Abstract
This study investigates the application of the Aerosol-to-Liquid Particle Extraction System (ALPXS), which uses wet electrostatic precipitation to collect airborne particles, for multi-element indoor stationary monitoring. Optimum conditions are determined for capturing airborne particles for metal determination by inductively coupled plasma–mass spectrometry (ICP-MS), for measuring field blanks, and for calculating limits of detection (LOD) and quantification (LOQ). Due to the relatively high flow rate (300 L min−1), a sampling duration of 1 hr to 2 hr was adequate to capture airborne particle-bound metals under the investigated experimental conditions. The performance of the ALPXS during a building renovation demonstrated signal-to-noise ratios appropriate for sampling airborne particles in environments with elevated metal concentrations, such as workplace settings. The ALPXS shows promise as a research tool for providing useful information on short-term variations (transient signals) and for trapping particles into aqueous solutions where needed for subsequent characterization. As the ALPXS does not provide size-specific samples, and its efficiency at different flow rates has yet to be quantified, the ALPXS would not replace standard filter-based protocols accepted for regulatory applications (e.g., exposure measurements), but rather would provide additional information if used in conjunction with filter based methods.
Implications
This study investigates the capability of the Aerosol-to-Liquid Particle Extraction System (ALPXS) for stationary sampling of airborne metals in indoor workplace environments, with subsequent analysis by ICP-MS. The high flow rate (300 L/min) permits a short sampling duration (< 2 hr). Results indicated that the ALPXS was capable of monitoring short-term changes in metal emissions during a renovation activity. This portable instrument may prove to be advantageous in occupational settings as a qualitative indicator of elevated concentrations of airborne metals at short time scales.
Introduction
New methods for sample collection and analysis are continuously being developed to improve the characterization of aerosols in the workplace and to ensure that airborne concentrations of hazardous substances do not exceed exposure limits prescribed by the responsible occupational health and safety authorities (Butler et al., Citation2013; Miller et al., Citation2010). Canadian authorities generally rely on standards recommended by both the American Conference of Governmental Industrial Hygienists (ACGIH) and National Institute for Occupational Safety and Health (NIOSH) for air sampling methods and sample analysis in the workplace (Canada Occupational Health and Safety, Citation2012). For the determination of airborne metal concentrations, NIOSH recommends the use of low-volume air pumps (1–4 L min−1) to collect air particles on filters (NIOSH, Citation2003). Sample collection generally occurs for 7 to 10 hr to ensure compliance with the occupation exposure limit (OEL), permissible exposure limit (PEL) or threshold limit value (TLV) (ASTM, Citation2008). The period of sample collection is selected to approximate exposure during a normal working day. Subsequent analysis of collected particulate matter by sensitive analytical instruments, such as inductively coupled plasma–mass spectrometry (ICP-MS), can provide results in the nanograms per cubic meter range, provided a sufficient mass of air particles can be collected. However, collection periods of 7 to 10 hr often result in insufficient sample to exceed ICP-MS detection limits. Even with a 24-hr sampling period, low air volumes can result in unreliable multi-element data caused by the analytical challenges of handling and preparing lightly-loaded filter samples (detailed by Rasmussen et al., Citation2007).
This study investigates the capability of wet electrostatic precipitation (with subsequent multi-element analysis by ICP-MS) as a method for monitoring short-term changes in airborne metal concentrations, which would complement standard filter-based protocols for stationary sampling. Air is drawn into the Aerosol-to-Liquid Particle Extraction System (ALPXS) at approximately 300 L min−1, which is a significantly greater volume than the 1–4 L min−1 typical of filter methods used in occupational hygiene applications. The potential usefulness of the ALPXS for capturing transient signals was demonstrated in a previous application (Rasmussen et al., Citation2013) in which aerosolized single-walled carbon nanotubes were collected for elemental analysis by ICP-MS. Developing capacity for monitoring “momentarily elevated” concentrations of airborne particles is particularly important for assessing workplace exposures to airborne carbon nanotubes (Hashimoto et al., Citation2013).
In the first stage of the present study, a method of measuring field blanks is developed for the purpose of calculating limits of detection (LOD) and limits of quantification (LOQ), and to investigate potential sources of inadvertent contamination. The next stage evaluates the optimal operating conditions of ALPXS using a series of controlled particle emission experiments, in which the ALPXS is placed inside a laboratory fume hood where aerosolized particles (a standard reference material) are released from a sieve shaker to generate a sustained source of airborne particles. In the final stage, the developed ALPXS/ICP-MS method is applied to a case study involving an indoor building renovation. In this application, the ALPXS method is used for monitoring airborne metal concentrations in close proximity to the renovation activity in comparison to indoor background concentrations. Potential advantages of a shorter sampling duration (i.e., 30 min) emerge in the study of transient signals that occur during the renovation activity.
Materials and Methods
Reagents and labware
Deionized (DI) water (18.2 MΩ cm−1 Milli-Q Element, Bedford, MA) was used as the sampling fluid in the ALPXS, and for preparation and dilution of samples, standards, and quality control solutions. Metal-free graduated polypropylene disposable centrifuge tubes (50 mL or 15 mL; Corning, Inc., Corning, NY) were used for preparation of standards and dilutions. Metal-free graduated (50-mL) disposable DigiPrep test tubes (SCP Science, Quebec, Canada) were used for ALPXS sampling and subsequent fluid evaporation. Upon completion of the evaporation process, the residue was acid digested with concentrated nitric acid (Seastar Chemical, Inc., Burnaby, British Columbia, Canada).
