Abstract
A survey of key indoor air quality (IAQ) parameters and resident health was carried out in 72 apartments within a single low-income senior housing building in Phoenix, Arizona. Air sampling was carried out simultaneously with a questionnaire on personal habits and general health of residents. Mean PM10 concentrations are 66±16, 58±13, and 24±3 μg/m3 and mean PM2.5 concentrations are 62±16, 53±13, and 20±2 μg/m3 for the living room, kitchen, and outdoor balcony, respectively. Median PM10 concentrations are 17, 18 and 17 μg/m3 and median PM2.5 concentrations are 13, 14, and 13 μg/m3, respectively. The initial results indicate that increased indoor particle concentrations coincide with residents who report smoking cigarettes. Indoor formaldehyde concentrations revealed median levels of 36.9, 38.8, and 4.3 ppb in the living room, kitchen, and balcony, respectively. Results show that 36% of living room samples and 44% of kitchen samples exceeded the Health Canada REL for chronic exposure to formaldehyde (40 ppb). Associations between occupants’ behavior, self-reported health conditions, and IAQ are evaluated.
Implications:
This study provides a characterization of indoor air quality (IAQ) of subsidized apartments for seniors in Phoenix, Arizona. It is important for policy makers to understand the environments in which low-income seniors live, as they are vulnerable to the health impacts from poor IAQ. Formaldehyde concentrations were found to exceed the Health Canada 8-hr reference exposure level (REL) for up to 44% of indoor samples. Particulate matter exposure was governed by resident behavior (i.e., smoking). Associations between occupants’ behavior, IAQ, and self-reported health conditions are evaluated. This work can provide a foundation for subsequent remediation of IAQ conditions.
Introduction
With urban populations spending a majority of their time indoors, understanding the sources of indoor pollutants with the ultimate goal of mitigating exposure is a vital concern (Lee et al., Citation2002; Wallace et al., Citation2006). Of particular interest is the control of pollutants that can impact people who are most vulnerable to exposure, including children, the elderly, and those with existing respiratory disease. Further, limited access to health care may limit intervention to overcome any health burden on low-income populations affected by air pollution. For this reason, low-income seniors are amongst those most impacted by, and least able to respond to, health burdens from indoor pollution (Williams et al., Citation2000).
With rising energy costs and concern about the impact of fossil-fuel-based energy on climate, energy efficiency retrofits have become more common, with billions of dollars from numerous sources available to implement energy savings in buildings. One common, low-cost/high-return approach to saving energy is sealing the building envelope to reduce building leakage, with the goal of lowering the amount of make-up air that must be conditioned and the associated energy used for air handling. However, this sealing of the building envelope may trap pollutants released from indoor sources, leading to increased exposure to pollutants for residents (Jones, Citation1999; Fisk, Citation2000). Identifying common indoor pollutant sources in multiunit residential buildings may help in the design and implementation of more effective energy efficiency interventions, which may include retrofits in association with different approaches to building ventilation, filtration, and air cleaning.
Particulate matter, or PM, is of great concern to the U.S. Environmental Protection Agency (EPA) because of the impact on heart and lung health (Dockery et al., Citation1993). Particles with an aerodynamic diameter less than 10 μm have the potential to pass through the throat and nose and into the lungs. Additionally, epidemiological studies have linked increased outdoor PM exposure to increased mortality and exacerbation of existing respiratory diseases (Wallace, Citation1996; Li et al., Citation2003; Englert, Citation2004; Davidson et al., Citation2005). Although much of the research studying health impacts of indoor air pollution has focused on volatile organic compounds, biological aerosols, or radon (Jones, Citation1999; Bernstein et al., Citation2008), there is growing evidence of the impact of indoor PM on health (Koenig et al., Citation2005). Although there are no established acceptable limits of indoor PM levels, the National Ambient Air Quality Standards (NAAQS) regulating ambient concentrations of particles in the United States over 24-hr averaging periods include standards for PM10 and PM2.5 (PM with an aerodynamic diameter <10 μm and <2.5 μm, respectively) at 150 and 35 μg/m3, respectively. An annual PM2.5 standard of 12 μg/m3 has recently been modified by the EPA, due to increasing health concerns of fine particle exposure (EPA, Citation2012).
