Abstract
A computational fluid dynamics (CFD) methodology for simulating the combustion process has been validated with experimental results. Three different types of experimental setups were used to validate the CFD model. These setups include an industrial-scale flare setups and two lab-scale flames. The CFD study also involved three different fuels: C3H6/CH4/Air/N2, C2H4/O2/Ar, and CH4/Air. In the first setup, flare efficiency data from the Texas Commission on Environmental Quality (TCEQ) 2010 field tests were used to validate the CFD model. In the second setup, a McKenna burner with flat flames was simulated. Temperature and mass fractions of important species were compared with the experimental data. Finally, results of an experimental study done at Sandia National Laboratories to generate a lifted jet flame were used for the purpose of validation. The reduced 50 species mechanism, LU 1.1, the realizable k-ϵ turbulence model, and the EDC turbulence–chemistry interaction model were used for this work. Flare efficiency, axial profiles of temperature, and mass fractions of various intermediate species obtained in the simulation were compared with experimental data and a good agreement between the profiles was clearly observed. In particular, the simulation match with the TCEQ 2010 flare tests has been significantly improved (within 5% of the data) compared to the results reported by Singh et al. in 2012. Validation of the speciated flat flame data supports the view that flares can be a primary source of formaldehyde emission.
Implications
Validated computational fluid dynamics (CFD) models can be a useful tool to predict destruction and removal efficiency (DRE) and combustion efficiency (CE) under steam/air assist conditions in the face of many other flare operating variables such as fuel composition, exit jet velocity, and crosswind. Augmented with rigorous combustion chemistry, CFD is also a powerful tool to predict flare emissions such as formaldehyde. In fact, this study implicates flares emissions as a primary source of formaldehyde emissions. The rigorous CFD simulations, together with available controlled flare test data, can be fitted into simple response surface models for quick engineering use.
Introduction
The most recent Texas Air Quality Studies (TxAQS 2000 and TxAQS II) revealed that air quality models (Comprehensive Air Quality Model with extensions [CAMx], Community Multi-Scale Air Quality [CMAQ]) often significantly underpredict observed peak O3 with the current volatile organic compounds (VOC) emission inventories in the Houston–Galveston–Brazoria area (HGB). It is generally believed that either unidentified VOC emission inventory sources exist or identified sources are significantly under-reported. One potential under-reported or non-reported emission source is due to flare operations (Texas Commission on Environmental Quality [TCEQ], Citation2007; Environ, Citation2006; ZEECO, Citation2007). The more common elevated flares are also subject to crosswind effects, which can decrease the combustion efficiency by reducing the residence time of the combustible materials. The TCEQ’s ongoing evaluation of flare operations, therefore, is an important element of a future State Implementation Plan (SIP) revision (TCEQ, Citation2009). Flare efficiencies depend on many factors. Specifically, both destruction and removal efficiency (DREs) and combustion efficiency (CE) depend on the heating value of the vent gas, vent gas species, exit velocity, steam injection, assisted air, fuel–air mixing, and wind speed (Pohl et al., Citation1984; Pohl and Soelberg, Citation1985; TCEQ, Citation2000, Citation2007; U.S. Environmental Protection Agency [EPA], Citation2009, Citation2012; Singh et al., 2013). As a result, DRE and CE can drop below the 98% threshold under certain high air/steam-assisted conditions even when the flare operation is in compliance with 40 CFR 60.18 (U.S. Government, Citation2009) as indicated in recent TCEQ and EPA reports (Allen and Torres, Citation2011; EPA, Citation2012). A photochemical model used by Al-Fadhli et al. (Citation2012) predicted an increase in ozone concentrations by more than 15 ppb at low DREs. Another issue is that the required flare turndown ratio (more than 15,000 to 1) to accommodate various operating modes makes it difficult to maintain high efficiency at all conditions (Baukal and Schwartz, Citation2001; Allen and Torres, Citation2011; Torres et al, Citation2012).
Computational fluid dynamics (CFD) simulations of the combustion process have received considerable attention due to their potential to closely emulate real-world combustion scenarios. Such studies include, but are not limited to, the simulation of internal combustion engines, industrial flares and burners, fire and explosion, industrial furnaces, and others. The focus of this work is to model industrial flares and laboratory flames of similar fuel species and to compare the combustion efficiency and speciated emissions with experimental data. In recent years, work on the simulation of open air combustion practices has been done using different models. For example, Smith (Citation2011) and Jatale et al. (Citation2012) recently validated their large eddy simulation (LES) model by comparing the CFD simulation results with experimentally measured flare efficiencies of a 4-inch diameter laboratory flare at the CANMET wind tunnel flare facility. Castiñeira and Edgar (Citation2008) used the probability density function (PDF) model to simulate small scale flares in a wind tunnel setup as well. The simulation results were validated with temperature and concentration profiles of major species. However, due to a limited number of species (16) considered in the reaction mechanism, some intermediate species such as OH, HCHO, or NO were not reported in Castiñeira’s work. On the other extreme, rigorous reaction mechanisms used for combustion modeling include as many as 700 reactions and more than 100 species (Wang et al., Citation2007). Even though the computational power to simulate such reactive flows has increased drastically in recent years, such models are still very computationally expensive. Hence, reduced mechanisms are necessary to make numerical modeling practical for turbulent combustion applications.
Lamar University’s research team has combined the GRI-3.0 mechanism (Smith et al., Citation2000) (optimized for methane) and the USC-I mechanism (Davis et al., Citation1999) (optimized for ethylene but without the NOx species) to obtain a mechanism containing 93 species and 600 reactions. This mechanism was reduced to create a series of 50-species mechanisms for the combustion of C1–C3 light hydrocarbons for use in Fluent-Chemkin. Mechanisms LU 1.0 and LU 1.1 were developed based on analysis of rate constant, maximum mass fraction, and number of reactions involved in the reaction pathway. In LU 1.1, CN was replaced by NO2 to model the 2010 John Zink flare test (Allen and Torres, Citation2011). LU1.1 (Lou et al., Citation2011; Singh et al., Citation2012, Citation2014) used in this study was validated with key laboratory test data, that is, laminar flame speed (Davis and Law, Citation1998), adiabatic flame temperature (Law et al., Citation2005), and ignition delay (Mayers and Bartle, Citation1969). The objective of this work was to further validate LU 1.1 and the CFD models against three sets of experimental data involving the combustion of methane, ethylene, propylene, and a combination of the three species. LU1.1 was validated by comparing the predicted concentration profiles of some important species with lab-scale flame experimental data. For any detailed combustion reaction mechanism, the ability to correctly predict concentrations of intermediate species is important. To evaluate the accuracy of LU1.1, mass fractions of species like NO, HCHO, and OH were measured along the height of the flame. These predicted concentration profiles were then plotted and compared against the experimental data. Due to the different scale and setup of the three experiments, the model was validated for both laminar and turbulent flames. The three experiments are discussed in the next section. One of the three setups was the Sandia flame in a vitiated coflow. Similar validation tests of Sandia Flames using various CFD models are reviewed here. The PDF flamelet model using mixture fraction theory and (CH4–Air) GRI reaction mechanism 2.11 were used to simulate the Sandia flame (Nik et al., Citation2010). Tyliszczak (Citation2013) used an unsteady-state LES model along with the PDF turbulence-chemistry model coupled with GRI 2.11 reaction mechanism to simulate the Sandia flame. The diameter of the lab-scale burner used for Sandia F flame was 18.9 mm. In Vujanovic (Citation2009), a reduced reaction mechanism was coupled with the steady-state laminar flamelet combustion model. In all of these studies, the accuracy of the modeling was limited by the PDF/flamelet model, which cannot predict slower reactions. In the present work, a rigorous combustion model, the eddy dissipation concept (EDC) model, was coupled with the 50-species LU 1.1 reaction mechanism.
