Abstract
Particulate matter (PM) has been becoming the principal urban pollutant in many major cities in China, and even all over the world. It is reported that the coal combustion process is one of the main sources of PM in the atmosphere. Therefore, an investigation of formation and emission of fine primary PM in coal combustion was conducted. First, the sources and classification of coal-fired primary PM were discussed; then their formation pathways during the coal combustion process were analyzed in detail. Accordingly, the emission control methods for fine particles generated from coal-fired power plants were put forward, and were classified as precombustion control, in-combustion control, and postcombustion control. Precombustion control refers to the processes for improving the coal quality before combustion, such as coal type selection and coal preparation. In-combustion control means to take measures for adjusting the combustion conditions and injection of additives during the combustion process to abate the formation of PM. Postcombustion control is the way that the fine PM are aggregated into larger ones by some agglomeration approaches and subsequently are removed by dust removal devices, or some high-performance modifications of conventional particle emission control devices (PECDs) can be taken for capturing fine particles. Finally, some general management suggestions are given for reducing fine PM emission in coal-fired power plants.
Implications
The analysis and discussions of coal properties and its combustion process are critical to recognizing the formation and emission of the fine primary PM in combustion. The measures of precombustion, in-combustion, and postcombustion control based on the analysis and discussions are favorable for abating the PM emission. Practically, some measures of implementation do need the support of national policies, even needing to sacrifice economy to gain environmental profit, but this is the very time to execute these, and high-performance PECDs, especially novel devices, should be used for removing fine PM in flue gas.
Introduction
The foggy and hazy weather in central and eastern China caused by airborne particulate matter (PM) has attracted worldwide attention since the beginning of 2013. In many major cities in China, the pollution caused by PM, instead of sulfur dioxide and nitrogen oxides, has become the major issue. Besides the negative impacts to climate like deterioration of the atmospheric visibility, the harm to human health resulting from exposure to ambient PM, such as respiratory problems and heart attacks, has also been confirmed by epidemiology (Lighty, Veranth, and Sarofim, Citation2000; Tucker, Citation2000). PM2.5, namely, the fine particles with aerodynamic diameters less than or equal to 2.5 μm, have been studied more frequently due to their greater potential to cause health problems than the coarse particles with aerodynamic diameters greater than 2.5 μm. Although accounting for only a small fraction of total PM, PM2.5 have high surface-to-volume ratios, which enable them to enrich sulfates, nitrates, acids, heavy metals, bacteria, and more on their surfaces (Davidson, Phalen, and Solomon, Citation2005). Furthermore, due to the facts that they can remain suspended for longer periods of time than coarse particles and that they can penetrate more deeply into the lungs after being inhaled into the respiratory system, PM2.5 have more potential to cause health effects than coarse particles (Pope et al., Citation2006).
Ambient PM can be classified into primary and secondary PM based on origin. Primary PM are emitted directly from sources, while secondary PM are formed in the atmosphere from gaseous emissions (Davidson et al., Citation2005). Studies on source attribution showed that in China, on a nationwide basis, the PM emission from coal-fired power plants is one of the major sources of primary PM in the ambient air (Yao et al., Citation2009), and accounted for 44.6% of the total PM emission of 8.55 million tonnes in 2005 (National Bureau of Statistics of China [NBSC], Citation2006). However, it is expected that China’s energy structure with priority given to coal is hard to change in the foreseeable future (Wang, Feng, and Tverberg, Citation2013). In 2010, coal accounted for more than 76.5% of China’s energy production and about 68.0% of its energy consumption (NBSC, Citation2011). It is projected that coal will continue to contribute 60–70% to China’s energy consumption to the middle of the 21st century, namely, 2050. Therefore, PM emission control in coal-fired power plants has become a hot topic in many environmental studies in recent years. However, particles generated from the coal combustion process in the submicrometer size range are much more difficult to collect with conventional particle emission control devices (PECDs) than larger particles (McElroy et al., Citation1982). Thus, study on the formation reduction and control of fine particles generated from coal-fired power plants has great significance to the improvement of ambient air quality.
Worldwide, in order to protect the ambient air from the pollution of fine particles, a number of countries have promulgated new national ambient air quality standard (NAAQS) for PM2.5 to limit its emission; for example, the U.S. Environmental Protection Agency (EPA) promulgated a new annual PM2.5 NAAQS of 12 μg/m3 in 2012. In China, a revised NAAQS was also put into effect in 2012, with an addition of a 24-hr PM2.5 standard of 75 μg/m3and an annual standard of 35 μg/m3 for secondary standard. Correspondingly, China’s Ministry of Environmental Protection (MEP) lowered the PM emission standard for thermal power plants from 50 mg/m3 to 30 mg/m3 (MEP, Citation2011). In order to be in compliance with this new emission standard, better emission control technologies and more strict management measures must be taken to reduce the emission of primary PM in coal-fired power plants. This paper starts with the constituent petrography of coal, followed by source analysis and classification of PM generated from coal combustion, as well as discussion on PM formation pathways during the coal combustion process. Then the emission control methods for fine PM in coal-fired power plants are elaborated and summarized. At the end, some conclusions are derived on how to improve the control of fine PM emission in coal-fired power plants. The details are illustrated in the following.
Formation Pathways of Primary Particulate Matter in Coal Combustion Process
Sources of coal-fired particulate matter
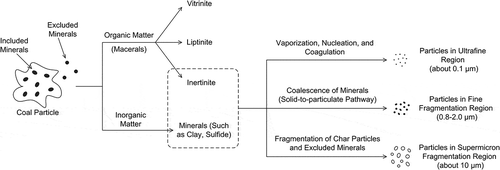
In terms of coal petrology, the petrography constituent and metamorphism degree of coal are two primary influencing factors to the particles’ burnout characteristics in the combustion process. Organic matter that consist of various components with distinct chemical and physical properties, which are referred to as macerals, and inorganic matter, which mostly occurs in the form of minerals, are two main constituents of coal (Jena, Biswal, and Rudramuniyappa, Citation2008). Currently, the coal macerals can be mainly classified into three different groups: vitrinite, inertinite, and liptinite, which are all organic matter with different properties (Jimenez et al., Citation1998). As active constituents, vitrinite and liptinite have good reactivity, making them easy to be burned in the heating process. However, opposite to vitrinite and liptinite, the inertinite has poor burnout characteristics and has limited change in the combustion process. In addition, since the clay, sulfide, carbonate, and oxide in coal are inorganic minerals that cannot be burned, and the release of volatile gaseous materials and combustion of fixed carbon are hindered by them, it can be concluded that during the coal combustion process, the inertinite and inorganic minerals in coal contribute to the formation of solid residue and fly ash particles.
