Abstract
Pseudevernia furfuracea (L.) Zopf biosorption efficiency for zinc(II) was determined. The biosorption efficiency of Zn(II) onto P. furfuracea was significantly affected by the parameters of pH, biomass concentration, stirring speed, contact time, and temperature. The maximum biosorption efficiency of P. furfuracea was 92% at 10 mg/L Zn(II), for 5 g/L lichen biomass dosage. The biosorption of Zn(II) ions onto biomass was better described by the Langmuir model and the pseudo-second-order kinetic. The obtained thermodynamic parameters from biosorption of Zn(II) ions onto biomass were feasible, exothermic, and spontaneous. The different desorbents were used to perform the desorption studies for Zn(II)-loaded biomass. Fourier transform infrared (FTIR) spectroscopy was used to determine the participating functional groups of P. furfuracea biomass in Zn (II) biosorption. The broad and strong bands at 3292–3304 cm−1 were due to bound hydroxyl (–OH) or amine (–NH) groups. The effective desorptions were obtained up to 96% with HNO3. P. furfuracea is an encouraging biosorbent for Zn(II) ions, with high metal biosorption and desorption capacities, availability, and low cost. It was believed that by using this new method in which biomass is used as a sorbent, the toxic pollutants could be selectively removed from aqueous solutions to desired low levels. The remarkable properties of lichens in the transformation and detoxification of organic and inorganic pollutants are well known, and many processes have received attention in the general area of environmental biotechnology and microbiology.
Implications:
The remarkable properties of lichens in the biosorption capacity of organic and inorganic pollutants are well known, and many processes have received attention in the general area of environmental biotechnology and microbiology.
Introduction
Rapid industrialization and increasing population have led to deterioration of some ecosystems, with the accumulation of heavy metals, synthetic compounds, waste nuclear liquids, and so on (Velea et al., Citation1995). Mining and metallurgical wastewaters are considered to be the major sources of metal contamination, and the need for economic and effective methods for the removal of metals has resulted in the development of new separation technologies (Schneider and Rubio, Citation1995). Precipitation, ion exchange, electrochemical processes, and/or membrane processes are commonly applied for the treatment of industrial effluents. However, the application of such processes is sometimes restricted because of technical or economic constraints (Jansson-Charrier et al., Citation1995). The search for new technologies involving the removal of toxic metals from waste waters has been directed toward biosorption, based on the metal binding capacities of various biological material.
Zinc (Zn), which may be found in wastewater discharges from acid mine drainage and galvanizing plants, is not biodegradable and travels through the food chain via bioaccumulation. Therefore, there is significant interest regarding Zn removal from wastewaters and its toxicity for humans at levels of 100–500 mg/day. The maximum acceptable concentration of Zn in drinking water is 5.0 mg/L (Kumar et al., Citation2006).
Biosorption is a promising technology not only for the removal of heavy metals but also for the recovery of metals from solution. The recovery of heavy metals from waste solutions is recently getting attention because of their increasing price. Biosorption is an ascending technology for the removal of metals from solutions through adsorption using biological materials. The accumulation of the heavy metals onto biological materials through metabolically mediated or physicochemical pathways of removal from wastewater can be defined as biosorption (Norton et al., Citation2004). Biosorption contains several mechanisms, such as ion exchange, chelation, precipitation, sorption by physical forces, and ion entrapment in inter- and intrafibrillar capillaries and spaces of the structural lignin and polysaccharide networks. Some biosorbents have broad spectrums without any specific selectivity for any particular metal ion, while others can be specific for some metal ion (Kumar et al., Citation2006).
The use of biosorbents for the removal of toxic pollutants or for the recovery of valuable resources from aqueous wastewaters is one of the most recent developments in environmental or bioresource technology. Since Hecker first reported a quantitative study on the copper uptake by fungal spores of Trilletia tritici and Ustilago crameri in 1902 (McCallan and Miller, Citation1956; Muraleedharan et al., Citation1991), more than 3,000 research articles on biosorption have been published in in differents journals from many different countries. In addition, about 70 review papers and some books have appeared about biosorption phenomena, equilibrium and kinetic modeling, reactor operation, and application to real industries (John Wase and Forster, Citation1997; Volesky, Citation2004. Further, microbial biomass types over wide range have been investigated in biosorption studies (Munoz et al., Citation2006). These include archaea, bacteria (Vijayaraghavan et al., Citation2007; Bueno et al., Citation2008), cyanobacteria (Kiran et al., Citation2007), algae (Mohan et al. Citation2007; Aksu and Dönmez Citation2006), including macroalgae, that is, seaweeds (Yang and Chen Citation2008), and fungi (Wang and Chen Citation2006; Bayramoğlu and Arica 2007) fruiting bodies (mushrooms, brackets, etc.) and lichens (Turhan et al., Citation2005; Dogan et al., Citation2006; Ekmekyapar et al., Citation2006; Citation2012; Ateş et al., Citation2007; Sarı et al., Citation2007; Pipiska et al., Citation2007; Kiliç et al., Citation2008; Citation2014; Uluözlü et al., Citation2008; Citation2010; Bingöl et al., Citation2009; Tüzen et al., Citation2009; Hamutoğlu et al., Citation2012). It is clear that the usage of biomass types that are efficient, cheap, and easy to grow or harvest is prefered. Hamutoğlu et al. (Citation2012) reviewed the usege of biological materials (bacteria, fungi, lichens, and plants) as biosorbents that were applied for the recovery of some metals. The advantages of these biosorbents—cost-effectiveness and easiness for removal of metal ions—were discussed in this report (Hamutoğlu et al., Citation2012).
Biomaterials over a wide range available in nature have been employed as biosorbents for desired pollutant removal. All kinds of microbial, plant, and animal biomass and their derivative products have received great interest in a variety of ways and in relation to a variety of substances (Volesky, Citation2003). However, in recent years attention has been driven toward lichen biomaterials. Lichens are usually slow-growing organisms consisting of a fungus and an alga or cyanobacterium, which combine in a symbiotic relationship with several unique physiological and morphological characteristics (Ateş et al., Citation2007). Lichens have been widely used as air pollution monitors because of their ability to strongly bind to and accumulate many metals (Hawksworth and Rose, Citation1976; Guidotti et al., Citation2009; Cansaran-Duman et al., Citation2009, Citation2011; Aslan et al., Citation2011). In recent years, genotoxicity studies with lichen species demonstrated the possible ecotoxicological impacts of such contamination by providing valuable information on environmental pollution and improving the process of risk assessment through analyses by molecular markers (Aras et al., Citation2010; Citation2012; Cansaran-Duman et al., Citation2011; Citation2012; Citation2013). Especially, metal ion binding properties of lichens have been found such that nonliving lichen biomass is able to bind metal ions to a greater degree than living lichens (Purvis et al., Citation2000), because the living plasma membrane excludes metals from entering the cell (Chettri et al., Citation1998). The mechanism of cation uptake by lichen is generally regarded as an abiotic process governed by surface complexation of cations with exposed functional groups on the lichen surface (Pipiska et al., Citation2007). Carboxylic and hydrocarboxlic acids and chitin have been suggested as metal binding ligands in lichen (Chettri et al., Citation1998; Bingöl et al., Citation2009).