To evaluate the reaction mechanism’s capability of modeling industrial scale flares, test cases from the TCEQ Flare Study project were modeled. Industrial flares with diameters ranging from 24 inches to 36 inches were used during these tests. To our knowledge, the previous results reported by Singh et al. in Citation2012 are the only validation of CFD simulations with controlled industrial-scale flares. Prior simulation studies (e.g., Castiñeira Citation2008) mainly modeled laboratory scale flares with flare diameters of up to 6 inches. Further, the TCEQ Flare Study tests modeled in this work and earlier by Singh et al. (Citation2012) were conducted at very low exit velocities and low heating values. These conditions represent flare operations in a stand-by mode and have importance in representing regular flare activities in handling fugitive emissions, venting, and pressure relief operations.
In addition to the setups mentioned already, the McKenna burner, a laminar flat flame burner, was also modeled. Such laminar flame burners are often used for accurately measuring the concentration of various VOC species. Using the measured concentration profiles, the reaction mechanism’s ability to predict the emission of HCHO during combustion process was validated. Both formaldehyde and acetaldehyde, important radical producing photochemical species (Seinfeld and Pandis, Citation2006), were also measured during the TCEQ’s 2010 Tulsa Flare tests (e.g., Test Number S4.1, Run1).
Numerical Simulation
All the already-mentioned case studies were simulated using the commercial CFD package ANSYS FLUENT 13.0. To reduce the computational time, Fluent was run using parallel computing settings; that is, each case was run on 8 or 12 local parallel processors.
Governing equations
Computational fluid dynamics mainly involves solving sets of transport equations using numerical methods like the Green–Gauss or the least square method. The governing transport equations are solved for mass, momentum (turbulence), energy and chemical species. Direct numerical simulation (DNS) for industrial flares is computationally not feasible because of the time dependent governing equations. Large eddy simulation (LES) modeling in which large eddies are explicitly computed in a time dependent simulation using the “filtered” Navier–Stokes equations can be applied for industrial flares, but it is computationally expensive. Therefore, in this study, the more popular Reynolds-averaged Navier–Stokes (RANS) equations, which govern the transport of the averaged flow quantities with the whole range of the scales of turbulence being modeled, are used to model turbulence. The RANS model is widely used for its reduced computational time and wide range of practical applications.
The basic governing equations to be solved for RANS combustion modeling are the mass, momentum (turbulence), energy, and chemical species.
The continuity equation for the RANS model is given in eq 1:
The ensemble-averaged momentum equation is written as
A common approach, the Boussinesq hypothesis (eq 4), is used to relate the Reynolds stresses to mean velocity gradients. This hypothesis is used for low computational cost associated with the solving of the turbulent viscosity, μt. It is used in k-є turbulent modeling, which is followed in this work. In this case, two additional transport equations, the turbulence kinetic energy k and the turbulence dissipation rate є, are solved and μt is computed as a function of k and є.
In many cases, the models based on the Boussinesq hypothesis perform well:
The turbulent kinetic energy k is defined as
For the k-є model, the eddy viscosity is calculated by the Prandtl–Kolamagorov relationship as follows:
The other governing equation to be solved for is the energy equation (eq 7). The general form of the energy equation is presented as
The last and the most important governing transport equation to be solved for industrial flare modeling is the species transport equation,
Computational domain
All the simulations were performed on three-dimensional (3-D) computational domains. These 3-D domains were created and meshed in GAMBIT 2.4.6. Both structured and unstructured cells were used to discretize the domain. The mesh near the fuel jet/burner/flare tip was kept very fine as compared to the rest of the domain. This helped in reducing the number of cells and hence the computational time. To select the optimum number of cells, grid independence studies were performed for all three models. Grid independence studies help to avoid the use of an excessive number of cells while preserving the accuracy of the final solution.
Boundary conditions
The boundary conditions specified in the CFD model are discussed here. Fuel inlet, pilot inlet, and crosswind were specified as “velocity inlets.” Each velocity-inlet surface was specified by mass fractions, temperature, and a velocity magnitude. The flow direction was kept normal to the surface. Turbulence of the velocity inlet surfaces were specified by turbulence intensity and hydraulic diameter. The bottom surface of all the domains, flare stack, and burner surfaces were set as a non-slip wall. For accurate prediction of turbulent flow near the flare stack boundary surface, the enhanced wall treatment function was used. All the surfaces from which the flows exit the domain were set as the pressure outlet. These pressure outlet surfaces were specified by a gauge pressure value of zero.
CFD models
A pressure-based solver with double precision was used for modeling. The turbulence was modeled using the realizable k-ϵ model. Radiation effects were neglected to reduce the computational costs. For the pressure–velocity coupling, the widely used SIMPLE (Patankar, Citation1980) algorithm was enabled. Discretization of gradients for constructing values of scalars at the cell faces were computed using the Green–Gauss cell-based method, and the pressure staggering option (PRESTO) was used for pressure discretization. For all the other equations, the first-order upwind scheme was used initially. However, for better accuracy, this was later changed to the second-order upwind scheme. Similarly, the underrelaxation factors were initially set at 0.5 (except for pressure, turbulent viscosity, and body forces, which were kept the same as default), and were then gradually brought to their default values with convergence. Other variables defined in the model are given in .
Table 1. Variables used in the model
Turbulence model
For modeling turbulence in the domain, the realizable k-ϵ model was used. The realizable k-ϵ model remains the most widely used model to simulate practical applications. It is derived from the standard k-ϵ turbulence model. Modified equations for calculating the turbulent viscosity and dissipation rate are introduced and added to the standard model. The realizable k-ϵ model is suitable for a wider range of applications, gives a more stable solution, and is computationally inexpensive at the same time. However, to closely emulate the open-air flaring process, the LES model should not be overlooked. It is a transient model that can simulate large eddies in a turbulent flow. However, due to its very high computational cost, it is not commonly used for modeling complex combustion processes involving hundreds of reactions. It normally takes hundreds of parallel processors and weeks of computational time to simulate few seconds of a combustion process. For this reason, the realizable k-ϵ model was preferred over the LES model in this work.