The majority of inorganic minerals in pulverized coal mainly have three occurrence modes: elements or cations dissolved in the inherent moisture of coal; minerals associated with the organic matter in coal; and discrete inorganic mineral grains in coal particles. They can be further classified by included minerals (minerals that are intimately surrounded by the carbon matrix) and excluded minerals (liberated minerals not or at least associated with coal carbon matter) (Yan, Gupta, and Wall, Citation2002). Inorganic minerals dispersed in coal particles generally refer to alkali metal and alkaline earth metal cations, represented by Fe3+, Mg2+, Ca2+, Na+, and K+, whose occurrence modes include clay minerals, carbide minerals, carbonate minerals, sulfate minerals, chloride minerals, silicate minerals, metal minerals, and so on. In addition, the coal contains a variety of trace elements of high volatility or semivolatility. In the whole coal combustion process, all this inorganic matter just decribed is transformed into gaseous, liquid, or solid residue and fly ash particles through complex physical or chemical reactions. Sample experiments on coal properties using chemical methods such as x-ray fluorescence also suggest that the prevalent inorganic minerals in coal are SiO2, Al2O3, Fe2O3, SO3, CaO, MgO, K2O, TiO2, Na2O, and others (Yan et al., Citation2002; Zhang and Ninomiya, Citation2006), along with trace elements such as As, Be, Cd, Co, Cr, Hg, Mn, Ni, Pb, Sb, and Se.
Classifications of coal-fired particulate matter
According to the formation pathways, PM dispersed in the atmosphere can be divided into two categories: primary PM and secondary PM. Primary PM is discharged into the ambient air directly from the combustion sources, including thermal power plants, industrial processes, heavy- and light-duty vehicles, biomass burning, and so on. Comparatively, the secondary PM is transformed from the photochemical reaction of primary discharged gaseous precursors in the ambient air indirectly, and its physical and chemical characteristics are totally different from those of primary PM (Verma et al., Citation2009). As mentioned earlier, with the occurrence of inertinite and inorganic minerals, the combustion process of coal is a major source of primary PM in the ambient air; therefore, this paper mainly focuses on the formation and emission control of primary PM directly discharged into the atmosphere from the coal-fired power plants.
Generally, in a coal-fired power plant, the aerodynamic diameter of the pulverized coal particles for combustion is within 200 μm, and the combustion process usually produces fly ash particles having a diameter ranging from 0.03 to 100 μm (Zhang, Ninomiya, and Yamashita, Citation2006). According to the aerodynamic size distribution (the following mentioned particle sizes are all aerodynamic sizes), these emitted coal-fired primary PM are divided into submicrometer particles (particulates with sizes less than 1 μm) and supermicrometer particles (particulates larger than 1μm). Supermicrometer particles not only include the coarse ones with aerodynamic diameters greater than 10 μm, but also encompass a part of the inhalable particles that have diameters less than 10 μm. Although fine particles like PM2.5 only contribute a small fraction to the total mass of fly ash particles, their ability to escape from the dust removal devices is especially strong, compared with the larger ones, which can be almost completely removed (McElroy et al., Citation1982). In addition, many epidemiology studies have been carried out to prove that the fine particles present potential risk to human health, relating to cardiopulmonary mortality, lung cancer, and so on, since they can enter into human lungs through respiratory tract (Schwartz, Dockery, and Neas, Citation1996). Therefore, the studies on formation and control of fine PM from coal-fired power plants have great significance to the health of human beings.
Formation of particulate matter during coal combustion
Abundant studies on particle formation mechanism in the past decades have put forward a classic bimodal particle size distribution consisting of a submicrometer mode and a supermicrometer mode during pulverized coal combustion, which was described as a combination effect of agglomeration of fused minerals, fragmentation of cenospheres, and vaporization and condensation of volatile minerals (Sarofim, Howard, and Padia, Citation1977; Flagan and Friedlander, Citation1978). However, more recent studies indicated that a trimodal particle size distribution could be more precise and thoughtful to describe the formation mechanism of coal-fired fly ash particles, including an ultrafine region with a peak at approximately 0.1 μm diameter, a fine fragmentation region between 0.8 and 2.0 μm diameter, and a supermicrometer fragmentation region at approximately 10 μm diameter and greater (McElroy et al., Citation1982; Linak, Miller, and Wendt, Citation2000).
In the combustion process, the formation pathway of particles in the ultrafine region can be described by the mechanism of inorganic minerals vaporization, nucleation, coagulation, condensation, and coalescence (Seames, Citation2003). First in the furnace zone, with an increasing temperature of coal, a substantial fraction of inorganic minerals (mainly high volatile matter) undergoes vaporization into vapor phase. After leaving the flame region downstream of the combustion zone, these mineral vapors cool down as the temperature decrease. Then under a supersaturated condition, homogeneous condensation of these vapors makes them nucleate into particles of only a few nanometers, which can be further coagulated into larger ones. Meanwhile, a part of the vapors heterogeneously condenses on the surface of the preexisting particles, which helps the enlargement of particles’ diameter. As a result of coalescence between the nuclei particles and preexisting particles, fine PM enriched by numerous mineral elements is formed, showing a distinct peak in the ultrafine region at about 0.1 μm (Senior, Helble, and Sarofim, Citation2000).
The generation of particles of approximately 10 μm in the supermicrometer fragmentation region can be explained by the mechanism of fragmentation of char particles and excluded minerals. During the early phase of pulverized coal combustion, abundant thin-walled cenospherical char particles with nonuniform pores are formed, into which oxygen can easily to enter. Since severe oxidation reactions and combustion occur on the inner and outer surface of them at the same time, the char particles swell extensively, and some small fragments tend to detach from the particles into residual fly ash (Liu et al., Citation2000). Excluded minerals that are not associated with carbonaceous matter in coal have little probability of collision with other minerals or coal particles (Yan et al., Citation2002). When the coal particles enter the furnace flame zone, a high temperature gradient brings about severe thermal expansion of excluded minerals. If the minerals have not been melted by the high temperature, this kind of internal stress would lead to the fragmentation of the mineral particles (Fix et al., Citation2013), which contributes to part of the formation of particles in the supermicrometer fragmentation region. Furthermore, decomposition of the excluded minerals may rapidly release some gases like CO2, H2O, and SO2 under high temperature, which also results in the change of minerals structure as well as fragmentation (Yan et al., Citation2002).
Several studies have suggested that coal-fired particles in the fine fragmentation region with diameter ranging from 0.8 to 2.0 μm are too large to be the result of a vapor-to-particulate growth process, and instead are formed by the direct transformation of refractory elements in raw coal (Linak et al., Citation2000; Zhang and Ninomiya, Citation2006). Hence their formation mechanism is still not very well understood compared with that of the other two regions. In the combustion process, loosening and shrinkage of the external surface of char particles could take place, while the inner surface remains unchanged. This causes the exposure of included mineral residues to the char particles surface, which can further be melted with a temperature of char particles as high as around 2000°C, exceeding the melting points of most refractory minerals (Yan et al., Citation2002). With the subsequent shrinkage of char particles, these molten included-mineral residues begin to coalesce with each other into ash particles in a solid-to-particulate pathway (Zhang and Ninomiya, Citation2006). This process appears to lead to the formation of PM of fine fragmentation mode.
