Abstract
The potential adverse health effects of PM2.5 (particulate matter with an aerodynamic diameter <2.5 μm) and vapor samples from three communities that neighbor railyards, Commerce (CM), Long Beach (LB), and San Bernardino (SB), were assessed by determination of chemical reactivities attributed to the induction of oxidative stress by air pollutants. The assays used were dithiothreitol (DTT)- and dihydrobenzoic acid (DHBA)-based procedures for prooxidant content and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) assay for electrophiles. Prooxidants and electrophiles have been proposed as the reactive chemical species responsible for the induction of oxidative stress by air pollution mixtures. The PM2.5 samples from CM and LB sites showed seasonal differences in reactivities, with higher levels in the winter, whereas the SB sample differences were reversed. The reactivities in the vapor samples were all very similar, except for the summer SB samples, which contained higher levels of both prooxidants and electrophiles. The results suggest that the observed reactivities reflect general geographical differences rather than direct effects of the railyards. Distributional differences in reactivities were also observed, with PM2.5 fractions containing most of the prooxidants (74–81%) and the vapor phase most of the electrophiles (82–96%). The high levels of the vapor-phase electrophiles and their potential for adverse biological effects point out the importance of the vapor phase in assessing the potential health effects of ambient air.
Implications: PM2.5 and its corresponding vapor phase, containing semivolatile organics, were collected in three communities in the Los Angeles Basin and examined with toxicologically relevant chemical assays. The PM2.5 phase contained most of the prooxidants and the vapor phase contained most of the electrophiles, whose content was highest in summer samples from a receptor site that reflected greater photochemical processing of the air parcel during its transport. As electrophiles initiate both adverse and adaptive responses to foreign substances by biological systems, their presence in the vapor phase emphasizes the importance of this phase in the overall health effects of ambient air.
Introduction
This laboratory has been studying the chemical properties of air pollutant mixtures to understand their relationship to the adverse health effects associated with air pollution. The chemical properties of particular interest are the capacity to generate reactive oxygen species, or prooxidant activity, and the capacity to form covalent bonds with functional groups on biological macromolecules, or electrophilic activity. Numerous studies have attributed several adverse health effects related to air pollution to the induction of oxidative stress (Baeza-Squiban et al., Citation1999; Donaldson et al., Citation2001; Li et al., Citation2002; Nel et al., Citation2001), a state in which the cell has a high proportion of oxidized species generated from an increase in reactive oxygen, typically mediated by prooxidants utilizing endogenous electron sources such as NADPH (nicotinamide adenine dinucleotide phosphate) and ascorbate. The second property, electrophilicity, whose relationship to air pollution toxicology has been far less studied, can cause irreversible inactivation of biological molecules, including those with regulatory functions (Jacobs and Marnett, Citation2010; Monks and Lau, Citation1992). Cellular changes, mediated by these reactive species, trigger multiple signaling pathways leading to inflammation and other forms of cellular distress (Donaldson and Tran, Citation2002; Schafer and Buettner, Citation2001; Squadrito et al., Citation2001). Inorganic and organic chemical species associated with ambient aerosols can participate in these reactions. For example, particulate transition metals such as iron and copper are capable of generating reactive oxygen species by catalyzing the transfer of electrons from a reducing source such as ascorbate to oxygen (Di Stefano et al., Citation2009) and organic species such as quinones can transfer electrons from NADPH to oxygen to generate superoxide and other reactive oxygen species (Kumagai et al., Citation1998b, Citation2002). Quinones and quinone-like compounds exhibit both prooxidant and electrophilic activities, altering activity of regulatory proteins and enzymes by both actions (Kumagai et al., Citation1998a, Citation2012; Shinyashiki et al., Citation2008). Quinones are found in the particle and vapor phases of ambient air (Cho et al., Citation2004; Chung et al., Citation2006; Delgado-Saborit et al., Citation2013; Jakober et al., Citation2007; Valavanidis et al., Citation2006) and are generated in vivo following exposure to their parent polycyclic aromatic hydrocarbons (PAHs; e.g., Kumagai et al., Citation2012; Waidyanatha and Rappaport, Citation2008).
This report describes results of an attempt to assess the role of railyards on toxicologically relevant reactivities of ambient air in neighboring communities by analysis of PM2.5 (particulate matter with an aerodynamic diameter <2.5 μm) and their corresponding vapor-phase organics in samples from three communities in the Los Angeles Basin, Long Beach (LB), Commerce (CM), and San Bernardino (SB) (). Collections took place during the months of November–December 2009 and July–August 2010, which are the cool and warm seasons in the basin, to provide a means of assessing seasonal differences. The assays measured prooxidant content with dithiothreitol (DTT)- and dihydroxybenzoate (DHBA)-based procedures and electrophile content with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-based procedure.