The aim of the present work is to investigate the adsorption properties of lichen biomass of Pseudevernia furfuracea (L.) Zopf for Zn(II) by batch adsorption techniques. Biosorption conditions like pH, biomass dosage, contact time, and temperature were also determined due to their signifigant effects. The Langmuir and Freundlich isotherm models were used to determine the biosorption mechanism and surface properties of the biomass. The Zn(II) biosorption mechanisms of P. furfuracea biomass were assessed in terms of thermodynamics and kinetics. This is the first report, to our knowledge, about the biosorption capacity of P. furfuracea toward Zn(II).
Material and Methods
Biosorbent preparation
The lichen biomass of Pseudevernia furfuracea was collected from Karabük, Yenice forest, Turkey, in November 2005. There is a rich and large forest ecosystem in terms of species that is protected according to World Wildlife Fund (WWF) in the city. The samples were taken from a few trees at a station where there is not any kind of contaminations. Pseudevernia furfuracea is used as a biosorbent for the biosorption of Zn(II) ions.
Preparation of stock solution
Five hundred milligrams per liter Zn(II) stock solution was prepared by dissolving the required quantity of ZnSO4·7H2O with deionized H2O. The dilutions were made according to the required concentrations.
Fourier transform infrared spectroscopy (FTIR)
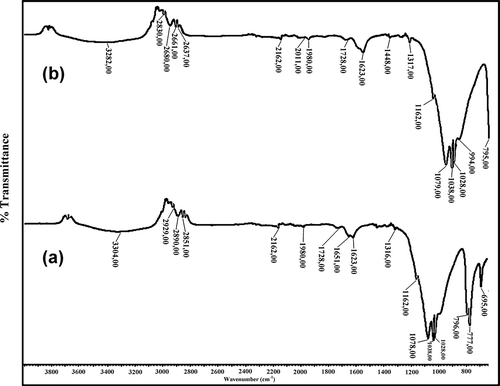
To identify the functional groups that were present on the biosorbent material, the method applied by Kılıç et al. (2013) was used. Fourier transform infrared spectroscopy (FTIR) spectra were recorded in the region of 300–3000 cm−1.
Biosorption studies
The experiments were performed in a metallic solution at room temperature (20ºC) with pH values in the range 2–6. Pseudevernia furfuracea was added to various concentrations of Zn(II) solution (5–100 mg/L). The biosorbent concentrations and stirring speeds were varied from 1 to 50 g/L and 50 to 250 rpm, respectively. The blend was stirred in a shaker at a constant speed for 60 min at 20ºC. The samples were taken at certain time intervals and filtered with 0.45-μm membrane for the removal of suspended biomass and analyzed for residual Zn(II) concentration. The Zn(II) concentration in the supernatant solution was determined using a flame atomic absorption spectrophotometer (GBC Avanta Ver 2.02) at 324 nm. All experiments were perform in a batch stirred system. All of the presented data are the mean values from three replicate experiments. The standard deviations were less than 5%.
Desorption studies
In order to carry out desorption studies, Pseudevernia furfuracea samples were added to a 10-mg/L Zn(II) solution and the mixture was stirred for 60 min at 150 rpm. Blue band filter paper was used to filter the system and washed with distilled water. Different desorbents including HCl (0.1 M), HNO3 (0.1 M), ethylenediamine tetraacetic acid (EDTA, 0.5 M), NaCl (0.1 M), and H2O were used in desorption studies of Zn(II)-loaded P. furfuracea. The solid to liquid ratio was kept at 5 g/L and the mixture was shaken on a rotary shaker for 15 min at 150 rpm. The reaction mixture was filtered and the supernatant was used to determine the Zn(II) concentration after desorption. The same procedure was used for the blank solution. The desorbed Zn(II) ions from P. furfuracea were determined using a flame atomic absorption spectrophotometer (GBC Avanta Ver 2.02) at 324 nm.
Results and Discussion
FTIR analysis
FTIR spectroscopy was used to determine the participating functional groups of Pseudevernia furfuracea biomass in Zn (II) biosorption . The broad and strong bands at 3292–3304 cm−1 were due to bound hydroxyl (–OH) or amine (–NH) groups. The peaks at 1649-1651 cm−1 were attributed to stretching vibration of a carboxyl group (–C=O). The bands obtained at 1038 cm−1 were attributed to CO stretching of alcohols and carboxylic acids. The peaks of the C–H groups were observed at 2929 cm−1. The changes in the adsorbtion bands are the result of functional groups participating in Zn(II) biosorption. The stretching vibration of OH group was shifted from 3304 to 3292 cm−1 for the Zn(II)-loaded biomass. These results revealed chemical interactions between the metal ions and the hydroxyl groups to occur on the biomass surface. The peaks at 1651 cm−1 and 1444cm−1 were attributed to stretching vibration of the carboxyl group (–C=O). The carboxyl peaks at 1651 cm−1 and 1444 cm−1 were shifted to 1649 and 1448 cm−1 for the Zn(II)-loaded biomass. These results indicate the carboxyl (–COOH) and hydroxyl (–OH) groups of the biomass to be mainly involved in the biosorption of Zn(II) onto P. furfuracea biomass. The peaks observed at 2929 cm−1 did not alter after biosorption of Zn(II), meaning the C–H group did not participate in the biosorption of Zn(II). The FTIR results obtained in the current study were similar to the data reported in previous studies.