All calibration and internal standards for ICP-MS analysis were prepared using High Purity Standards from Delta Scientific Laboratory Products Ltd. (Mississauga, Ontario). Internal standard solutions (50 μg L−1) were prepared using single element standard solutions of Sc, In, Y (when it was not an analyte), and Re (1000 mg L−1). Reference samples containing trace elements in water were prepared using TM 28.3 Standard (National Water Research Institute, Environment Canada) for ICP-MS quality assessment. Multi-element calibration verification standards were prepared at 0.5, 1.0, 5.0, 10.0, 20.0, and 50.0 ng mL−1 concentrations. Certified reference material NIST 1648a (Urban Particulate Matter obtained from the National Institute of Standards and Technology, Gaithersburg, MD) was used to evaluate digestion efficiency by measurement of elemental recoveries.
Instrumentation
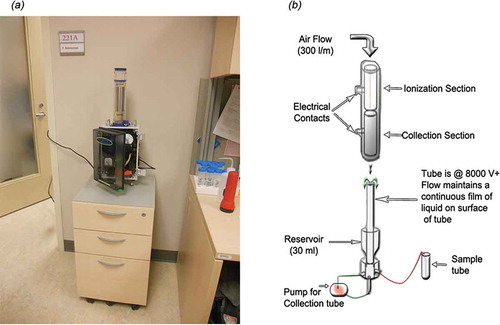
The ALPXS (Meinhard, Co., USA, patent US2012/0048748-A1) was used to collect airborne samples in indoor environments located at the University of Ottawa, Ontario, Canada. This portable device uses wet electrostatic precipitation to extract particles from air and may be operated using an AC supply or battery. The main components include an ionization electrode, collection electrode, sample tube (liquid reservoir), and a liquid pump. The electrodes are stainless steel (American Iron & Steel Industries designation number AISI-316). Ambient air is drawn into a tube via an opening on top of the ionization section ( and ). The adjustable intake air flow rate provides a stable airflow with a minimum pressure drop across the device. When the high voltage is turned “on,” –8,000 V direct current (DC) potential is applied to stainless-steel wire (d = 0.005 inches) in the center of the ionization section, which in turn creates the electrostatic field. Particles assume a negative charge when passing this section. The collection section located below consists of the counterelectrode (+8,000 V DC), a liquid pump, and a sampling tube. With the aid of the pump, fluid is constantly recirculated from the sampling tube and percolated smoothly over the counter electrode. The downward airflow created by the pump brings the negatively (–ve) charged particles towards the positive (+ve) counterelectrode on which the percolating fluid flow is maintained. Through this process the particles are continuously captured and concentrated in the sampling fluid. According to the manufacturer’s description (ALPXS, Citation2010), sample collection efficiency for particles less than 0.3 μm is greater than 90%, but no information about collection efficiency is provided for aerosols >0.3 μm. Sampling efficiency is not evaluated further in the present study.
Figure 1. (a) Monitoring office environment using ALPXS. (b) Schematic diagram of ALPXS. Modified from ALPXS (Citation2010).
Evaporation of liquids was accomplished with a Digi-Prep Hot Block in 50-mL metal-free graduated Digi-Prep sample tubes (SCP Science, Quebec, Canada). All metal components on the Hot Block are coated with polytetrafluoroethylene (PTFE) that prevents corrosion so that it reduces sample contamination during the evaporation process. Following complete evaporation, concentrated nitric acid was added to the residue and the samples were allowed to digest in an ultrasonic bath (Branson Ultrasonics, Danbury, CT).
The Elan DRC II inductively coupled plasma–mass spectrometer (ICP-MS; Perkin Elmer, Woodbridge, ON, Canada) was operated in both standard and dynamic reaction cell (DRC) mode for elemental analysis of test samples. The sample introduction system consisted of a cyclonic spray chamber (Glass Expansion, West Melbourne, Australia) and a Meinhard TQ-30-A3 concentric nebulizer (J. E. Meinhard Associates, Inc., Santa Ana, CA), and the interface consisted of a platinum skimmer and sampler cones. Instrument operation conditions are shown in . Corrections for molecular interactions were applied as in Elan software version 3.5.
Table 1. ICP-MS operating conditions
A sieve shaker (Model Ro-Tap RX-94; WS Tyler, Mentor, OH), operating inside a laboratory fume hood, was used to deliver a sustained source of airborne particulate material using NIST 1648a Urban Particulate Matter. The shaker was modified to operate at different shaking frequencies in order to change the sustained concentration of particulate material in the air. Increasing the shaking frequency resulted in an increase in the concentration of airborne particulate material inside the fume hood.
Indoor environmental conditions were monitored and recorded for all experiments using the DustTrak II aerosol monitor (model 8530; TSI, Shoreview, MN) and the Q-TRAK indoor air quality monitor with probe 980 (model 7575; TSI, Shoreview, MN, usa). The DustTrak II was used to monitor air particle concentrations between 0.001 and 400 mg m−3 (±0.1% resolution), ranging in size from 0.1 to 10 μm. The Q-TRAK was used to measure and record indoor temperature (range 0–60ºC; accuracy ±0.5ºC) and humidity (range 5–95%; accuracy ±3% including hysteresis) every 60 sec.
Operation of ALPXS
In order to calculate the concentration of metals in air (Cair in ng/m3; as shown in eq 1), it was necessary to determine the ALPXS air sample velocity (vinlet), area of the intake inlet (Ainlet) and total sample time (tcollection). The vinlet was measured by positioning an anemometer (45158, Extech Instruments, Nashua, NH) perpendicular to the ALPXS intake inlet, while blocking all other openings with polyethylene sheeting. Other parameters required in the calculation are the volume of solution used for ICP-MS analysis (Vsolution) and concentration of metals in solution (Csolution):
The ALPXS was cleaned before each sample collection run by wiping inside the ionization section with a long-handled flat swab dipped in 70% isopropyl alcohol. Care was taken not to touch the fine ionization electrode in the center. The inside of the counterelectrode was cleaned by passing a thin pipe cleaner through the electrode. After cleaning, the ALPXS was allowed to completely dry before commencing the sample run. The water recirculating tubing was replaced if carryover was observed from a previous sample run, particularly after use in locations with high air particle concentrations.