Methods
Reagents
Chicken muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH), NAD+, ethylenediaminetetraacetic acid (EDTA), glyceraldehyde-3-phosphate, dithiothreitol (DTT), 5,5ʹ-dithiobis(2-nitrobenzoic acid) (DTNB), sodium salicylate, ascorbic acid, diethylenetriaminepentaacetic acid (DTPA), citric acid trisodium salt dihydrate, and monohydrate were purchased from Sigma-Aldrich (St. Louis, MO). Other reagents were of the highest grade available and purchased from Fisher Scientific (Pittsburgh, PA).
Sample collection
Medium-volume samplers (Tisch model 1202; Cleves, OH) were placed in the selected locations, and PM2.5 and vapors were collected for 48 hr each week for a month during the summer and winter seasons for a total of eight samples at each site. Teflon-coated glass fiber filters (Pall Corp, East Hills, NY) were used for PM2.5 collection and XAD-4 resin beds (Acros, Thermo Fisher Scientific; Houston, TX) for the vapor phase. Sampling details and matrix cleaning procedures have been previously published (Eiguren-Fernandez et al., Citation2004). Estimates of the volume equivalent were based on the total air volume collected divided by the area of the filter, to obtain volume of air (m3) per square cm of filter. Then, for analysis of the PM on the filter, punches of known area were obtained and used in the extraction process.
Sample extraction
Aqueous suspensions of PM2.5 samples were obtained by sonicating filter punches in deionized water for 20 min. As this process causes partial decomposition of the filters, it was not possible to measure the mass on the filter. Accordingly, the volume equivalent of air was used to describe the final concentration of the particles in the aqueous suspension, which was 2.2–6.0 m3/mL.
XAD resin beds containing the trapped vapor-phase organic components (mostly volatile and semivolatile organic components) corresponding to each particle sample were extracted by sonication (30 min) with dichloromethane (DCM). The suspension was filtered through a 0.45-µm nylon filter (Millipore, Billerica, MA), volume reduced, and solvent evaporated into a known volume of dimethyl sulfoxide (DMSO), so the concentration for analysis could be expressed as m3 per mL of DMSO. The final concentration of the organic extract was approximately 300 m3/mL of DMSO. Blanks were prepared as described previously and used as controls (Eiguren-Fernandez et al., Citation2004).
Chemical assays
A summary of the assays used is given in .
Table 1. Assays used
DTT assay
This assay measures the prooxidant content of the sample based on its ability to transfer electrons from DTT to oxygen (Cho et al., Citation2005; Kumagai et al., Citation2002). In brief, the sample was incubated with DTT for varying times and quenched by addition of 5,5′-dithiobis(2-dinitro)benzoic acid (DTNB) and the remaining DTT measured by the absorption at 412 nm. Rates were calculated after averaging duplicate runs and were blank corrected. Since DTT can be oxidized by high concentrations of metal ions (Kachur et al., Citation1997; Netto and Stadtman, Citation1996), the contribution of metals to the DTT-based redox activity was also determined by adding the metal chelator diethylenetriaminepentaacetic acid (DTPA) (20 µM) to one set of the samples. Results are reported as nanomoles of DTT consumed per minute per m3 of air sample.
DHBA assay
This assay assesses the ability of transition metals associated with the ambient particles to catalyze the Fenton reaction in which hydrogen peroxide is converted to hydroxyl radical (Di Stefano et al., Citation2009). Briefly, aliquots of PM2.5 water suspensions were incubated for varying times with ascorbic acid and salicylate and the rates of formation of dihydroxybenzoic acids (DHBAs) determined following reaction termination by the addition of metaphosphoric acid. DHBA formation by the samples was completely blocked by the metal chelator DTPA, which forms an unreactive complex with metals and blocks the reaction. The concentrations of 2,3- and 2,5-DHBA were determined by high-performance liquid chromatography (HPLC)–electrochemical detection. All samples were run in duplicate and blank corrected.
The DHBA assay was performed only on the aqueous suspensions of filters, as the DCM extracts (representing the vapor-phase compounds) would be devoid of metals. Results are reported as nanomoles of dihydroxybenzoate formed per minute per m3 of air sample.
