Abstract
In this study, polyvinyl alcohol (PVA) and titania (TiO2) Degussa P-25 were mixed to generate TiO2 nonwoven filters using electrospinning. The wires of titanium dioxide and the nonwoven binding titania nanofibers were formed using 14 kV voltage and a distance of 15 cm. A single-factor experimental method was used to investigate the effects of parameters such as initial concentration, retention time, and light source on acetone removal by nonwoven binding titania nanofibers. Furthermore, the effects of parameters such as gas pressure, particle size, initial concentration, and retention time on the removal of particulates were also assessed. The results showed that the degradation efficiency increased with decreasing initial concentrations and increasing retention time. The best operational conditions during this study for the removal of acetone using the TiO2 nonwoven filters were a retention time of 100 sec, initial acetone concentration of 250 ppm, and ultraviolet (UV) light source of 254 nm. Under those conditions, 99% acetone removal efficiency was obtained. In addition, 90% particulate matter removal efficiency was reached when the particulate size was greater than 200 nm and the reaction time was longer than 5 minutes. The prepared TiO2/nanofiber has good performance for volatile organic compounds (VOCs) and particulate removal at the same time.
Implications: In this study, polyvinyl alcohol (PVA) and titania (TiO2) Degussa P-25 were mixed to generate TiO2 nonwoven filters using electrospinning. The results showed that the optimum operating conditions for the removal of acetone using the TiO2 nonwoven filters were a retention time of 100 sec, initial acetone concentration of 250 ppm, and UV light source of 254 nm. Under those conditions, 99% acetone removal efficiency was obtained.
Introduction
The removal of volatile organic compounds (VOCs) and particulate matter has been extensively studied because they can pose adverse effects to human health and the environment. Some of the treatment methods for the removal of VOCs include absorption, condensation, biological treatment, incineration, catalytic incineration, and photocatalytic incineration. These methods suffer from some problems, such as low removal efficiency and high costs. A feasible method is the photocatalytic oxidation method, which is being researched and used to improve treatment processes (Hoffmann et al., Citation1995; Ollis et al., Citation2000; Puma et al., Citation2009; Huang et al., Citation2010). The most commonly used catalyst for photocatalytic reactions is titanium dioxide (TiO2). The reaction rate that can be achieved with TiO2 is higher than for other typical catalysts, and this catalyst is preferred over others because is not poisonous. In addition, it is inexpensive and has good physical properties.
In recent years, many researchers have studied the development of electrospinning technology for biomedical applications (Doshi et al., Citation1995; Matthews et al., Citation2002; Zong et al., Citation2002). Electrospinning is a simple and easy method to control the fiber shape. The fibers produced by electrospinning show highly desired characteristics, such as large surface area, high porosity, and small pore size (Deitzel et al., Citation2001; Huang et al., Citation2003; Natthan et al., Citation2010).
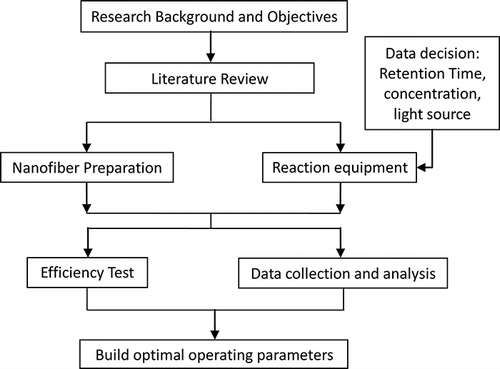
In this study, we developed a method to combine TiO2 with electrospinning technology. The use of this method to prepare nonwoven binding titania nanofibers can improve the surface area of the catalyst. The prepared catalyst was used to treat VOCs and reduce particulate emissions. The target VOC selected for this study was acetone. In this study, the effects of concentration, light source, and retention time on degradation of acetone were investigated. Furthermore, the effects of gas pressure, particle size, initial concentration, and retention time on the removal of particulates were also investigated. This research study followed the flow processes shown in .