The formation pathways of PM generated from coal combustion process that we have just discussed can be illustrated by . In the actual coal combustion process, many of these changes are simultaneous, so that the formation of primary PM is more likely a consequence of multiple mechanisms and processes.
Control Measures for Fine Primary Particulate Matter in Coal-Fired Power Plants
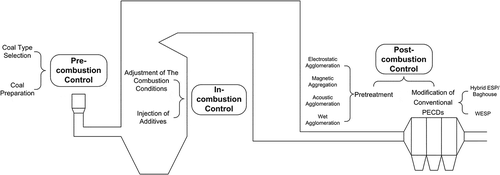
With a fundamental understanding of the formation pathways of primary PM in the pulverized coal combustion process, it is possible for us to take targeted measures to control the formation and reduce the emission of fly ash particles. Conventional PECDs in coal-fired plants, including cyclones, wet scrubbers, electrostatic precipitators (ESPs), and baghouses (or fabric filters), generally have good performance on the control of coarse fly ash particles. However, as noted previously, their removal efficiency of fine PM in the ultrafine and fine fragmentation regions is not very satisfying. Yao et al. (Citation2009) have summarized that for the large coal-fired power plants with capacity more than 500 MW, the removal efficiencies of total suspended particulates (TSP), PM10, and PM2.5 of ESPs are 99.76–99.89%, 98.58–99.62%, and 96.75–99.16%, respectively, while those of the baghouses are correspondingly 99.94%, 99.76%, and 99.72%. Even though possessing a relatively higher removal efficiency of PM2.5 compared with other conventional PECDs, the baghouses still cannot meet the needs of fine PM control in coal-fired plants. Although a series of previous studies has put forward various strategies to reduce the formation or promote the capture ability of fine PM during combustion process, in this paper we formulate our own systematic approaches for abating the formation and emission of fine PM in coal-fired power plants according to the preceding analysis and discussions. These control measures are divided into precombustion control, in-combustion control, and postcombustion control. The detailed discussions are as follows and the diagram is shown as .
Precombustion control
Several studies have indicated that the coal quality, including the properties of coal particles, as well as their morphology, can greatly affect the formation of PM during coal combustion process. As the first step to control PM emission, precombustion control refers to selecting appropriate type of coal and coal preparation method suitable for the combustion process. As a result, the formation of primary PM in the subsequent combustion process could be weakened.
Coal type selection
Not all types of coal are suitable for combustion in coal-fired power plants, since properties of coal, like pore structures, constituents of inorganic minerals, included and excluded minerals content, the modes of occurrence of elements, and so on, can greatly affect coal particles’ performance in the furnace zone. Different coal pore structures can result in different breakage degree of coal particles, different size distribution of primary PM, and different diffusion resistance, which determines the amount of volatile elements in the vapor phase participating in the condensation process. Breakage degree of coal particles can also be affected by mineral constituents and content. For example, pyrite and carbonate are easy to fragment and decompose under high temperatures (Yan et al., Citation2002), while little or negligible fragmentation of other minerals like silicate, quartz, and illite is observed (Raask, Citation1985).The modes of occurrence of elements in coal have impact on the accumulation characteristics of trace elements in primary PM. Taking the chromium (Cr) as an example, which is of high toxicity and of large emission amount in the coal combustion process, there are currently two point of views about its modes of occurrence in coal: combined with organic matter or with minerals. In their study on different types of coal in America by x-ray absorption fine structure (XAFS), Huggins et al. (Citation2000) pointed out that Cr occurs principally in two forms in bituminous coal: Cr3+associated with illite, and CrO(OH) associated with macerals. However, Shoji et al. (Citation2002) presented that the modes of occurrence of Cr in coal vary with the coal types. In the PM samples generated from eastern U.S. coal, only Cr3+ was observed, while Cr6+accounted for 10–30% in the fly ash from the western United States. Besides, laboratory studies that have been carried out in our former work (Lu and Li, Citation2006a) indicated that the emission amount of PM10, PM2.5, or PM1 after coal combustion is not just a function of coal ash content, fixed carbon, or volatile matter, but high ash content is indeed related to high values of PM2.5/PM10 ratios and PM1/PM2.5 ratios.
The preceding discussion reveals that coals with different properties will undergo different conversion processes, bringing in various emission characteristics of primary PM. Hence, appropriate screening of coal types in advance according to their properties could reduce the formation of fine PM in coal-fired power plants. The general method to determine the applicable coal type is a coal combustion test on a specific furnace. Furthermore, Goto et al. (Citation2009a, Citation2009b) have put forward a series of modified coal type selection methods, which take into account not only the combustion test results but also the combustion state in an actual coal-fired power plant.
Coal preparation
Studies by Ninomiya et al. (Citation2004) and our previous experimental study (Lu and Li, Citation2007b) both suggested that coal particle size affects PM emission significantly in the combustion process. Decreasing coal fineness leads to much more formation of fine PM, because of the direct transferring of more excluded minerals. For pulverized coal with particle size below 63 μm, the PM formed during combustion showed a bimodal size distribution, including a fine mode at about 0.5 μm resulting from fragmentation and vaporization, while for coals of 125–250 μm the PM emission formed a single-mode distribution at about 4 μm (Ninomiya et al., Citation2004). Moreover, finer coal combustion tends to emit PM containing more volatile trace elements (Lu and Li, Citation2007b). Therefore, it is extremely important to prepare the coal with appropriate fineness before combustion since the fineness of pulverized coal affects the combustion state and the capture efficiencies of PECDs, and potentially influences human health.
R90 is an engineering parameter used to define the coal fineness that affects the stability of combustion in the furnace. The smaller the value is, the finer is the pulverized coal. Studies by Liu et al. (Citation2003b) on fly ash samples collected at the entrance and exit of PECDs showed that when R90 decreased from 24.0% to 18.4%, the mass ratio of PM2.5 to the total particulates increased from 33.85% to 36.68%. This phenomenon reveals that during combustion the emission of fine PM, especially the PM2.5, is greatly affected by coal fineness, which is consistent with the work by Ninomiya et al. (Citation2004). Guo et al. (Citation2012) have concluded that when R90 > 45%, the pulverized coal is considered rough for the combustion process, while when R90 < 45%, the pulverized coal is sufficiently fine but the resistance of mill separator is large, and the power consumption for coal grinding is high. Thus, economics of both energy production and emissions should be considered in coal preparation.
In-combustion control
In-combustion control reduces PM emission by controlling the coal particles fragmentation degree and the vaporization degree of organic matter or refractory oxides in the furnace zone. Usually it is achieved through adjustment of the combustion conditions, and injection of additives into the furnace.
Adjustment of the combustion conditions
Important factors that affect PM formation and PM size distribution in combustion process are combustion temperature, burning time, and boiler load.