GAPDH assay
This assay measures the content of electrophiles in the sample, based on their ability to inhibit or inactivate the thiolate enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), through covalent bonding. Inhibition of GAPDH by vapor and PM2.5 samples was determined under anaerobic conditions according to the method described previously (Shinyashiki et al., Citation2008). In brief, a mixture of 1 unit of rabbit GAPDH was incubated with aliquots of the organic extracts of vapors and particles or water suspension under argon gas at 25 °C for 120 min. The reaction was quenched by adding an equal volume of cold DTT solution, and GAPDH activity, measured as the rate of nicotinamide adenine dinucleotide (NADH) formation, was monitored by its absorption at 340 nm. The ability to inactivate the enzyme is expressed as the equivalents of N-ethylmaleimide (NEM), the standard electrophile. Samples were run in triplicate and values reported as averages. Results are reported as NEM equivalents per m3.
Statistical analyses
Results from chemical assays are the average of four measurements,with the exception of one CM PM2.5 sample (upwind) that was lost during a GAPDH assay and one vapor-phase (downwind) sample. The analysis of variance (ANOVA) and Tukey post hoc test and Pearson correlation analyses were performed with Graph Pad Prism 6 (Graph Pad Software, San Diego, CA). Each set had 15 comparisons, with asterisks showing the following P values: ****P < 0.0001 or ***P < 0.001; **P < 0.01; and *P < 0.05.
Results and Discussion
Collections and site descriptions
The railyard communities included CM, which is in the center of the basin, about 16 mi east of the Pacific Ocean and 56 mi west of SB, a community in the eastern end of the basin, and LB, which is 7 mi south of CM and adjacent to the Pacific Ocean (). The railyards associated with the three communities are among the busiest in the country, operating 24 hr a day, 365 days a year, with each yard generating approximately the same levels of emissions (22–24 tons per year; Casteneda et al., Citation2008). In an attempt to assess direct railyard contributions to the air sample, upwind and downwind sites from each railyard were selected on the basis of the prevailing wind pattern during the daytime hours, as we reasoned that the railyard emissions would be highest during this time. The prevailing wind pattern in the Los Angeles Basin is relatively consistent. Daytime winds in the Los Angeles Basin are northeasterly between 10 and 15 mph, and evening winds are southwesterly between 5 and 6 mph during evening hours (Air Quality Management District, 1993).
The Los Angeles Basin does not exhibit major seasonal weather changes. Typically, the warm season, or summer, extends from July to October and the cool season, or winter, extends from November to June. With this in mind, collections were made in July (summer) and in November (winter). CM and LB have similar temperature variations, but SB temperatures tend to be more extreme, i.e., they are higher in the summer and lower in the winter. For example, the difference in temperature for the winter and summer months for CM and LB during this study were 13 and 15 °F, respectively, whereas that for SB was 30 °F.
A 48-hr collection protocol was used in this study to generate sufficient sample quantities to allow chemical reactivity analyses and cellular assays. To assess the differences between the two sites for each community, one-way analysis of variance was performed on the PM2.5 DTT assay data, followed by Tukey multiple comparison analysis. The results showed no significant differences between samples for the up- and downwind sites for any of the three communities. As a result, the data for the two sites were pooled to reflect the community.
The simultaneous collections of particles and vapors at the three communities chosen allowed quantitative comparisons of the potential for adverse health effects through the ability to generate reactive oxygen and formation of covalent bonds with reactive centers in cells by air samples. From that perspective, the results showed air masses from CM and LB to be similar in composition, likely reflecting a higher level of emission derived reactivities, with SB as a receptor area whose air mass had higher levels of reactive species capable of initiating adverse health effects.
Chemical properties of the air mass of the three communities
Since prooxidants and electrophiles are reactive chemical species whose concentration could be altered by chemical reactions during the collection process, it is possible for these species to undergo reactions during collection and storage. Thus, the actual values reported here are net reactivities, i.e., the reactivities remaining in the filter and vapor samples following collection and storage at 4 °C. The assumption made is that the values are proportional to the actual concentrations in the air sample and comparable because of consistent handling procedures.
Prooxidants
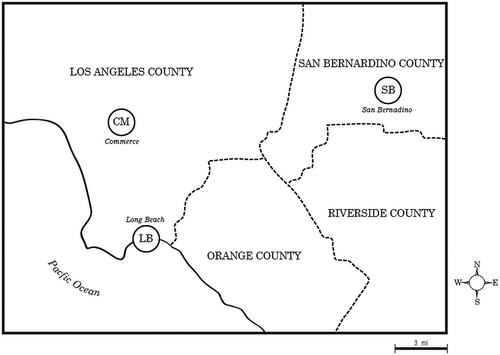
The DTT-based prooxidant content of aqueous suspensions of the PM phase and DMSO-based concentrates of dichloromethane extracts of the XAD resins were determined and are shown for each community in .