The ALPXS was placed on a leveled nonvibrating surface for all monitoring experiments. The sample tube was filled with 20–40 mL of deionized water, depending on the length of the collection cycle. For example, 40 mL of deionized water in the sampling tube was adequate to maintain the continuous recirculating water flow for a 1-hr cycle, allowing for 12 mL of evaporation loss. After securing the liquid tubing and testing all electrical connections (–ve/+ve high voltage [HV] and ground wires), the water flow control was used to create a smooth percolating water flow over the collection electrode of the instrument (). The water flow setting was changed from zero to “operate” and the airflow was measured using the anemometer. The ALPXS was operated without high voltage to obtain a “no-HV” field blank. A faint ozone smell was detected when the high-voltage circuit was turned on (“HV-on”), indicating proper functioning of the electrostatic capture mechanism. To ensure consistent results during air particle collection, it was important to ensure that a smooth water flow was maintained in the collection tube, avoiding an arch formation on the surface. The final volume in the sampling tube (dependent on evaporation during sampling) was recorded and used in subsequent calculations.
Experimental Design
Background samples were collected repetitively (for sampling periods of 30, 60, or 120 min depending on the experiment) by deploying the ALPXS in office and laboratory environments at University of Ottawa. For background measurements the ALPXS was operated in standard conditions in “HV-on” mode and air flowing into the collection chamber (˜ 300 L min−1).
For calculation of limits of detection (LOD) and limits of quantification (LOQ), the ALPXS was operated under the same conditions with the exception that “no-HV” mode was used, to minimize the entry of charged particles into the system. LODs and LOQs were established based on a 60-min sampling period, which was anticipated to be a typical sampling duration.
A series of four aerosolization experiments was conducted to determine optimal operating conditions and test the capabilities of the ALPXS to collect airborne particles of known composition in a simulated workplace setting. These experiments were designed to provide a sustained release of air particles of known composition (NIST 1648a Urban Particulate Matter) using an approximate mass of 0.05 g of NIST 1648a on a sieve shaker inside a self-ventilating fumehood. The ALPXS air intake was located approximately 0.5 m from the sieve shaker, and the fume hood ventilation was turned off during the monitoring period.
Subsequent to sampling indoor background locations, the capability of the ALPXS to collect air particles in workplace environments was tested by sampling in the vicinity of an indoor renovation involving the replacement of drywall following a flooding incident (“renovation experiment”). The air sampling intake was positioned approximately 2 m from the renovation activity, and each sampling run lasted 30 min. The sampling was repeated five times during two main renovation processes, which included (1) the cutting and replacing of damaged drywall, and (2) drying the repaired wall with a hot air blower followed by vacuum cleanup of the worksite.
Sample preparation and analysis of metals
All samples collected from the ALPXS were evaporated and digested before analysis by ICP-MS. The sample was evaporated to dryness using the DigiPrep HotBlock inside a clean trace metal-free fume hood to minimize contamination. A 2-mL aliquot of concentrated HNO3 (68% w/w) was added to each residue and the capped tubes were placed in an ultrasonic water bath at 60ºC for 2 hr. The digest was then transferred into a 15-mL disposable centrifuge tube and brought to a total of volume of 10 mL with DI water. The sample was mixed thoroughly using a vortex mixer. If sample residue was visible, the digest was centrifuged at 3000 rpm for 10 min. These samples were first screened by conducting a semiquantitative analysis by ICP-MS. The elements with concentrations higher than instrument detection limits (IDL) were selected for further analysis and comparison. Some samples needed dilution before analysis. Elements were determined in standard mode with the exception of Fe and Cr, which were determined in DRC mode (using ammonia as the reaction gas). The IDLs for each analyzed element, as calculated from three times the standard deviation (SD) of the blanks, were 0.04, 0.002, 0.07, 0.09, 0.25, 2.01, 0.01, 1.5, 0.02, 0.04, 0.01, 0.09, 0.03, 0.10, and 0.48 ng L−1 for As, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Sb, Sr, Ti, V, Y, and Zn respectively (note that IDL concentration units refer to aqueous solutions).
Results
Detection limits and background concentration
Field blanks collected at two indoor locations were used to assess detection levels during sample collection, preparation, and analysis. A limit of detection (LOD) and a limit of quantification (LOQ) for each element were calculated from the standard deviation (SD) of 13 “no-HV” ALPXS field blanks collected in an office (n = 10) and a laboratory (n = 3), which were both indoor locations where the experiments were carried out. The LOD was calculated as three times SD and the LOQ as 10 times SD. Note that the field blanks used for estimating LOD and LOQ are inclusive of ALPXS sample collection, sample digest, and subsequent analysis by ICP-MS. As shown in , these results were compared with the average of background measurements collected in the same office and laboratory environments. Mean background concentrations for most analyzed elements were greater than their LODs and less than their LOQs, with the exception of Fe, Pb, and Zn, which had results lower than their respective LODs (). LOD, LOQ, and indoor air background metal concentrations in are based on a 60-min collection period.
Table 2. LOD, LOQ, and indoor air background metal concentrations for analysed elements
Environmental background conditions were continuously recorded throughout the study, including air particle concentration (DustTrak II), humidity, and temperature (Q-TRAK). Over 13 days of testing, 5072 separate DustTrak II background measurements were made with the air particle concentration averaging 0.0053 mg m−3, ranging from 0.0036 to 0.0119 mg m−3. Temperature readings over the same period ranged from 21.8 to 23.2ºC (average 22.3ºC), and relative humidity ranged from 13.1% to 17.7% (average 16.3 %) as determined using the Q-TRAK.