Previous studies on the relatively nonvolatile refractory minerals, such as aluminum, iron, and calcium, suggested that increasing temperature leads to a growth of their volatilization (Seames, Citation2000). As mentioned earlier in the PM formation part, these compounds will then further undergo a series of transformations including nucleation, condensation, coagulation, and coalescence into fine PM. In addition, our previous work (Lu and Li, Citation2007a) indicates that PM emission amount increases with the increasing combustion temperature. Huang et al. (Citation2004) have analyzed that in the formation process of PM, higher combustion temperature increases the temperature gradient inside the coal particles and brings in larger thermal stress. This will result in more fragmentation of coke and excluded minerals into fine PM. However, combustion temperature affects the formation of PM in two opposite ways. On the one hand, under high combustion temperature, the coal particles swell extensively and bring in severe fragmentation and high vaporization rate of elements, which favors formation of more fine particles; on the other hand, higher temperature causes a greater possibility of coalescence between small particles into agglomerated ash, which will obviously reduce the fine particles amount (Lu and Li, Citation2007a). However, many studies indicate that the overall effect of high temperature is to increase the fine PM emission. Therefore, under the condition of high coal burnout level and high boiler thermal efficiency, it is feasible to reduce the formation and emission of fine PM through decreasing the combustion temperature or local temperature in the furnace.
Burning time has influence on PM formation similar to that of the temperature. In our earlier work (Lu and Li, Citation2008), a sampling probe together with an eight-stage Andersen particle impactor was employed to study the influence of burning time on features of coal-fired primary PM. Relationship of PM mass and size distribution indicated that the longer the burning time was, the more fine PM was produced. And in scanning electron microscope (SEM) images, fly ash particles with longer burning time were more irregular in shape, while shorter burning time brings in low crush degree, regular particle shape, and small deformation of coal. This can be explained, as longer burning time could cause not only severe fragmentation of coke and minerals, but also a more thorough vaporization of volatile matter and refractory oxides, which result in formation of more fine PM. But in an actual combustion process in a coal-fired furnace, longer burning time is of benefit to increasing coal particles’ burn-out rate and reducing unburned carbon content in fly ash. Hence the optimization of burning time should consider both PM emission and combustion efficiency.
In the studies on the emission of PM from coal-fired boiler by Liu et al. (Citation2003b), large ash particles were more likely to deposit or adhere to the furnace surfaces with decreasing boiler load due to the decrease of flue gas volume and velocity. Although the emission of total PM could be reduced, low boiler load aroused a larger ratio of fine PM to the total particulates (Liu et al., Citation2003b). In addition, most of the trace elements tend to deposit on the surface of smaller particles, and the smaller the diameter of fly ash is, the higher is the relative enrichment of trace elements (Huang et al., Citation2004). Hence with deficient oxygen and short residence time, coal particles in small load boiler cannot be burned sufficiently, bringing in a higher proportion of fine PM enriched with trace elements after combustion. Thus, considering the actual situation, it is reasonable to install large load boilers instead of the smaller ones when building new coal-fired power plants, and to gradually replace small load boilers with larger ones under the support of national policies.
Injection of additives
Injection of additives into the flame zone is regarded as a promising approach to reduce the fine PM formation. Smolik et al. (Citation2000) have investigated the composition of fly ash from the combustion of lignites with or without additives. It showed that the application of sorbents, including hydrated lime and limestone, increased the emission of coarse PM and decreased the emission of fine PM. The increased emission of coarse PM is attributed to a combined increase of fine particles scavenging by coarse fly ash and sorbents particles, and the decreased fine PM emission is considered as a result of reduced sulfur dioxide concentration. Our previous work (Lu and Li, Citation2006b) indicated that the addition of calcium oxide to the pulverized coal could also reduce the ratio of fine PM to the total particles. These studies suggest that the sorbent plays a critical role to the control of fine PM in the coal combustion process. In addition, a series of detailed studies has indicated that the injection of sorbents such as kaolinite, lime, limestone, calcium sulfate, silica, alumina, and bauxite could selectively reduce the emissions of metal elements such as lead, sodium, cadmium, copper, beryllium, nickel, mercury, and so on (Linak, Citation1995; Liu et al., Citation2003a; Gale and Wendt, Citation2002; Wendt and Lee, Citation2010). Therefore, sorbents also show a certain capturing ability for toxic metals in high-temperature combustors. The removal pathways and mechanisms have been extensively reviewed by Biswas and Wu (Citation1998).
Under normal combustion conditions, only a small fraction of particles ever has contact with another particle while suspended in the flue gas. But under high temperatures, with the injection of solid sorbents such as kaolinite, lime, and bauxite, additional agglomeration between fine coal particles and sorbents particles could occur (Yao et al., Citation2009). Besides, the sorbents with high porosity provide large surface areas for condensation and further chemical reactions of trace element vapors into large ash particles. Therefore, as a combined result of physical changes of particles and chemical reactions of trace elements, the injection of sorbents into the furnace zone leads to an increasing emission of coarse PM, while the emission of fine PM and trace elements are reduced. This change in the characteristics of PM emissions absolutely favors the latter particles removal process.
Postcombustion control
After the combustion process, by a series of physical and chemical methods or through technical modification of conventional PECDs, postcombustion control can be used to maximize the removal efficiency of fine PM that suspended in the flue gas. It includes various agglomeration approaches to aggregate the fine PM into larger ones, such as pretreating before particles are removed, and modifications of conventional PECDs to achieve high performance.
Electrostatic agglomeration
By utilizing a combination of an electrostatic agglomeration apparatus and electrostatic precipitator, the collection efficiency of conventional PECDs for fine PM in submicrometer size, which is usually extremely low, could be elevated. This new type of PECDs can be divided into three stages: The first stage collects coarse ash particles and charges fine particles in submicrometer size, the middle stage promotes fine-particles agglomeration, and the final stage collects the aggregated particles (Watanabe et al., Citation1995). By the installation in the middle stage of electrodes that are charged by alternating or direct current voltage, the agglomeration effect between fine particles could be strengthened. Experimental studies showed that after the application of electrostatic agglomeration, the mass percentage of submicrometer-sized particles in the emissions decreased 20%, and the collection efficiency of particles in the range of 0.06–12 μm increased to 98% (Watanabe et al., Citation1995). More recently, another type of electrostatic agglomeration with two stages has been developed, in which the particles are bipolarly charged and agglomerated simultaneously in the same stage (Xiang, Chen, and Colbeck, Citation2001). Xiang et al. (Citation2001) found that under the same experimental conditions, the capturing ability of the electrostatic agglomeration with two stages is stronger than that with three stages, with a respective 98.2% and 97.4% collection efficiency of silica flour. Therefore electrostatic agglomeration could be a promising technology to improve fine PM control in coal-fired power plants.