Effect of pH on Zn(II) biosorption
Many factors, such as pH, temperature, contact time, initial concentration of metal, and biomass, can influence metal sorption process. Solution pH is usually the most significant environmental factor for biosorption of heavy metal ions by Pseudevernia furfuracea biomass. Solution pH value strongly influences not only the solution chemistry of the heavy metals (hydrolysis, complexation by organic and/or inorganic ligands, redox reactions, precipitation, the speciation and the biosorption availability of the heavy metals) but also the site dissociation of the biomass surface (Wang and Chen, Citation2006, Citation2009). The solution pH affects the activity of functional groups, cell surface metal binding sites, and properties and solution chemistry of the metal ions (Huang et al., Citation2006). The effect of pH on the biosorption of Zn(II) ions onto P. furfuracea biomass was studied in the pH range 2–6 for an initial metal concentration of 10 mg/L Zn(II) solution at a stirring speed of 150 rpm. The results are presented in . Cation biosorption increases as the solution pH increases (Chang et al., Citation1997). The removed amounts of Zn(II) were 0.83% and 98%.
Figure 2. Effect of pH on biosorption efficiency (a) and initial Zn(II) concentration on Zn(II) uptake (qe = mg Zn(II)/g biomass) and biosorption efficiency (b). CO = 10 mg/L, T = 20ºC, m = 5 g/L, stirring speed = 150 rpm, contact time (t) = 60 min.
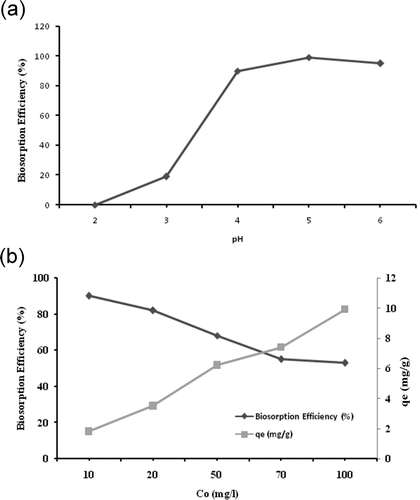
The heavy metal biosorption is highly pH dependent. The increase/decrease in biosorption levels with an increase/decrease in pH can be explained by availability of negatively/positively charged grups at the biosorbent surface, which is necessary for the biosorption of metals to proceed. Less removal at low pH value may be due to competition of protons (H+) and metal cations for the same sites on the biomass, resulting in an increase in protonated sites. As the pH is decreased, the overall cell wall of Pseudevernia furfuracea charge becomes positive and the repulsive forces limit the biosorption of Zn(II), resulting in reduced binding of cations, and consequently reduced uptake of zinc by the biomass (Chang et al., Citation1997). At higher pH values, the number of negatively charged groups on the biosorbent surface probably increases, becoming deprotonated and able to attract the zinc cations, resulting in enhancement of the biosorption process (Montanher et al., Citation2005; Velmurugan et al., Citation2010). The competition ability of metal ions with hydrogen ions for active sites on the biosorbent surface is directly related to the acidity of the solution (Lodeiro et al., Citation2006). Metal binding onto biomass during biosorption involves complex mechanisms, such as ion exchange, chelation, adsorption by physical forces, and ion entrapment in inter- and intrafibrillar capillaries and spaces of the cell structural network of a biosorbent (Chojnacka et al., Citation2005; Sarı and Tüzen, Citation2009a). Generally the optimum binding is observed at a pH of around 5.0 in previous studies, which is consistent with the current results (Kılıç et al., Citation2014). Little binding is seen below pH values of 2.0 for most metal ions. The FTIR spectroscopic analysis showed that the P. furfuracea has carboxyl, hydroxyl, and amine functional groups participating in metal ion binding, depending on the pH value of the solution The highest biosorption was obtained with at pH 5, and further experiments were performed with pH 5.0.
Effect of initial Zn (II) concentration on biosorption
Different initial metal ion concentrations were agitated with Pseudevernia furfuracea at a stirring speed of 150 rpm to determine the biosorption capacity and the biosorption efficiency (). The biosorption experiments were performed in triplicate, and there were no significant differences among replicates (P < 0.01). The initial Zn(II) concentrations were varied from 10 to 100 mg/L. The biosorption efficiency of P. furfuracea was 89% for 10 mg/L Zn(II) concentration. The rate of biosorption is a function of initial Zn(II) concentration. The increase in biosorption capacity with increasing initial Zn(II) concentration may be due to higher availability of Zn(II) ions in the solution for biosorption. Higher initial Zn(II) concentration also causes an increased concentration gradient, which provides higher probability of collision between Zn(II) ions and active sites of the biosorbent, thus leading to greater biosorption capacity (Shroff and Vaidya, Citation2011; Suazo-Madrid et al., Citation2011). The biosorption efficiency of P. furfuracea decreased with the increase of Zn(II) concentration. This result can be explained due to an increase in the number of ions competing for the available binding sites and also the lack of active sites on the biomass at higher concentrations. The biosorption capacity of P. furfuracea increased with the increase of initial Zn(II) concentration then became almost constant with the higher Zn(II) concentration and reached a saturation value. At higher concentrations, more unadsorbed metal ions are left in solution due to the lack of available binding sites (Ekmekyapar et al., Citation2006; Ucun et al., Citation2009).
Effect of biosorbent dosage on biosorption
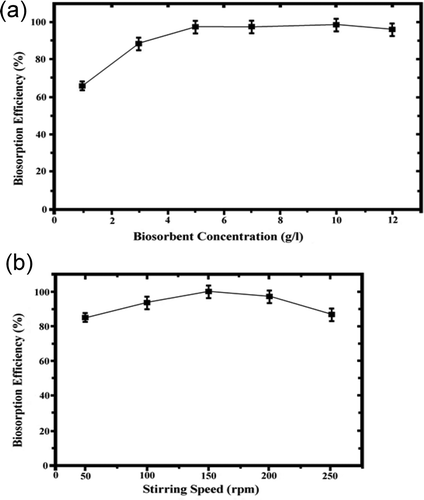
The influence of initial biosorbent dosage (1–12 g/L) on the biosorption efficiency of Pseudevernia furfuracea was studied for stable Zn(II) concentration. The experiments were performed at a stirring speed of 150 rpm. The results are shown in . The increase in biomass dosage from 1 to 5 g/L resulted in increase in biosorption efficiency due to increased surface area and availability of more binding sites for complexation of metal ions. Further increase in biosorbent dosage caused a decrease to clumping of the sorbent particles and loss of available surface area for Zn(II) uptake (Velmurugan et al., Citation2010). Further experiments were performed with 5 g/L biosorbent dosage.