Figure 2. Prooxidant content of PM2.5 and vapors. Particle- and vapor-phase DTT-based prooxidant contents are shown as the means and SDs of eight values except for one CM PM sample and one SB sample that were lost. (a) PM2.5 samples; (b) vapor samples. Asterisks represent significant differences between winter and summer values, with ***P < 0.001; ****P < 0.0001.
In contrast to previous diesel exhaust and ambient air studies (e.g., Li et al., Citation2003), which used methanol extracts of PM2.5 filters, we used aqueous suspensions. Methods that employ an organic extract concentrate the organic compounds in the PM, leaving the metals and other highly polar components in the particles, whereas the suspension will more realistically represent the PM and any components bound to it. The vapor-phase components analyzed are those substances trapped on XAD and subsequently extracted with DCM, a procedure that concentrates volatile or semivolatile organic compounds of low and moderate polarity.
Winter prooxidant values for PM2.5 from CM and LB were significantly higher than those in the summer (). This observation could reflect winter climate conditions, i.e., somewhat lower temperatures, wind speeds, and an inversion layer resulting in lower dilution and dispersion of pollutants, including prooxidants. The winter summer prooxidant differences for SB PM2.5 were muted, and although the difference was not significant, the trend was toward higher summer than winter values. The prooxidant content of the corresponding vapor phases () were all much lower (note scale differences of y-axis in ) than the PM2.5, with seasonal differences observed only for SB samples, which were markedly higher in the summer.
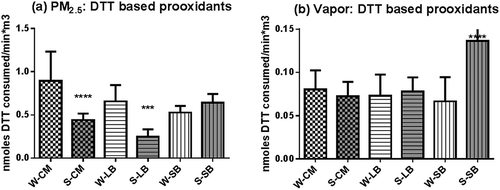
The contribution of metals to the prooxidant activities in the PM2.5 phase was assessed in two ways, the effect of chelation on DTT activity () and the capacity of the sample to catalyze the Fenton reaction (). The metal chelator, DTPA, at a concentration of 20 µM, reduced the DTT activity of the PM2.5 phase by up to 90%, and although this effect could imply that essentially all of the activity is metal based, it could also reflect cooperative prooxidant actions between metals and organic species in a humic-like substance (HULIS) complex (Ghio et al., Citation1996).
Figure 3. Metal-based prooxidants in PM2.5. (a) DTPA-sensitive component of the DTT-based prooxidant assay, obtained by subtraction of DTT activity determined in the presence of 20 µM DTPA from the total activity. (b) Ability of each sample to convert salicylate to dihydroxybenzoic acid. Asterisks show significant differences from winter samples at ***P < 0.001; ****P < 0.0001.
A more direct assay of metal-based reactions is the generation of dihydroxybenzoic acid (DHBA) by the sample in the presence of ascorbate, which is the result of metal catalysis of the so-called Fenton reaction (reaction 1):
The Fenton reaction generates hydroxyl radical by reduction of hydrogen peroxide and is catalyzed by transition metals (M++; reaction 1), in the presence of an electron source such as ascorbic acid. The highly reactive hydroxyl radical will react with any hydrogen source and, in the assay, converts salicylate (SA) to a mixture of dihydroxy benzoic acids (DHBA), measurable by HPLC and electrochemical detection (Di Stefano et al., Citation2009). Organic prooxidants such as quinones do not catalyze this reaction (Di Stefano et al., Citation2009). All of the PM2.5 samples exhibited the capacity to catalyze the reaction to varying degrees (), showing that PM2.5 contained redox-active metals. A direct comparison between the DTT/DTPA activity and the DHBA hydroxyl radical reaction cannot be made because of the differing potencies of metals to catalyze the reactions. Copper ions catalyze the Fenton reaction at rates about 15 times faster than iron ions, so when both ions are present at comparable concentrations, the major contributor in the DHBA reaction is the copper ion. Likewise, copper should be a major contributor in the DTT reaction, but the potency differences would likely be closer to 20 (Charrier and Anastasio, Citation2012). Based on results from a previous study (Di Stefano et al., Citation2009), the values shown reflect copper ion content equivalent to 0.15–0.28 nmoles/m3. Taken together, both the DTT/DTPA and DHBA assays indicate that metals do contribute to the overall prooxidant content of the PM2.5 fraction, as has been previously reported (Charrier and Anastasio, Citation2012; Verma et al., Citation2009, Citation2012). Since the vapor-phase samples do not contain metals, they were not assayed by this procedure.
