Abstract
When a contaminated site contains pollutants including both nonvolatile metals and Hg, one single remediation technology may not satisfactorily remove all contaminants. Therefore, in this study, chemical extraction and thermal treatment were combined as a remediation train to remove heavy metals, including Hg, from contaminated soil. A 0.2 M solution of ethylenediamine tetraacetic acid (EDTA) was shown to be the most effective reagent for extraction of considerable amounts of Cu, Pb, and Zn (>50%). Hg removal was ineffective using 0.2 M EDTA, but thermogravimetric analysis suggested that heating to 550°C with a heating rate of 5°C/min for a duration of 1 hr appeared to be an effective approach for Hg removal. With the employment of thermal treatment, up to 99% of Hg could be removed. However, executing thermal treatment prior to chemical extraction reduced the effectiveness of the subsequent EDTA extraction because nonvolatile heavy metals were immobilized in soil aggregates after the 550°C treatment. The remediation train of chemical extraction followed by thermal treatment appears to remediate soils that have been contaminated by many nonvolatile heavy metals and Hg.
Implications
A remediation train conjoining two or more techniques has been initialized to remove multiple metals. Better understandings of the impacts of treatment sequences, namely, which technique should be employed first on the soil properties and the decontamination efficiency, are in high demand. This study provides a strategy to remove multiple heavy metals including Hg from a contaminated soil. The interactions between thermal treatment and chemical extraction on repartitioning of heavy metals was revealed. The obtained results could offer an integrating strategy to remediate the soil contaminated with both heavy metals and volatile contaminants.
Introduction
Soils frequently act as the final sink for contaminants because of direct or indirect contacts with industrial waste discharge, landfills, and leachates. Among various contaminants, heavy metals in soils bring about significant challenges because they are immutable, not degradable, and persistent. Soil contamination with heavy metal remains an increasing problem in the world, and the cleanup of these soils is very time-consuming and challenging (Dermont et al., Citation2008a). Remediation of heavy metal-contaminated sites conventionally involves excavation of the contaminated soils, followed by immobilization of metals using solidification/stabilization technology, prior to refilling the treated materials on site or disposal in a permitted landfill site (Sims, Citation1990). However, these remediation approaches are no longer considered permanent environmental solutions because the target metals in soils cannot be eliminated by solidification or stabilization (Dermont et al., Citation2008b). Therefore, there is an urgent requirement for techniques that can remove target metals from soils, hence eliminating contaminations and the associated environmental risks. Thermal treatment and soil washing have been recommended as permanent treatment alternatives for removing Hg and other metals from contaminated soils (Dermont et al., Citation2008b; Wang et al., Citation2012).
Soil washing includes physical separation, chemical extraction, and various integrated processes combining both physical and chemical methods (Mamm et al., 1999). Physical separation is suitable for particulate forms of heavy metals in soils, whereas chemical extraction is primarily applicable when the target metals exist in ionic forms. Physical separation is suitable where particulate forms of metals are adsorbed and concentrated in fractions of specific particle sizes, for example, silt- and clay-sized (Griffiths, Citation1995). Chemical extraction and its related techniques aim to solubilize the metals in soils using aqueous extractants such as acids or chelating agents, and a number of studies have also examined the influence of type and concentration of agents, reaction time, and solid/liquid ratios for extraction operations for heavy metal removal (Abumaizar and Smith, Citation1999; Dermont et al., Citation2008b; Leštan et al., Citation2008). Acids and chelating agents are effective because of their abilities in enhancing mobility of heavy metals at low pH and solubilizing metals through complexation. Among acids and chelating agents, hydrochloric acid (HCl) and ethylenediamine tetraacetic acid (EDTA) have been widely investigated and employed to extract Cd, Cr, Cu, Ni, Pb, and Zn from soils (Cline and Reed, Citation1995; Reed et al., Citation1996; Sun et al., Citation2001; Ehsan et al., Citation2006). However, the efficiency of metal extraction depends on type, concentration, and fraction of heavy metals present, as well as on soil characteristics (Dermont et al., Citation2008a, Citation2008b).