Effect of stirring speed on biosorption
The effect of stirring speed within the range of 50–250 rpm for Zn(II) removal by Pseudevernia furfuracea was studied. The biosorption efficiency increased with stirring speed between 50 and 150 rpm due to an increase in the mass transfer rate (Benefield et al., Citation2009). Optimum Zn(II) removal was obtained with an agitation speed of 150 rpm as shown in . This was the result of maximum availability of binding sites for Zn(II) uptake. Also, the best homogeneity of suspension in biomass was obtained at a stirring speed of 150 rpm. The boundary-layer thickness decreases with the increase of stirring speed, which causes a decrease in surface film resistance. Thus, the metal ions are more easily adsorbed to biosorbent surface. At higher stir rates, the suspension is no longer homogeneous, which makes the removal process difficult (Velmurugan et al., Citation2010). Further experiments were performed at a stirring speed of 150 rpm.
Effect of time and temperature on biosorption
The rate of biosorption is another important parameter for the design of biosorption experiments. The experiments were carried out at different temperatures within the range 20–50ºC at a stirring speed of 150 rpm and different contact times of 0.16–52 hr, to optimize the time and temperature dependency of the biosorption process. The biosorption of Zn(II) ions onto the biomass was maximum after 60 min, followed by a decrease in removal of metal ions after the equilibrium time (). The results showed that biosorption of Zn(II) is fast and the equilibrium is achieved in 60 min.
Figure 4. Effect of contact time (a) and temperature (b) on biosorption. Initial metal concentration (CO) = 10 mg/L, pH 5.0, temperature (T) = 20ºC, biosorbent concentration (m) = 5 g/L, stirring speed = 150 rpm.
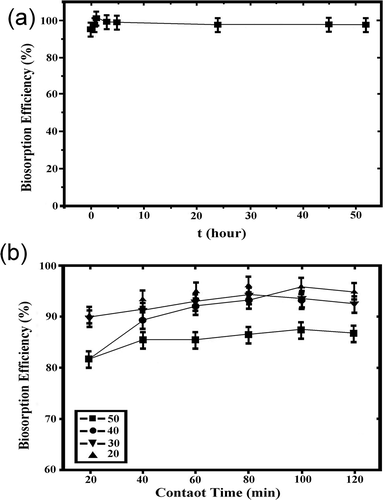
Physical adsorption that occured as a result of van der Waals forces is usually predominant at low temperature, characterized by a relatively low energy of adsorption, and the adsorbed molecule is not attached to a specific site at the surface.
The temperature of the medium also affects the removal efficiency of Zn(II) from the aqueous solution. The temperature dependencies of Zn(II) biosorption were determined at varying temperatures within the range 20–50ºC. The biosorption efficiency of Zn(II) decreased from 92% to 85% with the temperature increasing from 20 to 50ºC for 60 min (). The results indicated the exothermic nature of Zn(II) biosorption onto Pseudevernia furfuracea biomass. The increase in the desorption of Zn(II) from the interface to the solution may be result of a decrease in the biosorption of Zn(II) with temperature increase (Ekmekyapar et al., Citation2006; Ucun et al., Citation2009; Chubar et al., Citation2004; Sarı et al., Citation2007). Further biosorption experiments were performed at 20ºC.
Biosorption isotherm models
The equilibrium experiments were performed for Zn(II) with Pseudevernia furfuracea. The equilibrium data that give information on the nature of the interaction between sorbate and biosorbent under the system conditions are basic requirements for the design of biosorption systems and are known as biosorption isotherms. Two equilibrium models, namely, Langmuir and Freundlich isotherm models, were used to determine the biosorption mechanism and surface properties of the biomass (Langmuir, Citation1918; Freundlich, Citation1906). The Langmuir isotherm, the monolayer type of adsorption, which is a well-known model for the biosorption of a solute from an aqueus solution, indicates a reduction of the available specific homogeneous interaction sites within the sorbent as the metal ion concentration increases. This formula represents the Langmuir model:
The Freundlich model is given by
Figure 5. Langmuir isotherm (a) and Freundlich isotherm (b) plots for the biosorption of Zn(II) onto Pseudevernia furfuracea biomass. Biomass dosage: 5 g/L, contact time: 60 min; pH: 5; stirring speed: 150 rpm; temperature: 20ºC.
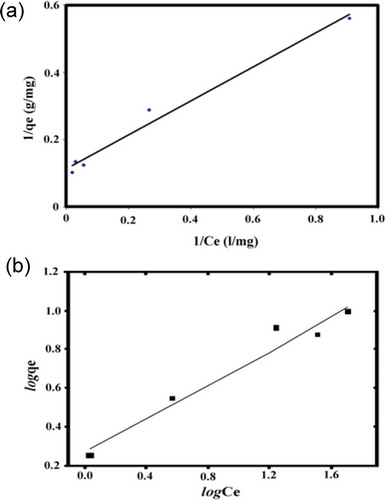
Table 1. Adsorption isotherm constants for biosorption of Zn(II) onto Pseudevernia furfuracea biomass
The Langmuir isotherm is suitable for defining the biosorption of Zn(II) by P. furfuracea since the linear regression coefficient (R2) value is high with a value of 0.983 (y = 0.5066x + 0.112). This shows the biosorption process taking place at the active sites on the surface of the biomass to be of monolayer character. The binding energy on the complete surface of the P. furfuracea is uniform. The RL value of 0.311 suggests good adsorption of Zn(II). The values of Kf and 1/n were found to be 1.85 and 0.43, respectively, for Zn(II) biosorption. The 1/n values were between 0 and 1, indicating that the biosorption of Zn(II) onto P. furfuracea was also favorable at experimental conditions.
The R2 value for the Freundlich model was found to be 0.9619 (y = 0.4387x + 0.2664), indicating that this model could not adequately describe the relationship between the amount of Zn(II) adsorbed by the biomass and its equilibrium concentration in the solution. The obtained qm value was higher than the experimental qe value.
The most efficient biosorption was recorded at 8.93 mg/g. The Zn(II) biosorption capacities of different adsorbents have been determined in previous studies (Katsou et al., Citation2010; Mahamadi, and Nharingo, Citation2010a, Citation2010b; Santos et al., Citation2010; Pérez Silva et al., Citation2009). The study designates that P. furfuracea is an encouraging biosorbent for removal of Zn(II) ions from aqueous solution.