Electrophiles
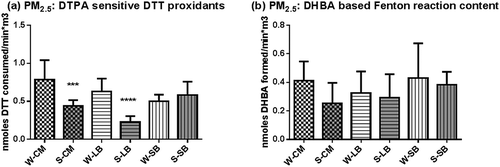
Electrophiles represent another class of reactive chemical species capable of eliciting biological responses by forming covalent bonds with key functional groups in biological macromolecules (Itoh et al., Citation2004; Jacobs and Marnett, Citation2010; Lopachin and Decaprio, Citation2005). We have assayed electrophiles in air samples by determining their ability to inactivate glyceraldehyde-3-phosphate dehydrogenase (GAPDH) through covalent bond formation with an active thiol group in the protein, and the results are shown in . Compounds capable of reacting in this assay include quinones and α,β-unsaturated carbonyl containing compounds as well as some metals such as zinc.
Figure 4. Electrophile content of PM2.5 and vapors. Particle- and vapor-phase electrophiles. (a) PM2.5- and (b) vapor-phase contents of samples, measured by the ability to inactivate glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The results shown are the means ± SDs of at least seven samples. Asterisks show significant differences from the winter values at ****P < 0.0001.
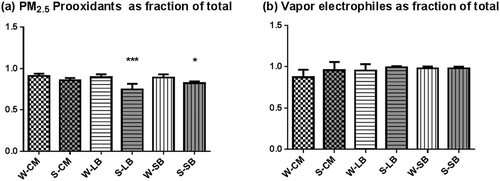
Particle-associated electrophile levels were found to be low at all sites and exhibited high variability, as shown by the wide standard deviations for each site (). Vapor-phase extracts contained the majority (80–100%) of the electrophiles (), which were particularly high in summer samples from San Bernardino. This difference could be attributed to atmospheric chemical and photochemical transformation processes that occurred during movement of the air mass from the central areas of the basin where the precursors were generated. Thus, the San Bernardino sites differ from the other two in terms both of seasonal differences and levels of summer vapor-phase electrophiles.
Prooxidant and electrophile distributions between phases
As noted above, the difference in distribution of prooxidants and electrophiles between the particle and vapor phases was quite marked, with particles containing 74–81% of the prooxidants () and the vapors containing 82–96% of the electrophiles (). shows high contributions of metals to the prooxidant content of PM2.5, which could explain their localization. The distribution of reactivities between vapor and particles for all samples were analyzed by one-way ANOVA and Tukey post hoc test for differences. The results showed significant differences only for the summer distribution of prooxidant content in the LB and SB samples, which were lower in the summer (). This difference likely reflects contributions of semivolatile organic prooxidants such as quinones generated by photochemical reactions, which occur more extensively in summer months. In a previous study of summer (June/July) air samples collected in the central area of Los Angeles (Shinkai et al., Citation2013), we found the reactive species content to be in the same range and distribution as those found in CM and LB.
Figure 5. Distribution of prooxidants and electrophiles.(a) Prooxidants in the PM2.5 fractions of the total DTT-based prooxidants. (b) Levels of electrophiles as fractions of the total. The values are the mean values of all samples with asterisks representative of significant differences between winter and summer values for a particular community. Asterisks shown significant differences from the corresponding winter values at ***P < 0.001; *P < 0.05.
Since the vapor-phase components are trapped by XAD resins and extracted with DCM, they are volatile or semivolatile organic compounds. The majority of these species contribute to the electrophilic properties of the air samples and exert biological effects by this mechanism. Electrophiles in these vapor samples collected in Riverside, California, another receptor community in the eastern end of the basin, formed covalent bonds with GAPDH at concentrations of 0.3 m3/mL, with an averaged molecular weight of 21–32 daltons for the binding species based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) data (Iwamoto et al., Citation2010), supporting the notion that vapor-phase electrophiles are low-molecular-weight compounds. Furthermore, under conditions that did not alter intracellular oxidation status, the samples stimulated macrophages and epithelial cells (Shinkai et al., Citation2013) to express proteins associated with adaptation and inflammation. Thus, from a toxicological perspective, these volatile electrophilic species are clearly important and should be examined concurrently with the particles to assess the overall health effects of the combined air sample. The notion of measuring volatile compounds has recently been raised in a review of the use of PM mass as a surrogate of air pollution (Hoare, Citation2014).