Experiental Methods
Nanofiber preparation
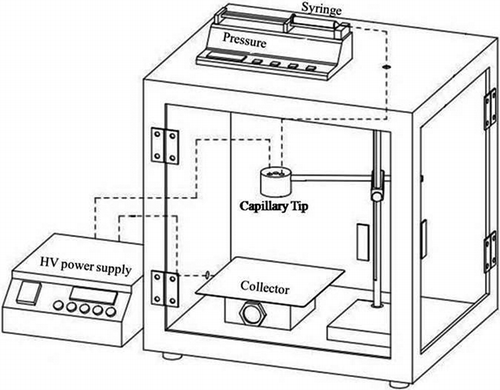
The appropriate amount of dispersing agent, dioctyl sulfossuccinate sodium salt, was added to 15 mL of deionized water and fully mixed. Then PVA and TiO2 were added to the mixed solution and stirred for 2 hr at 70ºC (Seok et al., Citation2008). shows the schematic diagram of an electrospinning system. The polymer solution was injected with a needle syringe equipped with 25 gauge at the nozzle. The electric potential was fixed at 14 kV. The nanofibers were collected as spun on an aluminum sheet on the collector. The solution was electrospun at room temperature and the collection distance was fixed at approximately 15 cm. The solution feed was driven by gravity and the electrostatic force that was generated during spinning.
Reaction system
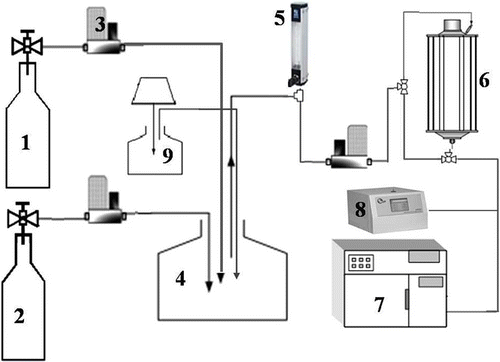
The schematic diagram of the test system is shown in . The system used compressed cylinders for air and acetone generation. The compressed air was used for the dilution of acetone. The particle generator was used for producing different size of particles. Three flow systems, including acetone, dilution air, and the particles, were mixed in a mixing chamber, stabilized under 27 ± 1.5°C, to achieve the desired acetone and particle concentration. The acetone concentrations used in this study were 250, 500, 1000, and 1500 ppm (by volume) with ±5% deviation, selected according to the industrial safety and health standard (1000 ppm). Concentrations in the stream were measured by gas chromatography with flame ionization detection (GC-FID; Perkin Elmer Co., Ltd, Autosystem Gas Chromatograph). Three retention times, 10, 40, and 100 sec, were used to understand the effect on removal efficiency. Two kinds of 8-W low-power light source, including 254 nm and 365 nm wavelength, were used to assess the effect of light intensity. The limitation of the small reactor is that it is only used for low flow rate and low velocity conditions. It is also suitable for VOCs removal in a plant.
The gas pressures of particle generation system used in this study were 10 and 30 psi to control particle size distribution since the generated particulate size is 100 and 200 nm under pressure 10 and 30 psi, respectively. Five retention times, including 2, 7, 12, 20, and 30 min, were examined to get optimum conditions. The removal efficiency (η) is calculated as follows:
The results of Ku et al. (Citation2001) showed that photocatalysis by ultraviolet (UV)/TiO2 process is technically feasible to decompose trichloroethylene in air streams. By combining the proposed reaction mechanism scheme, a semiempirical design equation can be used to predict the treatment performance of an annular photoreactor for the decomposition of gaseous trichloroethylene by the UV/TiO2 process.
