlich adsorption isotherm model (with correlation factor R2 of 0.948) was found to be better than the Langmuir isotherm (R2 of 0.836) for modeling CO2 adsorption onto A-Q-MCM materials. The results are summarized in .
Table 5. Summary of the Langmuir and Freundlich isotherm constants
Conclusion
The present results clearly demonstrate the feasibility and utility of employing spent quartz sand as a source of silica to manufacture mesoporous silica materials. The samples were characterized by XRF, BET, FTIR, EA, and SEM. From XRF analysis it was found that both spent quartz sand and Q-MCM have high concentrations of silica of roughly 99.9%. There were type IV adsorption isotherms in nitrogen adsorption-desorption curves. BET showed that Q-MCM has uniform pore sizes (0.907 cm3/g) and a high surface area (1028.5 m2/g). The concentration of amine impregnated onto A-Q-MCM was found to be about 2.39%. FT-IR spectrometry showed absorbance peaks at 2927 and 2852 cm−1, which correspond to the m(C–H) stretches on A-Q-MCM. Peaks at 3369 and 3304 cm−1 were due to m(N–H) stretches. The performance of the A-Q-MCM in five continuous CO2 sorption-desorption runs shows that the capacity of A-Q-MCM is almost stable and slightly deceasing. The mean adsorption capacity of the A-Q-MCM was found to be about 14.71 mg/g.
In the single CO2 adsorption experiments, the breakthrough time decreased with increasing inlet concentration of CO2. The logarithm breakthrough time is linear, inverse to the logarithm inlet concentration. It is important to predict breakthrough curves of various inlet concentrations by a simple model. This modeling suggested that it is possible to predict breakthrough behaviors under certain conditions. The saturated adsorption capacity rose with increase in testing concentrations. Also, the adsorption capacity of A-Q-MCM could be predicted well by Freundlich isothermal adsorption model.
Additional information
Notes on contributors
Yiteng Su
Yiteng Su is a postgraduate student in the Department of Environmental Engineering, National I-Lan University, I-Lan City, Taiwan, Republic of China. Yiteng Su and Dr. Angus Shiue contributed equally to this work.
Lihong Peng
Lihong Peng is an associate professor in the College of the Environment and Ecology, Xiamen University, Xiamen, P.R. China.
Angus Shiue
Angus Shiue is an assistant professor in Department of Environmental Engineering, National I-Lan University, I-Lan City, Taiwan, Republic of China.
Gui-Bing Hong
Gui-Bing Hong is an associate professor in the Department of Cosmetic Application and Management, St. Mary’s Medicine, I-Lan City, Taiwan, Republic of China.
Zhang Qian
Zhang Qian obtained her Ph.D. in the Graduate Institute of Environmental Engineering at National Taiwan University, Taipei, Taiwan, Republic of China.
Chang-Tang Chang
Chang-Tang Chang obtained his Ph.D. in the Graduate Institute of Environmental Engineering at National Taiwan University, Taipei, Taiwan, Republic of China and is a professor in the Department of Environmental Engineering, National I-Lan University, I-Lan City, Taiwan, Republic of China.
References
- Adam, F., and C.W. Kueh. 2014. Phenyl-amino sulfonic solid acid-MCM-41 complex: A highly active and selective catalyst for the synthesis of mono-alkylated products in the solvent free tert-butylation of phenol. J. Taiwan Inst. Chem. Eng. 45:713–723. doi:10.1016/j.jtice. 2013.07.008.