Magnetic aggregation
Separation by magnetic fields has been applied to pollution control processes, including particles reduction in power plants and coal purification. Coal-fired fly ash particles have a relatively high content of iron oxides such as Fe3O4 and γ-Fe2O3. In additional external magnetic fields, these ash particles with iron oxides are easy to magnetize, due to their high saturation magnetization. Ferromagnetic particles possess remnant magnetization, which makes these particles remain magnetized even after the removal of the external magnetic field (Kumar and Biswas, Citation2005). Through a combined effect of magnetic dipole force, Brownian motion, and shear forces, the collision and aggregation between fly ash particles can be enhanced, leading to a transformation from fine PM into coarse ones. Studies on magnetic aggregation of fly ash particles with size range of 0.023–9.314 μm by Li et al. (Citation2007a) indicated that for the midsized particles (0.1–1 μm), removal efficiencies are higher than those of the smaller (<0.1 μm) and bigger ones (>1 μm), and can be improved by prolonging their residence time in the magnetic field. According to the numerical simulation by Li et al. (Citation2007b), when the particle mass concentration is 40 g/m3, the total removal efficiency of PM10 reaches 80% by the magnetic aggregation method. The feasibility of this approach for the control of fine PM, therefore, has been demonstrated.
Acoustic agglomeration
Acoustic agglomeration is a preconditioning technology that has the potential to enlarge fine particles sizes in a gaseous medium. By using high-intensity sound waves to improve the turbulence intensity of the gas, this approach could promote the motion and collision between fine particles (Hoffmann, Citation1997). It can change the size distribution of fly ash particles in a relatively short time through agglomeration, making them more likely to be captured by the following PECDs, such as the ESPs and baghouses. In 1886, William Ostwald first put forward the idea of particle agglomeration in sound field, and its industrial applications have been reported since the 1940s (Denser and Neumann, Citation1949). In the sound field, the orthokinetic interaction and hydrodynamic interaction are believed to be the two most important effects involved in this agglomeration process. The orthokinetic interaction is based on the idea that the relative motion between suspended particles of different sizes produces the collision, and the hydrodynamic interaction results from hydrodynamic forces and the asymmetry of surrounding medium of particles (Hoffmann, Citation2000; Riera-Franco de Sarabia et al., Citation2000). Gallego-Juarez et al. (Citation1999) applied this process for reduction of particle emission from a semi-industrial pilot plant, resulting in an approximately 40% reduction of fine particles number concentration. In the experimental study on acoustic agglomeration of coal-fired fly ash particles by Liu et al. (Citation2009), they found that the optimum operating conditions of acoustic agglomeration can be achieved by adjusting the acoustic frequency, sound pressure level (SPL), and residence time. A reduction of 68.4% in total number concentration was gained under a SPL of 147 dB and a frequency of 1400 Hz.
Wet agglomeration
Wet agglomeration, or wet granulation, refers to the process of agglomerating particles into larger ones by spraying liquid binders toward the particles as they are agitated in a fluidized bed, high shear mixer, or similar devices. Different mechanisms have been used to describe the wet agglomeration process, and this has been reviewed in detail by Iveson et al. (Citation2001). When the liquid binders are sprayed into the agglomerating zone, they collide with dry particles and are distributed throughout them. Then the binders begin to wet the particles, forming the initial agglomerates. As the wetting process proceeds, the fluid penetrates into the pores of the particles surface, forms a nucleus, and migrates outward as the nucleus grows. Further growth of agglomerates occurs when fine particles collide with them and adhere to their surface. Therefore, through a combined effect of wetting, nucleation, and growth, the particles’ size distribution can be changed into a coarse mode, which is of benefit to the ultimate particles removal process. Application of wet agglomeration in a circulating fluidized bed (CFB) is thought to be a feasible and promising approach for fine PM control because of its ability to remove sulfur dioxide as well as fly ash particles in the flue gas at the same time. Investigations by Rambali et al. (Citation2001a, Citation2001b) showed that in a fluidized bed, the inlet air temperature, the inlet airflow rate, the spray rate, and the nozzle air pressure were the key process variables that determine the sizes of agglomerates, and the optimum values of these variables could be achieved by experimental design.
Modification of conventional particle emission control devices
As mentioned earlier, even though conventional PECDs such as ESPs and baghouses have outstanding performance in the removal of coarse PM from coal-fired flue gas, both of them have limitations that prevent them from achieving high removal efficiencies for fine PM in the submicrometer size. Thus, during the past decades, some modified technologies based on conventional PECDs have been developed, and their abilities for capturing fine particles in coal-fired power plants have been demonstrated by engineering applications, such as hybrid ESP/baghouse and wet electrostatic precipitators (WESP).
The baghouse has the advantage of possessing high removal efficiency with low pressure drop. Through the effects of inertial impaction, interception, and convective Brownian diffusion, the baghouse could filter most fly ash particles in the flue gas. For particles larger than 0.5 μm, inertial impaction plays a critical role in their removal process, and Brownian diffusion accounts for particles smaller than 0.05 μm. For particles in the size range of 0.05-0.5 μm, a baghouse has a minimum removal efficiency, since both inertial impaction and Brownian diffusion are weak in this size range (Hinds, Citation1982). Similarily, for ESP, the fractional penetration of particles ranging from 0.1 to 1.0 μm is greater than that of larger and smaller particles, due to their weak ability to be charged by both electrostatic forces and diffusion (Hinds, Citation1982). But through application of electrostatic forces, the removal efficiency of a baghouse can be significantly increased, since ash particles or both ash particles and fibers can be highly charged. Wang (Citation2001) has given a review on studies of the application of electrostatic forces in filtration, in which he summarized that there are mainly two ways of applying electrostatic forces to augment the removal efficiency of the baghouse: (1) charging the airborne particles and (2) bringing in an electric field in the filter. As the second way possesses a relatively higher efficiency with smaller collection space and lower pressure drop, it is more widely employed in engineering application.
Initially proposed by Electric Power Research Institute, an apparatus termed a compact hybrid particulate collector (COHPAC) is used for efficient removal of particulates. In the hybrid ESP/baghouse devices such as COHPAC, by incorporating a baghouse internally within an ESP, or incorporating an ESP and a baghouse in series, coarse particles are collected by the ESP, and the baghouse could capture the fine particles that have not been collected by the ESP (Chang, Citation1992; Filter Media Consulting, Inc., Citation1997). Hence, the removal efficiency of the baghouse can be improved significantly even operated at high filtration velocities, and the size of the baghouse can be significantly reduced as well. Industrial tests performed in power stations, for example, the operation of a COHPAC in Big Brown Station for more than 7000 hr, reveal the generally positive performance of particulate collection of the COHPAC, with very low stack opacity (below 5%) and high air-to-cloth (A/C) ratio (Filter Media Consulting, Inc., Citation1997). Besides, another technology named an advanced hybrid particulate collector (AHPC) is being developed at the Energy & Environment Research Center, which employs the ESPs and baghouse in the same housing and successfully combines their best features in a unique approach (Miller et al., Citation1997). AHPC uses a sophisticated fabric to ensure ultrahigh collection efficiency to 99.99% of fine PM, but is less prevalent because of its higher cost compared to conventional fabrics.
