Abstract
Studies were conducted at Great Smoky Mountains National Park (NP) (GRSM), Tennessee, Mount Rainier NP (MORA), Washington, and Acadia NP (ACAD), Maine, to evaluate assumptions used to estimate aerosol light extinction from chemical composition. The revised IMPROVE equation calculates light scattering from concentrations of PM2.5 sulfates, nitrates, organic carbon mass (OM), and soil. Organics are assumed to be nonhygroscopic. Organic carbon (OC) is converted to OM with a multiplier of 1.8. Experiments were conducted to evaluate assumptions on aerosol hydration state, the OM/OC ratio, OM hygroscopicity, and mass scattering efficiencies. Sulfates were neutralized by ammonium during winter at GRSM (W, winter) and at MORA during summer but were acidic at ACAD and GRSM (S, summer) during summer. Hygroscopic growth was mostly smooth and continuous, rarely exhibiting hysteresis. Deliquescence was not observed except infrequently during winter at GRSM (W). Water-soluble organic carbon (WSOC) was separated from bulk OC with solid-phase absorbents. The average OM/OC ratios were 2.0, 2.7, 2.1, and 2.2 at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Hygroscopic growth factors (GF) at relative humidity (RH) 90% for aerosols generated from WSOC extracts averaged 1.19, 1.06, 1.13, and 1.16 at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Thus, the assumption that OM is not hygroscopic may lead to underestimation of its contribution to light scattering.
Implications: Studies at IMPROVE sites conducted in U.S. national parks showed that aerosol organics comprise more PM2.5 mass and absorb more water as a function of relative humidity than is currently assumed by the IMPROVE equation for calculating chemical light extinction. Future strategies for reducing regional haze may therefore need to focus more heavily on understanding the origins and control of anthropogenic sources of organic aerosols.
Introduction
Aerosols affect visibility by scattering and absorbing light. Particle light scattering (Bsp) and absorption (Bap) have been estimated from aerosol size distributions and chemical composition (Hayasaka et al., Citation1992; Zhang et al., Citation1994; Lowenthal et al., Citation1995; McInnes et al., Citation1998; Malm et al., Citation2003, Citation2005; Pitchford et al., Citation2007). Assuming that the aerosol chemical components are externally mixed, chemical light scattering takes the form:
Systematic bias in the original IMPROVE equation was observed by Malm et al. (Citation2003), Lowenthal and Kumar (Citation2004), Ryan et al. (Citation2005), McMeeking et al. (Citation2005), and Malm and Hand (Citation2007). Equation 2 overestimated measured Bsp at low concentration and underestimated it at high concentration. In response, the IMPROVE equation was revised as follows (Pitchford et al., Citation2007):
The original OC multiplier of 1.4 was based on measurements in urban areas (White and Roberts, Citation1977). Aerosol studies at remote locations suggested that the OM/OC at such locations may exceed 2 (Turpin and Lim, Citation2001; Kiss et al., Citation2002; El-Zanan et al., Citation2005; Malm et al., Citation2005; Yu et al., Citation2005; Chen and Yu, Citation2007; Malm and Hand, Citation2007; Polidori et al., Citation2008; Lowenthal et al., Citation2009). The revised IMPROVE equation uses a consensus value of 1.8. The fRH are based on hygroscopic growth factors for (NH4)2SO4 calculated with the AIM thermodynamic model (Aerosol Inorganics Model: Clegg et al., Citation1998). The hygroscopic growth of pure (NH4)2SO4 is “hysteretic.” Dry particles deliquesce at a relative humidity (RH) of about 80% and continue to absorb water above this RH. Upon dehumidification, particles retain water in a nonequilibrium state below the deliquescence RH until they crystalize at approximately 37% RH. The revised IMPROVE equation assumes that (NH4)2SO4 and NH4NO3 are in their most hydrated state. While the revised IMPROVE equation assumes that organics are nonhygroscopic, experimental evidence suggests that this may not be the case (Gysel et al., Citation2004; Dinar et al., Citation2006, Citation2007; Hallar et al., Citation2013). On the other hand, Modini et al. (Citation2010) found that heating marine aerosols reduced their hygroscopic growth. This was attributed to inhibition of water uptake by volatile organic coatings. Chan and Chan (Citation2007) found that organic coatings increased the deliquescence RH and decreased the efflorescence RH for ammonium sulfate particles but that the equilibrium growth for ammonium sulfate was not affected.
A study was begun during summer (S) 2006 at Great Smoky Mountains National Park (NP) [GRSM (S)] to investigate assumptions underlying the PM2.5 scattering (Bsp) components of the revised IMPROVE equation: (1) (NH4)2SO4 and NH4NO3 are always in their most hydrated state; (2) organics are not hygroscopic; (3) the OM/OC ratio is 1.8; and (4) particle size and concentration are directly related (Lowenthal et al., Citation2009). Since then, similar studies have been conducted during winter 2008 at GRSM (W, winter), Mount Rainier NP (MORA) during summer 2009, and Acadia NP (ACAD) during summer 2011. This paper provides an overview of the entire study and a comprehensive summary and discussion.
Methods
Field sampling and laboratory measurements are described in detail by Lowenthal et al. (Citation2009). Site locations and sampling periods are shown in . The GRSM studies were conducted at the IMPROVE site at Look Rock at the western end of the park. The MORA study was conducted at the IMPROVE site at park headquarters, 16 km west of the Nisqually entrance to the park. The ACAD study was conducted at the Schoodic Education and Research Center in Acadia NP on the Schoodic Peninsula. This site is about 18 km east of the IMPROVE site on Mt. Desert Island in the western section of the park.
Table 1. Site locations and sample periods
Field measurements
PM2.5 aerosol sampling
Twenty-four-hour duration PM2.5 filter samples were collected daily starting at about 10:00 a.m., local time, with four Tisch Environmental, Inc., high-volume (hivol) samplers on Teflon Impregnated Glass Fiber filters (number 7215, TIGF) (Pall Corporation). Hivol samples were collected on Zefluor filters (number P5PJ001, Pall Corporation) from July 19, 2006, to July 31, 2006, during GRSM (S). Four 24-hr-duration hivol samples were required to collect sufficient organic mass (OM) for laboratory analysis. Twenty-four-hour-duration PM2.5 samples were also collected daily on 47-mm quartz-fiber filters (2500 Pallflex QAT-UP, Pall Corporation) in a medium-volume (medvol) sampler for determination of inorganic ions, elemental and organic carbon (EC and OC), and water-soluble organic carbon (WSOC). Inorganic ions and WSOC were also measured on the hivol filters. The filters were refrigerated before and after sampling. Field blanks were determined from filters handled similarly to the sample filters except that they were not placed in the samplers.