- Bacsik, Z., R. Atluri, A.E. Garcia-Bennett, and N. Hedin. 2010. Temperature-induced uptake of CO2 and formation of carbamates in mesocaged silica modified with n-propylamines. Langmuir 26:10013–10024. doi:10.1021/la1001495
- Beck, J.S., J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, and E.W. Sheppard. 1992. A new family of mesoporous molecular sieves prepared with liquid crystal templates, J. Am. Chem. Soc. 114:10834–10843. doi:10.1021/ja00053a020
- Belmabkhout, Y., R. Serna-Guerrero, and A. Sayari. 2010. Amine-bearing mesoporous silica for CO2 removal from dry and humid air. Chem. Eng. Sci. 65:3695–3698. doi:10.1016/j.ces.2010.02.044
- Bouhadjar, B., R. Hamacha, A. Morsli, and A. Bengueddach. 2013. Adsorption of Yellow dye on calcined or uncalcined Al-MCM-41 mesoporous materials. Arabian J. Chem 6(3). doi:10.1016/j.arabjc. 2013.07.049
- Chang, F.Y., K.J. Chao, H.H. Cheng, and C.-S. Tan. 2009. Adsorption of CO2 onto amine-grafted mesoporous silicas. Sep. Purif. Technol. 70:87–95. doi:10.1016/j.seppur.2009.08.016
- Cheng, Z., Y. Wu, N. Wang, W. Yang, and T. Xu. 2010. Development of a novel hollow fiber cation-exchange membrane from bromomethylated poly (2,6-dimethyl-1, 4-phenylene oxide) for removal of heavy-metal ions. Ind. Eng. Chem. Res. 49:3079–3087. doi:10.1021/ie901408c
- Danckwerts, P.V. 1979. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 34:443–446. doi:10.1016/0009-2509(79)85087-3
- Demessence, A., D.M. D’Alessandro, M.L. Foo, and J.R. Long. 2009. Strong CO2 binding in a water-stable, triazolate-bridged metal-organic framework functionalized with ethylenediamine. J. Am. Chem. Soc. 131: 8784–8786. doi:10.1021/ja903411w
- Evangelista, S.M., E. DeOliveira, G.R. Castro, F. Luiz, C.A. Zara, and G.S. Prado. 2007. Hexagonal mesoporous silica modified with 2-mercaptothiazoline for removing mercury from water solution. Surf. Sci. 601:2194–2202. doi:10.1016/j.susc.2007.03.020
- Grigoropoulou, G., P. Stathi, M.A. Karakassides, M. Louloudi, and L.Y. Deligiannakis. 2008. Functionalized SiO2 with N-, S-containing ligands for Pb (II) and Cd (II) adsorption. Colloid. Surface. A 320: 25–35.doi:1016/j.colsurfa.2008.01.007
- Hansen, J.E., and J. Houghton. 1998. Global warming: The complete briefing. Atmos. Chem. 30:409–412. doi:10.1023/A:1006043116343
- Harlick P.J.E., and A. Sayari. 2007. Applications of pore-expanded mesoporous silica. 5. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind. Eng. Chem. Res. 46:446–458. doi:10.1021/ie060774+
- Hassan H., and B.H. Hameed. 2011. Oxidative decolorization of Acid Red 1 solutions by Fe–zeolite Y type catalyst. Desalination. 276:45–52. doi:10.1016/j.desal.2011.03.18
- Hoffert M.I., K. Caldeira, and G. Benford. 2002. Advanced technology paths to global climate stability: Energy for a greenhouse planet. Science 298: 981–987. doi:10.1126/science.1072357
- Hu, C.Y., S.L. Lo, W.H. Kuan, and Y.D. Lee. 2008. Treatment of high fluoride-content wastewater by continuous electrocoagulation–flotation system with bipolar aluminum electrodes. Sep. Purif. Technol. 60:1–5. doi:10.1016/j.seppur.2007.07.040
- Jadhav, P.D., R.V. Chatti, R.B. Biniwale, N.K. Labhsetwar, S. Devotta, and S.S. Rayalu. 2007. Monoethanol amine modified zeolite 13X for CO2 adsorption at different temperatures. Energy Fuels 21:3555–3559. doi:10.1021/ef070038y
- Kakizawa, M., A. Yamasaki, and Y. Yanagisawa. 2001. A new CO2 disposal process via artificial weathering of calcium silicate accelerated by acetic acid. Energy 26:341–354. doi:10.1016/S0360-5442(01)00005-6