Combustion model
The turbulence–chemistry interaction model used in this work was the eddy dissipation concept (EDC) model. Due to their very low computational cost, non-premixed models like probability density function (PDF) flamelet models are the most commonly used combustion models. However, their fundamental assumption of infinitely fast chemistry renders them inapplicable for modeling low-Btu, low-exit-velocity flaring. On the other hand, the EDC model is one of the most rigorous chemistry–turbulence interaction models. The EDC model assumes that the reactions in the flame occur in small turbulent structures called fine scales. To generate these fine scales in a turbulent reacting flow, the model uses detailed reaction mechanisms. Unlike other combustion models, both the kinetic rates and mixing rates are calculated. The slower of the two is then used as the reaction rate. For this reason, the eddy dissipation concept is an ideal model for simulating turbulence combustion. It should be noted that all the cases run using the EDC approach were completed in two stages. Initially “cold flow” was simulated, meaning that combustion chemistry was disabled during this period. Once a converged cold flow was obtained, the region near the flare stack was patched with a temperature of 2000 K. The EDC chemistry modeling was then enabled and the combustion of the fuel started, which further raised the plume temperature.
Radiation model
Due to the high computational cost, radiation was ignored in the simulation. Radiation is generally used for modeling industrial furnaces, gas fired heaters, and other equipment where combustion is used for heat transfer and acts as source of heat energy. Most applications that utilize radiation models involve combustion in an enclosed domain. In open-air combustion systems such as industrial flares and lab-scale burners, the heat generated by the flare is of no use and is dissipated into the atmosphere. Using a radiation model can definitely improve the simulation results but requires a considerable amount of additional computational time.
Fluent postprocessing
Many specific results were obtained during the postprocessing step of the simulation to facilitate the comparison between the simulations and experimental data. For the TCEQ flare setup involving a full-scale flare, the predicted and experimental flare efficiencies were compared. The modeled efficiencies were calculated using the integral flow rates of various species over the inlet and outlet surfaces of the domain. For the lab-scale flames, the predicted axial profiles of concentration and temperature were compared to the experimental ones. A “profile line” at the center of the geometry was used in both cases. The profile line provided the measurements of temperature and mole/mass fractions of various species along the height of the flame.
Data Sources
TCEQ flare: Industrial-scale flare
The model to be validated was developed to simulate turbulent industrial flares under various operating conditions. Hence, the model was first checked against industrial-scale flare data obtained from the TCEQ 2010 Flare Study (Allen and Torres, Citation2011) at the John Zink facility in Tulsa, OK. A similar study (McDaniel, Citation1983; Pohl, Citation1984) was done under the auspices of the EPA in 1983–1984 and measured the effect of lower heating value (LHV), exit velocity, and other parameters on the flare performance. The 2010 TCEQ study was aimed to study the flare’s performance under low jet velocity, low Btu conditions (stand-by mode) using the state-of-the-art measurement technique. A 1.05-m-diameter flare was used to combust propylene gas, along with variable flows of Tulsa Natural Gas (TNG; Baukal and Schwartz, Citation2001) and nitrogen (N2). Operating parameters like air-to-fuel ratio, combustion-zone heating value, and vent gas flow rate were varied during the flare tests. The measurement of species concentration was done using redundant measurement systems. For example, an extractive sampling method with a moveable sample collector was used to collect plume samples during the tests to determine the flare efficiencies. Remote-sensing technologies like passive and active Fourier-transform infrared (PFTIR/AFTIR) spectroscopy and GasFindIR passive infrared cameras were also used to analyze the flare plume. Therefore, the test data are believed to be more accurate than previous studies. The data points to compare simulation results were taken from Appendix E of the TCEQ 2010 Flare Study Final Report.
Flare efficiencies
Two types of flare efficiencies were monitored and reported during the flare study: DRE and CE.
DRE (destruction and removal efficiency). DRE represents the percent of the fuel destroyed relative to the amount of fuel actually sent to the flare. Using C3H6 as an example, it can be written as
9
CE (combustion efficiency). CE, on the other hand, takes into consideration the percentage of fuel successfully converted into carbon dioxide, the final oxidation product. It is defined as
10
HCs plume in the preceding equation defines the amount of hydrocarbons present in the plume. Using the data provided in the TCEQ flare project final report, the flares were modeled in the CFD Software, ANSYS Fluent (Fluent Inc., Citation2011). A total of 10 cases from Appendix E of the TCEQ 2010 Flare Study Final Report were simulated. The details of cases modeled are provided in (Allen and Torres, 2010).
Table 2. Conditions used for TCEQ flare test cases
Flare geometry
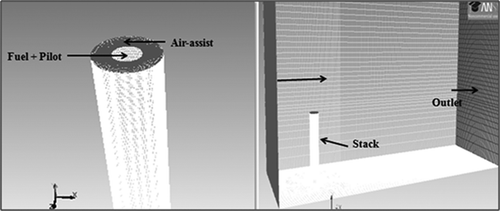
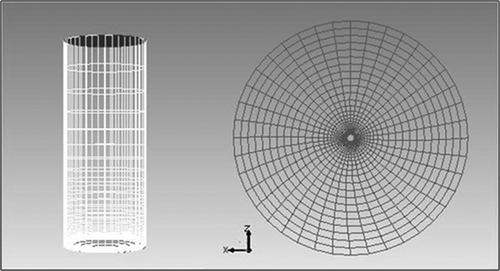
To keep the geometry simple, a rectangular domain was built in GAMBIT. The length, width, and height of the geometry were kept as 30 m, 10 m, and 30 m, respectively. The flare was located at 5 m from the left side and at the center of the width. The CFD domain for the simulation of the TCEQ flare is shown in . This configuration provided enough time for the combustion process to be completed inside the domain. The height of the flare stack was kept as 10 m and the diameter as 1.05 m. To avoid complex geometry, and hence an unnecessarily large number of cells, the fuel jet opening was modified. The jet used for previous study by Singh et al. (2011) had separate fuel and pilot gas openings. In this work, the fuel and the pilot gas were provided from a common outlet at the center. The air assist was injected from the outer ring of the flare stack opening and the crosswind direction was from left to right.
The largest velocity and species concentration gradients were located in regions near the stack. To increase the accuracy of the reacting flow profile, the mesh density near the stack was kept higher relative to other zones in the domain. The final grid of 840,000 cells was successfully checked for skewness. Skewness is a commonly used parameter to check the quality of the grid. The skewness of the grid used for this work was kept under 0.4. Initially, several grids were prepared for the model. The grid with minimum computational time and with minimal loss of accuracy was selected.