Organic carbon measurement is subject to a positive sampling artifact caused by adsorption of volatile organic compounds (VOC) by quartz filters during sampling (Turpin et al., Citation1994). Organic carbon sampling artifacts have been addressed using backup quartz filters and VOC denuders (e.g., Subramanian et al., Citation2004; Chow et al., Citation2006). While such sampling strategies were not employed in the GRSM studies, backup quartz-fiber filters were used in the MORA and ACAD studies. OC and WSOC on the backup filters was subtracted from the front quartz filter OC and WSOC concentrations to correct for the blank and positive artifact for each sample. The field blank was subtracted in the GRSM studies.
presents average field blank and backup filter OC and WSOC concentrations (µg/filter) for the four studies. The highest field blanks (as a percentage of the front filter concentration) were seen at GRSM (W) for OC measured on the front quartz medvol (29%) and WSOC measured on the Zefluor and TIGF hivol filters (29%). Medvol quartz backup OC and WSOC were 3–4 times higher than the corresponding field blanks. Lowenthal et al. (Citation2009) noted that subtracting the field blank in the absence of a backup filter may have underestimated the OC concentration at GRSM. shows that backup filter concentrations of OC and WSOC were higher than the corresponding field blanks at MORA and ACAD.
Table 2. Average field blank and backup (where applicable) filter OC and WSOC concentrations (µg/sample)
Ambient hydration state
The ambient hydration state during the four studies was determined in-situ with an ambient-state hygroscopic tandem differential mobility analyzer (AS-HTDMA) (Gasparini et al., Citation2004; Santarpia et al., Citation2004; Taylor et al., Citation2011). Taylor et al. (Citation2011) described the instrument and the procedure for inferring the ambient hydration state of particles and presented results for the GRSM (S) and GRSM (W) studies. Ambient particles are size selected with diameter Da at ambient RH (RHa) and temperature. The final particle diameter (Df) is then measured after drying to RH < 20% with a Nafion dryer, increasing the RH to RHa, increasing the RH to 85–95%, and decreasing the RH again to RHa. The ambient hydration state is inferred from the ratio of Df to Da after various RH conditioning regimes. Nonhygroscopic aerosols display Df equal to Da under all conditions. Hygroscopicity is indicated when Df at RHa or RH > 85% is greater than Df at dryness. For hygroscopic particles, nonhysteretic (continuous) growth is indicated when Df is equal to Da both after increasing the RH from dryness to RHa and after decreasing RH from 85 to 95% to RHa. Hysteretic growth for ambient particles in their least hydrated state (lower leg) is indicated when Df is equal to Da after raising the RH from dryness to RHa but greater than Da after the RH is reduced from 85 to 95% to RHa. Hysteretic growth for ambient particles in their most hydrated state (upper leg) displays Df equal to Da after humidification from dryness to RHa and also after dehumidification from RH>85% to RHa. This ideal model describes hygroscopic growth for pure ionic compounds. Real-world aerosols may only approximate this behavior. Indeed, organics have been shown to alter the hygroscopic behavior of pure salts (Cruz and Pandis, 2000). In practice, hysteretic behavior is described in terms of more hydrated (MH) and less hydrated (LH) states, that is, more closely aligned with the upper and lower legs, respectively, of the hysteresis loop (Taylor et al., Citation2011).
Dry particle size distribution
The dry particle size distribution was measured with a scanning mobility particle sizer (SMPS) (Lowenthal et al., Citation2009). The system contains a high-flow differential mobility analyzer (DMA). This design increases the particle count rate and thus the precision of the measurement (Stolzenburg et al., Citation1998). Particles were dried using a Nafion tube bundle. This DMA was also used in conjunction with a humidifier and a second DMA as a hygroscopic tandem differential mobility analyzer (HTDMA) to measure ambient hygroscopic growth factors, as described in the following section. Dry particle size distribution and growth factor scans were conducted alternately at different frequencies during the four studies. Dry size distributions were measured at a frequency of 17, 8, 5, and 3 times per day at generally regular intervals during the GRSM (S), GRSM (W), MORA, and ACAD studies, respectively.
Laboratory measurements
Soluble ion and carbon analysis
Laboratory analyses are described by Lowenthal et al. (Citation2009). Hivol and medvol samples were analyzed for SO42-, NH4+, and Cl- ions by ion chromatography. Ammonium ion (NH4+) was analyzed by automated colorimetry. Ammonium was measured using ion chromatography for the ACAD study. Elemental and organic carbon (EC and OC) were determined on quartz filters using thermal-optical reflectance (TOR) (Chow et al., Citation1993). WSOC during the GRSM (S) study was determined from the hivol filter water extracts using total organic carbon (TOC) analysis (Lowenthal et al., Citation2009). Twenty of the GRSM (S) samples were also analyzed for WSOC by TOR (Yang et al., Citation2003). The two methods agreed well:
In the MORA and ACAD studies, front quartz filter OC and WSOC were corrected for the blank and positive sampling artifact by subtracting the respective concentrations on the backup filter. The average ratios of backup to front WSOC were 0.23 ± 0.13 and 0.27 ± 0.13 at MORA and ACAD, respectively. The average ratios of backup to front OC were 0.17 ± 0.09 and 0.36 ± 0.22 at MORA and ACAD, respectively.
Isolation of WSOC
Two of the main goals of this project are to determine the OM/OC ratio and to determine the hygroscopic growth potential of WSOC. Both require isolating WSOC from inorganic ions in the water extract. This separation was done using solid-phase extraction (SPE) (Decesari et al., Citation2001; Varga et al., Citation2001; Alves et al., Citation2002; Duarte and Duarte, Citation2005; Rinehart et al., Citation2006) as described by Lowenthal et al. (Citation2009). The four daily hivol filter samples were combined and extracted in ultrapure water. The water extract was concentrated to about 15–20 ml. The filter material was dried and extracted in dichloromethane (DCM). In order to obtain sufficient WSOC mass for measuring hygroscopic growth, the daily hivol extracts were combined sequentially into 5, 5, 7, and 6 composites for GRSM (S), GRSM (W), MORA, and ACAD, respectively, based on the daily WSOC concentrations. WSOC and inorganic ions in the composite samples were separated using the method of Duarte and Duarte (Citation2005). The water extracts were applied in series to two columns containing XAD-8 and XAD-4 resins, respectively. XAD-8 collects higher molecular weight “humic acid like substances” (HULIS), while XAD-4 collects lower molecular weight, more water-soluble material. After washing the columns with water to remove inorganic ions, both columns are back-eluted with a methanol–water mixture. This process was repeated until at least 99% of the initial sulfate in the water extract was removed. The recovery (%) of WSOC was determined after each pass through the columns. The methanol–water eluates were evaporated under a gentle stream of ultra-high-purity nitrogen at room temperature to near dryness and redissolved in ultrapure water.