Among all heavy metals, Hg is probably the trickiest one to deal with because of its volatility and strong affinity for soil particles and organic matter. Studies reporting Hg removal using chemical extraction are rare in the literature, and some studies have queried the effectiveness of existing removal techniques (Wang et al., Citation2012). Thermal desorption is a promising technique that utilizes heat to enhance the volatility of Hg, hence eliminating Hg by transferring Hg into cryogenic condensers or other adsorptive materials (activated carbon, for instance) without burning the soil. The volume of contaminated soils can be reduced by the benefit of transferring Hg from soils to the aforementioned collectors, and the condensed Hg can be further stored or reused. Inorganic Hg usually exists in soil in the elemental state or as Hg(II) compounds such as HgS, HgO, and HgCO3. When heated to between 600 and 800ºC, these Hg compounds converted into gaseous elemental Hg and were therefore stripped off (Chang and Yen, Citation2006). Several Hg thermal desorption experiments have demonstrated the feasibility of Hg removal at temperatures ranging from 127 to 700ºC, but most Hg removal occurred at relatively high temperature (460–700ºC) (Chang and Yen, Citation2006; Kunkel et al., Citation2006). Massacci et al. (Citation2000) reported that Hg concentration in soil samples decreased from 217 to 0.01 mg/kg after 4 hr of roasting at 700ºC. With the use of thermal treatment at 470°C for 20 min, 99% of Hg was removed from a soil excavated from a former chloro-alkali plant that had contained 2400 mg Hg/kg (Taube et al., Citation2008). Huang et al. (Citation2011) also used thermal treatment to remediate Hg-contaminated soil; the results indicated that thermal decontamination at a temperature above 550ºC enabled the reduction of Hg from 1320 to 6 mg/kg (0.45% of the original concentration).
Globally, many sites have been contaminated by a mixture of Hg and other metals. We have elucidated that thermal decontamination at 550°C was effective in removing 99 to 100% of Hg. After heating at 550°C, the decomposed and fused soil clay minerals were concreted when subsequently cooled, which also resulted in immobilization of the nonvolatile metals. After heating at 550°C, Cr, Cu, Ni, Pb, and Zn that were originally associated with Fe/Mn oxides were transformed into acid-extractable, organic-matter-bound, and residual forms (Huang et al., Citation2011). A problem found in the earlier study was that thermal treatment alone made it difficult to remediate soils that had been contaminated by multiple heavy metals, including volatile and nonvolatile metals. In order to address this problem of remediation of soils contaminated by multiple heavy metals, a “remediation train” combining two or more techniques was employed. This study aims to provide a systematic strategy for removal of Hg and nonvolatile heavy metals from a contaminated soil. Preliminary experiments were carried out to determine optimal operational parameters of a remediation train to lower the cost and enhance the efficiency of removal. In addition, we aimed to show how the species of heavy metal influences the efficiency of thermal treatment and chemical extraction. The overall concept could be of interest to engineers or decision makers involved in remediation of soils contaminated by both heavy metals and volatile contaminants.
Materials and Methods
Soil sample collection and characterization
The study soil was collected from a contaminated site (22º33,ʹ30” N, 120º27,ʹ31” E) in Pingtung, Taiwan. The site consisted of an illegal dump with industrial hazardous solid wastes (which have since been removed), where leachate and residual heavy metals have accumulated in the soil after waste removal. Five duplicated soil samples (0–15 cm) were obtained from site within a 0.8-ha land area and mixed well into a single composite sample. The soil sample was air-dried, sieved (<2 mm), and stored in the dark for physiochemical analysis.
The soil pH was measured in a mixture of soil and deionized water (1:1, w/v) with a glass electrode (McLean, Citation1982). Total organic carbon (OC) content was determined using the Walkley–Black wet oxidation approach (Nelson and Sommers, Citation1982). The analysis of cation exchange capacity (CEC) was carried out using the ammonium acetate method (pH 7.0) (Rhoades, Citation1982). The carbonate content of the soil was analyzed by a gravimetric method (Nelson, Citation1982). Free Fe (Fed) and Mn (Mnd) were extracted by the dithionite–citrate–bicarbonate (DCB) method (Mehra and Jackson, Citation1960). The analysis of soil particle-size distribution was performed with the pipette method (Gee and Bauder, Citation1986).
The total concentration of Cr, Cu, Ni, Pb, and Zn was determined following aqua regia digestion (International Standards Organization [ISO], Citation1995). Three grams of sieved soil (<0.15 mm) was placed into a 100-mL beaker, and the beaker was covered with a watchglass and digested for 16 hr after the addition of 21 mL of HCl and 7 mL of HNO3. The beaker was then put on a hot plate and the sample was boiled for 2 hr. The digestion solution was filtered and diluted with deionized water to 100 mL after the addition of 10 mL of 10% HNO3. The total concentrations of heavy metals were measured using a flame atomic absorption spectrometer (FAAS, Hitachi Z-2300).
The analysis of total Hg was carried out following the acid digestion protocol (Stewart et al., Citation1982). A brief description is provided here for clarity. One gram of sieved soil (<0.15 mm) was mixed with 5 mL deionized water and 5 mL aqua regia in a 250-mL beaker, and the mixture was covered and boiled for 3 min. The mixture was subsequently heated at 95°C for 30 min after the addition of 50 mL of deionized water and 15 mL of potassium permanganate. The digestion solution was filtered and diluted with deionized water to 100 mL. Total Hg content was then measured using a cold vapor atomic absorption spectrometer (CVAAS, CETAC M-6100).