Formaldehyde is a pollutant of concern due to its prevalence indoors and its association with chronic and acute health effects. It is found in the additives used in wood-based building products and furnishings, such as particleboard (Hodgson et al., Citation2002; Singer et al., Citation2006; Destaillats et al., Citation2006, Citation2011; Sidheswaran et al., Citation2013). Acute formaldehyde exposures may lead to sensory irritation symptoms (eye, nose, and throat), as well as irritation of the upper respiratory system, nasal obstruction, pulmonary edema, and dyspnea. Chronic exposures have been linked with allergic sensitization, asthma symptoms, histopathological changes in respiratory epithelium, and decrements in lung function (Lawrence Berkeley National Laboratory [LBNL], Citation2008; Salthammer et al., Citation2010; California Environmental Protection Agency [CalEPA], Citation2007). In addition, formaldehyde is listed by the EPA as a probable carcinogen (group B1; EPA, Citation1999a), and the World Health Organization has classified formaldehyde as a human carcinogen (Cogliano et al., Citation2005). A recent assessment listed formaldehyde among the top five indoor pollutants leading to chronic health effects in U.S. residences (Logue et al., Citation2012). Several health-based exposure levels for formaldehyde have been established by regulatory agencies. In the United States, the CalEPA established an acute reference exposure level of 44 ppb and an 8-hr reference exposure level (chronic exposure) of 7 ppb (CalEPA, Citation2007). Similarly, Health Canada has established an 8-hr exposure limit of 40 ppb based on respiratory symptoms in children (Health Canada, Citation2006). Several other exposure levels established by other countries and agencies are summarized by Salthammer et al. (Citation2010).
With this background, we report a study characterizing a city-subsidized apartment complex for seniors in Phoenix, Arizona. The building is characterized by concentrations of indoor PM and volatile aldehydes. Although there are many contaminants of concern in the indoor environment, few can be tested in short time periods in a minimally invasive way. PM, formaldehyde, and acetaldehyde were chosen due to their high chance of detection, known impacts on human health, and availability of standards for comparison. In addition, PM is often used to investigate the impacts of environmental tobacco smoke, whereas formaldehyde can indicate the release of pollutants from building materials. Associations between occupants’ behavior, self-reported health conditions, and indoor air quality (IAQ; i.e., which sources or individual behaviors are linked to high measure PM and aldehydes) are evaluated.
Materials and Methods
Sampling campaign and health survey
A study was conducted at a local apartment complex, operated by the City of Phoenix Housing Department, for seniors who qualify for subsidized rent. Originally built in the early 1970s, this three-story apartment building contains 116 identical units. Air samples were collected in the self-contained apartment units, with simultaneous measurements of indoor air pollutants (PM and aldehydes) in the living room and kitchen and outdoor pollutant concentrations on the balcony of each unit. All units have 619 ft2 of livable space and are identical in interior layout and are all-electric homes (i.e., no fireplaces, gas stoves, etc.) with individual packaged terminal air conditioning (PTAC) units.
At the same time as air quality testing, a health survey of over 100 questions was given to the residents to solicit information about personal habits and health conditions of the apartment occupants. This questionnaire consisted of open-ended and fixed-response questions developed from applicable portions of the National Health Interview Survey (NHIS) and from the Behavioral Risk Factor Surveillance System (BRFSS) for Arizona as well as questions about personal habits and perceived air quality. Questions most relevant to this article involve smoking and cleaning behaviors, pet ownership, methods of odor reduction, and respiratory health. Performing the air quality measurements and administering the questionnaire simultaneously decreased the impact that a resident’s activities (i.e., cooking, smoking) can have on IAQ measurements.
The indoor air quality testing, presented here, is a subset of a larger-scale study in which cost efficiency and health benefits are also being analyzed (Ahrentzen et al., Citation2013). The larger study takes benefits such as reduced falls, quality of life, and fewer trips to the doctor into account, whereas the air quality portion mainly focuses on respiratory diseases and perception of air quality. A total of 72 apartments with 77 residents were studied during the program between June 10 and July 12, 2010. One-hour air quality samples were collected in each unit between the hours of 9 a.m. and 5 p.m. Repeated testing in a subset of units (7% replicate) ensured that no time-of-day bias impacted collected data. The summer season was selected for sampling, as local hot weather would result in the apartment units being sealed (i.e., windows closed) with air conditioning running. This ensured consistency between units and enabled the isolation of the impact of resident behavior on IAQ. Residents were asked not to cook, smoke, or clean for 2 hr prior to air quality measurements, in order to minimize introducing strong transient sources that would impact the air quality measurements. Although 1-hr sampling periods are relatively short and may be more susceptible to transient emission events, the sampling plan was carefully designed in order to minimize the impact of resident activity during sampling, thus reducing the potential impact of individual activities on indoor air quality. In addition, this sampling plan maximized the number of participants to ensure sufficient apartments for potential follow-up analysis. Short-duration samples allowed coordinating for a large number of units over the month-long sampling period, obtaining a representative data set for the building by testing units on different floors, wings, and orientations.