OM/OC ratio
The OM/OC ratio of the isolated WSOC in each composite sample was determined by spotting the XAD-treated water extract onto quartz filter punches. Each punch was pre- and postweighed to 1 µg to determine the mass of WSOC. The punch was then subjected to TOR analysis for organic carbon content (OC). The same procedure was used to determine the OM/OC ratio of organic material in the DCM extracts. A weighted average OM/OC ratio was estimated based on the average WSOC/OC ratio and the assumption that the DCM extract represents the non-water-soluble fraction, that is, 1 minus the average WSOC/OC ratio.
Hygroscopic growth of WSOC aerosols
A hygroscopic growth factor (GF) is the ratio of the diameter of a particle at a specified RH to that at dryness (DRH/D0). Growth curves were measured for particles generated from the isolated WSOC extracts using HTDMA, as described by Lowenthal et al. (Citation2009) and Hallar et al. (Citation2013). Aerosols were generated from the WSOC extracts using a TSI model 3076 constant-output atomizer. Growth curves were measured from dryness to 95% RH and from 95% RH to dryness. Particles with a dry diameter of 70 nm were selected by the first DMA, and humidified over a range of discrete RH where their hydrated sizes were measured. The uncertainty of measured growth factors is 0.02 (Lowental et al., Citation2009).
Results and Discussion
Chemical composition
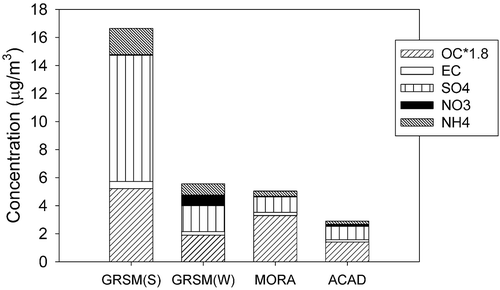
Average medvol chemical composition (sulfate [SO42-], nitrate [NO3-], ammonium [NH4+], OM, and EC) during the four studies is shown in . Chloride was detected in less than half of the samples and is not plotted in . presents the same data in numerical format. OM was estimated as 1.8 OC following the revised IMPROVE equation. Overall concentrations were higher at GRSM (S) than during the other studies. The lowest concentrations were found at ACAD. Sulfate was the dominant component at GRSM (S) (54%). OM was the dominant component at MORA (65%) and ACAD (48%), while OM and sulfate were roughly equivalent at GRSM (W). Average measurement uncertainties for OC, EC, SO42-, NO3-, and NH4+were 12, 21, 5, 26, and 5%, respectively. also presents average concentrations for subsets of samples where there were concurrent IMPROVE samples. IMPROVE samples are collected every third day, starting at midnight, local time. There were 10, 9, 9, and 8 such sample pairs for GRSM (S), GRSM (W), MORA, and ACAD, respectively. Average concentrations agreed well with the IMPROVE measurements.
Table 3. Average concentrations (µg/m3) during four field studies at three U.S. national parks
Figure 1. Average concentrations of OM (1.8OC), EC, sulfate, nitrate, and ammonium at GRSM (S), GRSM (W), MORA, and ACAD.
presents the study-average molar ratios of NH4+ to SO42- during the four studies. Assuming that NO3- was present as NH4NO3, measured NH4+ was corrected by subtracting NH4+ equivalent to NO3- (0.29 NO3-) from measured NH4+. The molar ratios of NH4+ to SO42- in ammonium sulfate (NH4)2SO4 and ammonium bisulfate (NH4HSO4) are 2 and 1, respectively. indicates that SO42- was acidic at GRSM (S) and ACAD, although the ratios were most variable at ACAD. Sulfate was nearly completely neutralized at GRSM (W) and MORA. If the atmosphere is chemically homogeneous during a 24-hr sample and all particles are at equilibrium with atmospheric gases, acidic SO42- would not be expected to coexist with NH4NO3. In this case, all NH4+ would be associated with SO42- and NO3- would be associated with other cations. also presents the molar ratios of (uncorrected measured) NH4+ to SO42-. Because NO3- concentrations are relatively low, the results are essentially the same. Sulfate was acidic at GRSM (S) and ACAD and neutralized at GRSM (W) and MORA. Ammonium sulfate in eq 2 overestimates sulfate compound mass and associated light extinction when sulfate is not completely neutralized by ammonia.
Table 4. Average molar ratios of NH4+ to SO42- during the four studies
The average ratios, expressed as percentages, of WSOC to OC were 22 ± 19, 21 ± 12, 77 ± 14, and 93 ± 21 at GRSM (S), GRSM (W), MORA, and ACAD, respectively. The high ratios at MORA and ACAD suggest that OM has the potential for exhibiting hygroscopic growth at those sites. The low ratios at GRSM may be biased because the field blank does not fully account for the positive adsorption artifact. Based on the average front filter OC and WSOC concentrations in , the WSOC/OC ratios at MORA calculated by subtracting the values of the field blank and backup filter from the front filter values are 0.78 (field blank) and 0.76 (backup filter). The corresponding WSOC/OC ratios at ACAD are 0.85 (field blank) and 0.93 (backup filter). Thus, the WSOC/OC ratios at MORA and ACAD are relatively insensitive to whether the field blank or backup filter is used to correct the front-filter WSOC and OC concentrations. If this was also the case at GRSM, then the relatively low WSOC/OC ratios there (0.22 and 0.21 during summer and winter, respectively) would not have changed dramatically had backup filters been available.
presents WSOC/OC ratios measured in previous studies at remote and urban sites. The WSOC/OC ratio depends on compound polarity and oxygen content. The ratio should be higher in aged aerosols at remote sites than in freshly emitted aerosols at urban sites. Zappoli et al. (Citation1999) speculated that the high ratio (0.77) at Aspvreten, Sweden, was due to a predominance of natural versus anthropogenic sources of organic aerosol, although no specific chemical evidence was presented. Kiss et al. (Citation2002) reported a high WSOC/OC ratio (0.71) at K-puszta, Hungary. Elemental analysis indicated an O:C ratio of 0.75 in isolated WSOC, and Fourier-transform infrared (FTIR) analysis identified oxygenated functional groups. Miyazaki et al. (Citation2006) found lower WSOC/OC ratios in Tokyo, Japan: 0.35 in summer and 0.20 in winter. WSOC was correlated with estimated secondary organic carbon (SOC) concentrations and the ratios of WSOC and SOC to OC were higher in summer than winter, presumably due to higher photochemical activity. Sullivan and Webber (Citation2006) measured WSOC/OC ratios of 0.47 in summer and 0.42 in winter in Atlanta, GA. The WSOC was separated into hydrophilic and hydrophobic components, each of which was divided into acidic, neutral, and basic compound classes. While the seasonal variation in WSOC/OC was small, there were seasonal differences in the WSOC chemistry. Hydrophilic and hydrophobic organic acids comprised 29 and 14% (43%), respectively, of WSOC in summer and 19 and 11% (30%), respectively, during winter. It was surmised that oxygen-rich organic acids were produced by secondary organic aerosol formation, which was enhanced during summer.