The values for each community were subjected to Pearson correlation analysis and the correlation coefficients and corresponding P values determined. Our purpose was to assess the possible relationships between ambient air from the three communities by comparing values for each day’s collection. A high correlation (P < 0.05) would suggest that the air contents have similar day-to-day changes.
Winter PM2.5 correlation coefficients between prooxidant contents from CM and LB were high (Pearson coefficient = 0.814, P = 0.014), but the corresponding coefficients for SB (vs. CM, Pearson coefficient = −0.538; vs. LB, Pearson coefficient = −0.335) were much lower. The higher correlation of prooxidant contents for winter samples from CM and LB could reflect similar primary emissions, which differ from those of SB, whose air mass includes a mixture of primary and secondary emissions. The DTPA-sensitive prooxidants () and Fenton reaction () summer prooxidants correlated with P values less than 0.05 for CM (0.71; P = 0.049) and LB (0.73; P = 0.038), suggesting that contributions to the overall prooxidant reactivity reflected similar proportions of reactive metals in the samples. The summer prooxidant content showed little correlation between communities, perhaps indicating greater and more variable atmospheric conditions between communities during the summer season.
In summary, the correlations between the communities observed are consistent with the hypothesis that the reactive components in PM2.5 from CM and LB air samples exhibit characteristics that may reflect source emissions, in contrast to those from SB, a receptor site whose air mass may reflect photochemical or other atmospheric reactions.
Conclusion
The results from these studies allowed quantitative comparisons of ambient air samples in three communities in the Los Angeles Basin in terms of their potential for adverse health effects. The comparisons included levels of prooxidants and electrophiles in both particulate and vapor phases, together with their seasonal differences. Both particulate and vapor samples contained the reactive species, with prooxidants concentrated in the particulate and electrophiles concentrated in the vapor phase. Levels of the two reactivities exhibited distinct seasonal differences, with PM2.5 prooxidants significantly higher in the winter samples and electrophiles showing a higher level only in the summer sample from SB. The localization of reactive organic compounds in the vapor phase, most notably electrophiles, points out their importance in the biological actions of ambient air and the necessity to monitor this phase as well as particles in the potential health assessment of ambient air.
Funding
This report was prepared as a result of work sponsored and paid for by the South Coast Air Quality Management (AQMD) under awards 12865 and 20090799. The opinions, findings, conclusions, and recommendations are those of the authors and do not necessarily represent the views of AQMD. AQMD, its officers, employees, contractors, and subcontractors make no warranty, expressed or implied, and assume no legal liability for the information in this report. AQMD has not approved or disapproved this report, nor has AQMD passed upon the accuracy or adequacy of the information contained herein. E.M.S. was supported by NIH research grant D43TW000623 funded by the Fogarty International Center. This work was also supported in part by the U.S. Environmental Protection Agency–funded Southern California Center for Airborne Particulate Matter (grant RD-83241301) and has not been subjected to the agency’s required peer and policy review and therefore does not necessarily reflect the views of the agency. No official endorsement should be inferred.
Acknowledgment
The authors thank the multiple homeowners who allowed the intrusions associated with air sample collections on their property.
Additional information
Funding
Notes on contributors
Arantza Eiguren-Fernandez
Arantza Eiguren-Fernandez, Ph.D., was an assistant researcher in the Department of Environmental Health Sciences, Center for Health Sciences, University of California–Los Angeles, Los Angeles, CA, USA and is currently a research scientist at Aerosol Dynamics Inc., Berkeley, CA, USA.
Emma Di Stefano
Emma Di Stefano, M.S., was a staff research associate in the Department of Environmental Health Sciences, Center for Health Sciences, University of California–Los Angeles, Los Angeles, CA, USA. She is currently retired.
Debra A. Schmitz
Debra A. Schmitz, B.S., is a staff research associate and John R. Froines, Ph.D., is professor emeritus in the Department of Environmental Health Sciences, Center for Health Sciences, University of California–Los Angeles, Los Angeles, CA, USA.
Aline Lefol Nani Guarieiro
Aline Lefol Nani Guarieiro, Ph.D., is currently a research scientist at INCT em Energia e Ambiente, Salvador, BA, Brazil.
Erika M. Salinas
Erika M. Salinas, M.S., is currently a research scientist at Quimica Aplicada, Universidad Autonoma Metropolitana, México City, México.
Elina Nasser
Elina Nasser, B.S., was outreach coordinator and liaison with communities involved. Her current address is Austin, TX, USA.
John R. Froines
Arthur K. Cho, Ph.D., is professor emeritus in the Departments of Molecular and Medical Pharmacology and Environmental Health Sciences, Center for Health Sciences, University of California–Los Angeles, Los Angeles, CA, USA.