Kinetics of biosorption
In this study, Lagergren’s pseudo-first-order and pseudo-second-order model were applied to the experimental data in order to identify the biosorption kinetics of Zn(II) ions onto Pseudevernia furfuracea biomass (Lagergren, Citation1898; Ho and McKay, Citation2000). The linearized form of the pseudo-first-order rate equation by Lagergren is given as
The linear equation of the pseudo-second-order kinetic model can be presented as
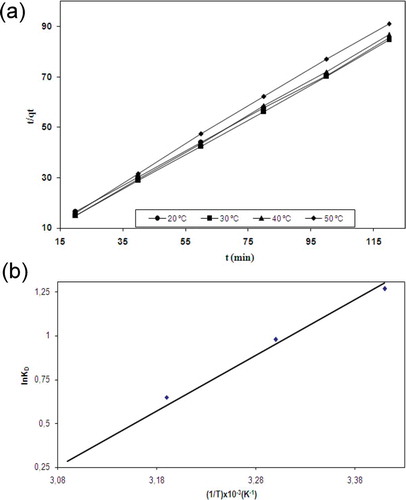
The rate constant k1 was calculated from the slope of the ln(qe − qt) versus t plot (). A relatively low correlation coefficient was obtained by the Langergren model. In addition, the experimental qe values did not agree with the calculated data. Accordingly, the biosorption of Zn(II) onto P. furfuracea cannot be considered as a pseudo-first-order kinetic.
Table 2. Kinetic parameters obtained from pseudo-first-order and pseudo-second-order versions for biosorption of Zn(II) onto Pseudevernia furfuracea biomass at different temperatures
The pseudo-second-order model was tested for the understanding of the biosorption kinetics of Zn(II) onto P. furfuracea biomass. Pseudo-second-order kinetic plots at varying temperatures for biosorption of Zn (II) onto P. furfuracea biomass are presented in . The rate at which the adsorption sites are covered is proportional to the square of the number of unoccupied sites, which is proportional to the adsorption rate of metal ions. The results shows that the pseudo-second-order model accurately represents the experimental behavior due to the correlation factors of the pseudo-second-order kinetics model in the range of 0.9988 and 0.9995. The pseudo-second-order model was used to describe the different metal ions biosorption by different bioadsorbents (Kılıç et al., Citation2014; Sarı and Tüzen, Citation2009a; Sarı et al., Citation2007; Aravindhan et al., Citation2007; Sarı and Tüzen, Citation2009b). The experimental data are better fitted by the second-order equation than by the pseudo-first-order equation. The pseudo-second-order kinetic model provided a good correlation for the biosorption of Zn(II) onto P. furfuracea compared to the pseudo-first-order model.
Biosorption thermodynamics
Zn(II) ions loaded onto Pseudevernia furfuracea biomass were calculated with the thermodynamic parameters. The following equations were used to calculate the thermodynamic parameters:
The thermodynamic data are shown in . The enthalpy (ΔH°) and entropy (ΔS°) can be calculated from the slope and intercept of the plot of ln KD versus 1/T, respectively. The obtained ΔG° values for biosorption of Zn(II) ions onto P. furfuracea biomass were –3.093, –2.468, –1.691, and –0.644 kJ/mol at 20, 30, 40, and 50ºC, respectively. According to the negative values of ΔG, the biosorption of Zn(II) ions onto P. furfuracea biomass was spontaneous and feasible. The increase in the ΔG values with increasing temperature shows a decline in feasibility of biosorption at higher temperatures. Enthalpy and the entropy values are shown in . ΔH° was found to be –26.51 J/mol for the biosorption of Zn(II) ions onto biomass. The negative ΔH° value displayed at 20–50ºC shows the exothermic natüre of the process. ΔS° was obtained as –79.57 J/mol K for the biosorption of Zn(II) ions onto P. furfuracea biomass. The negative ΔS° value indicates a decrease in the randomness at the solid/solution interface during the biosorption process (Kılıç et al., Citation2014).
Table 3. Thermodynamic parameters for the biosorption of Zn(II) onto Pseudevernia furfuracea biomass
Desorption
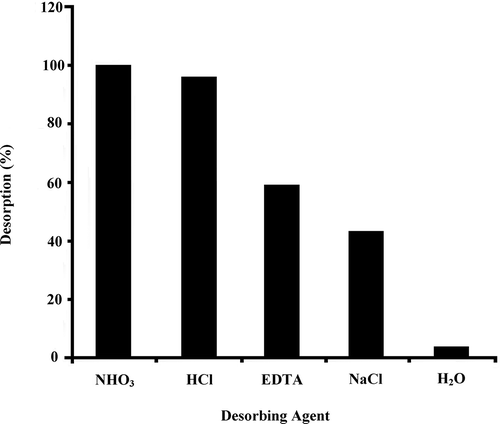
Heavy metal pollution is a serious environmental problem in the world, especially in developing countries. Among different treatment technologies, biosorption seems a promising alternative method. Good recovery after metal sorption is also important for commercially. Also biosorption is a promising technology not only for the removal of heavy metals but also for the recovery of metals from solution. The recovery of heavy metals from waste solutions is recently getting attention because of their increasing price. Their available deposit is limited, and industries need more consumption of heavy metals especially precious metals (Won et al., Citation2014). Pseudevernia furfuracea are potent and effective for heavy metal removal from aqueous solution. For this purpose, different desorbing agents such as HCl, HNO3 EDTA, NaCl, and H2O were used for the desorption studies of Zn(II)-loaded P. furfuracea to determine the best desorbing agent .Results of desorption studies showed that the highest recovery of Zn(II) was obtained with HCl, HNO3, and EDTA after 15 min at room temperature (). In the presence of HCl, HNO3 EDTA, NaCl, and H2O, 88, 96, 54, 40, and 4% of asorbed Zn(II) was released, respectively. Almost negligible desorption results were observed with distilled water (H2O). Effective desorption was obtained in HNO3. Pseudevernia furfuracea biomass is potent and effective for heavy metal removal from aqueous solution. From the investigations carried out in our lab, it was observed that the utilization of lichens will be a promising alternative to conventional adsorbents used for the treatment of contaminated aqueous solutions.
Conclusion
Heavy metal pollution is a serious environmental problem in the world, especially in developing countries. Among different treatment technologies, biosorption seems a promising alternative method. Using 5 g/L lichen biomass and 10 mg/L Zn(II) yielded the maximum biosorption efficiency with the value of 92%. The biosorption of Zn(II) ions onto biomass was beter described by the Langmuir model and the pseudo-second-order kinetic. The obtained thermodynamic parameters from biosorption of Zn(II) ions onto biomass was feasible, exothermic, and spontaneous. An effective desorption of 96% was obtained with HNO3. Pseudevernia furfuracea is efficient to capture metal cations through chelation and/or electrostatic interactions. Pseudevernia furfuracea can be easily modified by many methods to fabricate desirable biosorbents with good sorption and desorption capacity.