McKenna flame: Laminar premixed combustion
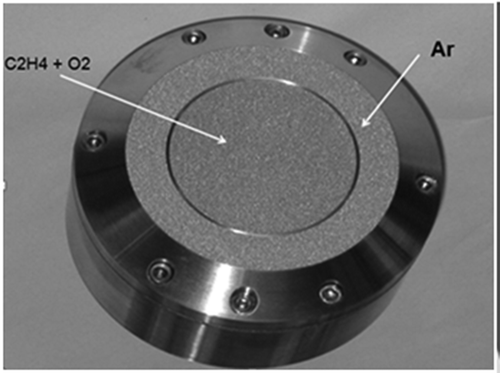
Laminar flat flames are one of the flames commonly used to study combustion chemistry. Flat flame burners like the McKenna burner (Holthuis & Associates, Citation2013) are often used to produce such flames. Stable flames from such burners provide an accurate measurement of temperature and concentration of species formed during the combustion process. A number of researchers (Zhang et al., Citation2006; Bhargava and Westmoreland., Citation1998) have used these burners to study lab-scale flames. Zhang et al. at the National Synchrotron Radiation Laboratory, China, have studied low-pressure C2H4/O2/Ar laminar flames. Synchrotron photoionization and molecular beam mass spectrometry (MBMS) techniques were used to measure species concentrations. In this lab scale experimental setup, a 6 cm diameter McKenna burner was used during the experiment. The burner used by Zhang et al. is shown in . The conditions used for this McKenna flame are shown in . The pore Reynolds number (d*v*ρ/μ) for the modeled flame is 0.1065, which is well within the laminar region. The Richardson number (g*d/v2) for the same flame is 2.96E-03. Note that the diameter of the micropores in a McKenna burner is given as 1.0 E–4 m (Holthuis & Associates, Citation2013). Also, the Richardson number is the ratio of buoyancy forces to inertia forces, where a number less than 1 indicates a predominance of inertial forces (Pohl et al, Citation1984). The two numbers were calculated using the data given in .
Table 3. Conditions used for McKenna flame
Table 4. Conditions used to calculate the Reynolds/ Richardson numbers
Geometry
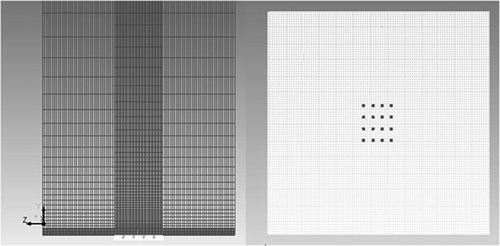
Modeling lab-scale or industrial-scale flames using a rigorous EDC (eddy dissipation concept) model is computationally very expensive. The modeling becomes more complex when a comprehensive reaction mechanism like LU1.1, consisting of 50 species and about 400 reactions, is employed. Hence, for the sake of simplifying the model, the porous surface of the burner was replaced with small jet flames spread evenly across the burner. These small flames coerced together to form a single laminar and flat flame. The idea was to generate a flame having a uniform axial profile of temperature and the species’ concentrations. The temperature and the species concentration profiles were measured along the length of the flame. As there were no radial variations in the species concentration or temperature, the shape of the burner (square or round) does not affect these profiles. The geometry used for simulating the flat flame burner is shown in . A square geometry was used for both the burner and the full domain. Due to the laminar characteristic of the flame, the grid was finely meshed along the height of the flame (normal to the burner surface). The rest of the domain had a relatively coarser mesh. The grid had 611,008 cells and was successfully checked for skewness.
Sandia flame: Jet flame in a vitiated coflow
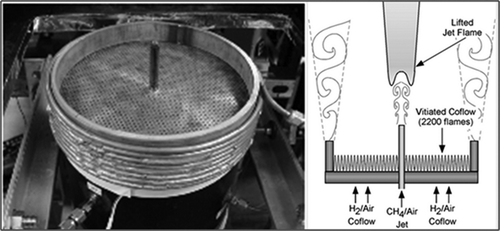
Unlike the previous experimental setup, this study simulated a high jet velocity and a more turbulent flow. The experiment was performed by Cabra et al. (Citation2005) at the Combustion Research Facility, Sandia National Laboratory, USA. In addition to the flow conditions, the fuel composition used was also different. In their work, Cabra et al. used CH4 and air as the reactants for combustion. The setup included a central burner surrounded by a perforated plate. The actual experimental setup is shown in . The jet fuel composition was kept at 33% CH4 and 66% air at a jet velocity of 100 m/sec. The premixed fuel was maintained at a temperature of 320 K. The perforated plate acted as a source of “coaxial flow of hot combustion products from a lean premixed flame.” The combustion products were obtained from a lean H2/Air flame. The coaxial flow was sent at a velocity of 5.4 m/sec and a temperature of 1350 K. The idea of providing a coaxial flow was to avoid any interference from the surrounding air. By keeping the composition and temperature of the coaxial flow similar to the hot combustion products, any interaction in the combustion chemistry could be avoided. Also, the coaxial flow reduced the mixing of surrounding air, which could have led to a leaner fuel and brought down the flame temperature. Using this setup, Cabra et al. were able to maintain a very stable and uniform flame. Raman/Rayleigh/laser-induced fluorescence (LIF) measurement methods were used to analyze the flame temperature and major species concentrations.
Instead of pure O2 (as used in the McKenna flame setup), air was used for the combustion process. Due to the addition of N2 in the fuel, formation of NOx was also involved in the combustion chemistry. Since almost all flares use air as their oxidation medium, the Sandia flame is a closer representation of the combustion chemistry during flaring. Also, the N2 in the fuel provided an opportunity to measure the concentration of NO, which is an important intermediate in the combustion process.
Geometry
A cylindrical domain was used for the simulation of this flame. All of the geometry configurations and boundary conditions were kept the same as the experimental setup. The details are given in and the geometry used is shown in . The grid had 86,360 cells and was successfully checked for skewness.
Table 5. Conditions used for Sandia flame
Results
The simulation results obtained in the form of flare efficiencies, temperature profiles, and concentrations of species (CO2, CO, OH, H2O, O2, CH2O, and NO) were compared with the experimental data. The details are given next.