References
- Baeza-Squiban, A., V. Bonvallot, S. Boland, and F. Marano. 1999. Airborne particles evoke an inflammatory response in human airway epithelium. Activation of transcription factors. Cell. Biol. Toxicol. 15:375–380. doi:10.1023/A:1007653900063
- Casteneda, H., E. Yang, A. Mahmood, S. Cutts, and A. Mitchell. 2008. Health Risk Assessment for the BNSF Railway San Bernadino Railyard. Sacramento, CA: Air Resources Board, California Environmental Protection Agency.
- Charrier, J.G., and C. Anastasio. 2012. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. Discuss. 12:11317–11350. doi:10.5194/acpd-12-11317-2012
- Cho, A., E. Di Stefano, Y. Ying, C.E. Rodriguez, D. Schmitz, Y. Kumagai, A.H. Miguel, A. Eiguren-Fernandez, T. Kobayashi, and E.F. Avol Jr. 2004. Determination of four quinones in diesel exhaust particles, SRM 1649a and atmospheric PM2.5. Aerosol Sci. Technol. 38:68–81. doi:10.1080/02786820390229471
- Cho, A.K., C. Sioutas, A.H. Miguel, Y. Kumagai, D.A. Schmitz, M. Singh, A. Eiguren-Fernandez, and J.R. Froines. 2005. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 99:40–47. doi:10.1016/j.envres.2005.01.003
- Chung, M.Y., R.A. Lazaro, D. Lim, J. Jackson, J. Lyon, D. Rendulic, and A.S. Hasson. 2006. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environ. Sci. Technol. 40:4880–4886. doi:10.1021/es0515957
- Delgado-Saborit, J.M., M.S. Alam, K.J. Godri Pollitt, and R.M. Harrison. 2013. Analysis of atmospheric concentrations of quinones and polycyclic aromatic hydrocarbons in vapour and particulate phases. Atmos. Environ. 77:974–982. doi:10.1016/j.atmosenv.2013.05.080
- Di Stefano, E., A. Eiguren-Fernandez, R.J. Delfino, C. Sioutas, J. Froines, and A.K. Cho. 2009. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhal. Toxicol. 21: 731–738.
- Donaldson, K., V. Stone, A. Clouter, L. Renwick, and W. MacNee. 2001. Ultrafine particles. Occup. Environ. Med. 58:211–216. doi:10.1136/oem.58.3.211
- Donaldson, K., and C.L. Tran 2002. Inflammation caused by particles and fibers. Inhal. Toxicol. 14:5–27. doi:10.1080/089583701753338613
- Eiguren-Fernandez, A., A.H. Miguel, J.R. Froines, S. Thurairatnam, and E.L. Avol. 2004. Seasonal and spatial variation of polycyclic aromatic hydrocarbons in vapor-phase and PM2.5 in southern California urban and rural communities. Aerosol Sci. Technol. 38:447–455. doi:10.1080/02786820490449511
- Ghio, A., J. Stonehuerner, R. Pritchard, C. Piantadosi, D. Quigley, K. Dreher, and D. Costa. 1996. Humic-like substances in air pollution particulates correalte with concentrations of transition metals and oxidant generation. Inhal. Toxicol. 8: 479–494. doi:10.3109/08958379609005441
- Hoare, J.L. 2014. New directions: Questions surrounding suspended particle mass used as a surrogate for air quality and for regulatory control of ambient urban air pollution. Atmos. Environ. 91:175–177. doi:10.1016/j.atmosenv.2014.04.004
- Itoh, K., K.I. Tong, and M. Yamamoto. 2004. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 36:1208–1213. doi:10.1016/j.freeradbiomed.2004.02.075
- Iwamoto, N., A. Nishiyama, A. Eiguren-Fernandez, W. Hinds, Y. Kumagai, J. Froines, A. Cho, and M. Shinyashiki. 2010. Biochemical and cellular effects of electrophiles present in ambient air samples. Atmos. Environ. 44:1483–1489. doi:10.1016/j.atmosenv.2010.01.045
- Jacobs, A.T., and L.J. Marnett. 2010. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 43:673–683. doi:10.1021/ar900286y
- Jakober, C.A., S.G. Riddle, M.A. Robert, H. Destaillats, M.J. Charles, P.G. Green, and M.J. Kleeman. 2007. Ouinone emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol. 41: 4548–4554. doi:10.1021/es062967u