Particulate matter measurements
Indoor air quality sampling included real-time measurement of PM using a TSI DustTrak DRX (model 8533; TSI, Inc., Shoreview, MN) sampler. This instrument contains a light-scattering laser photometer to detect various particle sizes, including PM1, PM2.5, PM4, PM10, and PMTotal. The maximum size measured for the PMTotal is approximately 15 microns based on manufacturer specifications. Three samplers were deployed to the apartment kitchen, living room, and balcony to simultaneously collect particle data over a 1-hr period during which the resident was given the health survey. By sampling both indoor and outdoor air, we are able to calculate indoor/outdoor ratios, with the goal of quantifying the impact of infiltration of outdoor particles versus indoor sources on indoor air quality. Dusttraks were labeled and used in a consistent manner among units, were calibrated prior to the study, and were tested for reproducibility by collocated sampling. Although Dustraks have been shown to overestimate PM compared with gravimetric measurements, the use of a consistent sampling platform was designed to minimize bias due to sampling technique (Jenkins et al., Citation2004).
Aldehyde measurements
Samples of indoor and outdoor formaldehyde and acetaldehyde were collected using commercial samplers containing dinitrophenylhydrazine (DNPH)-coated silica gel (Sep-Pak XPoSure Aldehyde Sampler, catalog no. WAT047205; Waters Corp., Milford, MA). The cartridges were preceded by an ozone scrubber (Sep-Pak Ozone Scrubber, catalog no. WAT054420; Waters) to eliminate ozone from the incoming air. Air was drawn through the samplers by means of pumps operating at <2 L min−1 (determined with a precision better than ±3%). Samples were collected over 1-hr periods using portable gas pumps (Universal XR Pump, model PCXR4; SKC Inc., Eighty Four, PA). The sampling flow of each pump was calibrated in the laboratory before and after the sampling period using a bubble flow meter and a primary air flow calibrator (Gilibrator-2; Sensidyne, St. Petersburg, FL). Three samples were collected simultaneously with and in close proximity to the PM samplers in the living room, kitchen, and balcony.
After collection, each DNPH cartridge was capped, labeled, and stored at 4 °C until it was extracted and analyzed. Acetonitrile extracts were analyzed by high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection at 360 nm following a EPA method (EPA, Citation1999b). The concentration value reported in each case corresponded to a time-integrated average over the sampling period. Calibration curves for quantification were determined with authentic standards of the dinitrophenylhydrazones of formaldehyde and acetaldehyde (Sigma-Aldrich, St. Louis, MO). The detection limit for each volatile carbonyl was typically 10 ng or lower, corresponding to air concentrations of <0.1 ppb. Laboratory and field blank samples (at least three laboratory and six field blanks) were also analyzed, showing nondetectable values of the three analytes.
Results and Discussion
Occupant questionnaire outcomes
To characterize the demographics of the apartment units sampled, key variables expected to impact sources of air pollution in the apartment (i.e., smoking, use of candles, etc.) as well as those with self-reported existing respiratory disease that might lead to residents taking active steps to mitigate indoor pollution, are summarized in . The demographics of those residents who participated in our study aid in the interpretation of the indoor air quality data. Eleven to thirteen percent of residents reported an existing respiratory disease (i.e., asthma or emphysema), 14% owned pets, and 64% used something to change the smell of the air at least once a week (candles, incense, air freshener, or other such as scented plug-ins, Lysol-type sprays, and carpet fresheners).
Table 1. Participant responses to indoor source and respiratory health-related questions
Particulate matter
For initial comparison, measured levels of indoor PM often far exceeded measured outdoor concentrations, an indication of the importance of indoor PM sources for the units participating in the study. As can be seen in , indoor particle concentrations averages are higher and more widely variable than outdoor PM concentration averages, although part of the difference may be due to varying particle morphology between indoor and outdoor PM, which alters instrument response. When comparing living room PM10 to outdoor PM10, there is a mean difference of 42 (μg/m3) (paired t test: t = 2.665, P < 0.01) and a low correlation of 0.29 (P < 0.05). The differences between measured particle concentrations in the kitchen and living room for each unit are not statistically significant (values had a linear regression correlation of 0.993); therefore, we will use the living room data to be representative of indoor PM levels.