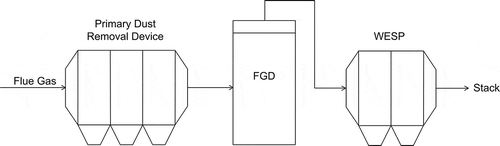
By capturing pollutants from a flowing fluid, WESP is recognized as the most reliable device for simultaneous removal of submicrometer sized fly ash particles and gaseous pollutants, for example, SO2. WESP is generally composed of an inlet diffuser, a transverse electrostatic discharge section, an extended discharge section, a mist eliminator section, and an outlet section (Bakke, Citation1976). The whole WESP system is installed downstream of the FGD, as shown in . In the WESP, the flue gas to be treated is saturated with water vapor. By spraying into the inlet section, some of the coarse particles will be removed and the gas absorption process will be started. This technology allows the collected particles and moisture to form flowing slurry that helps to keep the dust collection plates clean, which can obviously lower the probability of dust reentrainment and reduce the concentration of fine PM. It possesses some advantages over other conventional PECDs, such as moderate energy consumption, robust performance scarcely affected by particles properties, and organic droplets removing ability (Bakke, Citation1975; Saiyasitpanich et al., Citation2007). With more than 1000 WESPs currently in operation worldwide, WESP commercial utilization has been proved (Kumar and Mansour, Citation1998). The WESP system utilized in the AES Deepwater plant in Texas, which follows a dry ESP and a wet FGD, possesses a collection efficiency of particulates in the range of 95–97% and a collection efficiency of sulfuric acid greater than 90%. Without dry ESP installed, the WESP used in the plant of Northern States Power/Xcel Energy achieved a collection efficiency of particulates over 90% and a stack opacity below 10% (Staehle et al., Citation2003). The additional benefits such as keeping SO3 at very low levels and possible benefits in mercury, NH3, and HCl control will make WESP a desirable choice when considering the available options for fine PM control.
Conclusion
The emission of primary PM, especially the fine PM, from pulverized coal combustion process in coal-fired power plant is related to the coal type, coal fineness, combustion conditions, injection of additives, capturing ability of PECDs, and so on.
Accordingly, the measures of precombustion control and in-combustion control are analyzed, and it revealed that these measures are somehow capable of abating the formation of fine particles. Therefore, some precombustion control and in-combustion control measures, for example, injection of additives, should be considered to take for controlling the fine particles problem in practice.
Implementation of some measures does need the support of national policies, such as coal type selection. Selection of coal type may require that high-quality coal be distributed to the targeted regions. Besides, combustion conditions and boiler load are critical factors for power generation, as well as for emission of fine particles. In order to abate the fine particle emission, coal combustion efficiency should be sacrificed to get environmental benefits, and large load boilers should be considered instead of the smaller ones when building new coal-fired power plants.
In current practice, “economic fineness” is always employed for pulverized coal due to consideration of both good combustion performance of the coal and economic benefit. However, an “environmental fineness,” which can bring in less environment impacts, should also be considered for pulverized coal based on our discussion.
For postcombustion control of fine PM in flue gas, pretreatment by fine particles agglomeration is essential and favorable for subsequent capture by PECDs. A hybrid system of ESP/baghouse and the WESP are proved to be more capable of trapping fine particles. However, new modification or combined particle removal systems should be continuously developed, tested, and applied in order to reach a higher removal efficiency and to stay in compliance with the increasingly strict emission standard of fine PM.
Funding
This work is supported by the National Natural Science Foundation of China (grant 51176047, grant 51376063) and the National Natural Science Foundation of Hebei Province (grant E2012502048).
Additional information
Notes on contributors
Jianyi Lu
Jianyi Lu is a professor and Xudan Ren is a master’s degree candidate at the School of Environmental Science and Engineering, North China Electric Power University, Baoding city, Hebei Province, P.R. China.
Xudan Ren
Jianyi Lu is a professor and Xudan Ren is a master’s degree candidate at the School of Environmental Science and Engineering, North China Electric Power University, Baoding city, Hebei Province, P.R. China.
References
- Bakke, E. 1975. Wet electrostatic precipitators for control of submicron particles. J. Air Pollut. Control Assoc. 25(2): 163–167. doi:10.1080/00022470.1975.10470067
- Bakke, E. 1976. U.S. Patent 3,958,961.
- Biswas, P., and C.Y. Wu. 1998. Control of toxic metal emissions from combustors using sorbents: a review. J. Air Waste Manage. Assoc. 48(2): 113–127. doi:10.1080/10473289.1998.10463657
- Chang, R. 1992. U.S. Patent 5,158,580.
- Davidson, C.I., R.F. Phalen, and P.A. Solomon. 2005. Airborne particulate matter and human health: A review. Aerosol Sci. Technol. 39(8): 737–749. doi:10.1080/02786820500191348
- Denser, H.W., and E. Neumann. 1949. Industrial sonic agglomeration and collection systems. Ind. Eng. Chem. 41(11): 2439–2442. doi:10.1021/ie50479a023
- Fix, G., W. Seames, M. Mann, S. Benson, and D. Miller. 2013. The effect of combustion temperature on coal ash fine-fragmentation mode formation mechanisms. Fuel 113:140–147. doi:10.1016/j.fuel.2013.05.096
- Filter Media Consulting, Inc. 1997. Concept with a future: COHPAC. Filtr. Sep. 34(2): 137, 139. doi:10.1016/S0015-1882(97)84806-9
- Flagan, R.C., and S.K. Friedlander. 1978. Particle formation in pulverized coal combustion: A review. Recent Development in Aerosol Science. New York: Wiley-Interscience.
- Gale, T.K., and J.O.L. Wendt. 2002. High-temperature interactions between multiple-metals and kaolinite. Combust. Flame 131:299–307. doi:10.1016/S0010-2180(02)00404-2
- Gallego-Juarez, J.A., E. Riera-Franco de Sarabia, G. Rodriguez-Corral, T.L. Hoffmann, J.C. Galvez-Moraleda, J.J. Rodriguez-Maroto, F.J. Gomez-Moreno, A. Bahillo-Ruiz, and M. Martin-Espigares. 1999. Application of acoustic agglomeration to reduce fine particle emissions from coal combustion plants. Environ. Sci. Technol. 33(21): 3843–3849. doi:10.1021/es990002n
- Goto, S., K. Masuda, M. Nakanura, M. Sueyoshi, T. Future, Y. Uchida, and H. Hatazaki. 2009a. A study of coal type selection for a coal fired power plant considering coal and fly ash properties. Int. J. Innov. Comput. Information Control 5(1): 215–223.