Optimization of ALPXS operating conditions
A series of four aerosolization experiments was conducted inside a laboratory fume hood using a sieve shaker to disperse particulate material containing known concentrations of metals (NIST 1648a) into the air, with the purpose of optimizing operating conditions for monitoring metals in indoor air.
The first experiment investigated the capability of the ALPXS to detect aerosolized NIST particles compared to the background aerosol (signal-to-noise ratio), with a sampling duration of 30 min. The test was repeated a total of three times using identical conditions. indicates that mean elemental concentrations from the aerosolization experiment exceeded laboratory background concentrations, and that all measurements exceeded LOD, with the exception of background values for Pb and Zn. Relative standard deviations (RSD) for the aerosolization experiment ranged from 20% to 32% for most elements in (Ti, V, Cr, Mn, C, Sr, Cd, and Sb), but were higher (39–45% RSD) for Pb, Zn, Cu, and Ni. Sources of variability may include preferential aerosolization of lighter particles of the NIST 1648a source material during shaking, the heterogeneity of metal distribution across different particles (Salcedo et al., Citation2014), and the difficulty in maintaining a consistent mass of source material. These inherent limitations associated with using the sieve shaker, as the aerosolizer impacted our ability to quantitatively assess the reproducibility of the ALPXS. However, overall metal concentrations for aerosolized samples exceed background samples anywhere from 2 times (Sb) to 400 times (Ti), indicating an overall strong signal-to-noise ratio and successful extraction of dispersed NIST particles by the ALPXS.
Table 3. Mean (SD) airborne elemental concentrations (ng m−3) in the aerosolization experiment using NIST 1648a and operating the ALPXS for 30 min sampling duration (n = 3)
The second experiment used elemental ratios to further assess the capability of the ALPXS to monitor airborne metals at a level of accuracy and reproducibility that can distinguish background air metal concentrations from particle-bound metals emitted from a work-related process (sieving). This experiment involved 7 separate aerosolization runs using the sieve shaker as the source of emitted NIST 1648a particles, with ALPXS sample collection periods ranging from 30 to 60 minutes. Elemental ratios from aerosolized NIST 1648a collected using the ALPXS were compared to observed bulk NIST 1648a ratios in . The observed bulk NIST 1648a ratios were obtained by digesting the powder using procedures identical to those used for digestion of ALPXS samples, followed by ICP-MS analysis. Also shown are the elemental ratios of the laboratory background aerosols. The resulting ratios are presented in as mean and standard deviation (SD) for laboratory background aerosol samples, aerosolized NIST 1648a samples collected by the ALPXS, and bulk NIST 1648a powder (nonaerosolized). In comparing aerosolized NIST samples with bulk NIST, Sr/V ratios are extremely close with less than a 4% relative difference between the two ratios. The greatest relative difference is with Ni/Sr, where the aerosolized NIST ratio is approximately nine times greater than the bulk NIST ratio. Overall there is excellent agreement between Sr/V, Sb/V, Co/V, V/Cd, Cr/V, and Cd/Mn when comparing aerosolized and bulk 1648a ratios. In contrast, shows that elemental ratios for background aerosols differ from the NIST aerosols and bulk powder, with background ratios for Sr/V, Sb/V, Co/V, Cr/V, Pb/V, Cu/V, and Ni/Sr ranging from approximately 3 times to 260 times higher than either set of NIST ratios. These differences provide evidence that the air particles captured by the ALPXS during the aerosolization experiment originates predominately from the NIST 1648a source material rather than background aerosols, and that elemental signatures can be used to distinguish particle source emissions from background aerosols.
Table 4. Comparison of elemental ratios for aerosolized NIST 1648a, sampled by ALPXS and bulk NIST-1648a powder
The ratios Pb/V, Ti/Mn, Zn/Sr, and Cu/V are in the same order of magnitude for aerosolized NIST and the bulk NIST powder, but the standard deviation for all of these ratios in the aerosolized NIST is relatively high. Again, this points to a lack of homogeneous dispersion of NIST 1648a during the aerosolization experiment, associated with the dispersion mechanism and heterogeneity of metal distribution within the NIST particles captured by the ALPXS. The greatest difference between aerosolized and bulk NIST ratios was observed with Ni/Sr results, where the aerosol ratio was eight times greater than the bulk NIST 1648a (). Such a large discrepancy may also be caused by potential source(s) of Ni contamination during ALPXS sample collection, discussed later.
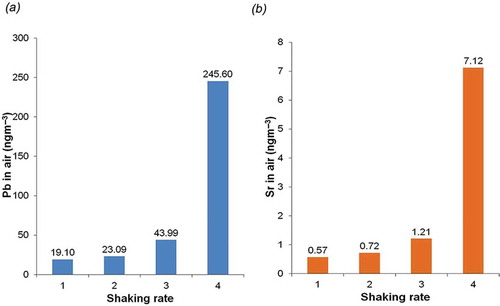
The third aerosolization experiment is a qualitative test of the ability of the ALPXS to detect increases in airborne metal concentrations resulting from increasing particle emission rates. shows how Pb and Sr concentrations increased as the sieve shaker frequency was increased over four separate sample runs. Similar results were obtained for the other elements (not shown). The sampling duration for each run was 60 min. The intensity of shaking was increased for each of the four runs (illustrated by levels 1 to 4 on the x-axis of , with 4 representing the maximum intensity), with the lowest shaking rate (lowest particle concentration) occurring in the first run and the highest shaking rate (highest particle concentration) occurring in the fourth run. Note that Pb and Sr act as airborne tracers of the released particles related to the source, as their air concentrations increase with higher shaking rates, in direct association with the increase in NIST 1648a particles emitted from the source (). further shows that as the rate of shaking increased (from rate 1 to rate 4), the Pb/Sr ratio remained consistent within experimental error (34:1 for rate 1 compared to 35:1 for rate 4) and was also similar to the Pb/Sr ratio in the NISTa bulk powder (26/1; ). Similarly, in a study of Y and Ni impurities in single wall carbon nanotubes (Rasmussen et al., Citation2013), the ALPXS/ICP-MS method detected air concentrations of Y and Ni in approximately the same proportion as observed in the bulk powder (Y/Ni ratio ≈ 1/5).