TCEQ flare tests
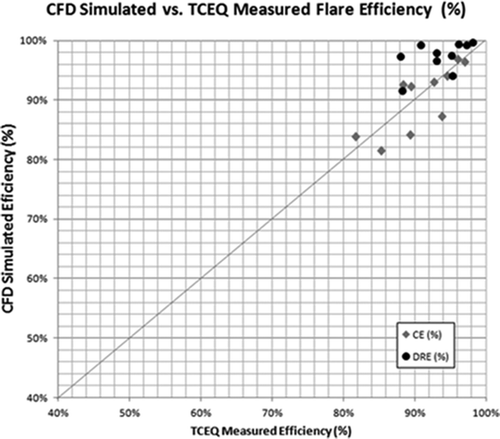
The experimental data collected during the 2010 TCEQ flare tests included mainly flare efficiencies. The predicted flare destruction efficiencies, DRE and CE, are compared with field measurements in and , respectively. A good agreement was found for both flare efficiencies: The average absolute error for DRE was 4.50% with maximum error being around 10% (see ), while the absolute average error for CE was 3.04% with maximum error reaching around 6.7% (see ). In the past, the same test cases were modeled and reported in the 2011 Texas AQRP (Air Quality Research Program) 10-022 report (for A/F mass ratio < 28) (Chen et al., Citation2011). In that report, the average absolute error in the DRE prediction was 13.3%. The EDC model used in this work employed a new and simplified geometry. The new model improves DRE of air-assisted low LHV/low jet velocity propylene/TNG flares significantly compared to the DRE values reported in the AQRP report (Chen et al., Citation2011). It also drastically improves CE prediction compared to the average absolute error of 29.8% in the 2011 AQRP 10-022 report (for A/F mass ratio < 28). The results clearly validate the CFD model used, given the uncertainties in both the field measurements and the numerical simulations.
Table 6. Comparison of TCEQ measured and simulated DRE (%)
Table 7. Comparison of TCEQ measured and simulated CE (%)
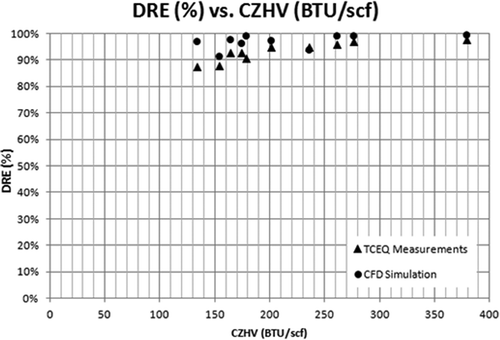
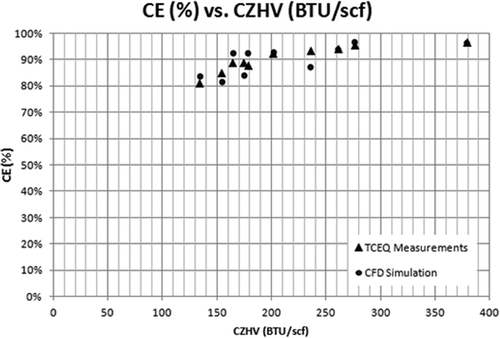
presents the same comparisons in the form of calculated versus experimental values for both DRE and CE. As observed from the plot, the model closely predicts the flare efficiencies. CE tends to have an evenly distributed error, while DRE tends to have a higher predicted value. In and , the DRE and CE versus combustion zone heating value (CZHV) are presented. CZHV is the resultant heating value of the fuel gas when any assist medium like air/steam is also taken into account. CZHV in this work was calculated using eq (11). For higher CZHVs, the model gives more accurate results compared with lower CZHVs. For this test case, the error is the largest (about 10%) for DREs predicted at the lowest heating values:
McKenna flame burner
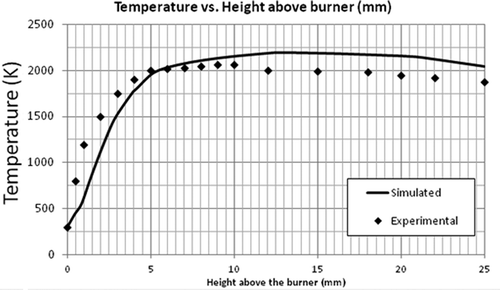
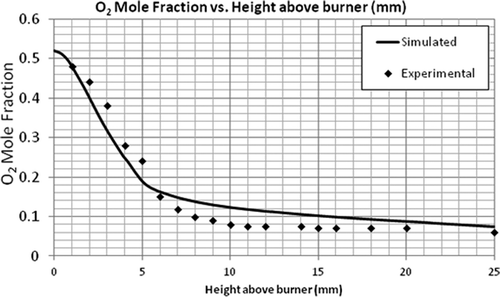
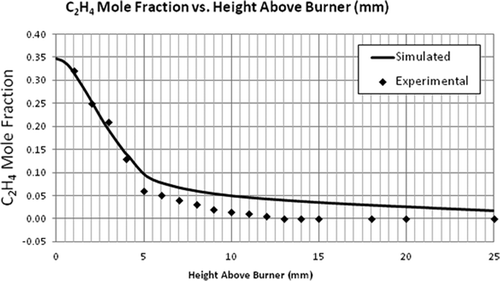
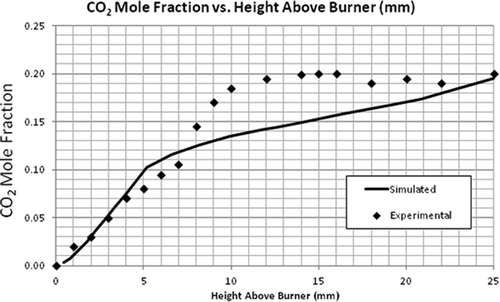
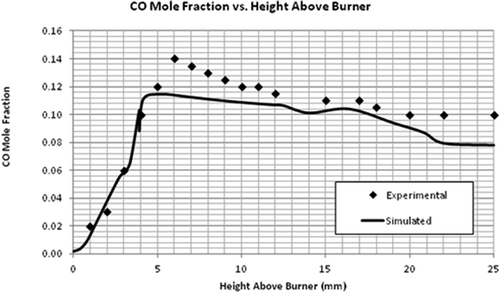
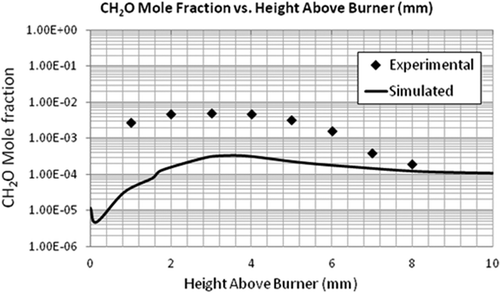
Simulation of the flat flame burner using the LU 1.1 mechanism was performed and the validation of the numerical model using this flame was done by comparing the axial profiles of temperature and species concentration. The most important parameter to be observed is temperature as the combustion chemistry is highly dependent on it. It can be seen in that the general trend of the simulated temperature over the length of the flame was correct. – compare the mole fractions of O2, C2H4, and CO2. A good agreement was found between the predicted and experimental profiles of these major species concentrations. Although the exit mole fractions (10 mm above burner) are the same, there is some discrepancy in the CO2 and CH2O concentrations at the middle of the flame. For the radical-producing CH2O, the EDC model underpredicts the maximum mole fraction by a factor of 10 and the exiting mole fraction by a factor of 1.6 (see ). These discrepancies can be seen as a cumulative result of uncertainties in the reaction mechanism and the CFD model.
