Abstract
Nitrogen oxides (NOx) emitted from combustion processes have elevated concentrations in large urban areas. They cause a range of adverse health effects, acid rain, and are precursors to formation of other atmospheric pollutants, such as ozone, peroxyacetyl nitrate, and inorganic aerosols. Photocatalytic materials containing a semi-conductor that can be activated by sunlight, such as titanium dioxide, have been studied for their ability to remove NOx. The study presented herein aims to elucidate the environmental parameters that most influence the NOx removal efficiency of photocatalytic coatings in hot and humid climate conditions. Concrete samples coated with a commercially available photocatalytic coating (a stucco) and an uncoated sample have been tested in a reactor simulating reasonable summertime outdoor sunlight, relative humidity and temperature conditions in southeast Texas. Two-level full factorial experiments were completed on each sample for five parameters. It was found that contact time, relative humidity and temperature significantly influenced both NO and NO2 removal. Elevated concentrations of organic pollutants reduced NO removal by the coating. Ultra-violet light intensity did not significantly influence removal of NO or NO2, however, ultra-violet light intensity was involved in a two-factor interaction that significantly influenced removal of both NO and NO2.
Implications: The potential for removal of oxides of NOx in realistic outdoor conditions by a photocatalytic coating (a stucco) applied on concrete was studied through full factorial experimentation. Results suggest that locations offering longer contact time between the pollutant and the reactive surface would lead to greater removal. The presence of elevated relative humidity reduces the NOx removal effectiveness of the coating. Concentrations of organic pollutants in the vicinity of the material did not significantly influence its removal effectiveness, making it potentially suitable as a control strategy for NO2 near roadways, where elevated NO2 levels are observed in combination with volatile organic compounds (VOCs).
Introduction
Nitrogen oxides (NOx) are ubiquitous in urban environments. They are produced by various fossil fuel combustion processes found in industries, power generation facilities, and vehicles. In the United States, emissions from highway vehicles represent 31% of NOx emissions (U.S. Environmental Protection Agency [EPA], Citation2011). Oxides of nitrogen are responsible for acid rain, which damages structures and vegetation. They are also precursors to the formation of ozone and other pollutants through photochemical processes (Seinfeld and Pandis, Citation2006). Exposure to NOx has been associated with adverse health effects, including increased airway reactivity, decreased pulmonary function (Bauer et al., Citation1986; Mohsenin, Citation1987; Frampton et al., Citation1991), onset of asthma (Gauderman et al., Citation2005; Clark et al., Citation2009), arrhythmia (Peters et al., Citation2000), and lung cancer (Nafstad et al., Citation2004; Raaschou-Nielsen and Reynolds, Citation2006). Populations living near roadways are routinely exposed to elevated levels of NOx and are subject to greater adverse health effects than the general population (Brunekreef et al., Citation1997; Krämer et al., Citation2000; Janssen et al., Citation2003; Thoma et al., Citation2008; EPA, Citation2010).
Finding new ways to remove NOx originating on roadways from urban atmospheres could be very beneficial for both the environment and the health of populations in these areas. Photocatalytic materials have been studied for years because of their capacity to remove NOx through oxidation reactions (Fujishima and Honda, Citation1972; Allen et al., Citation2005; Ballari et al., Citation2010b; Laufs et al., Citation2010). Built structures near roadways are candidates for the application of this technology. They are in close proximity to a NOx emission source, and enhanced dispersion of pollutants from roadways due to mechanical shear of air caused by motor vehicles increases opportunity for contact. In contrast to other stationary sources such as industries or power plants, the pollutant source is close to the ground, so it is possible to take advantage of surrounding structures for passive removal. It is also possible to implement new structures for the purpose of pollution reduction, with other added benefits (e.g., noise reduction and enhanced roadway safety). Application of photocatalytic material is attractive because, once it is applied, it could potentially remove NOx without necessitating energy input. Moreover, because the material acts as a catalyst, it could theoretically be used indefinitely without replacement as long as it can be regenerated.