- Khatri, R.A., S.S.C. Chuang, Y. Soong, and M. Gray. 2006. Thermal and chemical stability of regenerable solid amine sorbent for CO2 capture. Energy Fuels 20:1514–1520. doi:10.1021/ef050402y
- Lee, C., S. Liu, L. Juang, C. Wang, K. Lin, and M. Lyu. 2007. Application of MCM-41 for dyes removal from wastewater. J. Hazard. Mater. 147: 997–1005. doi:10.1016/j.jhazmat.2007.01.130
- Li, Y., W. Wang, Z. Zhan, M. Woo, C. Wu, and P. Biswas. 2010. Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts. Appl. Catal. B Environ. 100:386–392. doi:10.1016/j.apcatb.2010.08.015
- Plaza, M.G., C. Pevida, A. Arenillas, F. Rubiera, and J.J. Pis. 2007. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 86:2204–2212. doi:10.1016/j.fuel.2007.06.001
- Saha, D., Z.B. Bao, F. Jia, and S.G. Deng. 2010. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 44:1820–1826. doi:10.1021/es9032309
- Saha, D., and S.G. Deng. 2010. Adsorption equilibrium and kinetics of CO2, CH4, N2O, and NH3 on ordered mesoporous carbon. J. Colloid Interface Sci. 345:402–409. doi:10.1016/j.jcis.2010.01.076
- Sanz, R., G. Calleja, A. Arencibia, and E.S. Sanz-Pérez. 2010. CO2 adsorption on branched polyethyleneimine-impregnated mesoporous silica SBA-15. Appl. Surf. Sci. 256:5323–5328. doi:10.1016/j.apsusc.2009.12.070
- Sathitsuksanoh, N. 2007. Sequestration of CO2 by chemically reactive aqueous K2CO3 in high efficiency adsorbents using microfibrous media entrapped support particulates. Master’s thesis, Graduate Faculty, Auburn University, Auburn, Alabama, May 10, 2007.
- Son, W.J., J.S. Choi., and W.S. Ahn. 2008. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Micropor. Mesopor. Mater. 113: 31–40. doi:10.1016/j.micromeso.2007.10.049
- Wu, H.Y., X.-L. Zhang, C.-Y. Yang, X. Chen, and X.-C. Zheng. 2013. Alkali-hydrothermal synthesis and characterization of W-MCM-41 mesoporous materials with various Si/W molar ratios. Appl. Surf. Sci. 270:590–595. doi:10.1016/j.apsusc.2013.01.090
- Xu, X., C. Song, J.M. Andresen, B.G. Miller, and A.W. Scaroni. 2002. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 16:1463–1469. doi:10.1021/ef020058u
- Xu, X.L., X.X. Zhao, L.B. Sun, and X.Q. Liu. 2009. Adsorption separation of carbon dioxide, methane and nitrogen on monoethanol amine modified b-zeolite. J. Nat. Gas Chem. 18:167–172. doi:10.1016/S1003-9953(08)60098-5
- Ying, J.Y., C.P. Mehnert., and M.S. Wong. 1999. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Edit. 38: 56–77. doi:10.1002/(SICI)1521
- Yue, M.B., L.B. Sun., and Y. Cao. 2008. Efficient CO2 capturer derived from as-synthesized MCM-41 modified with amine. Chem. Eur. J. 14: 3442–3451. doi:10.1002/chem.200701467
- Yu, L., J. Gong, C. Zeng, and L. Zhang. 2013. Synthesis of binderless zeolite X microspheres and their CO2 adsorption properties. Sep. Purif. Technol. 118:188–195. doi:10.1016/j.seppur.2013.06.035
- Zeleňák, V., M. Badaničová, D. Halamova, J. Čejka, A. Zukal, N. Murafa, and G. Goerigk. 2008a. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 144:336–342. doi:10.1016/j.cej.2008.07.025
- Zeleňák, V., D. Halamova, L. Gaberova, E. Bloch, and P. Llewellyn. 2008b. Amine-modified SBA-12 mesoporous silica for carbon dioxide capture: Effect of amine basicity on sorption properties. Micropor. Mesopor. Mater. 116:358–364. doi:10.1016/j.micromeso.2008.04.023
- Zhao, D., Q. Huo, J. Feng, F.C. Bradley, and D.S. Galen. 1998. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 120:6024–6036. doi:10.1021/ja974025i