Table 2. Particulate matter concentrations for the living room, kitchen, and balcony (outdoor)
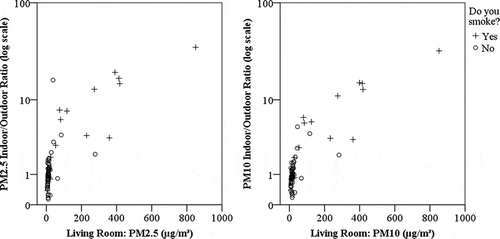
The mean indoor/outdoor (I/O) ratios for PM2.5 and PM10 for all units studied are 3.0 (σ = 5.6) and 2.5 (σ = 4.8), respectively. The difference of the means from unity is statistically significant (t = 2.945, P < 0.005 and t = 2.699, P < 0.01, respectively). The median I/O ratio is 1.0 for both PM2.5 and PM10. However, if participant data are separated by those who smoke and those who do not, the impact of smoking as a PM source is clearly evident. The mean values of the I/O ratios for nonsmoking participants are 1.4 (σ = 2.1) and 1.1 (σ = 0.8) and the median values are 1.0 and 0.9 for PM2.5 and PM10, respectively. In addition, the difference of the means from unity is not statistically significant. Smoking participants have mean I/O ratios of 8.5 (σ = 9.5) and 7.4 (σ = 8.3) and median I/O ratios of 4.9 and 4.5, respectively, and the difference of the means from unity is statistically significant (t = 3.169, P < 0.006 and t = 3.057, P < 0.008, respectively). Variances assumed unequal, the differences in the mean I/O ratios for units where residents report smoking versus those units where residents do not report smoking is statistically significant (independent-samples t test: t = 3.00 and t = 3.01, P < 0.01). shows the relationship between measured indoor PM2.5 and PM10 and the log of the respective I/O ratio, with data points differentiating between units occupied by residents who smoke and those who do not. From this plot, it is clear that the units with the most elevated ratio of indoor to outdoor PM also have the highest concentrations of indoor PM and tend to be occupied by persons who reported that they smoke.
Figure 1. Concentration vs. indoor/outdoor ratio, PM2.5 (left) and PM10 (right). Circles are nonsmoking units and plus signs are smoking units.
summarizes all data collected in the study for particle concentrations at each monitoring location broken down by particle size for fine (PM2.5) and coarse PM (PM2.5–10). shows that the majority of particle mass are measured in the fine particle fraction, which may indicate the importance of particle sources such as combustion as opposed to pet dander and other mechanical entrainment of dust, which typically produce coarse mode particles.
Figure 2. Fine (dashed lines) and coarse (solid black) particle concentrations in the living room, kitchen, and balcony (outdoor) for residents who report they do not smoke (left) and those that report they do smoke (right). Note the differences in scale.
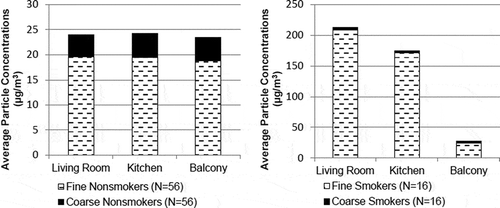
The data also suggest that infiltration of outdoor particles is not expected to be the dominant source of indoor particles, based on the relative concentration of particles. This is evident in both and , with the average I/O ratios being greater than 1 and balcony PM concentrations being much lower than the elevated indoor PM levels in units in which residents smoke. For these reasons, it is important to focus on the potential sources of indoor particles in the home, including smoking and use of air fresheners, candles, and incense.
As previously mentioned, the NAAQS regulating outdoor concentrations of particles in the United States is set at a 24-hr average concentration of 150 μg/m3 for PM10 and 35 μg/m3 for PM2.5 as well as an annual PM2.5 limit of 12 μg/m3. Although not directly applicable to indoor PM concentrations, this is used as a screening level to identify units where the indoor PM levels might be considered to directly impact health. In , we report the number of units that exceed each of these three standards.
Table 3. Number of units with PM concentrations above and below the EPA National Ambient Air Quality Standards, split by occupant reported smoking habits
Smoking had a clear impact on indoor PM levels in the units in which residents indicated that they smoke (n = 16). Mean values of living room PM10 were 213 ± 58 μg/m3 for smokers versus 24 ± 5 μg/m3 for nonsmokers (n = 56). Mean values of living room PM2.5 were 209 ± 58 μg/m3 for smokers versus 20 ± 5 μg/m3 for nonsmokers. Apart from smoking, elevated indoor PM can originate from combustion sources (i.e., candles and incense), air fresheners, or the presence of pets, and each of these sources has been shown in prior research to impact PM concentrations inside the home (Géhin et al Citation2008). In , the outlying data points correspond to units occupied by participants who reported using air fresheners, candles, and/or incense. Among nonsmokers, the most commonly indicated potential source of indoor particles was air fresheners (n = 28 out of 56). Although nonsmoking units in which products were used to change the smell in their homes (n = 33) have a higher average PM compared with nonsmoking units where no additional potential PM source was reported as used (n = 23), this difference was not statistically significant (t = 1.3, P < 0.2). The average PM10 concentration for units occupied by a nonsmoker who also reported none of these alternative sources of PM (i.e., use of candles, incense, or air fresheners or owning a pet) was 17 μg/m3, whereas units occupied by nonsmokers reporting one or more of these alternative sources had a mean PM10 of 24 μg/m3.
Figure 3. PM2.5 (left) and PM10 (right) concentrations (μg/m3), separated by smokers (n = 16) and nonsmokers (n = 56). The bold line within the box indicates the median. The top and bottom of the boxes indicate the 75th and 25th percentiles, respectively. Asterisks and circles denote outliers.