Sequential extraction procedures of metals
Fractionation of heavy metals can yield useful information on the mobility and bioavailability of metals and therefore the potential risk to the environment. Consequently, the fractionation of heavy metals is frequently carried out before the appropriate remediation methods are chosen. The sequential extraction procedure (SEP) developed by Neculita et al. (Citation2005) was used to determine the mobility of Hg in the soil, while the mobility of Cr, Cu, Ni, Pb, and Zn was determined using the SEP developed by Tessier et al. (Citation1979). The Hg fractionation followed the procedure of Neculita et al. (Citation2005), with a modification that potassium permanganate digestion was substituted by aqua regia to determine the residual fraction. The fractions of sequential extraction for Hg in this study were as water-soluble (F1), exchangeable (F2), organic (F3), and residual (F4) (Neculita et al., Citation2005), and the Hg concentration of each fraction was determined with the CVAAS. The sequential extraction procedure used for fractionation of Cr, Cu, Ni, Pb, and Zn was developed and described by Tessier et al. (Citation1979); exchangeable (F1), carbonate (F2), Fe-Mn oxide (F3), organic (F4), and residual (F5) fractions were distinguished using various extractants (Tessier et al., Citation1979). The extractant for the residual fraction was also replaced with aqua regia in this study. The metal concentration in each solution was determined by FAAS.
Thermogravimetric analysis (TGA)
To understand the thermal characteristics of a soil sample prior to thermal desorption, the thermogravimetric data of the soil sample were obtained using a thermobalance (Thermo Cahn TG-2121) equipped with a downstream filter with active charcoal to adsorb gaseous Hg released from samples. Approximately 20 mg soil was placed in an open platinum crucible hung in the furnace and precisely weighted with the thermobalance. Thermogravimetric analysis (TGA) was then performed from room temperature to 900°C with a heating rate of 5 or 10°C/min at N2 flow of 90 cm3/min.
Remediation train
Chemical extraction and thermal desorption were combined and organized as two approaches of remediation train. One combination involved chemical extraction followed by thermal treatment, and the other was the inverse order of the previous combination. The heavy metal removal and the impacts of one approach on the subsequent approach were examined to evaluate the decontamination efficiency of each remediation train.
Regarding chemical extraction, 6 g soil was mixed with 60 mL of three reagents, HCl, CaCl2, and EDTA, at various concentrations (0.05, 0.1, and 0.2 M) and was then shaken in an 80-mL polypropylene centrifuge tube for 1 hr using a mechanical orbital shaker at 150 rpm (amplitude of 5 cm) at room temperature. The supernatant was obtained by centrifuging at 3000 rpm (1000 × g) for 10 min and then analyzed for Cr, Cu, Ni, Pb, and Zn using the FAAS. To examine the removal of Hg, solid residue was air-dried and analyzed following the total Hg analysis protocol and the use of CVAAS.
For thermal treatment, 10 g of bulk soil was placed in a vertical stainless-steel fixed-bed tubular reactor and thermally treated at various temperatures and durations based on the results obtained from TGA. The reactor was equipped with a filter containing active charcoal to collect desorbed gaseous Hg, and the cylindrical chamber of reactor was 25 mm diameter. The experiment was carried out at a 60 mL/min N2 flow, from room temperature to 550°C, with a heating rate of 5°C/min, and temperature was maintained at 550°C for 1 hr. Total Hg was analyzed prior to and after thermal desorption to assess the removal efficiency of the thermal decontamination.
Results and Discussion
Soil characteristics
presents the characteristics of the soil sample and the heavy metal contents. The soil had an alkaline pH because of the dumped solid waste, which included a considerable amount of CaCO3. The CEC value was 14 cmol/kg. However, the organic carbon content was low. Since the soil was dumped with both Hg-contaminated deposits and other hazardous materials, the soil contained considerable amounts of coexisting heavy metals, including Cr, Cu, Ni, Pb, and Zn. The soil pollution control limits (SPCLs) in the Soil and Groundwater Pollution Remediation Act (SGPRA) of Taiwan for Hg, Cr, Cu, Ni, Pb, and Zn are 20, 250, 400, 200, 2000, and 2000 mg/kg, respectively. With the exception of Zn, all the metals in the soil exceeded the SPCLs, and thus this site has been listed in the pollution remediation sites according to the SGPRA. However, no comprehensive remediation action has been performed at the site to date.