It should be noted that even though the maximum mole fractions predicted for some of the species were not equal to the experimental values, the exit mole fractions were about the same. The model to be validated is aimed for flare combustion. Since only the exit mole fractions are used to calculate the flare efficiencies, the model can be used for that purpose. Further, even though the CFD model underpredicts the formaldehyde concentrations, the mere existence of formaldehyde (and maybe at a higher concentration) as an incomplete combustion product supports the view that flares can be a primary source of formaldehyde emission.
Sandia flame: High-velocity jet flow
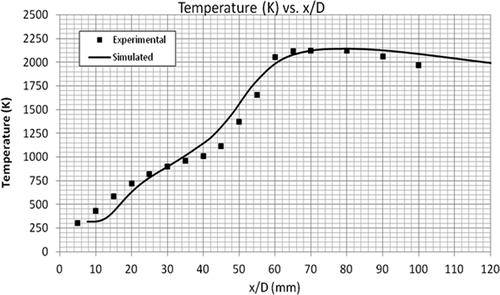
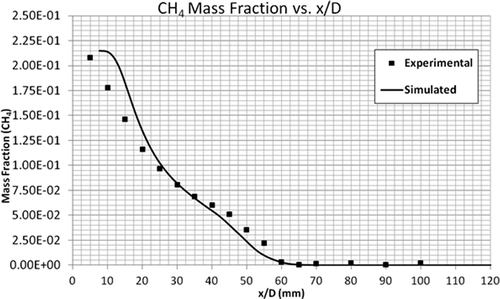
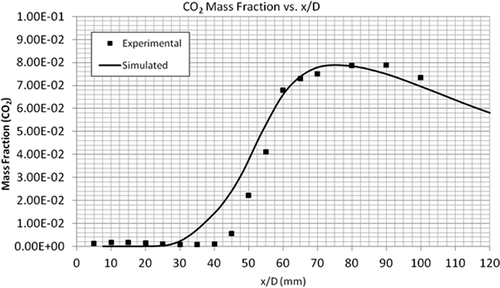
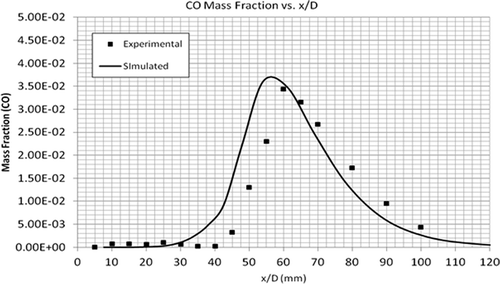
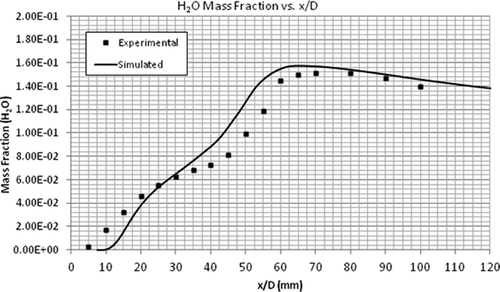
compares the temperature profiles of the simulated and experimental flames. The simulation predicted the general trend of observed data very well compared to the experimental flame data. compares the CH4 mass fractions along the height of the flame. It can be seen that the combustion of CH4 is slow for the first few millimeters above the flame. However, at an x/D value of 20 mm, the simulated and experimental results are in total agreement. As shown by –, the mass fractions of CO2, CO, and H2O are in very good agreement with the experimental data. The maximum mass fractions of almost all these products occur at the same height above the burner, that is, x/D = 60 mm. In summary, the EDC model accurately predicts the profiles of temperature and concentrations of major species (CH4, CO2, CO, and H2O).
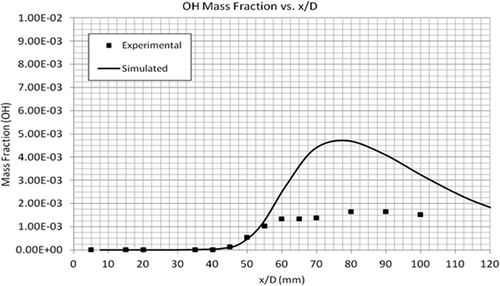
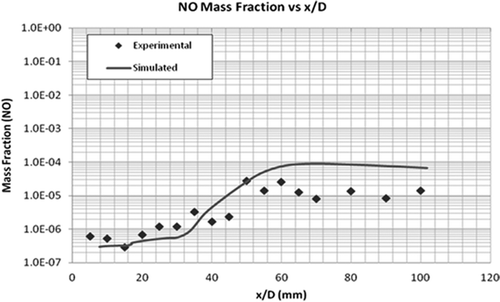
Two important radicals, OH and NO, were also compared with the experimental data. OH and NO are by-products of the combustion process and an important source for the formation of atmospheric O3 (Seinfeld and Pandis, Citation2006). The model predicts the mass fraction of trace species NO and OH (at the core of the flame) within a factor of 3.5, as shown in and . Similar to CO2, the exit mole fractions of both the radicals are equal to the measured values. The discrepancy can be seen around the middle of the flame, which again is due to the uncertainty in the reaction mechanism.
Discussion
Though the simulation results were reasonably close to the experimental data, there is some room for improvement. The modeling of lab-scale and industrial-scale flares can be improved by using more rigorous models. The most important factor affecting the simulation results is the kinetic modeling. CFD models and sufficient computational resources allow the use of a more detailed kinetic mechanism, which can improve the accuracy of predicted concentration profiles. Improvements can be made in the geometry of the flare tip. The ring-shaped geometry used as the fuel and pilot gas source can be optimized by increasing the flow area to improve the prediction of DREs. In this work, the open air flaring process was modeled using steady-state models. For further improvements, unsteady-state models like LES models can be used.
In general, the CFD model used to simulate the three flames provided good results. In this work, a comprehensive reaction mechanism LU1.1 was used in conjunction with the most rigorous turbulence model and turbulence–chemistry interaction models available to simulate industrial-scale flares at the stand-by mode for the first time. The methodology proves to be applicable to industrial-scale flaring in that the errors of the predicted DREs and CEs are within 5% of the measured efficiencies. For lab-scale flames, concentrations of major species, for example, C2H4, CH4, CO2, CO, and H2O, were accurately predicted. On the other hand, the study also observed some discrepancies in the prediction of certain radicals and trace species such as HCHO, OH, and NO.