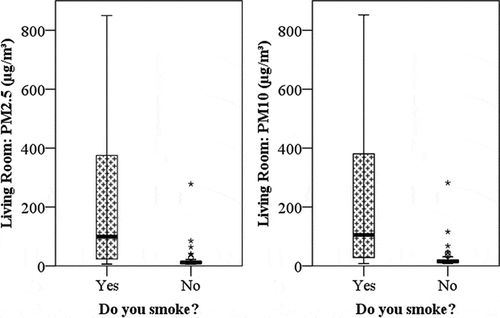
Figure 4. PM10 concentrations and respiratory problems separated by smokers (texture) and nonsmokers (solid black). n indicates the number of samples for each group. The bold line within the box indicates the median. The top and bottom of the boxes indicate the 75th and 25th percentiles, respectively. Asterisks and circles denote outliers.
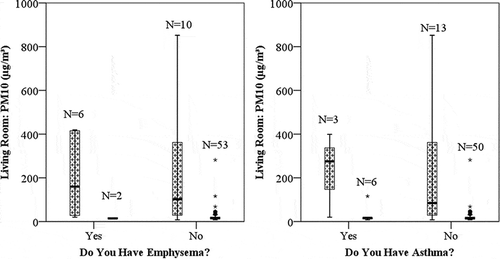
One potential hypothesis to test is that persons with existing respiratory problems may mitigate sources of indoor pollutants to limit their exposure to particles in the home. Based on the data collected as part of our current study, this hypothesis is not supported. Occupants who indicated that they had either emphysema or asthma had higher average particle concentrations than those who did not have these respiratory problems, as seen in . Units occupied by participants reporting emphysema had a mean [median] PM10 level of 154 [57] μg/m3 versus 56 [16] μg/m3 (no report of emphysema), but these differences were not statistically significant (t = 1.5, P < 0.2). Occupants reporting asthma had a mean [median] PM10 level of 98 [18] μg/m3 versus 61 [16] μg/m3 (no report of asthma), but these differences were also not statistically significant (t = 0.7, P < 0.5). These results are likely complicated by the correlation between persons reporting respiratory disease and those who smoke, as 75% of persons with emphysema and 33% of persons with asthma are smokers, compared with the entire study population where only 22% smoke.
Aldehyde measurements
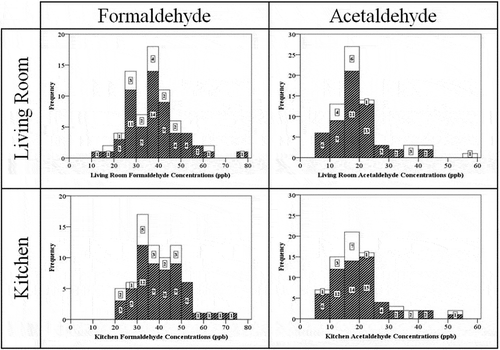
summarizes formaldehyde and acetaldehyde concentrations measured in the living room, the kitchen, and the balcony. We also illustrate the cumulative frequency of the data in . Indoor formaldehyde concentrations spanned the range of 10–80 ppb, with a median of 36.9 ppb in the living room and 38.8 ppb in the kitchen. No major differences were observed between the two indoor samples, which are highly correlated due to their close proximity (paired-sample correlation = 0.857, P < 0.001).
Table 4. Volatile aldehyde concentrations and indoor/outdoor ratios
Figure 5. Cumulative frequency of formaldehyde concentrations (top) and acetaldehyde concentrations (bottom) measured in the living room (dash-dot), kitchen (short dash), and outdoors (long dash).
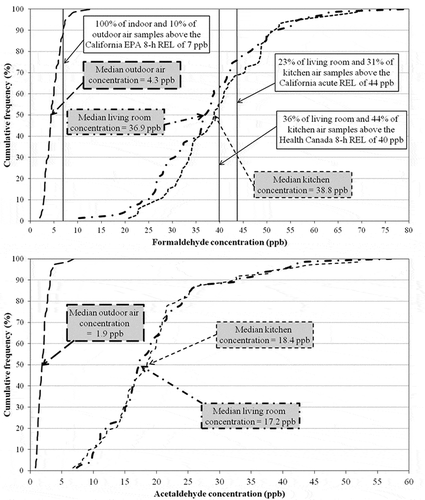
Median formaldehyde concentrations were much higher than the 8-hr reference exposure level (REL) established by the CalEPA (7 ppb), comparable to the 8-hr REL proposed by Health Canada (40 ppb), and were slightly lower than the CalEPA 1-hr REL (44 ppb) (CalEPA, Citation2007; Health Canada, Citation2006). By contrast, acetaldehyde levels were below the health-based exposure levels (the CalEPA 8-hr REL is 160 ppb and 1-hr REL is 260 ppb for acetaldehyde). Median acetaldehyde levels for the living room and kitchen are 17.2 and 18.4 ppb, respectively.