Table 1. Characteristics of study soil
Initial heavy metal fractionation of soil sample
The fractional totals of Cr, Cu, Ni, Pb, and Zn in the soil ranged from 89.9 to 104% of the total metals. Therefore, the recovery of metal was not problematic during the SEP (). A typical SEP employs progressively stronger solvents to sequentially solubilize various metal fractions. Unlike the residual fraction, the other fractions are well recognized as potential labile pools of heavy metals in soils (Hseu, Citation2006). Therefore, the labile heavy metals were not in large quantities in the soil sample initially; the exchangeable Cr, Cu, Ni, Pb, and Zn were the lowest in all fractions. The contents of Fe/Mn oxide-bound Cu, Ni, and Pb were relatively larger than those in other fractions, representing that a greater amount of metals in the soil may become soluble when Fe/Mn gets dissolved or oxidized if the soil environment changes (Chuan et al., Citation1996). The predominant fractions of Cr and Zn were the residual fraction, suggesting low mobility of Cr and Zn in the soil.
Table 2. Chemical fractions of Cr, Cu, Ni, Pb, and Zn in the initial soil
The analytical recovery of the Hg fractions in the soil was 109% (), which was overestimated because of the high standard deviation. High Hg concentrations associated with high standard deviations led to the overestimated summation of Hg fractions, and a recovery over 100% was obtained as a result. However, the recovery within 100 ± 10% still indicated that the modified sequential extraction procedure was capable of representing Hg fractionation. The predominant fraction of Hg in the soil was the residual fraction, containing 96.1% of the total Hg. This result suggests that Hg in the soil is highly immobile and would not be easily removed using chemical reagents.
Table 3. Chemical fractions (mg/kg) of Hg in the initial soil
Heavy metal removal by chemical extraction
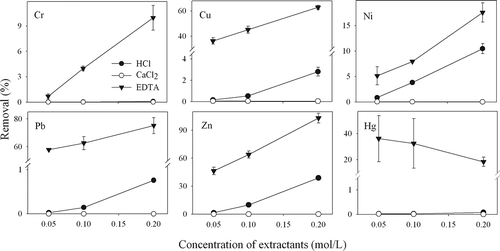
Results from the chemical extraction using HCl and EDTA showed that the increasing molarity of reagents enhanced the extractability of metals, while the removal of metal by CaCl2 was very limited (). Taking EDTA extraction, for instance, the removal of Cu by 0.05, 0.1, and 0.2 M EDTA was 36.3, 45.2, and 62.9%, respectively. EDTA possessed the most efficient removal of heavy metals among the three extractants, with the largest removal (>50%) for Cu, Pb, and Zn in particular. These experimental results were comparable to those from Lee and Kao (Citation2004), in which the effects of extracting reagents and metal speciation on heavy metal removal were studied; EDTA showed greater removal efficiencies for Cu, Zn, and Pb than HCl did. Nevertheless, results from our study may not be directly compared to those in Lee and Kao (Citation2004), due to the difference in soil properties and the concentration of chelating reagents. Very little of the heavy metals in the soil was extracted by CaCl2 (<1%), which accorded with the low mobility of metals in the soil ( and ). Unlike CaCl2, HCl greatly lowered the pH of soil and then enhanced the leachability of heavy metals, Hg was thus more effectively removed with HCl compared to CaCl2.
At 0.2 M, EDTA was not effective in Cr removal from the soil, only causing 10% removal (). The low efficiency of Cr removal was well explained by the low mobility of Cr suggested by the SEP results (). In contrast, despite the low leachability of Zn in the soil, Zn was thoroughly extracted by 0.2 M EDTA. These results suggest that Zn in the residual fraction becomes soluble when the soil is treated with 0.2 M EDTA. The results could be explained by the reports by Sun et al. (Citation2001) and Barona et al. (Citation2001), in which EDTA-extractable metals contributed from different fractions in various soil samples. The removal of Hg by EDTA extraction was not representative and the removal did not follow the increase of EDTA molarity. The standard deviations of Hg removal with diluted EDTA solutions were high, rendering the spread of data points over a large range from 20 to 60% of removal, and the data points were varied in triplicates or quadruplicates. The high standard deviation of Hg removal with EDTA treatments resulted from the inhomogeneity of Hg partition in the soil sample. The efficiency of Hg removal using EDTA depended on the portion of soil sample chosen at random for extraction.
Additionally, the greatest removal of Hg was only 36.2%, indicating that EDTA was not capable of removing Hg when Hg mainly existed as the residual fraction in soil. To remediate Hg-contaminated soil in this study, chemical extraction was shown to be ineffective; other remediation technologies such as thermal desorption should be examined and employed.