Additional information
Notes on contributors
Zeynep Kılıç
Zeynep Kılıç is Ph.D. student and Sümer Aras is a research professor at Ankara University, in the Science Faculty, Department of Biology, Biotechnology Section, Ankara, Turkey.
Orhan Atakol
Orhan Atakol and Emel Emregul are research professors at Ankara University, in the Science Faculty, Department of Chemistry, Ankara, Turkey.
Sümer Aras
Zeynep Kılıç is Ph.D. student and Sümer Aras is a research professor at Ankara University, in the Science Faculty, Department of Biology, Biotechnology Section, Ankara, Turkey.
Demet Cansaran-Duman
Demet Cansaran-Duman is a research professor at Ankara University, in the Biotechnology Institute, Ankara, Turkey.
Emel Emregul
Orhan Atakol and Emel Emregul are research professors at Ankara University, in the Science Faculty, Department of Chemistry, Ankara, Turkey.
References
- Aksu, Z., and G. Dönmez. 2006. Binary biosorption of cadmium(II) and nickel(II) onto dried Chlorella vulgaris: Co-ion effect on monocomponent isotherm parameters. Process Biochem. 41:860–68. doi:10.1016/j.procbio.2005.10.025
- Aras, S., T. Beyaztaş, D. Cansaran-Duman, and E. Gökçe. 2012. Evaluation of genotoxicity of Pseudevernia furfuracea (L.) Zopf by RAPD analysis. Gen. Mol. Res. 10(4): 3760–70. doi:10.4238/2011.December.15.4
- Aras, S., Ç. Kanlıtepe, D. Cansaran-Duman, M.G. Halıcı, and T. Beyaztaş. 2010. Assessment of air pollution genotoxicity by molecular markers in the exposed samples of Pseudevernia furfuracea (L.) Zopf in the province of Kayseri (Central Anatolia). J. Environ. Monit. 12:536–43. doi:10.1039/b906717e
- Aravindhan, R., J.R. Rao, and B.U. Nair. 2007. Removal of basic yellow dye from aqueous solution by sorption on green alga Caulerpa scalpelliformis. J. Hazard. Mater. 142:68–76. doi:10.1016/j.jhazmat.2006.07.058
- Aslan, A., A. Çiçek, K. Yazıcı, Y. Karagöz, M. Turan, F. Akkuş, and O. S. Yıldırım. 2011. The assessment of lichens as bioindicator of heavy metal pollution from motor vehicles activites. Afr. J. Agric. Res. 6(7): 1698–706.
- Ateş, A., A. Yıldız, N. Yıldız, and A. Çalımlı. 2007. Heavy metal removal from aqueous solution by Pseudevernia furfuracea (L.) Zopf. Anal. Chim. 97: 385–93. doi:10.1002/adic.200790023
- Bayramoglu, G., and M.Y. Arica. 2007. Biosorption of benzidine based textile dyes Direct Blue 1 and Direct Red 128 using native and heat-treated biomass of Trametes versicolor. J. Hazard. Mater. 143:135–43. doi:10.1016/j.jhazmat.2006.09.002
- Benefield, L.D., J.F. Judkins, Jr., and B.L. Weand. 2009. Process chemistry for water and wastewater treatment. Englewood Cliffs, NJ: Prentice Hall Inc.
- Bingöl, A., A. Aslan, and A. Çakıcı. 2009. Biosorption of chromate anions from aqueous solution by a cationic surfactant-modified lichen (Cladonia rangiformis (L.). J. Hazard. Mater. 161: 747–52. doi:10.1016/j.jhazmat.2008.04.018
- Bueno, B.Y.M., M.L. Torem, F. Molina, and L.M.S. de Mesquita. 2008. Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies. Mineral Eng. 21:65–75. doi:10.1016/j.mineng.2007.08.013
- Cansaran-Duman, D., E. Altunkaynak, and S. Aras. 2013. Heavy metal accumulation and genotoxicity indicator capacity of the lichen species, Ramalina pollinaria collected from around the iron-steel factory in Karabük, Turkey. Turk. J. Bot. doi:10.3906/bot-1201–19
- Cansaran-Duman, D., O. Atakol, I. Atasoy, D. Kahya, S. Aras, and T. Beyaztaş. 2009. Heavy metal accumulation in Pseudevernia furfuracea (L.) Zopf from the Karabük Iron-Steel Factory in Karabük, Turkey. Naturforsch. C 64:717–23.
- Cansaran-Duman, D., S. Aras, O. Atakol, and I. Atasoy. 2012. Accumulation of trace elements and the assessment of the genotoxicity in the lichen Pseudevernia furfuracea transplanted to a polluted site in Ankara. Ekoloji 21(85): 1–14. doi:10.5053/ekoloji
- Cansaran-Duman, D., T. Beyaztaş, O. Atakol, and S. Aras. 2011. Assesment of the air pollution genotoxicity by RAPD in Evernia prunastri L. Ach. Province of Iron-Steel Factory in Karabük, Turkey. J. Environ. Sci. 23(7): 1171–78. doi:10.1016/S1001-0742(10)60505-0
- Chang, J.S., R. Law, and C.C. Chang. 1997. Biosorption of lead, copper and cadmium by biomass of P. aeruginosa PU21. Water Res. 31:1651. doi:10.1016/S0043-1354(97)00008-0
- Chettri, M.K., C.M. Cook, E. Vardaka, T. Sawidis, and T. Lanaras. 1998. The effect of Cu, Zn and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis. Environ. Exp. Bot. 39: 1–10. doi:10.1016/S0098-8472(97)00024-5
- Chojnacka, K., A. Chojnacki, and H. Gorecka. 2005. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere 59:75–84. doi:10.1016/j.chemosphere.2004.10.005
- Chubar, N., J.R. Carvalho, and M.J.N. Correia. 2004. Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II). Coll. Surfactants A Physicochem. Eng. Aspects 230:57–65. doi:10.1016/j.colsurfa.2003.09.014
- Dogan, C.E., K. Turhan, G. Akçin, and A. Aslan. 2006. Biosorption of Au(III) and Cu(II) from aqueous solution by a non-living Cetraria islandica (L.) Ach. Ann. Chim. 2006:229–236. doi:10.1002/adic.200690022
- Ekmekyapar, F., A. Aslan, Y.K. Bayhan, and A. Çakıcı. 2012. Biosorption of Pb(II) by nonliving lichen biomass of Cladonia rangiformis Hoffm. Int. J. Environ. Res. 6(2): 417–24.