Photocatalysis is a process that occurs at the surface of a semiconductor exposed to light (Fujishima et al., Citation1972). When a photon with energy equal or larger than the band gap of the semiconductor is absorbed, an electron (e−) from the valence band is promoted to the conduction band. The result is the generation of a “hole” in the valence band (h+). h+ and e− are strong oxidizing and reducing agents, respectively. The electron-hole pair may react with electron donors or acceptors adsorbed to the semiconductor surface (Fujishima et al., Citation1972; Hoffmann et al., Citation1995; Chen and Poon, Citation2009). Oxygen and water adsorbed at the semiconductor surface are catalyzed to form reactive species, superoxide anions (O2−) and hydroxyl radicals (OH•). Hydroxyl radicals and superoxide anions are strong oxidizing and reducing agents, respectively. They can react with pollutant molecules adsorbed to the photocatalytic surface, such as NOx or hydrocarbons (Dalton et al., Citation2002; Allen et al., Citation2005; Ohko et al., Citation2009; Ballari et al., Citation2010b; Laufs et al., Citation2010). In the case of NOx, the product of the reactions involving NO, NO2, and hydroxyl radicals is nitric acid, which remains adsorbed on the photocatalytic surface until it is washed off by water (Devahasdin et al., Citation2003; Ballari et al., Citation2010b; Hunger et al., Citation2010).
Titanium dioxide is the most widely used semiconductor for photocatalysis applications. It is inexpensive, chemically and biologically inert, and stable with respect to photo and chemical corrosion (Hoffmann et al., Citation1995). Titanium dioxide has three crystalline forms (anatase, rutile, and brookite); mixtures of anatase and rutile have been found to be most photoactive, whereas anatase is the most photoactive single-crystal form (Scanlon et al., Citation2013).
Researchers have previously studied environmental parameters that can affect the photocatalytic removal of NOx. Removal increases nonlinearly with light intensity (Ollis et al., Citation1991; Lim et al., Citation2000; Bengtsson and Castellote, Citation2010), but decreases with increasing relative humidity (Hüsken et al., Citation2009; Dylla et al., Citation2010; Laufs et al., Citation2010; Ballari et al., Citation2011). Relative humidity might also influence NOx removal through reactions taking place in water films at the material surface (Svensson et al., Citation1987; Jenkin et al., Citation1988; Saliba et al., Citation2001; Finlayson-Pitts et al., Citation2002). Some researchers have also observed that for NO and NO2 concentrations less than 1 ppm, the conversion rate does not depend on the initial concentration injected, even when both chemicals are introduced at the same time (Bengtsson and Castellote, Citation2010; Laufs et al., Citation2010). But others have observed removal to increase with decreasing concentration in air (Hüsken et al., Citation2009). However, most researchers have not investigated NOx removals with realistic outdoor air mixtures that contain other pollutants such as ozone or volatile organic compounds (VOCs). Of those who have, none have studied the simultaneous effect of environmental conditions such as light intensity, temperature, and relative humidity.
This study aimed to elucidate the environmental parameters that have a significant effect on the NOx removal efficiency of a photocatalytic coating when tested under realistic near-roadway conditions, so that it is possible to determine where such materials could be used with the most benefits. In the past, researchers have studied a wide range of photocatalytic products, from TiO2 slurries (Lim et al., Citation2000; Devahasdin et al., Citation2003; Ohko et al., Citation2009; Bengtsson and Castellote, Citation2010), to paints (Maggos et al., Citation2007a; Laufs et al., Citation2010), paving stones (Ballari et al., Citation2010a; Hüsken et al., Citation2009; Ballari et al., Citation2011), or concrete containing TiO2 in its cement matrix (Beeldens, Citation2006; Chen and Poon, Citation2009; Dylla et al., Citation2010, Citation2011). In this study, we focused on a TiO2-containing stucco coating applied to concrete. The application of a stucco coating provides advantages over concrete that includes TiO2 in the cement. Since stucco is applied in a thin surface layer, a smaller amount of semiconductor material is needed for the same exposed surface area as concrete that contains TiO2 through the full depth. Since TiO2-containing cement is expensive, the stucco coating is a more cost-effective solution for new structures. Moreover, stucco coatings can also be applied on existing concrete structures, including buildings, reducing the cost of implementation of this technology on existing near-roadway structures.