Figure 2. Lead (a) and strontium (b) tracers of airborne particles released during sieving. Rate of shaking was increased incrementally with 60 min of monitoring at each increment, using ALPXS and ICP-MS determination.
The purpose of the fourth and final test in the aerosolization suite of experiments was to examine the potential influence of changing the ALPXS sampling duration. The sample collection period was increased from 30 min to 45 min to 60 min to 120 min in four separate trials (), with sampling repeated three times for each of the four trials, keeping all other conditions constant. Overall, elemental concentrations in the digests (ng L−1) increased as sampling time increased (not shown in ). Mean air concentration (ng m−3) of elements such as Zn, Pb, V, and Sr remained relatively constant whether the duration was 30, 45, 60, or 120 min (). However, mean air concentrations for some elements were higher in 30-min samples (e.g., 36.3 ng m−3 for Cu; ) than in longer duration samples (14.6–18.4 ng m−3 for Cu; ). Based on detailed observations of the complex heterogeneous matrix of NIST 1648a, which includes a variety of organic and mineral phases at the microscale (Salcedo et al., Citation2014), it is possible that higher release rates for some metals in the first 30 min () may be related to the preferential aerosolization of lighter organic-rich NIST 1648a particles. indicates an overall decrease in standard deviation after the 30-min test, suggesting that elemental releases from the sieve shaker became more constant over longer monitoring periods. Although Co reproducibility appeared to improve with longer sample collection, reproducibility of the remaining metals did not consistently increase or decrease with longer sample collection. There were no observable trends for either mean concentration or standard deviation for As, Cd, Mn, Pb, Sr, Ti, V, or Zn, relative to sample duration ().
Table 5. Capability of the ALPXS to sample airborne particles (ng m−3) over different sampling durations (30, 45, 60, and 120 min)
Results presented in suggest an optimum collection duration of 45–60 minutes for the conditions of this test. Overall, this test showed no significant improvement in results with longer sampling duration (120 min). High metal concentrations at the outset appear to be more related to the delivery system than the sampling system. While this was adequate for the purposes of the present study, future work will focus on devising an aerosolization source that provides more controlled release of particulate material into the air.
Monitoring emissions of airborne metals during building renovation
To apply the ALPXS/ICP-MS method for the purpose of monitoring emissions during the building renovation, the ALPXS unit was set up 2 m away from the construction activity. Five separate samples were collected consecutively, with each sample run lasting 30 min. The results for three elements (Sr, Ni, and Cr) are shown in . On the x-axis, stage zero (0) represents the background concentration for each metal. Stage 1 represents the first sample collected during renovation and stage 5 represents the final sample (approximately 2.5 hr later). Collection of samples for stages 1 and 2 was done during the removal and replacement of damaged drywall, and for stages 3 to 5 collection was done during drying and vacuuming the construction area. Strontium (Sr) shows the most variability, with the highest concentration of 9.46 ng m−3 occurring during the removal and replacement of damaged drywall (stages 1 and 2). The Sr concentration drops significantly during the drying and vacuuming (stages 3 to 5), reaching a minimum value of 1.85 ng m−3 during stage 5 (). It is concluded that the increase in Sr concentration by 30-fold is likely associated with emission of particles from the drywall, of which strontium is a significant constituent (U.S. EPA, Citation2009; Hirsch et al., Citation2010).
Figure 3. Variation of metal concentration of Sr, Ni, and Cr during stages of indoor renovation monitoring. Renovation activity included cutting and replacing of damaged drywall (stages 1 and 2) and drying the repaired wall with an air blower followed by vacuum clean-up of the workspace (stages 3 to 5). Stage zero (0) represents the background concentration.
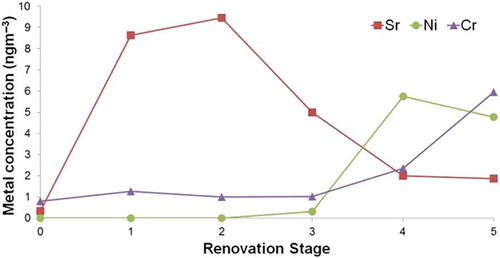
In contrast, Cr and Ni concentrations remained low during stages 1 to 3 and then increased in stages 4 and 5 (). In the case of Ni, the concentration was below detection (LOD = 1.14 ng m−3) until stage 4, where it reached its highest concentration of 5.74 ng m−3. Cr followed a similar pattern, reaching its highest concentration in stage 5 (5.94 ng m−3). The sixfold increase in Cr and Ni concentrations during drying and vacuuming () is likely related to metal release by mechanical abrasion of metallic parts within the equipment (blowers used in drying and the vacuum cleaner used in cleanup of the renovation area). Overall, the use of ALPXS for time-series monitoring of air quality during the renovation activity demonstrated its ability to detect short-term increases and decreases in airborne metal concentrations over the five consecutive stages with an appropriate level of sensitivity, responsiveness, and absence of any apparent carryover effect.
Nine other elements were monitored throughout the renovation (not shown in ), including Cd, Cu, Mn, and Zn, which increased after the second stage renovation in a pattern similar to Cr and Ni; Sb, which increased in stages 1–2 and decreased in stages 4–5, similar to Sr; As, Fe, and V, which did not show a specific pattern; and Pb, which was below the detection limit.