TGA test and thermal decontamination with fixed-bed reactor
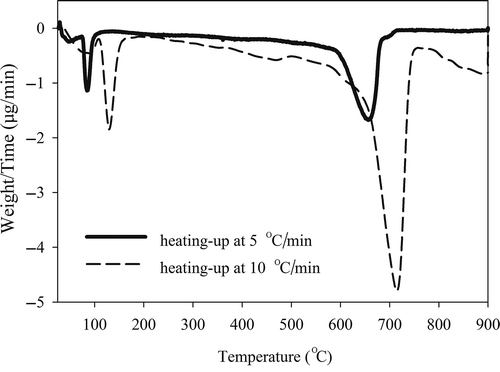
The TGA and differential thermogravimetric (DTG) curves present the thermal characteristics corresponding to physical and chemical changes of soil from the mass losses versus temperature. The obtained characteristic temperatures are helpful not only to set up the parameters of thermal treatment, but also to avoid damaging the soil structure at high temperature (Huang et al., Citation2011). The TGA and DTG results of the soil during heating at 5 (solid line) and 10°C/min (dash line) are shown in . With a 5°C/min heating rate, two significant DTG peaks were observed, at 70–110°C and 580–710°C, respectively, attributed to the loss of soil water and the dehydration of soil minerals (Dixon, Citation1977). A gradual decrease in mass (about 10 wt%) within the temperature interval of 120–550°C was also observed. The mass reduction may be mainly attributed to the thermal degradation of organic matter. Desorption of Hg from the soil can also occur in this temperature range. It is known that Hg(0) and Hg(I) can vaporize at 150–200 and 200°C, respectively, but for Hg(II) an even higher temperature of 300°C is required for vaporization (de Percine, Citation1995). Therefore, Hg species may consecutively evolve within this wide temperature interval. However, the evolution of Hg species could not be resolved from the TGA results in this study because of the insignificant decrease within the interval of 120 to 550°C and the small amount of Hg (180 mg/kg) compared to organic matter (1.3 wt%).
TGA operated at 10°C/min caused a desorption postponement, so the two significant DTG peaks at 5°C/min heating shifted to 105–160 and 600–760°C at 10°C/min. The desorption postponement due to the increased heating rate may lead to a reduction in the effectiveness of thermal decontamination at the lower temperature range. Also, a greater heating rate can consume more energy. Consequently, the 5°C/min heating rate is more suitable for the subsequent fixed-bed thermal decontamination. Based on the thermogravimetric results, the temperature for fixed-bed thermal treatment was set at 550°C for 1 hr with a heating rate of 5°C/min. Controlling the temperature at 550°C is also expected to cause less damage on the soil structure (Huang et al., Citation2011).
Remediation train combining chemical extraction and thermal desorption
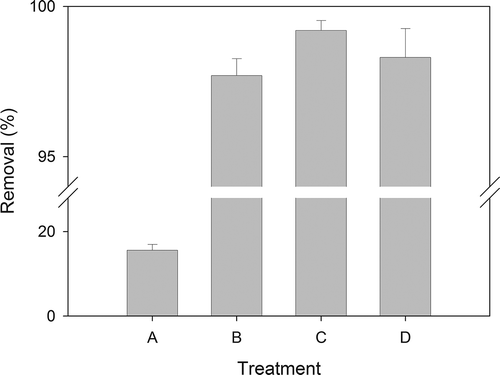
The first combination of remediation train involved chemical extraction using 0.2 M EDTA, mainly for Cr, Cu, Ni, Pb, and Zn removal, followed by thermal treatment for Hg. Using 0.2 M EDTA extraction alone led to a poor removal of Hg (treatment A in ), which was then improved up to 97.7% with subsequent thermal desorption (treatment C in ), suggesting that 550°C with a heating rate of 5°C/min was more effectual in decontaminating Hg from soil than 0.2 M EDTA extraction. Hg removal with the second combination of remediation that had thermal desorption prior to chemical extraction did not improve significantly (treatment D in ), compared to the removal with thermal desorption alone or the first combination of remediation train. Hg decontamination was shown to be less affected by the actual sequence of thermal desorption and chemical extractions.
Figure 3. Hg removal under single and remediation treatments: (A) chemical extraction, (B) thermal treatment, (C) chemical extraction followed by thermal treatment, and (D) thermal treatment followed by chemical extraction.