- Ekmekyapar, F., A. Aslan, A. Bayhan, and A. Çakıcı. 2006. Biosorption of copper(II) by nonliving lichen biomass of Cladonia rangiformis Hoffm. J. Hazard. Mater. B 137:293–98. doi:10.1016/j.jhazmat.2006.02.003
- Freundlich, H.M.F. 1906. Über die adsorption in lösungen. Z. Physik. Chem. (Leipzig) 57A:385–470.
- Guidotti, M., D. Stella, C. Dominici, G. Blasi, M. Owczarek, M. Vitali, and C. Protano. 2009. Monitoring of Traffic-Related Pollution in a Province of Central Italy with Transplanted Lichen Pseudevernia furfuracea. Bull. Environ. Contam. Toxicol. 83:852–58. doi:10.1007/s00128-009-9792-7
- Gupta, V.K., A. Rastogi, V.K. Saini, and J. Neeraj. 2006. Biosorption of copper(II) from aqueous solutions by Spirogyra species. J. Colloid Interface Sci. 296(1): 59–63. doi:10.1016/j.jcis.2005.08.033
- Hamutoglu, R., A.B. Dinçsoy, D. Cansaran-Duman, and S. Aras. 2012. Biyosorpsiyon, Adsorpsiyon, Fitoremediasyon Yöntemleri ve Uygulamaları. Turk. Hij. Den. Biyol. Derg. 4:235–53. doi:10.5505/TurkHijyen.2012.94914
- Hawksworth, D.L., and F. Rose. 1976. Lichens as Pollution Monitors. London, UK: Edward Arnold Ltd.
- Ho, Y.S., and G. McKay. 2000. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 34:735–42. doi:10.1016/S0043-1354(99)00232-8
- Huang, M.R., Q.Y. Peng, and X.G. Li. 2006. Rapid and effective adsorption of lead ions on fine poly(phenylenediamine) microparticles. Chem. Eur. J. 12:4341–50. doi:10.1002/(ISSN)1521-3765
- Jansson-Charrier, M., E. Guibal, R. Surjous, and P. Le Cloirec. 1995. Continuous removal of uranium by biosorption onto chitosan: Application to an industrial effluent. In Biohydrometallurgical Processing, ed. C.A. Jerez, T. Vargas, H. Toledo, and J.V. Wiertz, 267–276. Santiago, Chile: University of Chile.
- John Wase, D.A., and C.F. Forster. 1997. Biosorbents for Metal Ions. Boca Raton, FL: CRC Press.
- Katsou, E., S. Malamis, and K. Haralambous. 2010. Examination of zinc uptake in a combined system using sludge, minerals and ultrafiltration membranes. J. Hazard. Mater. 182(1–3): 27–38. doi:10.1016/j.jhazmat.2010.05.101
- Kılıç, Z., O. Atakol, S. Aras, D. Cansaran-Duman, P. Çelikkol, and E. Emregül. 2014. Evaluation of different isotherm models, kinetic, thermodynamic and copper biosorption efficiency of Lobaria pulmonaria (L.) Hoffm. J. Air Waste Manage. Assoc. 64(1): 115–23. doi:10.1080/10962247.2013.831383
- Kılıç, Z., O. Atakol, E. Emregül, P. Çelikkol, S. Aras, and D. Cansaran-Duman. 2008. Biosorption characteristics of zinc(II) from aqueous solutions by Pseudevernia furfuracea. Presented at 6th International Conference of Chemical Societies of South Eastern European Countries, 10–14 September, Sofia, Bulgaria.
- Kiran, B., A. Kaushik, and C.P. Kaushik. 2007. Response surface methodological approach for optimizing removal of Cr(VI) from aqueous solution using immobilized cyanobacterium. Chem. Eng. J. 126:147–53. doi:10.1016/j.cej.2006.09.002
- Kumar, Y.P., P. King, and V.S.K.R. Prasad. 2006. Zinc biosorption on Tectona grandis L. leaves biomass: equilibrium and kinetic studies. Chem. Eng. J. 124:63–70. doi:10.1016/j.cej.2006.07.010
- Lagergren, S. 1898. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetenskapsakad. Handl. 24:1–39.
- Langmuir, I. 1918. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am. Chem. Soc. 40:1361–403. doi:10.1021/ja02242a004
- Lodeiro, P., J.L. Barriada, R. Herrero, and M.E. Sastre de Vicente. 2006. The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: Kinetic and equilibrium studies; Environ. Pollut. 142: 264–73. doi:10.1016/j.envpol.2005.10.001
- Mahamadi, C., and T. Nharingo. 2010a. Utilization of water hyacinth weed (Eichhornia crassipes) for the removal of Pb(II), Cd(II) and Zn(II) from aquatic environments: An adsorption isotherm study. Environ Technol. 31(11): 1221–28. doi:10.1080/09593331003646604
- Mahamadi, C., and T. Nharingo. 2010b. Competitive adsorption of Pb2+, Cd 2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems; Bioresource Technol. 101(3): 859–64. doi:10.1016/j.biortech.2009.08.097
- McCallan, S.E.A.; Miller, L. P. Innate toxicity of fungicides. In: Metcalf RL ( eds.) Advanced in Pest Control Research, Interscience, NY, USA, 1956.
- Mohan, S.V., S.V. Ramanaiah, B. Rajkumar, and P.N. Sarma. 2007. Removal of fluoride from aqueous phase by biosorption onto algal biosorbent Spirogyra sp.-IO2: Sorption mechanism elucidation. J. Hazard. Mater. 141:465–74. doi:10.1016/j.jhazmat.2006.07.008
- Montanher, S.F., E.A. Oliveira, and M.C. Rollemberg. 2005. Removal of metal ions from aqueous solutions by sorption onto rice bran. J. Hazard. Mater. 117(2–3):207–11. doi:10.1016/j.jhazmat.2004.09.015
- Munoz, R., M.T. Alvarez, A. Munoz, E. Terrazas, B. Guieysse, and B. Mattiasson. 2006. Sequential removal of heavymetals ions and organic pollutants using an algal–bacterial consortium. Chemosphere 63:903-–11. doi:10.1016/j.chemosphere.2005.09.062
- Muraleedharan, T.R., L. Iyengar, and C. Venkobachar. 1991. Biosorption: An attractive alternative for metal removal and recovery. Curr. Sci. 61:379–85.