Discussion
The performance of the ALPXS during the building renovation application in this study demonstrated signal-to-noise ratios appropriate for sampling airborne particles in environments with elevated metal concentrations, such as workplace settings. The present study did not quantify sampling efficiency, but an earlier investigation of a prototype model (Kesavan and Hottell, Citation2004) suggests that the sampling efficiency may be only about 50% at 300 L/min. Using 0.5- and 1-μm PSL microspheres and 5-nm fluorescent oleic acid particles, their results showed that the efficiency of the prototype (ALPES) was 44.0%, 39.6%, and 57.4% at particle sizes of 0.1, 1, and 5 μm respectively (Kesavan and Hottell, Citation2004). Therefore, where quantitative measurements of airborne metal concentrations are needed, side-by-side monitoring with an accepted filter-based method would be recommended.
Our experiments indicate that the ALPXS shows promise for capturing short-term variations (transient signals), as demonstrated in the renovation study, with the caveat that it should be used in conjunction with traditional filter-based methods where a size-specific quantitative exposure measurement is required, for example, to demonstrate regulatory compliance. The ALPXS is also useful for trapping particles into aqueous solutions where needed for subsequent characterization. LODs determined for the ALPXS () were low enough to detect indoor background metal concentrations for many elements, despite the relatively short sampling times (30–120 min). Note that the units of measurement for typical workplace exposure limits are in the mg m−3 range (see ), which is orders of magnitude higher than the LODs determined for the ALPXS in the present study (; measured in ng m−3). Thus, the ALPXS would be capable of collecting useful short-term samples of airborne particle-bound metals to assist in characterizing temporal changes in workplace air quality, where concentrations approach the levels in .
Table 6. Compilation of time-weighted average exposure limits for inorganic airborne pollutants published by four different government agencies in Canada and the United States
The high air flow rate of the ALPXS (300 L min−3) permits shorter sampling times compared to filter based methods used in occupational safety and health applications (). For example, to sample the volume of the air sample recommended by NIOSH (Citation2003) for filter-based sampling (200–2000 L) would require a maximum of 7 min using the ALPXS, as compared to 8 hr required by the filter-based method (operated at 4 L min−1). The shortest sampling duration investigated in this study was 30 min, for which metal concentrations were well above detection for aerosolization experiments (). Shorter sample durations of 15, 10, and even 5 min are used in occupational safety and health to determine ceiling TLV and PEL (ASTM, Citation2008), with the ceiling defined as the highest exposure levels for workers at any instant in time. In this regard, the ALPXS has the potential to identify short-term changes (peak vs. background) in metal concentrations, even where the concentration range may be orders of magnitude below exposure limits. This is partially supported by results from the aerosolization experiment in which air concentrations for many elements did not significantly change for 30, 45, 60, and 120 min sample durations. Theoretically, the sample period could be reduced further, but testing would require improvements to the dispersal method used in the present study, to ensure a constant release of particulate material throughout the monitoring period. In addition, to determine the lower limits for sample duration using the ALPXS, future investigations will require element specific testing at known air concentrations.
Extending the sampling time for the ALPXS beyond 120 min may require modifications to the instrument, due to the observation of significant evaporation of the fluid during sample collection (12 mL hr−1) in the present study. Extrapolating to 8 hr, it is expected there would be close to 100 mL of evaporation. Currently, the instrument door will not close if the volume of the solution container is larger than 50 mL; therefore, unattended operation would not be possible for an 8-hr sample period due to evaporation. Accommodating a larger container would require modifications to the instrument.
Estimates for LOD and LOQ were based on the collection and analysis of field blank samples, collected while operating the ALPXS in “no-HV” mode. It is likely that these were overestimates of the true field blank, as air is continuously drawn into the instrument during the collection of the “no-HV” blank sample, which undoubtedly includes the passive entrainment of air particles into the percolating fluid. This is a limitation of the instrument design. Ideally, to establish the field blank for LOD or LOQ calculation, the instrument should operate with the circulating fluid and HV turned on and the air intake turned off. However, the current design does not permit the air intake to be turned off when operating the ALPXS. Modification of the ALPXS to be capable of operating without airflow would facilitate the collection of true field blanks.
The potential for metal contamination of samples from the instrument itself needs further investigation. Of the metals tested in this study, Cu, Fe, Zn, Pb, and Ni contamination was initially detected in the ALPXS solution when the instrument was first installed and tested. Contamination levels decreased with each subsequent blank run, although low-level contamination persisted to some degree, resulting in higher LODs for these metals (). Of these five metals, it is possible that the AISI-316 stainless-steel electrode electrodes could be a source of Ni. AISI-316 is composed of Mn (<2%), Si (<1%), Cr (16–18%), Mo (2–3%), and Ni (10–14%). Tests performed by Haudrechy et al. (Citation1993) indicated that AISI-316 released Ni (<0.03 μg cm−2 per week) in both acidic (pH 4.5) and neutral (pH 6.6) solutions of synthetic saliva. Based on this reported rate of leaching, it is possible that Ni from the ALPXS electrode could contribute up to 0.09 ng m−3 for every 2 hr of operation. It is likely that any fluid left on the electrode would continue to leach Ni during inoperative periods, which underscores the importance of thoroughly cleaning the instrument before every sample collection run. We observed that the greatest discrepancy in elemental ratios occurred for Ni/Sr ratios when comparing aerosolized NIST 1648a with bulk NIST 1648a (3.33 vs. 0.41, respectively, in ). However, the large variability caused by the sieve shaker method for aerosolizing particles in the method development section of this study is a limitation of the method of delivery, and is not a reflection of the ALPXS collection efficiency. Further work is required to quantitatively evaluate sources of variability and contamination during the operation of the ALPXS in specific environments. For example, the potential contribution of metals from the electrode could be significant in a clean environment, whereas this contribution may become negligible when the ALPXS is operated in an environment with elevated airborne metal levels.