Results and Discussion
Acetone removal
Effect of retention time on acetone removal
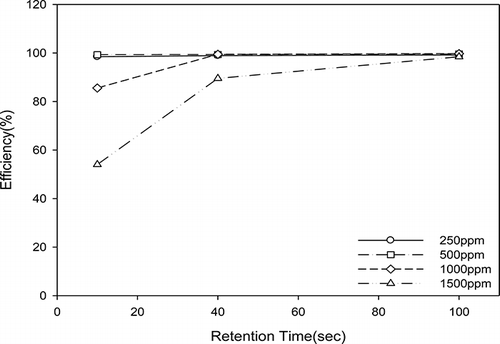
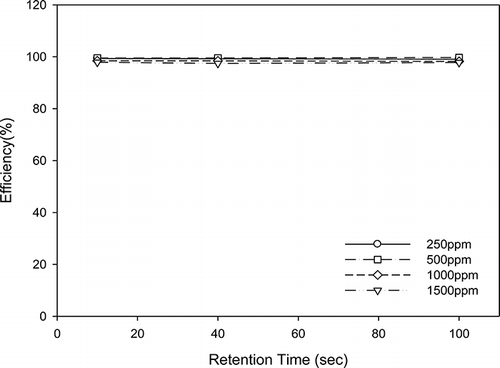
The degradation efficiency of acetone using TiO2/nanofibers was increased with increasing retention time. This effect can be observed in for all concentrations under the light source of UV-365 nm. For example, for an initial acetone concentration of 1500 ppm, the removal efficiency increased from approximately 50% to over 99% when the retention time was increased from 10 sec to 100 sec. A similar effect is observed for a concentration of 1000 ppm. For the lower concentrations of 500 and 250 ppm, the removal efficiency was over 99% regardless of the retention time, meaning that a retention time of 10 sec is sufficient to achieve high removal efficiencies at low concentrations.
Effect of light source on acetone removal
The removal efficiency increased when light sources of lower wavelengths were used. For example, using an initial concentration of acetone of 1500 ppm and a retention time of 10 sec resulted in a removal efficiency of approximately 50% under the light source of UV-365 nm. However, this removal efficiency increased to more than 99% when a light source of UV-254 nm was used under the same initial concentration and retention time conditions, as shown in and . shows that a light source of UV-254 nm results in more than 99% acetone removal efficiency regardless of the retention time or initial concentration. TiO2/nanofiber easily absorbs UV light. Furthermore, the power of the shorter wavelength is larger than that of the longer wavelength. Therefore, the removal efficiency at UV-254 nm is higher than that at UV-365 nm.
Effect of initial concentration on acetone removal
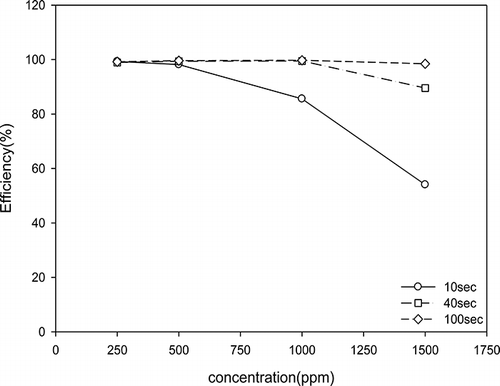
The degradation efficiency of acetone using TiO2/nanofibers decreased with increasing initial acetone concentration as shown in . For example, when the retention time is held constant at 10 sec, the degradation efficiency is 98% for 250 ppm and only 54% for 1500 ppm. At 100 sec retention time, the removal efficiency is not affected by the initial concentration, as the removal achieved is high for all concentrations. This means that the retention time of 100 sec is long enough for high removal efficiency of acetone at the highest concentration tested. The decrease in removal efficiency as a function of increasing concentration was caused by the activity sites of the photocatalyst being occupied with higher VOCs concentration, reducing degradation efficiency. The results of Avasarala et al. (Citation2010) showed that the decrease in degradation by increasing pollutant concentration is due to the reduction of the generation of radicals (•OH) on the catalyst surface as the active sites are covered by the pollutant molecules.