The formaldehyde levels measured in this study were significantly higher than those described in surveys conducted in U.S. commercial buildings and homes. Hodgson and Levin (Citation2003) reported a median formaldehyde level of 17 ppb in North American residences, with a 95th percentile of 61 ppb. Offermann (Citation2009) determined a median of 29 ppb formaldehyde in new homes in California. Liu (Citation2006) found indoor median formaldehyde and acetaldehyde levels to be 20.1 and 18.6 μg/m3, respectively. The higher levels observed in the studied building are likely associated with the combination of strong sources (e.g., building materials, occupant activities) and relatively low air exchange rates during the summer season.
In this study, the median outdoor concentration was 4.3 ppb for formaldehyde and 1.9 ppb for acetaldehyde, consistent with values previously reported in the literature for similar studies (Hun et al., Citation2010; Offermann et al., Citation2009). The high indoor/outdoor (I/O) concentration ratios (8 < I/O < 10) reported in indicate the prevalence of indoor sources for these pollutants. The difference of the means from unity is statistically significant (t = 18.026, P < 0.001 and t = 17.466, P < 0.001 for formaldehyde and acetaldehyde, respectively).
The distribution of formaldehyde and acetaldehyde concentrations across subsets of smoking and nonsmoking apartments is shown in . In contrast to the strong influence of smoking on PM levels, there was no statistically significant difference in volatile aldehyde levels between units where residents report smoking versus units where residents do not report smoking (t = −0.66, P < 0.6 and t = −1.82, P < 0.10 for formaldehyde in the living room and kitchen, respectively). Formaldehyde mean concentrations were in the range of 35–41 ppb (comprising both indoor measurements), with standard deviation between 8 and 12 ppb. Units in which residents indicated that they smoked showed slightly lower levels of formaldehyde than nonsmoking units; however, the differences (between 2 and 5 ppb) were smaller than the standard deviation of the data. Similarly, acetaldehyde mean indoor levels were in the range of 18–24 ppb, with standard deviations between 7 and 14 ppb. Acetaldehyde levels in smoking apartments were higher by a small margin of 3–5 ppb, which was also smaller than the standard deviation of the data and not statistically significant (t = 2.10, P < 0.04 and t = 0.8, P < 0.5 for the living room and kitchen, respectively).
Conclusion
The present work reports key indoor air quality parameters, including PM levels and aldehyde concentrations, for a low-income senior apartment complex. The air quality sampling was combined with a health questionnaire to garner information on the personal habits and general health of residents. With over 70 residences sampled, this large data set describes associations between occupants’ behavior, indoor air quality, and health, and provides a foundation for the subsequent evaluation of the impact of different interventions (e.g., building retrofits, increased ventilation, filtration, air cleaning) on indoor air quality.
The initial results indicate that elevated indoor particle concentrations are directly linked to residents who smoke; however, residents were only asked if they smoked, not if they smoked indoors. Data do not indicate that outdoor particles are infiltrating to the indoor environment. While smoking impacted indoor PM levels, there was no statistically significant difference on indoor aldehyde levels for residents who smoked compared with those who reported that they did not smoke. For all units, formaldehyde levels were greatly elevated, with 36% of living room samples and 44% of kitchen samples exceeding the Health Canada REL for chronic exposure to formaldehyde, of 40 ppb. No statistically significant correlation was found between measured indoor PM and aldehyde concentrations.
Although this study allowed us to sample many units, 1-hr sampling leads to some research limitations. For example, although data presented here represent typical concentrations in the summer, it may not hold true for the fall, winter, and spring seasons. Additionally, even though cross-contamination between different units could impact measured indoor air quality, as has been shown by Bohac et al. (Citation2011), air exchange between units was not quantified in this study. This could be an important factor if the environmental tobacco smoke of one neighbor was infiltrating the unit of a nonsmoker, thus increasing particle concentrations. Another limitation is that no longer-term (day-long or week-long) indoor air samples were collected.
Acknowledgments
The authors gratefully acknowledge the assistance of the staff of the City of Phoenix Housing Department, Patrick Montgomery, Drew Bryck, Ernesto Fonseca, John Ball, Kim Shea, William Johnson, Mookesh Patel, and Amandine Montalbano.
Funding
Funding was provided by the U.S. Department of Housing and Urban Development.
Additional information
Notes on contributors
Sarah E. Frey
Sarah E. Frey is a Ph.D. Candidate in the Department of Chemistry and Biochemistry at Arizona State University in Tempe, AZ.
Hugo Destaillats
Hugo Destaillats is a staff scientist at the Environmental Energy Technologies Division and Deputy Leader of the Indoor Environment Group at Lawrence Berkeley National Laboratory in Berkeley, CA.