Table 5. Measurements of the WSOC/OC ratio in previous studies and in this study
Hallar et al. (Citation2013) reported an average WSOC/OC ratio of 0.89 at Storm Peak Laboratory (3210 m ASL), a remote continental site in northwestern Colorado. Water extracts of daily samples were combined for detailed chemical analysis of WSOC. A high-molecular-weight WSOC fraction was isolated from an 8-day composite sample using SPE (see earlier discussion). More than 4000 negative ion molecular formulas with an average O:C ratio of 0.53 were identified with ultra-high-resolution mass spectroscopy (Mazzoleni et al., Citation2012). The corresponding lower molecular weight fraction, not isolated by SPE, was analyzed for 47 organic acids, lignin derivatives, sugars, sugar alcohols, and sugar-anhydrates (Samburova et al., Citation2013). These compounds accounted for 17% of the WSOC and had an average O:C ratio of 1.22. Assuming that the high-molecular-weight fraction accounted for the remaining WSOC (83%), the overall average O:C ratio was 0.65. By contrast, Aitken et al. (2008) reported an average O:C ratio of 0.41 in Mexico City aerosols.
Ambient hydration state
AS-HTDMA measurements, as described in the preceding and by Taylor et al. (Citation2011), were conducted for five monodisperse ambient particle diameters: 0.025, 0.05, 0.1, 0.2, and 0.4 µm. presents the results as percentages of AS-HTDMA measurements for each particle size that indicated the presence of more- and less-hydrated states (MH and LH, respectively). These indicate hysteretic behavior where LH approximates the lower (deliquescence) branch and MH the upper (efflorescence) branch. While there was some variation as a function of particle size, for example, 0.4 µm at GRSM (S), the “total” column in illustrates the principal trend: hygroscopic growth was nonhysteretic (smooth and continuous uptake and loss of water with increasing and decreasing RH, respectively) 88, 50, 95, and >99% of the time at GRSM (S), GRSM (W), MORA, and ACAD, respectively. When hysteretic behavior was observed, it was mainly on the upper leg except for GRSM (W), where the LH state was observed 17% of the time. These results support the IMPROVE assumption that ambient hygroscopic growth approximates the most hydrated state of pure (NH4)2SO4.
Table 6. The frequency of detection (percent of sample periods) of alternative hydration states
The primary determinant of hydration state, especially the phase transitions resulting in hysteresis, is chemical composition. In the case of sulfate-dominated aerosol, such as was observed during the GRSM projects, hysteresis is governed mainly by the degree of sulfate neutralization. Fully neutralized, pure ammonium sulfate effloresces and deliquesces at ~40 and 80% RH, respectively. Generally, the RH of these transitions decreases with increasing acidity; sulfuric acid displays no hydration phase transitions under tropospheric conditions. Secondary influences on sulfate aerosol hydration behavior are caused by the presence of other inorganic ions and soluble organic species.
OM/OC ratios
In order to accurately estimate the OM/OC ratio (and WSOC hygroscopic growth), the XAD cleaning process for separating WSOC from inorganic ions must be highly efficient. It is assumed that the DCM extracts contain only OM and that the OM extracted in DCM is representative of the non-water-soluble fraction. The XAD cleaning efficiency was determined by comparing the sample-equivalent concentrations of sulfate in the initial and final XAD-treated water extracts. The cleaning efficiencies were extremely high: 99.6 ± 0.3, 99.2 ± 0.2, 99.8 ± 0.2, and 99.8 ± 0.1% at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Two passes through the XAD columns were needed to achieve a sulfate removal efficiency >99% in the GRSM (S) study. Average WSOC recoveries ranged from 46 to 100%, consistent with recoveries of 63–71% reported by Duarte and Duarte (Citation2005). Incomplete recovery from the XAD columns is due to irreversible retention of high-molecular-weight OM by the columns and coelution of low-molecular-weight compounds with the inorganic ions (Hallar et al., Citation2013; Samburova et al., Citation2013).
Average OM/OC ratios are presented for the four studies in . The standard deviations represent the variability among composite samples in each study. The average OM/OC ratio was estimated from the OM/OC ratios in the DCM and water extracts using weighting based on the ratios of WSOC/OC summarized in . It is assumed that the fraction of particulate OC in the DCM extract is 1 minus WSOC/OC. All of the ratios presented in for DCM and water extracts are 1.8 or higher. The average ratios for WSOC range from 2.1 at MORA to 2.4 at GRSM (W). The DCM OM/OC ratios were lower than the OM/OC ratios in WSOC at GRSM (S) and ACAD, equal to the WSOC OM/OC ratio at MORA, and larger than the WSOC OM/OC ratio at GRSM (W) (2.8 vs. 2.4, respectively). The high DCM ratio of 2.8 at GRSM (W) results in the largest weighted average of 2.7. Because high OM/OC ratios are thought to result from polar compounds containing relatively higher amounts of oxygen, it is not clear what accounts for the high DCM ratio at GRSM during winter. Excluding GRSM (W), weighted-average OM/OC ratios ranged from 2.0 to 2.2.
Table 7. Average OM/OC ratios in DCM and water extracts during the four studies
The OM/OC ratios in material that was not recovered from the XAD columns or that passed through them along with the inorganic ions are unknown for this study. However, a similar study was conducted at Storm Peak Laboratory (3210 m ASL) in northwestern Colorado during summer 2010. As described earlier, Samburova et al. (Citation2013) measured the composition of ~50 sugar, sugar anhydrate, sugar alcohol, organic acid, and lignin derivative compounds that passed through the XAD columns along with inorganic ions during the water wash step. Based on their molecular formulas and concentrations, the OM/OC ratio of these compounds was 2.87. The OM/OC ratio of the high-molecular-weight water-soluble compounds retained by the XAD columns was estimated to be 1.87 based on the molecular formulas identified with ultra-high-resolution mass spectroscopy (Mazzoleni et al., Citation2012). The overall mass-weighted OM/OC ratio was 2.04. The OM/OC ratios estimated in the current study are operationally defined and represent material “extractable” in DCM and water. The implicit assumption is that the OM/OC ratios in the extracted OM represent those in the OM that was not recovered. Nonetheless, these results are consistent with OM/OC ratios estimated in previous studies by a variety of methods, for example, Yu et al. (Citation2005) (1.9), Turpin and Lim (Citation2001) (2.1), Kondo et al. (Citation2007) (1.7–2.2), Chen and Yu (Citation2007) (1.9–2.2), El Zanan et al. (Citation2005) (2.0), Krivácsy et al. (Citation2001) (1.9), and Kiss et al. (Citation2002) (1.93).