Particulate matter removal
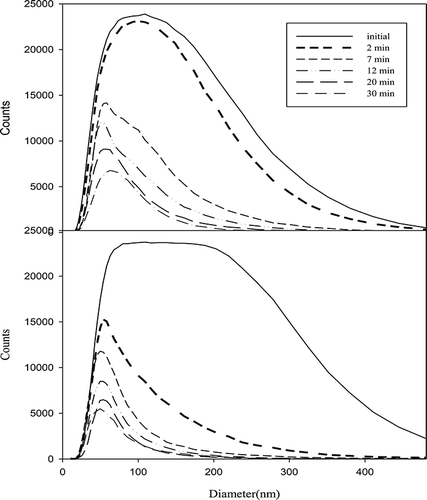
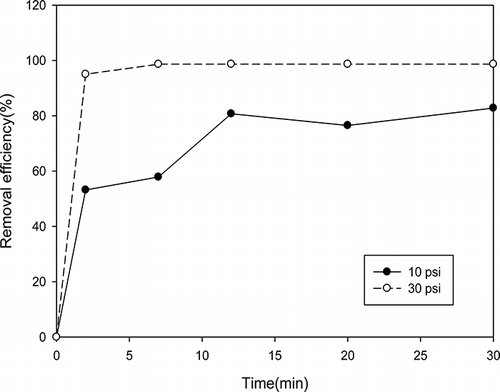
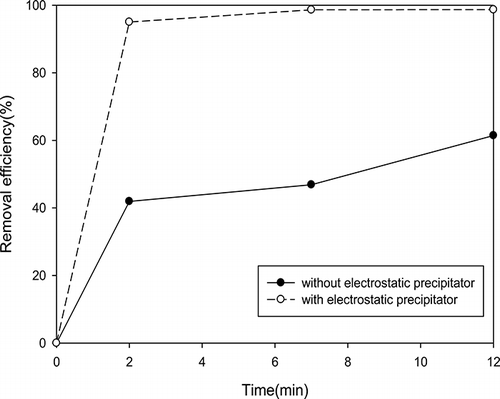
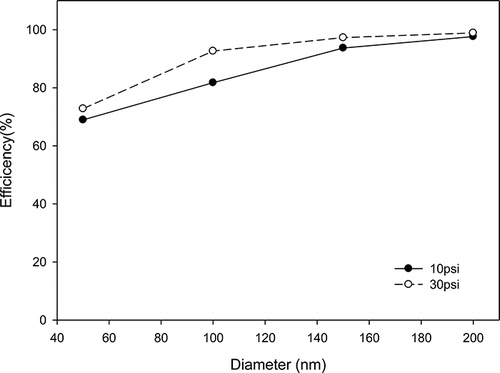
In order to comprehensively investigate the performance of nanofibers, it is important to separate VOCs and particulate matter removal. First, the prepared nanofibers were used to remove particulate matter. Then photocatalytic degradation of VOCs was also measured through the photocatalytic reaction device. Two kinds of gas pressure, including 10 and 30 psi, were used to control particle size. Furthermore, the comparison between before and after filtration was also examined to understand the performance of nanofibers, as shown in , , , and 10. The results showed that the removal efficiency of larger particles under 30 psi is higher than that of smaller particles under 10 psi with the same nanofibers. The particulate matter removal efficiency was dependent on particulate size as shown in . The removal efficiency was increased with increasing particulate size. For example, the removal efficiency could be up to 90% when the particulate size was larger than 150 nm. The VOCs reactor can capture particles without using nanofibers. However, the particle removal efficiency was under 60%, although the retention time was controlled 12 min. In contrast, the particle removal efficiency could reach 98% with nanofibers, although the retention time was controlled within 2 min.
Summary
In this work, porous nanofibers by electrospinning technology with TiO2 were synthesized. Electrospinning is an easy and efficient technology to synthesize nanofibers combined with TiO2. TiO2/nanofibers revealed a large surface area, high porosity, and small pore size, leading to an obvious enhancement of the photocatalytic activity. The results showed that the degradation efficiency for the removal of acetone was above 99% with light source at UV-254 nm. At higher wavelengths, with light source at UV-365 nm, the degradation efficiency of acetone with photocatalytic reaction decreased with the increasing initial acetone concentrations and increased with increasing retention time. It was also shown that particulate matter can be removed with nanofiber combined photocatalytic catalysts. The removal efficiency of particulate matter with nanofiber decreased with the increasing gas pressure. The removal efficiency of particulate matter can be greater than 98% with nanofibers. This suggests increasing the surface area of TiO2 nanofibers to increase removal efficiency. It also suggests assessing the performance of nanofibers under visible light for application in the field.
Funding
The authors are grateful for support by NSC 101-2120-S-197-001. This work was also supported by the Center of Nanotechnology, National Ilan University.