- Norton, L., K. Baskaran, and T. McKenzie. 2004. Biosorption of zinc from aqueous solutions using biosolids. Adv. Environ. Res. 8:629–35. doi:10.1016/S1093-0191(03)00035-2
- Pérez Silva, R.M., A. Abalos Rodríguez, J.M. Gómez Montes De Oca, and D. Cantero Moreno. 2009. Biosorption of chromium, copper, manganese and zinc by Pseudomonas aeruginosa AT18 isolated from a site contaminated with petroleum. Bioresource Technol. 100(4): 1533–38. doi:10.1016/j.biortech.2008.06.057
- Pipiska, M., M. Hornik, L. Vrtoch, J. Augustin, and J. Lesny. 2007. Biosorption of Co2+ ions by lichen Hypogymnia physodes from aqueous solution. Biologia 2007:276–82. doi:10.2478/s11756-007-0047-y
- Purvis, O.W., B.J. Williamson, K. Bartok, and N. Zoltani. 2000. Bioaccumulation of lead by the lichen Acarospara smaragdula from smelter emissions. Res. N. Phytol. 147:591–99. doi:10.1046/j.1469-8137.2000.00719.x
- Santos, A., P. Barton, E. Cartmell, F. Coulon, R.S. Crane, P. Hillis, J.N. Lester, T. Stephenson, and S.J. Judd. 2010. Fate and behaviour of copper and zinc in secondary biological wastewater treatment processes: II. Removal at varying sludge age. Environ. Technol. 31(7): 725–43. doi:10.1080/09593330.2010.481315
- Sarı, A., M. Tüzen, O.D. Uluözlü, and M. Soylak. 2007. Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem. Eng. J. 37:151–58. doi:10.1016/j.bej.2007.04.007
- Sarı A., and M. Tüzen. 2009a. Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J. Hazard. Mater. 164:1004–11. doi:10.1016/j.jhazmat.2008.09.002
- Sarı, A., and M. Tüzen. 2009b. Biosorption of As(III) and As(V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: Equilibrium and kinetic studies. J. Hazard. Mater. 164:1372–78. doi:10.1016/j.jhazmat.2008.09.047
- Schneider, I.A.H., and J. Rubio. 1995. New trends in biosorption of heavy metals by freshwater macrophytes. In Biohydrometallurgical Processing, ed. C.A. Jerez, T. Vargas, H. Toledo, and J.V. Wiertz, 318–327. Santiago, Chile: University of Chile.
- Shroff, K.A., and V.K. Vaidya. 2011. Kinetics and equilibrium studies on biosorption of nickel from aqueous solution bydead fungal biomass of Mucor hiemalis. Chem. Eng. J. 171: 1234–45. doi:10.1016/j.cej.2011.05.034
- Suazo-Madrid, A., L. Morales-Barrera, E. Aranda-García, and E. Cristiani-Urbina. 2011. Nickel(II) biosorption by Rhodotorula glutinis. J. Ind. Microbiol. Biotechnol. 38: 51–64. doi:10.1007/s10295-010-0828-0
- Susmita, S.G.; G. Krishna, and K.G. Bhattacharyya. 2006. Adsorption of Ni(II) on clays. J. Colloid Interface Sci. 2006, 295:21–32. doi:10.1016/j.jcis.2005.07.073
- Turhan, K., C. Ekinci-Dogan, G. Akçin, and A. Aslan. 2005. Biosorppption of Au(III) and Cu(II) from aqueous solution by a non-living Usnea longissima biomass. Fresenius Environ. Bull. 14:1129–35.
- Tüzen, M., A. Sarı, D. Mendil, and M. Soylak. 2009. Biosorptive removal of mercury(II) from aqueous solution using lichen (Xanthoparmelia conspersa) biomass: Kinetic and equilibrium studies. J. Hazard. Mater. 169:263–70. doi:10.1016/j.jhazmat.2009.03.096
- Ucun, H., O. Aksakal, and E. Yıldız. 2009. Copper(II) and zinc(II) biosorption on Pinus sylvestris L. J. Hazard. Mater. 161:1040–45. doi:10.1016/j.jhazmat.2008.04.050
- Uluözlü, O.D., A. Sarı, and M. Tüzen. 2010. Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chem. Eng. J. 163:382–88. doi:10.1016/j.cej.2010.08.022
- Uluözlü, O.D., A. Sarı, M. Tüzen, and M. Soylak. 2008. Biosorption of Pb(II) and Cr(III) from aqueous solution by lichen (Parmelina tiliaceae) biomass. Bioresource Technol. 99:2972–3298. doi:10.1016/j.biortech.2007.06.052
- Velea, I., A. Voicu, and I. Lazar. 1995. Biosorption of some metallic ions from industrial effluents using fungal strains and bacterial exopolysaccharides. In Biohydrometallurgical Processing, ed. C.A. Jerez, T. Vargas, H. Toledo, and J.V. Wiertz. University of Chile.
- Velmurugan, P., J. Shim, Y. You, S. Choi, S. Kamala-Kannan, K.J. Lee, H.M. Kim, and B.T. Oh. 2010. Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. J. Hazard. Mater. 182(1–3): 317–24.
- Vijayaraghavan, K., M.H. Han, S.B. Choi, and Y.S. Yun. 2007. Biosorption of reactive black 5 by Corynebacterium glutamicum biomass immobilized in alginate and polysulfone matrices. Chemosphere 68:1838–45. doi:10.1016/j.chemosphere.2007.03.030
- Volesky, B. 2003. Potential of biosorption. In Sorption and Biosorption, ed. B. Volesky, 138–142. Montreal, Canada: BV Sorbex, Inc.
- Volesky, B. 2004. Sorption and Biosorption. Quebec, Canada: BVSorbex, Inc.
- Won, S.W., P. Kotte, W. Wei, A. Lim, and Y.S. Yun. 2014. Biosorbents for recovery of precious metals. Bioresource Technol. doi:10.1016/j.biortech.2014.01.121
- Wang, J.L., and C. Chen. 2006. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 24:427–51. doi:10.1016/j.biotechadv.2006.03.001
- Wang, J.L., and C. Chen. 2009. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 27:195–226. doi:10.1016/j.biotechadv.2008.11.002
- Yang, L., and J.P. Chen. 2008. Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresource Technol. 99:297–307. doi:10.1016/j.biortech.2006.12.021