- Goto, S., S. Katafuchi, M. Sueyoshi, T. Future, Y. Uchida, H. Hatazaki, and M. Nakanura. 2009b. Coal type selection for thermal power plants through combustion state estimation. Paper presented at the ICCAS-SICE International Joint Conference, Fukuoka, Japan, August 18–21.
- Guo, X., A. Lv, and F. Xiao. 2012. The research on design parameters of fan mill direct pulverizing system. Energy Procedia 17( Part B): 1620–1626. doi:10.1016/j.egypro.2012.02.289
- Hinds, W.C. 1982. Aerosol technology: Properties, behavior, and measurement of airborne particles. New York, NY: John Wiley & Sons.
- Hoffmann, T.L. 1997. An extended kernel for acoustic agglomeration simulation based on the acoustic wake effect. J. Aerosol Sci. 28(6): 919–936. doi:10.1016/S0021-8502(96)00489-2
- Hoffmann, T.L. 2000. Environmental implications of acoustic aerosol agglomeration. Ultrasonics 38(1–8): 353–357. doi:10.1016/S0041-624X(99)00184-5
- Huang, Y., B. Jin, Z. Zhong, R. Xiao, Z. Tang, and H. Ren. 2004. Trace elements (Mn, Cr, Pb, Se, Zn, Cd and Hg) in emissions from a pulverized coal boiler. Fuel Process. Technol. 86(1): 23–32. doi:10.1016/j.fuproc.2003.10.022
- Huggins, F.E., N. Shah, and G.P. Huffman, A. Kolker, S. Crowley, C.A. Palmer, and R.B. Finkelman. 2000. Mode of occurrence of chromium in four US coals. Fuel Process. Technol. 63(2–3): 79–92. doi:10.1016/S0378-3820(99)00090-9
- Iveson, S.M., J.D. Litster, K. Hapgood, and B.J. Ennis. 2001. Nucleation, growth and breakage phenomena in agitated wet granulation processes: A review. Powder Technol. 117(1–2): 3–39. doi:10.1016/S0032-5910(01)00313-8
- Jena, M.S., S.K. Biswal, and M.V. Rudramuniyappa. 2008. Study on flotation characteristics of oxidised Indian high ash sub-bituminous coal. Int. J. Miner. Process. 87(1–2): 42–50. doi:10.1016/j.minpro.2008.01.004
- Jimenez, A., M.J. Iglesias, F. Laggoun-Defarge, and I. Suarez-Ruiz. 1998. Study of physical and chemical properties of vitrinite. Inferences on depositional and coalification controls. Chem. Geol. 150(3–4): 197–221. doi:10.1016/S0009-2541(98)00048-5
- Kumar, P., and P. Biswas. 2005. Analytical expressions of the collision frequency function for aggregation of magnetic particles. J. Aerosol Sci. 36(4): 455–469. doi:10.1016/j.jaerosci.2004.10.008
- Kumar, K.S., and A. Mansour. 1998. A review of recent developments in particulate control in the copper and nickel industry. Paper presented at the 1998 TMS Annual Meeting, San Antonio, TX, February 16–19.
- Li, Y.W., C.S. Zhao, X. Wu, D.F. Lu, and S. Han. 2007a. Aggregation mechanism of fine fly ash particles in uniform magnetic field. Korean J. Chem. Eng. 24(2): 319–327. doi:10.1007/s11814-007-5053-9
- Li, Y.W., X. Wu, C.S. Zhao, D.F. Lu, and S. Han. 2007b. Experimental study on aggregation of PM10 from coal combustion by magnetic seeding in uniform magnetic field [in Chinese]. Proc. Chin. Soc. Electr. Eng. 27:23–28.
- Lighty, J.S., J.M. Veranth, and A.F. Sarofim. 2000. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manage. Assoc. 50(9):1565–1618. doi:10.1080/10473289.2000.10464197
- Linak, W.P. 1995. Sorbent capture of nickel, lead, and cadmium in a laboratory swirl flame incinerator. Combust. Flame 100(1–2): 241–250. doi:10.1016/0010-2180(94)00073-2
- Linak, W.P., C.A. Miller, and J.O.L. Wendt. 2000. Comparison of particle size distributions and elemental partitioning from the combustion of pulverized coal and residual fuel oil. J. Air Waste Manage. Assoc. 50(8): 1532–1544. doi:10.1080/10473289.2000.10464171
- Liu, G., H. Wu, R.P. Gupta, J.A. Lucas, A.G. Tate, and T.F. Wall. 2000. Modeling the fragmentation of non-uniform porous char particles during pulverized coal combustion. Fuel 79(6): 627–633. doi:10.1016/S0016-2361(99)00186-6
- Liu, J., C. Zheng, H. Zeng, J. Zhang, and X. Lu. 2003a. Effects of solid adsorbents on the emission of heavy metals during coal combustion [in Chinese]. J. Environ. Sci. 24(5): 23–27.
- Liu, J.Z., H. Fan, J. Zhou, X. Cao, and K. Cen. 2003b. Experimental studies on the emission of PM10 and PM2.5 from coal-fired boiler [in Chinese]. Proc. Chin. Soc. Electr. Eng. 23(1): 145–149.
- Liu, J., G. Zhang, J. Zhou, J. Wang, W. Zhao, and K. Cen. 2009. Experimental study of acoustic agglomeration of coal-fired fly ash particles at low frequencies. Powder Technol. 193(1): 20–25. doi:10.1016/j.powtec.2009.02.002
- Lu, J.Y., and D.K. Li. 2006a. Emission features of primary particulate matters after different pulverized coals combustion [in Chinese]. J. Combust. Sci. Technol. 12(6): 514–518.
- Lu, J.Y., and D.K. Li. 2006b. Study of the effect of CaO addition on primary particle characteristics after pulverized coal combustion [in Chinese]. J. Eng. Therm. Energy Power 21(4): 373–377.
- Lu, J.Y., and D.K. Li. 2007a. Study on primary PM features influenced by pulverized coal combustion at different burning temperature [in Chinese]. Proc. Chin. Soc. Electr. Eng. 27(20): 24–29.
- Lu, J.Y., and D.K. Li. 2007b. Coal fineness effect on primary particulate matter features during pulverized coal combustion [in Chinese]. J. Environ. Sci. 28(9): 1944–1948.
- Lu, J.Y., and D.K. Li. 2008. Influence of burning time on primary particulate matter features during pulverized coal combustion [in Chinese]. J. Combust. Sci. Technol. 14(1): 55–60.
- McElroy, M.W., R.C. Carr, D.S. Ensor, and G.R. Markowski. 1982. Distribution of fine particles from coal combustion. Science. 215:13–19. doi:10.1126/science.215.4528.13
- Ministry of Environment Protection of China. 2011. Emission Standard of Air Pollutants for Thermal Power Plants GB13223—2011. Beijing: Ministry of Environment Protection of China. http://www.zhb.gov.cn.