The two possible reasons for these discrepancies are the uncertainties in the original reaction mechanisms and error in predicting the correct rate of reactions at high temperatures (>2000 K). The latter may be a result of reduction of the combined mechanism. Although the reaction mechanism was successful in predicting the temperature profile and most of the exit mole fractions, there is still some scope for improvement. A more comprehensive reaction mechanism that may include all the species and reactions needed to accurately predict intermediates can be employed. In addition to the reaction mechanism, inclusion of radiation in the model can also help in improving the accuracy of the results. These changes can definitely help in better prediction of the combustion process, but will demand significantly higher computational resources.
Even though the CFD model underpredicts the formaldehyde concentrations compared to the McKenna flat flame, the mere existence of formaldehyde as an incomplete combustion product supports the view that flares can be a primary source of formaldehyde emission. In fact, formaldehyde concentrations were measured in the range of 200–1200 ppbv in the 2010 flare study (Test S4.1R1). Acetaldehyde was also detected in the similar concentration levels (Allen and Torres, Citation2011). These data and their ratio to CO and propylene can be utilized in future flare modeling work.
Conclusion
The EDC model with a new and simplified geometry improves the accuracy of DRE calculations for air-assisted low LHV/low jet velocity propylene/TNG flare tests with an average error of 4.6%, compared to 13.3% published in the 2011 AQRP 10-022 final report (for A/F mass ratio < 28; Singh et al., 2013). The EDC model also improves the CE prediction accuracy with an average error of 2.6%, compared to 29.8% in the 2011 AQRP 10-022 report (for A/F mass ratio < 28).
For the lab-scale CH4/Air mixture (Sandia) flame, the EDC model accurately predicts the profiles of temperature and concentrations of major species (CH4, CO2, CO). The model predicts the mass fraction of trace species NO and OH (at the core of the flame) within a factor of 3.5. In the case of C2H4/O2/Ar flame, a good agreement was found between the predicted and experimental profiles of temperature, C2H4, CO2, and other major species concentrations. The EDC model underpredicted the maximum and exiting model fractions of CH2O by a factor of 10 and 1.6, respectively. OH, another intermediate species, was found to be overpredicted in the model by a factor of 5.
Funding
This work is supported by the State of Texas and the authors gratefully acknowledge financial support from TCEQ Supplemental Environmental Program (SEP agreement 2009-009) and the Texas Air Research Center (TARC grant 079LUB0096A).
Additional information
Notes on contributors
Kanwar Devesh Singh
Kanwar Devesh Singh and Preeti Gangadharan each hold a Ph.D. in chemical engineering from Lamar University, Beaumont, TX.
Preeti Gangadharan
Kanwar Devesh Singh and Preeti Gangadharan each hold a Ph.D. in chemical engineering from Lamar University, Beaumont, TX.
Daniel H. Chen
Daniel H. Chen and Helen H. Lou are professors and university scholars, and Peyton Richmond is an associate professor at Dan F. Smith Department of Chemical Engineering, Lamar University, Beaumont, TX.
Helen H. Lou
Daniel H. Chen and Helen H. Lou are professors and university scholars, and Peyton Richmond is an associate professor at Dan F. Smith Department of Chemical Engineering, Lamar University, Beaumont, TX.
Xianchang Li
Xianchang Li is an associate professor in the Department of Mechanical Engineering, Lamar University, Beaumont, TX.
Peyton Richmond
Daniel H. Chen and Helen H. Lou are professors and university scholars, and Peyton Richmond is an associate professor at Dan F. Smith Department of Chemical Engineering, Lamar University, Beaumont, TX.
References
- Al-Fadhli, F.M., Y. Kimura, E.C. McDonal Buller, and D.T. Allen. 2012. Impact of flare destruction efficiency and products of incomplete combustion on ozone formation in Houston, Texas. Ind. Eng. Chem. Res. 51:12663–12673. doi:10.1021/ie201400z
- Allen, D.T., and V.M. Torres. 2011. TCEQ flare study final report. TCEQ PGA No. 582-8-86245-FY09-04 and Task Order No. UTA10-000924-LOAT-RP9. Austin, TX: The University of Texas at Austin, The Center for Energy and Environmental Resources.
- Baukal, C.E., and R.E. Schwartz. 2001. The John Zink Combustion Handbook. New York, NY: CRC Press.
- Bhargava, A., and P.R. Westmoreland. 1998. Measured flame structure and kinetics in a fuel-rich ethylene flame. Combust. Flame 113:333–347. doi:10.1016/S0010-2180(97)00208-3
- Bhargava, A., and P.R. Westmoreland. 1998. MBMS analysis of a fuel-lean ethylene flame. Combust. Flame115(4): 456–467. doi:10.1016/S0010-2180(98)00018-2
- Cabra, R., J.Y. Chen, R.W. Dibble, A.N. Karpetis, and R.S. Barlow. 2005. Lifted methane–air jet flames in a vitiated co-flow. Combust. Flame143(4): 491–506. doi:10.1016/j.combustflame.2005.08.019
- Castiñeira, D., and T.F. Edgar. 2008. Computational fluid dynamics simulation of wind-tunnel experiments on flare combustion systems. Energy Fuels 22:1698–1706. doi:10.1021/ef700545j
- Chen, D., H. H. Lou, X. Li, K. Li, and C.B. Martin. 2011. Development of speciated industrial flare emission inventories for air quality modeling in Texas. Air Quality Research Program (AQRP) Project # 10-022, Final Report. Lamar University, Beaumont, TX, December 14.
- Chen, D., K. Singh, P. Gangadharan, H. Lou, X. Li, and P. Richmond. 2013. CFD study of flare operating parameters. Paper 271b, AIChE Annual Meeting, November 3–8, San Francisco, CA.
- Davis, S.G., C.K. Law, and H. Wang. 1999. Reaction mechanism of C3 fuel combustion. http://ignis.usc.edu/Mechanisms/C3/c3.html
- Davis, S.G., and C.K. Law, 1998. Determination of and fuel structure effects on laminar flame speeds of C1 to C8 hydrocarbons. Combust. Sci. Technol. 140(1): 427–449. doi:10.1080/00102209808915781
- Environ International Corporation. 2006. Comprehensive air quality model with extension (CAMx) preprocessors. http://www.camx.com/down/support.php
- Fluent, Inc. 2011. FLUENT 13.0 User’s Guide. http://aerojet.engr.ucdavis.edu/fluenthelp/ (accessed September 22, 2014).
- Holthuis & Associates. 2013. McKenna flat flame burner. http://www.flatflame.com
- Jatale, A., P. Smith, J. Thornock, and S. Smith. 2012. A validation of flare combustion efficiency simulations. American Flame Research Committee, Salt Lake City, UT.
- Law, C.K., A. Makino, and T.F. Lu. 2005. On the off-stoichiometric peaking of adiabatic flame temperature with equivalence ratio. Presented at the 4th Joint Meeting of the U.S. Sections of the Combustion Institute, Philadelphia, Pennsylvania, March 21.