Additional information
Notes on contributors
Yi-Hsuan Chuang
Chang-Tang Chang, Ph.D., is a professor and Yi-Hsuan Chuang is a master in the Department of Environmental Engineering, National I-Lan University, I-Lan City, Taiwan.
Gui-Bing Hong
Chang-Tang Chang, Ph.D., is a professor and Yi-Hsuan Chuang is a master in the Department of Environmental Engineering, National I-Lan University, I-Lan City, Taiwan.
Chang-Tang Chang
Gui-Bing Hong, Ph.D., is an associate professor in the Department of Cosmetic Application and Management, St. Mary’s Medicine, Nursing and Management College, Yilan County.
References
- Avasarala, B.K., S.R. Tirukkovalluri, and S. Bojja. 2010. Photocatalytic degradation of monocrotophos pesticide-An endocrine disruptor by magnesium doped titania. J. Hazard. Mater. 186(2–3): 1234–1240. doi:10.1016/j.jhazmat.2010.11.132
- Deitzel, J.M., J. Kleinmeyer, D. Harris, and N.C. Beck Tan. 2001. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42: 261–272. doi:10.1016/S0032-3861(00)00250-0
- Doshi, J., and D.H. Reneker. 1995. Electrospinning process and applications of electrospun fibers. J. Electrostatics 35(2–3): 151–160. doi:10.1109/IAS.1993.299067
- Hoffmann, M.R., S.T. Martin, W. Choi, and D.W. Bahnemannt. 1995. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95(1): 69–96. doi:10.1021/cr00033a004
- Huang, C.H., I.K. Wang, Y.M. Lin, Y.H. Tseng, and C.M. Lu. 2010. Visible light photocatalytic degradation of nitric oxides on PtOx-modified TiO2 via sol-gel and impregnation method. J. Mol. Cat. A Chem. 316(1–2): 163–170. doi:10.1016/j.molcata.2009.10.015
- Huang, Z.M., Y.Z. Zhang, M. Kotaki, and S. Ramakrishna. 2003. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Sci. Technol. 63: 2223–2253. doi:10.1016/S0266-3538(03)00178-7
- Ku, Y., C.M. Ma, and Y.S. Shen. 2001. Decomposition of gaseous trichloroethylene in a Photoreactor with TiO2-coated nonwoven fiber textile. Appl. Catal. B Environ. 34:181–190. doi:10.1016/S0926-3373(01)00216-8
- Matthews, J.A., G.E. Wnek, D.C. Simpson, and G.L. Bowlin. 2002. Electrospinning of collagen nanofibers. Biomacromolecules 3(2): 232–238. doi:10.1021/bm015533u
- Natthan, C., O. Praneet, R. Theerasak, N. Tanasait, and S. Pitt. 2010. Preparation and characterization of chitosan-hydroxybenzotriazole/polyvinyl alcohol blend nanofibers by the electrospinning technique. Carbohydr. Polymers 81:675–680. doi:10.1016/j.carbpol.2010.03.031
- Ollis, DF. 2000. Photocatalytic purification and remediation of contaminated air and water. C. R. Acad. Sci. Serie II Fascicule C Chim. 3(6): 405–411. doi:10.1016/S1387-1609(00)01169-5
- Puma, G.L., I.S. Estivill, T.N. Obee, and S.O. Hay. 2009. Kinetics rate model of the photocatalytic oxidation of trichloroethylene in air over TiO2 thin films. Sep. Purif. Technol. 67(2): 226–232. doi:10.1016/j.seppur.2009.03.011
- Seok, J.D., K.S. Cham, L. Geun, J.L. Sung, and K. Hoyoung. 2008. Development of photocatalytic TiO2 nanofibers by electrospinning and its application to degradation of dye pollutants. J. Hazard. Mater. 154(1–3): 118–127. doi:10.1016/j.jhazmat.2007.09.118
- Zong, X., K. Kim, D. Fang, S. Ran, B.S. Hsiao, and B. Chu. 2002. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43(16): 4403–4412. doi:10.1016/S0032-3861(02)00275-6