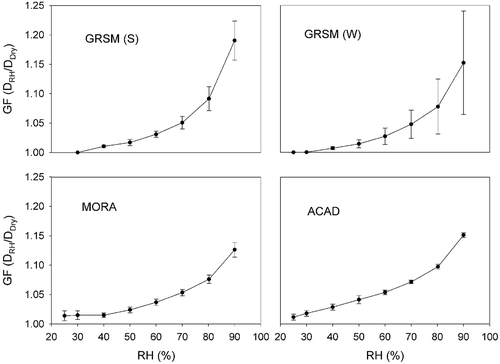
WSOC hygroscopic growth factors
Hygroscopic growth curves were measured as already described for aerosols generated from water extracts containing isolated WSOC. GFs were determined in scans from low to high RH and from high to low RH. The results were similar to those presented by Lowenthal et al. (Citation2009) for GRSM (S). The up-scans and down-scans were nearly identical and the growth of WSOC was continuous in both cases. Average down-scan GFs are shown for the four studies in . The results for GRSM (W) were the most variable. Average growth factors at 90% RH (GF90) were 1.19, 1.15, 1.13, and 1.15 at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Average growth factors at 80% RH (GF80) were 1.09, 1.08, 1.08, and 1.10 at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Gysel et al. (Citation2004) reported GF90 ranging from 1.08 to 1.17 for WSOC isolated from aerosol samples collected at K-puszta. For comparison, the GF90 for pure (NH4)2SO4 at 90% RH is 1.74 (Tang and Munkelwitz, Citation1994). While organic hygroscopicity is relatively small compared to that of inorganic constituents like ammonium sulfate, its contribution to particle growth and light extinction is not zero and becomes more significant at locations like MORA and ACAD where OM dominates aerosol mass and the WSOC/OC ratio is high.
Relationship between the dry particle size distribution and concentration
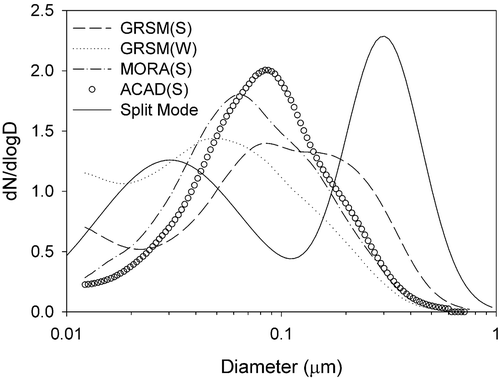
The split component model in the revised IMPROVE equation is based on observations that dry mass scattering efficiencies increase with concentration (Pitchford et al., Citation2007). Gas-to-particle conversion under dry or in-cloud conditions is expected to increase both particle size and concentrations of sulfates, nitrates, and organics. presents average SMPS size distributions from each of the four studies. Individual size distributions were normalized to their total number concentration before averaging. The revised IMPROVE equation assumes small and large lognormal size modes with geometric mass mean diameters of 0.2 and 0.5 µm, respectively, and corresponding geometric standard deviations of 2.2 and 1.5, respectively (Pitchford et al., Citation2007). These mass size distributions were converted to their corresponding number distributions by dividing the mass by diameter cubed. Small and large mode number distributions with concentrations normalized to their total number concentration are plotted in for comparison with the average measured distributions. Note that the average measured distributions during all four studies lie between the small and large modes specified for the revised IMPROVE equation. This suggests a range of ambient size modes from day to day, as opposed to two distinct small and large modes of varying amplitude.
Figure 3. Average dry size distributions during the four studies. Individual size distributions were normalized to the total number concentration before averaging.
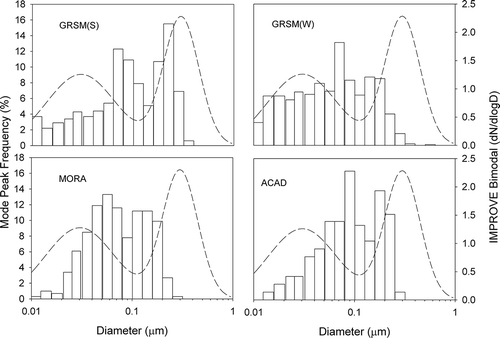
Each measured SMPS size distribution was fitted to multiple lognormal distributions. Only fitted modes accounting for greater than 10% of the total measured particle concentration were considered. The frequency distributions of the peak diameters of the fitted modes during each study are plotted in . The small and large (IMPROVE) distributions are also plotted, as in . There were few cases where the modal peaks corresponded with the large IMPROVE size mode. At MORA and ACAD, most of the modal peak diameters fell between the small and large IMPROVE size modes. However, at GRSM, a significant fraction of the modal peaks corresponded to the small IMPROVE mode, especially during the winter study. This suggests that aerosols at GRSM are less aged and that emissions sources are closer than at the other sites. These observations demonstrate that the split component model is operationally, if not physically, reasonable.
Conclusion
Light extinction by atmospheric aerosols depends on particle size and chemical composition. Chemical light extinction in the IMPROVE network in U.S. national parks is estimated from the bulk chemical composition of PM2.5 filter samples. Mass scattering efficiencies are applied to chemical concentrations to derive light extinction. The revised IMPROVE equation specifies mass scattering efficiencies for sulfates, nitrates, and organics, which increase with increasing concentration. This is based on the assumption that particle growth caused by gas-to-particle conversion during transport leads to higher concentration as well as larger mass scattering efficiency. Light scattering is enhanced by hygroscopic growth, and growth factors that vary as a function of relative humidity (RH) are applied to light scattering attributed to chemical constituents such as sulfate and nitrate. However, the IMPROVE equation assumes that organic matter (OM) is not hygroscopic. The IMPROVE equation also assumes that hygroscopic growth follows the upper leg of the (NH4)2SO4 growth curve. A long-term study was conducted at Great Smoky Mountains National Park during summer [GRSM (S)] and winter [GRSM (W)], and at Mount Rainier National Park (MORA) and Acadia National Park (ACAD) during summer to evaluate these assumptions.
Chemical composition varied significantly among the sites. Sulfates were dominant at GRSM (S), while OM was dominant at MORA and ACAD. Ammonium is not measured in the IMPROVE network and sulfate is assumed to be completely neutralized as (NH4)2SO4. The results of the current study show that sulfates were nearly completely neutralized at GRSM (W) and MORA, where average molar ratios of ammonium to sulfate were 1.76 and 1.80, respectively, but were acidic at GRSM (S) and ACAD, where average molar ratios of ammonium to sulfate were 1.16 and 1.00, respectively. The IMPROVE equation would overestimate ammoniated sulfate mass under these conditions.