- Lou, H.H., C.B. Martin, D. Chen, X. Li, K.Y. Li, H. Vaid, A.T. Kumar, K.D. Singh, and D.P. Bean, Jr. 2011. A reduced reaction mechanism for the simulation in ethylene flare combustion. Clean Technol. Environ. Policy 14(2): 229–239. doi:10.1007/s10098-011-0394-9
- Mayers, B.F,. and E.R. Bartle, 1969. Reaction and ignition delay times in the oxidation of propane. AIAA J. 7(10): 1862–1869. doi:10.2514/3.5473
- McDaniel, M. 1983. Flare efficiency study. U.S. Environmental Protection Agency, Report No. 600/2-83-052. July. http://www.epa.gov/ttn/chief/ap42/ch13/related/ref_01c13s05_jan1995.pdf (accessed September 22, 2014).
- Nik, M.B., S.L. Yilmaz, P. Givi, M.R. Sheikhi, and S.B.Pope. 2010. Simulation of Sandia Flame D using Velocity Scalar filtered density function, AIAA J. 48(7): 1513–1522. doi:10.2514/1.J050154
- Patankar, S.V. 1980. Numerical Heat Transfer and Fluid Flow. New York, NY: McGraw-Hill.
- Pohl, J., R. Payne, and J. Lee. 1984. Evaluation of the efficiency of industrial flares: Test results. EPA-600/2-84-095. Prepared for U.S. EPA Office of Research and Development by Energy and Environmental Research Corporation. May. http://www.tceq.state.tx.us/assets/public/implementation/air/rules/Flare/Resource_2.pdf (accessed September 22, 2014).
- Pohl, J., and N. Soelberg. 1985. Evaluation of the efficiency of industrial flares: Flare head design and gas composition. EPA-600/2-85-106. Prepared for EPA Office of Air Quality Planning and Standards. September. http://nepis.epa.gov/Exe/ZyPDF.cgi/P1003QL1.PDF?Dockey=P1003QL1.PDF (accessed September 22, 2014).
- Seinfeld, J. H., and S. Pandis. 2006. Atmospheric Chemistry and Physics—From Air Pollution to Climate Change, 2nd ed. New York, NY: John Wiley and Sons.
- Singh, K.D., T. Dabade, H. Vaid, P. Gangadharan, D. Chen, H. H. Lou, K.Y. Li, X. Li, and C.B. Martin. 2012. Computational fluid dynamics modeling of industrial flares operated in a stand-by mode. Ind. Eng. Chem. Res. 51(39): 12611–12620. doi:10.1021/ie300639f
- Singh, K.D., P. Gangadharan, D. Chen, H. Lou, X. Li, P. Richmond. 2014. Parametric study of ethylene flare operations and validation of a reduced combustion mechanism. Eng. Appl. Comput. Fluid Mech. 8(2): 2011–228.
- Smith, G.P., G.M. Golden, M. Frenklach, N.W. Moriarty, B. Eiteneer, M. Goldenberg, T. Bowman, R.K. Hanson, S. Song, W.C. Gardiner, V.V. Lissianski, and Z. Qin, 2000. GRI-Mech. http://www.me.berkeley.edu/gri_mech
- Smith, P. J., J. Thornock, D. Hinckley, and M. Hradisky. 2011. Large eddy simulation of industrial flares, SC’11 Companion, November 12–18, Seattle, WA. ACM 978-1-4503-1030-7/11/11.
- Texas Commission on Environmental Quality. 2000. Air permit technical guidance for chemical sources: Flares and vapors oxidizers. Air Permits Division, Texas Commission on Environmental Quality. October. http://www.tceq.state.tx.us/assets/public/comm_exec/pubs/rg/rg360/rg36007/techsupp_4.pdf (accessed September 22, 2014).
- Texas Commission on Environmental Quality. 2007. Technical supplement 4: Flares. TCEQ Publication RG-360A. January. http://www.tceq.texas.gov/assets/public/comm_exec/pubs/rg/rg360/rg36010/rg-360a.pdf
- Texas Commission on Environmental Quality. 2009. Comprehensive flare study project. PGA No. 582- 8-862-45-FY09-04, Tracking no. 2008-81. http://www.tceq.state.tx.us/assets/public/implementation/air/rules/Flare/Flare_Research_Final_QAPP.pdf (accessed September 22, 2014).
- University of Texas at Austin. 2010. Quality Assurance Project Plan, Texas Commission on Environmental Quality, Comprehensive Flare Study, PGA No. 582-8-862-45-FY09-04. http://www.tceq.state.tx.us/assets/public/implementation/air/rules/Flare/Flare_Research_Final_QAPP.pdf (accessed September 22, 2014).
- Torres, V., S. Herndon, Z. Kodesh, and D.T. Allen. 2012. Industrial flare performance at low flow conditions. 1. Study overview. Ind. Eng. Chem. Res. 51:12559–12568. doi:10.1021/ie202674t
- Tyliszczak, A. 2013. LES-CMC & LES-flamelet simulation of non-premixed methane flame (Sandia F). J. Theor. Appl. Mech. 51(4): 859–871.
- U.S. Environmental Protection Agency. 2012. Parameters for properly designed and operated flares. Report for Flare Review Panel, EPA Office of Air Quality Planning and Standards (OAQPS), April. http://www.epa.gov/ttn/atw/flare/2012flaretechreport.pdf (accessed September 22, 2014).
- U.S. Government. 2009. Code of Federal Regulations—Standards of performance for new stationary sources, general control device and work practice requirements. 40CFR § 60.18. http://edocket.access.gpo.gov/cfr_2009/julqtr/pdf/40cfr60.18.pdf (accessed September 22, 2014).
- Vujanovic, M., N. Duic, and R. Tatschl. 2009. Validation of reduced mechanisms for nitrogen chemistry in numerical simulation of a turbulent non-premixed flame. React. Kinetics Catal. Lett. 96(1): 125–138. doi:10.1007/s11144-009-5463-2
- Wang, H., X. You, A.V. Joshi, S.G. Davis, A. Laskin, F. Egolfopoulos, and C.K. Law. 2007. USC Mech Version II. High-temperature combustion reaction model of H2/CO/C1-C4 compounds. http://ignis.usc.edu/USC_Mech_II.html (accessed September 22, 2014).
- Zhang, Q., Y. Li, Z. Tian, T. Zhang, J. Wang, and F. Qi. 2006. Experimental study of premixed stoichiometric ethylene/oxygen/argon flame. Chin. J. Chem. Phys. 19(5): 379–385. doi:10.1360/cjcp2006.19(5).379.7
- ZEECO. 2007. Flare system emissions. Control. Texas Technology. Conference. http://texasiof.ces.utexas.edu/texasshowcase/pdfs/presentations/b2/ssmith.pdf (accessed September 22, 2014).