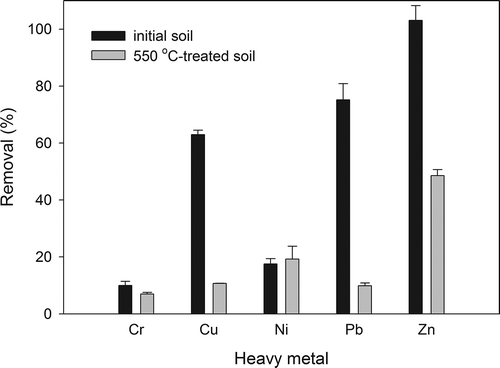
Carrying out the first combination of remediation train (chemical extraction followed by thermal desorption), thermal desorption after EDTA extraction did not further eliminate Cr, Cu, Ni, Pb, and Zn but slightly concentrated the metals instead as moisture and some clay minerals were dehydrated and lost at 550°C but metals remained (data not shown). For the alternative order, when thermal desorption at 550°C had taken place prior to EDTA extraction, the 0.2 M EDTA extractable Cu, Pb, and Zn were decreased dramatically by 50 to 80% (). This resulted largely from transformation and immobilization of heavy metals after thermal treatment (Chou et al., Citation2009; Laurent et al., Citation2009; Huang et al., Citation2011). The negative influence of 550°C treatment on subsequent EDTA extraction clearly illustrated that thermal treatment should not have been employed before carrying out chemical extraction. However, thermal treatment has indeed shown its ability in immobilizing specific heavy metals in soils, compared with other soil amendments, for example, zeolite, for the solidification or stabilization of heavy metals (Shi et al., Citation2009).
Overall, using the treatment train combining 0.2 M EDTA extraction followed by thermal desorption at 550°C for 1 hr, Hg and Zn were reduced nearly 100%, and Cu and Pb were reduced 60–80%. By contrast, the removal of Cr and Ni was only less than 20%. Unlike chemical extraction and thermal desorption, many in situ techniques involving amendments do not alter soil properties severely and have been used to stabilize heavy metals in soils or sediments. Fly ash and its mixture with kaolinite hugely reduced water-soluble Zn and Ni in a sediment sample that initially contained 30 and 45% extractable Zn and Ni, respectively (Tomasevic et al., Citation2013). In addition, the calcareous fly ashes were shown to have a good ability (up to 100% efficiency) in adsorbing aquatic Ni, Cu, Pb, Cd, and Zn (Itskos et al., Citation2010). Another common amendment, zeolite, has high specific surface area and is porous, offers adsorptive sites for pollutants in solutions, but also precipitates heavy metals by supplying alkalinity (Shi et al., Citation2009), where 100% of Cd, Cr, Cu, Ni, Pb, and Zn in wastewater can be removed by zeolite manufactured by fly ash (Koukouzas et al., Citation2010). However, for soils that have been contaminated by heavy metals of high concentration, a promising strategy is to eliminate the heavy metals in soils, rather than stabilizing and storing heavy metals underground. Amendments will be more useful to deal with aqueous samples or soils with low contents of pollutants.
Consequently, we propose here that extraction of nonvolatile heavy metals should be carried out prior to thermal desorption to remediate soils that have been contaminated by both nonvolatile and volatile heavy metals (e.g., Hg) and potentially volatile organic pollutants.
Conclusion
Thermal desorption and chemical extraction were combined as a remediation train, as an attempt to remove Hg and nonvolatile heavy metals from contaminated soil. The optimum conditions for thermal desorption and chemical extraction depended on the soil properties and the fractionation of heavy metals. In our study, the 0.2 M EDTA concentration chosen to extract metals effectively removed considerable amounts of Cu, Pb, and Zn (>50%). However, most Hg was not extractable; further treatment for Hg removal, like thermal desorption, was thus required. Based on TGA and fixed-bed thermal desorption experiments, heating at 550°C for 1 hr with a heating rate of 5°C/min appeared to be appropriate for Hg removal. With the employment of thermal desorption, 99% of Hg in soil could be eliminated. Unfortunately, thermal desorption prior to chemical extraction had a negative influence on subsequent chemical extraction because nonvolatile heavy metals were immobilized in soil after 550°C heating. We suggest that the nonvolatile metals should be removed before the employment of thermal decontamination that aims to remove Hg. The remediation train combining chemical extraction followed by thermal desorption appears to successfully decontaminate Cu, Pb, Zn, and Hg from the test soil. The present study provides demonstration of an effective remediation train applied to remediation of Hg and multiple heavy metal-contaminated soil.
Funding
The authors thank the Environmental Protection Administration and the National Science Council of Taiwan for financially supporting this study under contracts EPA-99-GA103-03-A236-2 and NSC101-2622-E-020-011-CC3, respectively.
Disclaimer
The opinions expressed in this paper are not necessarily those of the sponsors.
Acknowledgment
The authors greatly acknowledge Dr. Gregory Jacobson from the University of Waikato for his help correcting syntax errors and refining this paper.