- Miller, S.J., G.L. Schelkoph, G.E. Dunham, K. Walker, and H. Krigmont. 1997. Advanced hybrid particulate collector, A new concept for air toxics and fine-particle control. Paper presented at the Advanced Coal-Based Power and Environmental Systems ’97 Conference, Pittsburgh, PA, July 22–24.
- National Bureau of Statistics of China. 2006. China statistical yearbook 2006. Beijing, China: China Statistics Press.
- National Bureau of Statistics of China. 2011. China Statistical Yearbook 2011. Beijing, China: China Statistics Press.
- Ninomiya, Y., L. Zhang, A. Sato, and Z. Dong. 2004. Influence of coal particle size on particulate matter emission and its chemical species produced during coal combustion. Fuel Process. Technol. 85(8–10): 1065–1088. doi:10.1016/j.fuproc.2003.10.012
- Pope, C.A. III, and D.W. Dockery. 2006. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage. Assoc. 56(6): 709–742. doi:10.1080/10473289.2006.10464485
- Raask, E. 1985. Mineral impurities in coal combustion: behavior, problems and remedial measures. New York, NY: Hemisphere.
- Rambali, B., L. Baert, D. Thone, and D.L. Massart. 2001a. Using experimental design to optimize the process parameters in fluidized bed granulation. Drug Dev. Ind. Pharm. 27(1): 47–55. doi:10.1081/DDC-100000127
- Rambali, B., L. Baert, and D.L. Massart. 2001b. Using experimental design to optimize the process parameters in fluidized bed granulation on a semi-full scale. Int. J. Pharm. 220(1–2): 149–160. doi:10.1016/S0378-5173(01)00658-5
- Riera-Franco de Sarabia, E., J.A. Gallego-Juarez, G. Rodriguez-Corral, L. Elvia-Segura, and I. Gonzalez-Gomez. 2000. Application of high-power ultrasound to enhance fluid/solid particle separation processes. Ultrasonics 38(1–8): 642–646. doi:10.1016/S0041-624X(99)00129-8
- Saiyasitpanich, P., T.C. Keener, S.J. Khang, and M. Lu. 2007. Removal of diesel particulate matter (DPM) in a tubular wet electrostatic precipitator. J. Electrostat. 65(10–11):618–624. doi:10.1016/j.elstat.2007.01.005
- Sarofim, A.F., J.B. Howard, and A.S. Padia. 1977. The physical transformation of the mineral matter in pulverized coal under simulated combustion conditions. Combust. Sci. Technol. 16(3–6): 187–204. doi:10.1080/00102207708946804
- Schwartz, J., D.W. Dockery, and L.M. Neas. 1996. Is daily mortality associated specifically with fine particles? J Air Waste Manage. Assoc. 46(10): 927–939. doi:10.1080/10473289.1996.10467528
- Seames, W.S. 2000. The partitioning of trace elements during pulverized coal combustion. PhD dissertation, Chemical and Environmental Engineering, University of Arizona, Tucson, AZ.
- Seames, W.S. 2003. An initial study of the fine fragmentation fly ash particle mode generated during pulverized coal combustion. Fuel Process. Technol. 81(2): 109–125. doi:10.1016/S0378-3820(03)00006-7
- Senior, C.L., J.J. Helble, and A.F. Sarofim. 2000. Emissions of mercury, trace elements, and fine particles from stationary combustion sources. Fuel Process. Technol. 65–66:263–288. doi:10.1016/S0378-3820(00)00082-5
- Shoji, T., F.E. Huggins, and G.P. Huffman, W.P. Linak, and C.A. Miller. 2002. XAFS spectroscopy analysis of selected elements in fine particulate matter derived from coal combustion. Energy Fuels. 16(2): 325–329. doi:10.1021/ef010200b
- Smolik, J., J. Schwaz, V. Vesely, I. Sykorova, J. Kucera, and V. Havranek. 2000. Influence of calcareous sorbents on particulate emissions from fluidized bed combustion of lignite. Aerosol Sci. Technol. 33(6): 544–556. doi:10.1080/02786820050195386
- Staehle, R.C., R.J. Triscori, K.S. Kumar, G. Ross, and R. Cothron. 2003. Wet electrostatic precipitators for high efficiency control of fine particulates and sulfuric acid mist. Paper presented at the Institute of Clean Air Companies (ICAC) Forum, Nashville, TN, October 14–15.
- Tucker, W.G. 2000. An overview of PM2.5 sources and control strategies. Fuel Process. Technol. 65–66:379–392. doi:10.1016/S0378-3820(99)00105-8
- Verma, V., Z. Ning, A.K. Cho, J.J. Schauer, M.M. Shafer, and C. Sioutas. 2009. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 43(40): 6360–6368. doi:10.1016/j.atmosenv.2009.09.019
- Wang, C.S. 2001. Electrostatic forces in fibrous filters—A review. Powder Technol. 118(1–2): 166–170. doi:10.1016/S0032-5910(01)00307-2
- Wang, J., L. Feng, and G.E. Tverberg. 2013. An analysis of China’s coal supply and its impact on China’s future economic growth. Energy Policy 57: 542–551. doi:10.1016/j.enpol.2013.02.034
- Watanabe, T., F. Tochikubo, Y. Koizumi, T. Tsuchida, J. Hautanen, and E.I. Kauppinen. 1995. Submicron particle agglomeration by an electrostatic agglomerator. J. Electrostat. 34(4): 367–383. doi:10.1016/0304-3886(95)93833-5
- Wendt, J.O.L., and S.J. Lee. 2010. High-temperature sorbents for Hg, Cd, Pb, and other trace metals: Mechanisms and applications. Fuel 89(4): 895–903. doi:10.1016/j.fuel.2009.01.028
- Xiang, X., B. Chen, and I. Colbeck. 2001. Bipolar charged aerosol agglomeration and collection by a two zone agglomerator. J. Environ. Sci. 13:276–279.
- Yan, L., R.P. Gupta, and T.F. Wall. 2002. A mathematical model of ash formation during pulverized coal combustion. Fuel 81(3): 337–344. doi:10.1016/S0016-2361(01)00166-1
- Yao, Q., S.Q. Li, H.W. Xu, J.K. Zhuo, and Q. Song. 2009. Studies on formation and control of combustion particulate matter in China: A review. Energy 34(9): 1296–1309. doi:10.1016/j.energy.2009.03.013
- Zhang, L., and Y. Ninomiya. 2006. Emission of suspended PM10 from laboratory-scale coal combustion and its correlation with coal mineral properties. Fuel 85(2): 194–203. doi:10.1016/j.fuel.2005.03.034
- Zhang, L., Y. Ninomiya, and T. Yamashita. 2006. Formation of submicron particulate matter (PM1) during coal combustion and influence of reaction temperature. Fuel 85(10–11): 1446–1457. doi:10.1016/j.fuel.2006.01.009