The IMPROVE equation assumes that hygroscopic growth of ammonium sulfate and ammonium nitrate follows the upper branch of the ammonium sulfate hysteresis loop. This assumption was examined by subjecting size-segregated (0.025, 0.05, 0.1, 0.2, and 0.4 µm diameter) ambient aerosols to drying and humidification cycles to determine their ambient hygroscopic state. Hysteretic behavior was observed 50% of the time at GRSM (W) for all particle sizes. Growth followed the upper branch of a hysteresis loop in roughly two-thirds of these cases and the lower branch in one-third. GRSM (W) was the only study where deliquescence was observed. However, growth was smooth and continuous with increasing or decreasing RH for the other 50% of sample periods. Hysteretic growth was observed at GRSM (S) in 7 to 16% of the measurements but this was always on the upper branch (more hydrated state). The remainder of the measurements exhibited smooth and continuous growth. This was also the case at MORA, although the percentage of measurements exhibiting hysteresis on the upper branch ranged from 4 to 5%. No deliquescence was observed. Growth was always smooth and continuous at ACAD; no hysteretic behavior was observed. Hysteretic behavior at GRSM (W) is likely related to complete neutralization of sulfate at this site, although the presence of organics (34% of the sum of species) clearly modifies the hygroscopic behavior of the pure salt(s). Sulfate is also completely neutralized at MORA, but since OM is the dominant aerosol component (65% of the sum of species), hysteresis is rarely observed. Our results support the IMPROVE assumption that hygroscopic growth of sulfates and nitrates is characterized by their most hydrated state, assuming they are present as pure salts.
Organic carbon (OC) is routinely measured using thermal techniques, as is the case in the IMPROVE network. The revised IMPROVE equation converts OC to OM assuming a factor of 1.8 to account for unmeasured oxygen, hydrogen, sulfur, and other species in organic material.
In this study, the OM/OC ratio was measured directly by isolating OM extracted in water and organic solvent and measuring its mass and OC content. The average OM/OC ratios at GRSM (S), GRSM (W), MORA, and ACAD were 2.0 ± 0.3, 2.7 ± 0.3, 2.1 ± 0.2, and 2.2 ± 0.2, respectively. WSOC is expected to contain more oxygenated material than compounds extractable in organic solvents. A higher ratio of WSOC/OC would be expected to correspond to higher OM/OC ratios, but in both GRSM studies, water-soluble organic carbon (WSOC) was only 21–22% of total OC. In any case, the overall result of this study points to an OM/OC ratio of 2 or greater, suggesting that the revised IMPROVE equation underestimates OM mass by about 10%, at least, at these sites.
The potential contribution of OM to hygroscopic growth depends on the magnitude of the water-soluble fraction. In this study, it was found that the average ratios of WSOC to OC were 22, 21, 77, and 93% at GRSM (S), GRSM (W), MORA, and ACAD, respectively. Thus, hygroscopic growth of OM could be significant at MORA and ACAD, where OM is the main component of particle mass. Hygroscopic growth factors (GF) were measured directly on particles derived from isolated WSOC. Average growth factors at 90% and 80% RH ranged from 1.13 to 1.19 and from 1.08 to 1.10, respectively, during the four studies. While hygroscopic growth of organic material is smaller than that associated with inorganic compounds, it is not zero and may significantly increase particle size and thus light extinction at sites such as MORA and ACAD where OM is a significant component of particle mass and where most of the OM is water-soluble.
The revised IMPROVE equation employs a split mode model to account for the increase in dry mass scattering efficiency with concentration. However, measured particle size distributions do not appear to correspond with the small and large size modes of that model but generally lie between them. This suggests that aerosol distributions grow to greater or lesser extents along a continuum. It would be difficult if not impossible to generalize the evolution of particle size distributions across the IMPROVE network. The linear combination of small and large modes in the revised IMPROVE equation appears to be a reasonable empirical device that explains observed Bsp better than the fixed dry scattering efficiencies in the original IMPROVE equation.
Future work will apply the results of this study to estimating light scattering using long-term aerosol data at the three study sites and to the other U.S. national parks in the IMPROVE network. Since neither ammonium nor the WSOC/OC ratio is routinely measured, a complex sensitivity test matrix must be constructed. For example, the OM/OC ratio can vary from 1.8 to our experimental value of 2.1. The water-soluble organic fraction can be assumed to range from zero to 100%. While sulfates were generally acidic at the study sites, this is not necessarily the case at other IMPROVE sites. The effects of assuming fully or partially neutralized sulfate will be quantified. Finally, estimated and measured Bsp will be compared at sites where such measurements are available. The feasibility of revising the IMPROVE equation will be explored.
Funding
This work was supported by the EPRI in Palo Alto, CA. This research would not have been possible without the cooperation of the National Park Service and its personnel at Great Smoky Mountains NP, Mount Rainier NP, and Acadia NP.
Additional information
Funding
Notes on contributors
Douglas Lowenthal
Douglas Lowenthal, Barbara Zielinska, and Vera Samburova are research professors at the Desert Research Institute, Nevada System of Higher Education, in Reno, NV.
Barbara Zielinska
Douglas Lowenthal, Barbara Zielinska, and Vera Samburova are research professors at the Desert Research Institute, Nevada System of Higher Education, in Reno, NV.
Vera Samburova
Douglas Lowenthal, Barbara Zielinska, and Vera Samburova are research professors at the Desert Research Institute, Nevada System of Higher Education, in Reno, NV.
Don Collins
Don Collins is a professor of Atmospheric Sciences and director of the Environmental Programs in Geosciences at Texas A&M University, College Station, TX.
Nathan Taylor
Nathan Taylor is a graduate student in Atmospheric Sciences at Texas A&M University, College Station, TX.
Naresh Kumar
Naresh Kumar is a senior program manager at EPRI, Palo Alto, CA.