Despite the already-mentioned limitations of the sieve shaker as a particle emission source, elemental ratios for aerosolized NIST 1648a particles collected by the ALPXS were generally consistent with those of the bulk NIST 1648a parent material (). Elemental ratios characterizing background aerosols were significantly different from the NIST 1648a ratios () and showed that the ALPXS can be used to identify contamination sources in the workplace. Such a capability to differentiate process-related emissions from background aerosols in a workplace is important for the development of control strategies (Methner et al., Citation2007; Yeganeh et al., Citation2008).
Monitoring emissions during the renovation (described earlier) demonstrated that the ALPXS/ICP-MS method developed in this study has an adequate signal-to-noise ratio to capture short-term (transient) changes in trace metal concentrations in air. Other recently developed technologies operate on the basis of vapour condensation to capture particles into liquid (PIL) (Weber et al., 2001) but have not been coupled to ICP-MS for monitoring trace metals. PIL samplers have been implemented to monitor water-soluble Fe(II) in ambient air (Rastogi et al., Citation2009; Oakes et al., Citation2010); to monitor major ionic components (Mg, Ca, K, Na) of aerosols (Weber et al., 2001); and have been coupled with ion chromatography for online speciation (Orsini et al., Citation2003; Li et al., Citation2004). Li et al. (Citation2004) reported that a comparison of PIL and filter sampling yielded a slope of 0.59 (R2 = .75) for nitrates and ammonium. In the present study it was not possible to conduct a side-by-side comparison of ALPXS and filter-based methods due to equipment limitations, notably the confined space of the self-ventilating hood and the lack of filter sampling equipment capable of capturing 30-min indoor air samples suitable for trace metal determination.
Summary and Conclusion
This study investigated wet electrostatic precipitation (with subsequent multi-element analysis by ICP-MS) as a potential method to capture temporal variations in airborne metal concentrations, to complement information provided by standard filter-based protocols. As a first step, it was necessary to establish optimal operating conditions for using the ALPXS in conjunction with ICP-MS, as the ALPXS has only recently become commercially available, and then to collect measurements for the estimation of limits of detection (LOD) and limits of quantification (LOQ). The high flow rate of the ALPXS (300 L min−1) provided the advantage of relatively short sampling times, and enabled the collection of a series of 1-hr or 2-hr air samples. The method proved capable of capturing transient trace metal emissions during an indoor renovation, demonstrating its potential for investigating short-term changes in airborne metal concentrations and for using elemental signatures to distinguish indoor background aerosols from particles released from work-related processes. Sampling efficiency of the ALPXS was not evaluated in the present study. As the ALPXS does not provide size-specific samples, and its efficiency at different flow rates has yet to be quantified, the ALPXS would be useful as a qualitative indicator of the presence of elevated concentrations of airborne metals, but not as a substitute for standard filter-based protocols accepted for regulatory applications (e.g., exposure measurements). Instead, results of the present study show that the ALPXS could provide additional complementary information if used in conjunction with filter-based methods. The ALPXS could be improved by adding the capability to turn off the intake airflow during field blank sample collection. This would be useful to minimize the introduction of airborne particulate matter into the circulating fluid, so that contamination arising from the instrument itself could be isolated and identified. Also, extending unattended sampling for ALPXS beyond 120 min may require instrument modifications, based on the observation of significant solution evaporation during sample collection (12 mL hr−1).
Funding
The authors gratefully acknowledge support from Health Canada’s Clean Air Regulatory Agenda and the Chemicals Management Plan.
Acknowledgment
The authors thank Jianjun Niu and Luyza Avramescu of Health Canada and four anonymous reviewers for valuable review comments.
Additional information
Notes on contributors
Innocent Jayawardene
Pat E. Rasmussen (Health Canada research scientist) is an adjunct professor at University of Ottawa and works with Innocent Jayawardene (chemist) and Marc Chénier (technologist) in the Exposure and Biomonitoring Division, Healthy Environment and Consumer Safety Branch, Health Canada.
Pat E. Rasmussen
Pat E. Rasmussen (Health Canada research scientist) is an adjunct professor at University of Ottawa and works with Innocent Jayawardene (chemist) and Marc Chénier (technologist) in the Exposure and Biomonitoring Division, Healthy Environment and Consumer Safety Branch, Health Canada.
Marc Chenier
Pat E. Rasmussen (Health Canada research scientist) is an adjunct professor at University of Ottawa and works with Innocent Jayawardene (chemist) and Marc Chénier (technologist) in the Exposure and Biomonitoring Division, Healthy Environment and Consumer Safety Branch, Health Canada.
H. David Gardner
H. David Gardner is a database and statistical analyst at University of Ottawa.
References
- ALPXS [Aerosol-to-Liquid. Particle Extraction. System]. 2010. Wickersham Jr C E Air Quality Analyzer USA Patent US2012/0048748-A1. http://www.freepatentsonline.com/20120048748.pdf ( accessed April 4, 2014).
- American Standard for Testing and Materials. 2008. Standard guide for air sampling strategies for worker and workplace protection. American Standard for Testing and Materials, E1370 − 96, 1–9. http://www.astm.org/DATABASE.CART/HISTORICAL/E1370-96R08.htm (accessed on June 9, 2014).
- British Columbia Occupational Health and Safety. 2013. http://www2.worksafebc.com/Publications/OHSRegulation/GuidelinePart5.asp#SectionNumber:G5.57 ( accessed April 4, 2014).