Sebastian Cohn
Sebastian Cohn is a researcher in the Indoor Environment Group at Lawrence Berkeley National Laboratory in Berkeley, CA.
Sherry Ahrentzen
Sherry Ahrentzen is a professor in the M.E. Rinker, Sr., School of Building Construction at the University of Florida in Gainesville, FL.
Matthew P. Fraser
Matthew P. Fraser is a professor in the School of Sustainable Engineering and the Built Environment as well as the Executive Director of the Quantum Energy and Sustainable Solar Technologies (QESST) Engineering Research Center at Arizona State University in Tempe, AZ.
References
- Ahrentzen, S., J. Ball, H. Destaillats, S. Dwyer, J. Erickson, E. Fonseca, M.P. Fraser, S.E. Frey, W. Johnson, M. Patel, and K. Shea 2013. The Green Apple Research Project: Health Outcomes of a Green Housing Retrofit for Older Adults in Phoenix, Arizona. Final report for HUD Office of Healthy Homes and Lead Hazard Control (grant number: AZLHH0200-09), Washington, DC.
- Bernstein, J.A., N. Alexis, H. Bacchus, I.L. Bernstein, P. Fritz, E. Horner, N. Li, S. Mason, A. Nel, J. Oullette, K. Reijula, T. Reponen, J. Seltzer, A. Smith, and S.M. Tarlo. 2008. The health effects of nonindustrial indoor air pollution. J. Allergy Clin. Immun. 121:585–591. doi:10.1016/j.jaci.2007.10.045
- Bohac, D.L., M.J. Hewett, S.K. Hammond, and D.T. Grimsrud. 2011. Secondhand smoke transfer and reductions by air sealing and ventilation in multiunit buildings: PFT and nicotine verification. Indoor Air 21:36–34. doi:10.1111/ina.2010.21.issue-1
- California Environmental Protection Agency. 2007. Formaldehyde reference exposure levels. Public review draft. http://www.oehha.ca.gov/air/hot_spots/pdf/FormaldehydePR.pdf (accessed August 6, 2014).
- Cogliano, V.J., Y. Grosse, R.A. Baan, K. Straif, M.B. Secretan, and F. El Ghissassi. 2005. Meeting report: Summary of IARC monographs on formaldehyde, 2-butoxyethanol and 1-tert-butoxy-2-propanol. Environ. Health Perspect. 113:1205–1208. doi:10.1289/ehp.7542
- Davidson, C.I., R.F. Phalen, and P.A. Solomon. 2005. Airborne particulate matter and human health: A review. Aerosol Sci. Technol. 39:737–749. doi:10.1080/02786820500191348
- Destaillats, H., W. Chen, M.G. Apte, N. Li, M. Spears, J. Almosni, G. Brunner, J. Zhang, and W.J. Fisk. 2011. Secondary pollutants from ozone reactions with ventilation filters and degradation of filter media additives. Atmos. Environ. 45:3561–3568. doi:10.1016/j.atmosenv.2011.03.066
- Destaillats, H., M.M. Lunden, B.C. Singer, B.K. Coleman, A.T. Hodgson, C.J. Weschler, and W.W. Nazaroff. 2006. Indoor secondary pollutants from household product emissions in the presence of ozone. A bench scale study. Environ. Sci. Technol. 40:4421–4428. doi:10.1021/es052198z
- Dockery, D.W., C.A. Pope III, X. Xu, J.D. Spengler, J.H. Ware, M.E. Fay, B.G. Ferris, and F.E. Speizer. 1993. An association between air pollution and mortality in six US cities. N. Engl. J. Med. 329: 1753–1759. doi:10.1056/NEJM199312093292401
- Englert, N. 2004. Fine particles and human health—A review of epidemiological studies. Toxicol. Lett. 149:235–242. doi:10.1016/j.toxlet.2003.12.035
- Fisk, W.J. 2000. Health and productivity gains from better indoor environments and their relationship with building energy efficiency. Annu. Rev. Energy Environ. 25:537–566. doi:10.1146/energy.2000.25.issue-1
- Géhin, E., O. Ramalho, and S. Kirchner. 2008. Size distribution and emission rate measurement of fine and ultrafine particle from indoor human activities. Atmos. Environ. 42:8341–8352. doi:10.1016/j.atmosenv.2008.07.021
- Health Canada. 2006. Residential indoor air quality guideline. http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/air/formaldehyde_e.pdf (accessed August 6, 2014).
- Hodgson, A.T., and H. Levin. 2003. Classification of measured indoor volatile organic compounds based on noncancer health and comfort considerations. LBNL report no. 53308. http://energy.lbl.gov/ie/pdf/LBNL-53308.pdf (accessed August 6, 2014).