References
- Alves, C., A. Carvalho, and C. Pio. 2002. Mass balance of organic carbon fractions in atmospheric aerosols. J. Geophys. Res. 107:ICC 7-1–ICC 7-9. D21, 8345. doi:10.1029/2001JD000616
- Chan, M.N., and C.K. Chan. 2007. Mass transfer effects on the hygroscopic growth of ammonium sulfate particles with a water-insoluble coating. Atmos. Environ. 41:4423–33. doi:10.1016/j.atmosenv.2007.01.047
- Chen, X., and J.Z. Yu. 2007. Measurement of organic mass to organic carbon ratio in ambient aerosol samples using a gravimetric technique in combination with chemical analysis. Atmos. Environ. 41:8857–64. doi:10.1016/j.atmosenv.2007.08.023
- Chow, J.C., J.G. Watson, L.C. Pritchett, W.R. Pierson, C.A. Frazier, and R.G. Purcell. 1993. The DRI thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. air quality studies. Atmos. Environ. 27A:1185–202. doi:10.1016/0960-1686(93)90245-T
- Chow, J.C., J.G. Watson, D.H. Lowenthal, L.-W. Chen, and K. Magliano. 2006. Particulate carbon measurements in California’s San Joaquin Valley. Chemosphere 62:337–48. doi:10.1016/j.chemosphere.2005.04.094
- Clegg, S.L., P. Brimblecombe, and A.S. Wexler. 1998. A thermodynamic model of H-NH4-SO4-NO3-H2O at tropospheric temperatures. J. Phys. Chem. 102A:2137–54. doi:10.1021/jp973042r
- Decesari, S., M.C. Facchini, E. Matta, F. Lettini, M. Mircea, S. Fuzzi, E. Tagliavini, and J.-P. Putaud. 2001. Chemical features and seasonal variation of fine aerosol water-soluble compounds in the Po Valley, Italy. Atmos. Environ. 35:3691–99. doi:10.1016/S1352-2310(00)00509-4
- Dinar, E., I. Taraniuk, E.R. Graber, S. Katsman, T. Moise, T. Anttila, T.F. Mentel, and Y. Rudich. 2006. Cloud condensation nuclei properties of model and atmospheric HULIS. Atmos. Chem. Phys. 6:2465–81. doi:10.5194/acp-6-2465-2006
- Dinar, E., I. Taraniuk, E.R. Graber, T. Anttila, T.F. Mentel, and Y. Rudich. 2007. Hygroscopic growth of atmospheric and model humic-like substances. J. Geophys. Res. 112:D05211. doi:10.1029/2006JD007442
- Duarte, R.M.B.O., and A.C. Duarte. 2005. Application of non-ionic solid sorbents (XAD resins) for the isolation and fractionation of water-soluble organic compounds from atmospheric aerosols. J. Atmos. Chem. 51:79–93. doi:10.1007/s10874-005-8091-x
- El-Zanan, H.S., D.H. Lowenthal, B. Zielinska, J.C. Chow, and N. Kumar. 2005. Determination of the organic aerosol mass to organic carbon ratio in IMPROVE samples. Chemosphere 60:485–96. doi:10.1016/j.chemosphere.2005.01.005
- Gasparini, R., R. Li, and D.R. Collins. 2004. Integration of size distributions and size-resolved hygroscopicity measured during the Houston Supersite for compositional categorization of the aerosol. Atmos. Environ. 38:3285–303. doi:10.1016/j.atmosenv.2004.03.019
- Gysel, M., E. Weingartner, S. Nyeki, D. Paulsen, U. Baltensperger, I. Galambos, and G. Kiss. 2004. Hygroscopic properties of water-soluble matter and humic-like organics in atmospheric fine aerosol. Atmos. Chem. Phys. 4:35–50. doi:10.5194/acp-4-35-2004
- Hallar, A.G., D.H. Lowenthal, S.L. Clegg, V. Samburova, N. Taylor, L.R. Mazzoleni, B.K. Zielinska, T.B. Kristensen, G. Chirokova, I.B. McCubbin, C. Dodson, and D. Collins. 2013. Chemical and hygroscopic properties of aerosol organics at Storm Peak Laboratory. J. Geophys. Res. 118:4767–79. doi:10.1002/jgrd.50373
- Hayasaka, T., T. Nakajima, S. Ohta, and M. Tanaka. 1992. Optical and chemical properties of urban aerosols in Sendai and Sapporo, Japan. Atmos. Environ. 26A:2055–62. doi:10.1016/0960-1686(92)90090-8
- Kiss, G., B. Varga, I. Galambos, and I. Ganszky. 2002. Characterization of water-soluble organic matter isolated from atmospheric fine aerosol. J. Geophys. Res. 107:D21, 8339. doi:10.1029/2001JD000603
- Kondo, Y., Y. Miyazaki, N. Takegawa, T. Miyakawa, R.J. Weber, J.L. Jiminez, Q. Zhang, and D.R. Worsnop. 2007. Oxygenated and water-soluble organic aerosols in Tokyo. J. Geophys. Res. 112:D01203. doi:10.1029/2006JD007056
- Krivácsy, Z., A. Hoffer, Z. Sárvári, D. Temesi, D., U. Baltensperger, S. Nyeki, E. Weingartner, S. Kleefeld, and S.G. Jennings. 2001. Role of organic and black carbon in the chemical composition of atmospheric aerosol at European background sites. Atmos. Environ. 35:6231–44. doi:10.1016/S1352-2310(01)00467-8
- Lowenthal, D.H., C.F. Rogers, P. Saxena, J.G. Watson, and J.C. Chow. 1995. Sensitivity estimated light extinction coefficients to model assumptions and measurement errors. Atmos. Environ. 29:751–66. doi:10.1016/1352-2310(94)00340-Q
- Lowenthal, D.H., and N. Kumar. 2004. Variation of mass scattering efficiencies in IMPROVE. J. Air Waste Manage. Assoc. 54:926–34. doi:10.1080/10473289.2004.10470969
- Lowenthal, D.H., B. Zielinska, B. Mason, S. Samy, V. Samburova, D. Collins, C. Spencer, N. Taylor, J. Allen, and N.K. Kumar. 2009. Aerosol characterization studies at Great Smoky Mountains National Park, summer 2006. J. Geophys. Res. 114:D08206. doi:10.1029/2008JD011274
- Malm, W.C. 2000. Spatial and seasonal patterns and temporal variability of haze and its constituents in the United States, IMPROVE Report III. Cooperative Institute for Atmosphere, Fort Collins, CO, May. http://vista.cira.colostate.edu/improve/Publications/Reports/2000/PDF/IMPROVEReport_2000.pdf.