Additional information
Notes on contributors
Zeng-Yei Hseu
Zeng-Yei Hseu is a professor at the Department of Environmental Science and Engineering, National Pingtung University of Science and Technology, Taiwan.
Yu-Tuan Huang
Yu-Tuan Huang is a doctoral student at the Department of Earth and Ocean Sciences, University of Waikato, New Zealand.
Hsing-Cheng Hsi
Hsing-Cheng Hsi is an associate professor at the Graduate Institute of Environmental Engineering, National Taiwan University, Taiwan.
References
- Abumaizar, R. J., and E. H. Smith. 1999. Heavy metal contaminants removal by soil washing. J. Hazard. Mater. 70:71–86. doi:10.1016/S0304-3894(99)00149-1
- Barona, A., I. Aranguiz, and A. Elías. 2001. Metal associations in soils before and after EDTA extractive decontamination: Implications for the effectiveness of further clean-up procedures. Environ. Pollut. 113:79–85. doi:10.1016/S0269-7491(00)00158-5
- Chang, T. C., and J. H. Yen. 2006. On-site mercury-contaminated soils remediation by using thermal desorption technology. J. Hazard. Mater. 128: 208–17. doi:10.1016/j.jhazmat.2005.07.053
- Chou, J. D., M. Y. Wey, and S. H. Chang. 2009. Evaluation of the distribution patterns of Pb, Cu and Cd from MSWI fly ash during thermal treatment by sequential extraction procedure. J. Hazard. Mater. 162:1000–6. doi:10.1016/j.jhazmat.2008.05.155
- Chuan, M. S., G. Y. Shu, and J. C. Liu. 1996. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 90:543–56. doi:10.1007/BF00282668
- Cline S. R., and B. E. Reed. 1995. Lead removal from soils via bench-scale soil washing techniques. J. Environ. Eng. 121:700–5. doi:10.1061/(ASCE)0733-9372(1995)121:10(700)
- de Percine, P. R. 1995. Application of thermal desorption technologies to hazardous waste sites. J. Hazard. Mater. 40: 203–9. doi:10.1016/0304-3894(94)00085-U
- Dermont, G., M. Bergeron, G. Mercier, and M. Richer-Laflèche. 2008a. Metal-contaminated soils: remediation practices and treatment technologies. Practice Period. Hazard Toxic Radio Waste Magage. 12:188–209. doi:10.1061/(ASCE)1090-025X(2008)12:3(188)
- Dermont, G., M. Bergeron, G. Mercier, and M. Richer-Laflèche. 2008b. Soil washing for metal removal: a review of physical/chemical technologies and field applications. J. Hazard. Mater. 152:1–31. doi:10.1016/j.jhazmat.2007.10.043
- Dixon, J. B. 1977. Kaolinite and serpentine group minerals. In Minerals in Soil Environments, ed. J. B. Dixon, J. A. Kittrick, M. H. Milford, and J. L. White, 357–403. Madison, WI: Soil Science Society of America.
- Ehsan, S., S. O. Prasher, and W. D. Marshall. 2006. A washing procedure to mobilize mixed contaminants from soil: II. Heavy metals. J. Environ. Qual. 35: 2084–91. doi:10.2134/jeq2005.0475
- Gee, G. W., and J. W. Bauder. 1986. Particle-size analysis. In Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd ed., ed. A. Klut, 383–411. Agronomy Monograph 9. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Griffiths, R. A. 1995. Soil-washing technology and practice. J. Hazard. Mater. 40:175-90. doi:10.1016/0304-3894(94)00064-N
- Hseu, Z. Y. 2006. Extractability and bioavailability of zinc over time in three tropical soils incubated with biosolids. Chemosphere 63:762–71. doi:10.1016/j.chemosphere.2005.08.014
- Huang, Y. T., Z. Y. Hseu, and H. C. Hsi. 2011. Influences of thermal decontamination on mercury removal, soil properties, and repartitioning of coexisting heavy metals. Chemosphere 84:1244–49. doi:10.1016/j.chemosphere.2011.05.015
- International Standards Organization. 1995. Soil quality: Extraction of trace elements soluble in aqua-regia. ISO 11466. Geneva, Switzerland: ISO.