- Hodgson, A.T., D. Beal, and J.E.R. McIlyaine. 2002. Sources of formaldehyde, other aldehydes and terpenes in a new manufactured house. Indoor Air 12:235–242. doi:10.1034/j.1600-0668.2002.01129.x
- Hun, D.E., R.L. Corsi, M.T. Morandi, and J.A. Siegel. 2010. Formaldehyde in residences: Long-term indoor concentrations and influencing factors. Indoor Air 20:196–203. doi:10.1111/ina.2010.20.issue-3
- Jenkins, R.A., R.H. Ilgner, and B.A. Tomkins. 2004. Development and application of protocols for the determination of response of real-time particle monitors to common indoor aerosols. J. Air Waste Manage. Assoc. 54:229–241. doi:10.1080/10473289.2004.10470892
- Jones, A.P. 1999. Indoor air quality and health. Atmos. Environ. 33:4535–4564. doi:10.1016/S1352-2310(99)00272-1
- Koenig, J.Q., T.F. Mar, R.W. Allen, K. Jansen, T. Lumley, J.H. Sullivan, C.A. Trenga, T.V. Larson, and L.J.S. Liu. 2005. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ. Health Perspect. 113:499–503. doi:10.1289/ehp.7511
- Lawrence Berkeley National Laboratory. 2008. Indoor air quality scientific findings resource bank. http://www.iaqscience.lbl.gov/voc-sensory.html (accessed August 6, 2014).
- Lee, S.C., W.-M. Li, and C.-H. Ao. 2002. Investigation of indoor air quality at residential homes in Hong Kong—Case study. Atmos. Environ. 36:225–237. doi:10.1016/S1352-2310(01)00435-6
- Li, N., M.Q. Hao, R.F. Phalen, W.C. Hinds, and A.E. Nel. 2003. Particulate air pollutants and asthma—A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 109:250–265. doi:10.1016/j.clim.2003.08.006
- Liu, W., J. Zhang, L. Zhang, B.J. Turpin, C.P. Weisel, M.T. Morandi, T.H. Stock, S. Colome, and L.R. Korn. 2006. Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas of the United States. Atmos. Environ. 40:2202–2214. doi:10.1016/j.atmosenv.2005.12.005
- Logue, J. M., P.N. Price, M.H. Sherman, and B.C. Singer. 2012. A method to estimate the chronic health impact of air pollutants in US residences. Environ. Health Perspect. 120:216–222.
- Offermann, F.J. 2009. Ventilation and Indoor Air Quality in New Homes. California Air Resources Board and California Energy Commission, PIER Energy Related Environmental Research Program. Collaborative Report. CEC 500 2009 085. http://www.arb.ca.gov/research/apr/past/04-310.pdf (accessed August 6, 2014).
- Salthammer, T., S. Mentese, and R. Marutzky. 2010. Formaldehyde in the indoor environment. Chem. Rev. 110:2536–2572. doi:10.1021/cr800399g
- Sidheswaran, M., W. Chen, A. Chang, R. Miller, S. Cohn, D. Sullivan, W.J. Fisk, K. Kumagai, and H. Destaillats 2013. Formaldehyde emissions from ventilation filters under different relative humidity conditions. Environ. Sci. Technol. 47:5336–5343. doi:10.1021/es400290p
- Singer, B.C., H. Destaillats, A.T. Hodgson, and W.W. Nazaroff. 2006. Cleaning products and air fresheners: Emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 16:179–191. doi:10.1111/ina.2006.16.issue-3
- U.S. Environmental Protection Agency. 1999a. Integrated Risk Information System (IRIS) on Formaldehyde. Washington, DC: National Center for Environmental Assessment, Office of Research and Development.
- U.S. Environmental Protection Agency. 1999b. Compendium Method TO-11A—Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by HPLC [Active Sampling Methodology]. Cincinnati, OH: Office of Research and Development, U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 2012. Revised Air Quality Standards for Particle Pollution and Updates to the Air Quality Index (AQI). The National Ambient Air Quality Standards for Particle Pollution. http://www.epa.gov/airquality/particlepollution/2012/decfsstandards.pdf (accessed August 6, 2014).
- Wallace, L. 1996. Indoor particles: A review. J. Air Waste Manage. Assoc. 46:98–126. doi:10.1080/10473289.1996.10467451
- Wallace, L., R. Williams, A. Rea, and C. Croghan. 2006. Continuous weeklong measurements of personal exposures and indoor concentrations of fine particles for 37 health-impaired North Carolina residents for up to four seasons. Atmos. Environ. 40:399–414. doi:10.1016/j.atmosenv.2005.08.042
- Williams, R., J. Suggs, J. Creason, R. Rodes, P. Lawless, R. Kwok, R. Zweidinger, and L. Sheldon. 2000. The 1998 Baltimore particulate matter epidemiology-exposure study: Part 2—Personal exposure assessment associated with an elderly study population. J. Expos. Anal. Environ. Epidemol. 10:533–543. doi:10.1038/sj.jea.7500108