- Malm, W.C., and J.L. Hand. 2007. An examination of the physical and optical properties of aerosols collected in the IMPROVE program. Atmos. Environ. 41:3407–27. doi:10.1016/j.atmosenv.2006.12.012
- Malm, W.C., J.F., Sisler, D. Huffman, R.A. Eldred, and T.A. Cahill. 1994. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J. Geophys. Res. 99:1347–70. doi:10.1029/93JD02916
- Malm, W.C., D.E. Day, S.M. Kreidenweis, J.L. Collett, and T. Lee. 2003. Humidity-dependent optical properties of fine particles during the Big Bend Regional Aerosol and Visibility Study. J. Geophys. Res. 108:4279. doi:10.1029/2002JD002998
- Malm, W.C., D.E. Day, C. Carrico, S.M. Kreidenweis, J.L. Collett, Jr., G. McMeeking, J. Lee, and J. Carillo. 2005. Intercomparison and closure calculations using measurements of aerosol species and optical properties during the Yosemite Aerosol Characterization Study. J. Geophys. Res. 110:4302. doi:10.1029/2004JD005494
- Mazzoleni, L.R., P. Saranjampour, M. M. Dalbec, V. Samburova, A. G. Hallar, B. Zielinska, D. H. Lowenthal, and S. Kohl. 2012. Identification of water-soluble organic carbon in non-urban aerosols using ultra-high-resolution FT-ICR mass spectrometry: Organic anions. Environ. Chem. 9:285–97. doi:10.1071/EN11167
- McInnes, L., M.H. Bergin, J. Ogren, and S. Schwartz. 1998. Apportionment of light scattering and hygroscopic growth to aerosol composition. Geophys. Res. Lett. 25(4):513–16. doi:10.1029/98GL00127
- McMeeking, G.R., S.M. Kreidenweis, C.M. Carrico, T. Lee, J.L Collett, Jr., and W.C. Malm. 2005. Observations of smoke influenced aerosol during the Yosemite Aerosol Characterization Study, Part I: Size distributions and chemical composition. J. Geophys. Res. 110:D09206. doi:10.1029/2004JD005389
- Miyazaki, Y., Y. Kondo, N. Takegawa, Y. Komazaki, M. Fukuda, K. Kawamura, M. Mochida, K. Okuzawa, and R.J. Weber. 2006. Time-resolved measurements of water-soluble organic carbon in Tokyo. J. Geophys. Res. 111:D23, 2156–202. doi:10.1029/2006JD007125
- Modini, R.L., G.R. Johnson, C. He, and Z.D. Ristovski. 2010. Observation of the suppression of water uptake by marine particles. Atmos. Res. 98(2–4):219–28. doi:10.1016/j.atmosres.2010.03.025
- Pitchford, M., W. Malm, B. Schichtel, B., N. Kumar, D. Lowenthal, and J. Hand. 2007. Revised algorithm for estimating light extinction from IMPROVE particle speciation data. J. Air Waste Manage. Assoc. 57:1326–36. doi:10.3155/1047-3289.57.11.1326
- Polidori, A., B.J. Turpin, C.I. Davidson, L.A. Rodenburg, and F. Maimone. 2008. Organic PM2.5: Fractionation by polarity, FTIR spectroscopy, and OM/OC ratio for the Pittsburgh aerosol. Aerosol Sci. Technol. 42:233–46. doi:10.1080/02786820801958767
- Rinehart, L.R., E.M. Fujita, J.C. Chow, K.L. Magliano, K.L., and B. Zielinska. 2006. Spatial distribution of PM2.5 associated organic compounds in central California. Atmos. Environ. 40: 290–303. doi:10.1016/j.atmosenv.2005.09.035
- Ryan, P.A., D. Lowenthal, and N. Kumar. 2005. Improved light extinction reconstruction in Interagency Monitoring of Protected Visual Environments. J. Air Waste Manage. Assoc. 55:1751–59. doi:10.1080/10473289.2005.10464768
- Samburova, V., A.G. Hallar, L.R. Mazzoleni, P. Saranjampour, D. Lowenthal, S. Kohl, and B. Zielinska. 2013. Composition of water-soluble organic carbon in non-urban atmospheric aerosol collected at the Storm Peak Laboratory. Environ. Chem. 10:370–80. doi:10.1071/EN13079
- Santarpia, J.L., R. Li, and D.R. Collins. 2004. Direct measurement of the hydration state of ambient aerosol populations. J. Geophys. Res. 109:D18209. doi:10.1029/2004JD004653
- Stolzenburg, M., N. Kreisberg, and S. Hering. 1998. Atmospheric size distributions measured by differential mobility optical particle size spectrometry. Aerosol Sci. Technol. 29:402– 18. doi:10.1080/02786829808965579
- Subramanian, R., A.Y. Khlystov, J.C. Cabada, and A.L. Robinson. 2004. Positive and negative artifacts in particulate organic carbon measurements with denuded and undenuded sampler configurations. Aerosol Sci. Technol. 38(S1):27–48. doi:10.1080/02786820390229354
- Sullivan, A.P., and R.J. Weber. 2006. Chemical characterization of the ambient organic aerosol soluble in water: 2. Isolation of acid, neutral, and basic fractions by modified size-exclusion chromatography. J. Geophys. Res. 111:D05315. doi:10.1029/2005JD006486
- Tang, I.N., and H.R. Munkelwitz. 1994. Water activities, densities, and refractive indices of aqueous sulfates and sodium nitrate droplets of atmospheric importance. J. Geophys. Res. 99:18,801–8. doi:10.1029/94JD01345
- Taylor, N.F., D.R.Collins, C.W. Spencer, D.H. Lowenthal, B. Zielinska, V. Samburova, and N. Kumar. 2011. Measurement of ambient aerosol hydration state at Great Smoky Mountains National Park in the southeastern United States. Atmos. Chem. Phys. 11:12,085–107. doi:10.5194/acp-11-12085-2011
- Turpin, B.J., J.J. Huntzicker, and S.V. Hering. 1994. Investigation of organic aerosol sampling artifacts in the Los Angeles Basin. Atmos. Environ. 28:3061–71. doi:10.1016/1352-2310(94)00133-6
- Turpin, B.J., and H.-J. Lim. 2001. Contributions to PM2.5 mass concentrations: revisiting common assumptions for estimating organic mass. Aerosol. Sci. Technol. 35:602–10. doi:10.1080/02786820119445
- Varga, B., G. Kiss, I. Ganszky, A. Gelencser, and Z. Krivácsy. 2001. Isolation of water-soluble organic matter from atmospheric aerosol. Talanta 55:561–72. doi:10.1016/S0039-9140(01)00446-5
- White, W.H. 1986. On the theoretical and empirical basis for apportioning extinction by aerosols: a critical review. Atmos. Environ. 20:1659–72. doi:10.1016/0004-6981(86)90113-7
- White, W.H., and P.T. Roberts. 1977. On the nature and origins of visibility-reducing aerosols in the Los Angeles air basin. Atmos. Environ. 11:803–12. doi:10.1016/0004-6981(77)90042-7
- Yang, H., Q. Li, and J. Yu. 2003. Comparison of two methods for the determination of water-soluble organic carbon in atmospheric particles. Atmos. Environ. 37:865–70. doi:10.1016/S1352-2310(02)00953-6
- Yu, L.E., M.L. Shulman, R. Kopperud, and L.M. Hildemann. 2005. Fine organic aerosols collected in a humid, rural location (Great Smoky Mountains, Tennessee, USA): Chemical and temporal characteristics. Atmos. Environ. 39:6037–50. doi:10.1016/j.atmosenv.2005.06.043
- Zappoli, S., A. Andracchio, S. Fuzzi, M.C. Facchini, A. Gelencser, G. Kiss, Z. Krivácsy, A. Molnar, E. Meszaros, H.C. Hansson, K. Rosman, and Y. Zebuhr. 1999. Inorganic, organic and macromolecular components in different areas of Europe in relationship with solubility. Atmos. Environ. 33:2733–43. doi:10.1016/S1352-2310(98)00362-8
- Zhang, X.Q., B.J. Turpin, P.H. McMurry, S.V. Hering, and M.R. Stolzenburg. 1994. Mie theory evaluation of species contributions to 1990 wintertime visibility reduction in the Grand Canyon. J. Air Waste Manage. Assoc. 44:153–62. doi:10.1080/1073161X.1994.10467244
- Zhang, Q., M.R. Alfarra, D.R. Worsnop, J.D. Allan, H. Coe, M.R. Canagaratna, and J.L. Jimenez. 2005. Deconvolution and quantification of hydrocarbon-like and oxygenated organic aerosols based on aerosol mass spectrometry. Environ. Sci. Technol. 39:4938–52. doi:10.1021/es048568l