- Kachur, A.V., K.D. Held, C.J. Koch, and J.E. Biaglow. 1997. Mechanism of production of hydroxyl radicals in the copper-catalyzed oxidation of dithiothreitol. Radiat. Res. 147:409–415. doi:10.2307/3579496
- Kumagai, Y., S. Koide, K. Taguchi, A. Endo, Y. Nakai, T. Yoshikawa, and N. Shimojo. 2002. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 15:483–489. doi:10.1021/tx0100993
- Kumagai, Y., K. Midorikawa, Y. Nakai, T. Yoshikawa, K. Kushida, S. Homma-Takeda, and N. Shimojo. 1998a. Inhibition of nitric oxide formation and superoxide generation during reduction of LY83583 by neuronal nitric oxide synthase. Eur. J. Pharmacol. 360:213–218. doi:10.1016/S0014-2999(98)00666-9
- Kumagai, Y., H. Nakajima, K. Midorikawa, S. Homma-Takeda, and N. Shimojo. 1998b. Inhibition of nitric oxide formation by neuronal nitric oxide synthase by quinones: Nitric oxide synthase as a quinone reductase. Chem. Res. Toxicol. 11:608–613. doi:10.1021/tx970119u
- Kumagai, Y., Y. Shinkai, T. Miura, and A.K. Cho. 2012. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 52:221–247. doi:10.1146/annurev-pharmtox-010611-134517
- Li, N., S. Kim, M. Wang, J. Froines, C. Sioutas, and A. Nel. 2002. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 14: 459–486. doi:10.1080/089583701753678571
- Li, N., C. Sioutas, A. Cho, D. Schmitz, C. Misra, J. Sempf, M. Wang, T. Oberley, J. Froines, and A. Nel. 2003. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 111:455–460.
- Lopachin, R.M., and A.P. Decaprio. 2005. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol. Sci. 86:214–225. doi:10.1093/toxsci/kfi197
- Monks, T.J., and S.S. Lau 1992. Toxicology of quinone-thioethers. Crit. Rev. Toxicol. 22:243–270. doi:10.3109/10408449209146309
- Nel, A.E., D. Diaz-Sanchez, and N. Li. 2001. The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Curr. Opin. Pulm. Med. 7:20–26. doi:10.1097/00063198-200101000-00004
- Netto, L.E., and E.R. Stadtman. 1996. The iron-catalyzed oxidation of dithiothreitol is a biphasic process: Hydrogen peroxide is involved in the initiation of a free radical chain of reactions. Arch. Biochem. Biophys. 333: 233–242. doi:10.1006/abbi.1996.0386
- Schafer, F.Q., and G.R. Buettner. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30:1191–1212. doi:10.1016/S0891-5849(01)00480-4
- Shinkai, Y., S. Nakajima, A. Eiguren-Fernandez, E.D. Stefano, D.A. Schmitz, J.R. Froines, A.K. Cho, and Y. Kumagai. 2013. Ambient vapor samples activate the Nrf2-ARE pathway in human bronchial epithelial BEAS-2B cells. Environ. Toxicol. 25:1222–1230. doi:10.1002/tox.21860
- Shinyashiki, M., C.E. Rodriguez, E.W. Di Stefano, C. Sioutas, R.J. Delfino, Y. Kumagai, J.R. Froines, and A.K. Cho. 2008. On the interaction between glyceraldehyde-3-phosphate dehydrogenase and airborne particles: Evidence for electrophilic species. Atmos. Environ. 42:517–529. doi:10.1016/j.atmosenv.2007.09.047
- Squadrito, G.L., R. Cueto, B. Dellinger, and W.A. Pryor. 2001. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Biol. Med. 31:1132–1138. doi:10.1016/S0891-5849(01)00703-1
- Valavanidis, A., K. Fiotakis, T. Vlahogianni, V. Papadimitriou, and V. Pantikaki. 2006. Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environ. Chem. 3:118–123. doi:10.1071/EN05089
- Verma, V., Z. Ning, A.K. Cho, J.J. Schauer, M.M.S. Shafer, and C. Sioutas. 2009. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos. Environ. 43: 6360–6368. doi:10.1016/j.atmosenv.2009.09.019
- Verma, V., R. Rico-Martinez, N. Kotra, L. King, J. Liu, T.W. Snell, and R.J. Weber. 2012. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ. Sci. Technol. 46:11384–11392. doi:10.1021/es302484r
- Waidyanatha, S., and S.M. Rappaport. 2008. Hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone in Swiss Webster mice. Chem. Biol. Interact. 172:105–114. doi:10.1016/j.cbi.2008.01.004