- Itskos, G., N. Koukouzas, C. Vasilatos, I. Megremi, and A. Moutsatsou. 2010. Comparative uptake study of toxic elements from aqueous media by the different particle-size-fractions of fly ash. J. Hazard. Mater. 183:787–92. doi:10.1016/j.jhazmat.2010.07.095
- Koukouzas, N., C. Vasilatos, G. Itskos, I. Mitsis, and A. Moutsatsou. 2010. Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials. J. Hazard. Mater. 173:581–88. doi:10.1016/j.jhazmat.2009.08.126
- Kunkel, A. M., J. J. Seibert, L. J. Elliott, R. Kelley, L. E. Katz, and G. A. Pope. 2006. Remediation of elemental mercury using in situ thermal desorption (ISTD). Environ. Sci. Technol. 40:2384–89. doi:10.1021/es0503581
- Laurent, J., M. Pierra, M. Casellas, and C. Dagot. 2009. Fate of cadmium in activated sludge after changing its physico-chemical properties by thermal treatment. Chemosphere 77:771–77. doi:10.1016/j.chemosphere.2009.08.024
- Lee, C. S., and M. M. Kao. 2004. Effects of extracting reagents and metal speciation on the removal of heavy metal contaminated soils by chemical extraction. J. Environ. Sci. Health Part A 39:1233–49. doi:10.1081/ESE-120030328
- Leštan, D., C. L. Luo, and X. D. Li. 2008. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 153: 3–13. doi:10.1016/j.envpol.2007.11.015
- Mann, M. J. 1999. Full-scale and pilot-scale soil washing. J. Hazard. Mater. 66:119–36. doi:10.1016/S0304-3894(98)00207-6
- Massacci, P., L. Piga, and M. Ferrini. 2000. Applications of physical and thermal treatment for the removal of mercury from contaminated materials. Miner. Eng. 13:963–67. doi:10.1016/S0892-6875(00)00081-9
- McLean, E. O. 1982. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed., ed. A. L. Page, R. H., Miller, and D. R. Keeney, 199–224. Agronomy Monograph 9. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Mehra, O. P., and M. L. Jackson. 1960. Iron oxides removed from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. Clays Clay Miner. 7:317–27. doi:10.1346/CCMN.1958.0070122
- Neculita, C. M., G. J. Zagury, and L. Deschênes. 2005. Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J. Environ. Qual. 34:255–62. doi:10.2134/jeq2005.0255
- Nelson, D. W., and L. E. Sommers. 1982. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed., ed. A. L. Page, R. H., Miller, and D. R. Keeney, 539–77. Agronomy Monograph 9. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Nelson, R. E. 1982. Carbonate and gypsum. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed., ed. A. L. Page, R. H., Miller, and D. R. Keeney, 181–97. Agronomy Monograph 9. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Reed, B. E., P. C. Carriere, and R. Moore. 1996. Flushing of a Pb(II) contaminated soil using HCl, EDTA, and CaCl2. J. Environ. Eng. 122:48–50. doi:10.1061/(ASCE)0733-9372(1996)122:1(48)
- Rhoades, J. D. 1982. Cation exchange capacity. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed., ed. A. L. Page, R. H., Miller, and D. R. Keeney, 149–57. Agronomy Monograph 9. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Shi, W.-Y., H.-B. Shao, H. Li, M.-A. Shao, and S. Du. 2009. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J. Hazard. Mater. 170:1–6. doi:10.1016/j.jhazmat.2009.04.097
- Sims, R. C. 1990. Soil remediation techniques at uncontrolled hazardous waste sites. J. Air Waste Manage. Assoc. 40:704–32. doi:10.1080/10473289.1990.10466716
- Stewart, J. W. B., and J. R. Bettany. 1982. Mercury. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Methods, 2nd ed., ed. A. L. Page, R. H. Miller, and D. R. Keeney, 367–84. Madison, WI: Agronomy Society of America and Soil Science Society of America.
- Sun, B., F. J. Zhao, E. Lombi, and S. P. McGrath. 2001. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 113:111–20. doi:10.1016/S0269-7491(00)00176-7
- Taube, F., T. Pommer, A. Larsson, A. Shchukarev, and A. Nordin. 2008. Soil remediation—Mercury speciation in soil and vapor phase during thermal treatment. Water Air Soil Pollut. 193:155–63. doi:10.1007/s11270-008-9679-y
- Tessier, A., P. G. C. Campbell, and M. Bisson. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51:844–51. doi:10.1021/ac50043a017
- Tomasevic, D. D., M. B. Dalmacija, M. D. Prica, B. D. Dalmacija, D. V. Kerkez, M. R. Bečelić-Tomin, and S. D. Roncevic. 2013. Use of fly ash for remediation of metals polluted sediment—Green remediation. Chemosphere 92:1490–97. doi:10.1016/j.chemosphere.2013.03.063
- Wang, J., X. Feng, C. W. N. Anderson, Y. Xing, and L. Shang. 2012. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 221-222: 1–18. doi:10.1016/j.jhazmat.2012.04.035