- Butler, O.T., W.R.L. Cairns, J.M. Cook, and C.M. Davidson. 2013. Atomic spectrometry update. Environmental analysis. J. Anal. Atom. Spectrom. 28(2): 177–216. doi:10.1039/c2ja90077g
- Canadian Occupational Health and Safety. 2012. Canadian Occupational Health and Safety regulations, SOR/86-304, 243 pp. http://laws-lois.justice.gc.ca/PDF/SOR-86-304.pdf ( accessed April 4, 2014).
- Government of Quebec. 2013. Regulation respecting occupational health and safety, updated April, 2013. http://www2.publicationsduquebec.gouv.qc.ca/documents/lr/txtspc/S-2.1R13_EN_00010802.pdf ( accessed April 4, 2014).
- Hashimoto, N., I. Ogura, M Kotake, A. Kishimoto, and K. Honda. 2013. Evaluating the capabilities of portable black carbon monitors and photometers for measuring airborne carbon nanotubes. J. Nanopart. Res. 15: 2033. doi:10.1007/s11051-013-2033-3
- Haudrechy, P., J. Foussereau, B. Mantout, and B. Baroux. 1993. Nickel release from 304 and 316 stainless steels in synthetic sweat. Comparison with nickel and nickel-plated metals. Consequences on allergic contact dermatitis. Corrosion Sci. 35(1–4): 329–336. doi:10.1016/0010-938X(93)90164-C
- Hirsch, J., S.R. Lowry, and M. Dowd. 2010. X-ray fluorescence and FT-IR identification of strontium and carbonate in domestic and imported gypsum drywall. Spectroscopy 25(7): 30–37.
- Kesavan, J., and K.A. Hottell. 2004. Characteristics, sampling, efficiencies, and possible improvements to aerosol to liquid particle extraction system. (ALPES). U.S. Army Research, Development and Engineering Command. ECBC-TN-017, 1–15. http://www.researchgate.net/publication/235186130 (accessed on June 9, 2014).
- Li, Z., P.K. Hopke, L. Husain, Z. Qureshi, V.A. Dutkiewicz, J.J. Schwab, F. Drewnick, and K.L. Demerjian. 2004. Sources of fine particle composition in New York city. Atmos. Environ. 38:6521–29. doi:10.1016/j.atmosenv.2004.08.040
- Methner, M.M., M.E. Birch, D.E. Evans, B.K. Ku, K. Crouch, and M.D. Hoover. 2007. Identification and characterization of potential sources of worker exposure to carbon nanofibers during polymer composite laboratory operations. J. Occup. Environ. Hyg. 4(12): D125–D130.
- Miller, A., G. Frey, G. King, and C. Sunderman. 2010. A handheld electrostatic precipitator for sampling airborne particles and nanoparticles. Aerosol Sci. Technol. 44(6): 417–427. doi:10.1080/02786821003692063
- National Institute for Occupational Safety and Health. 2003. NIOSH manual of analytical methods (NINAM), ELEMENTS (ICP): METHOD 7300, 4th ed., DHHS (NIOSH) Publication 94-113 (August, 1994), 1st Suppl. Pub. 96-135, 2nd Suppl. Pub. 98-119, 3rd Suppl. 2003-154, 1–8. http://www.cdc.gov/niosh/docs/2003-154/pdfs/7300.pdf (accessed on June 9, 2014).
- National Institute for Occupational Safety and Health. 2007. NIOSH pocket guide to chemical hazards. DHHS (NIOSH) publication no. 2005-149, 424. http://www.cdc.gov/niosh/docs/2005-149/pdfs/2005-149.pdf (accessed on June 9, 2014).
- Oakes, M., N. Rastogi, J.M. Brian, M. Shafer, J.J. Schauer, E.S. Edgerton, and R.J. Weber. 2010. Characterization of soluble iron in urban aerosols using near real time data. J. Geophys. Res. 115: D15302. doi:10.1029/2009JD012532
- Orsini, D. A., Y. Ma, A. Sullivan, B. Sierau, K. Baumann, and R.J. Weber. 2003. Refinements to the particle-into-liquid sampler (PILS) for ground and airborne measurements of water soluble aerosol composition. Atmos. Environ. 37: 1243–59. doi:10.1016/S1352-2310(02)01015-4
- Rasmussen, P.E., I. Jayawardene, H.D. Gardner, M. Chénier, C. Levesque, and J. Niu. 2013. Metal impurities provide useful tracers for identifying exposures to airborne single-wall carbon nanotubes released from work-related processes. J. Phys. Conf. Ser. 429(1): 1–8. doi:10.1088/1742-6596/429/1/012007
- Rasmussen, P.E., A.J. Wheeler, N.M. Hassan, A. Filiatreault, and M. Lanouette. 2007. Monitoring personal, indoor, and outdoor exposures to metals in airborne particulate matter: risk of contamination during sampling, handling and analysis. Atmos. Environ. 41(28): 5897–907. doi:10.1016/j.atmosenv.2007.03.018
- Rastogi, N., M.M. Oakes, J.J. Schauer, M.M. Shafer, B.J. Majestic, and R.J. Weber. 2009. New technique for online measurement of water-soluble Fe-II in atmospheric aerosols. Environ. Sci. Technol. 43(7): 2425–30. doi:10.1021/es8031902
- Salcedo D., J.P. Bernal, O. Pérez-Arvizub, and E. Lounejeva. 2014. Assessment of sample preparation methods for the analysis of trace elements in airborne particulate matter. J. Anal. Atom. Spectrom. 29:753–61. doi:10.1039/c3ja50375e
- U.S. Environmental Protection Agency. 2009. Dry wall sampling analysis. http://www.epa.gov/oswer/docs/chinesedrywall.pdf
- Yeganeh, B., C.M. Kull, M.S. Hull, and L.C. Marr. 2008. Characterization of airborne particles during production of carbonaceous nanomaterials. Environ. Sci. Technol. 42(12): 4600–6. doi:1021/es703043c