In this paper, a commercially available photocatalytic coating was studied under simultaneously varying environmental conditions, to elucidate where such materials could be applied to new or existing structures with most benefits to remove pollutants emitted from nearby roadways.
Experimental Methods
Materials
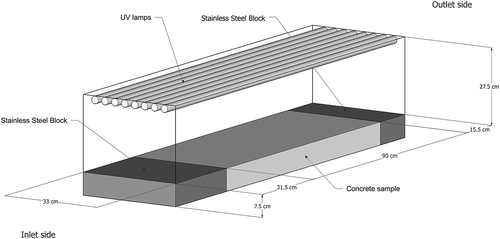
The stucco cement (TX Active; ESSROC, Nazareth, PA, USA) tested in this study is a commercially available product. It is composed of portland cement, admixtures, and 4.98% by mass of solids of TiO2. It was applied on concrete slabs manufactured following mixture proportions provided by a commercial concrete precast company in Texas for use in highway barriers: 25% fly ash/75% Type III cement, water to cementitious materials ratio by mass of 0.34, limestone coarse and fine aggregate, and Plastiment and Sika 2110 admixtures (Sika Corporation U.S., Lyndhurst, NJ, USA). The concrete samples were 7.5 cm thick and 33 cm wide. Two different lengths were fabricated to vary the contact time during chamber testing. Full-size samples were 90 cm in length and half-size samples were 45 cm in length.
Sample preparation
The stucco cement was mixed with sand (meeting American Society for Testing and Materials [ASTM] C926) and water to achieve the desired consistency (by weight: 67% sand, 13% water, 20% stucco cement). Two-thirds of the mixing water and one-half of the total sand volume were mixed first, and the stucco cement was then added. After incorporating the remaining water and sand, the stucco was mixed for at least 5 min until uniformity was achieved. The stucco was applied with a hand trowel in a 5-mm-thick layer on concrete slabs for a TiO2 loading of 0.015 kg/m2. Prepared samples were left to cure for several weeks in a 50% relative humidity (RH) room at 23 °C until testing. The concrete samples were allowed to cure for 1 week in a 100% RH room and to dry for 2 weeks in a 50% RH room, both at 23 °C, before the photocatalytic coating was applied. Uncoated concrete samples were also manufactured and tested for comparison. A picture of a full-size stucco-coated concrete specimen is available in the supplemental file for reference.
Laboratory system
Chamber system
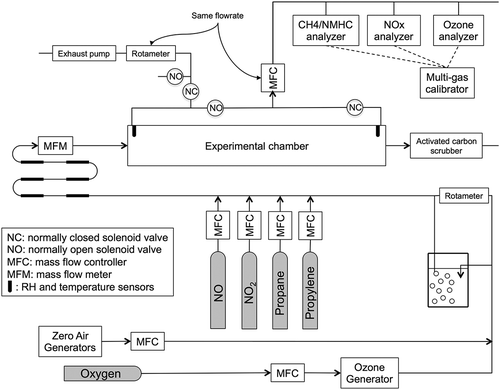
The experimental chamber used for testing of the photocatalytic coating is presented in . There are a few standards related to testing of photocatalytic materials (International Organization for Standardization [ISO], Citation2007; Japanese Industrial Standards [JIS], Citation2010; Ente Nazionale Italiano di Unificazione [UNI], Citation2010), which were developed to test different materials under a single, uniform condition. In this study, the goal was to examine the effect of varying environmental conditions on NOx removal by a coating. For that reason, a custom reactor was designed to recreate outdoor conditions and to provide a consistent airflow pattern above the material surface for various airflow rates. A 150-L stainless steel electro-polished chamber was used to test coated concrete samples, with dimensions 33 cm × 137 cm × 35 cm. Stainless steel blocks were made to fit at each end of the samples in order to create a flat surface extending the length of the chamber (). Once a sample and the stainless steel blocks were placed in the chamber, the air volume was 115 L. Eight ultraviolet (UV) lamps (40 W, 280–400 nm, λmax = 310 nm; model QFS-40; Q-Lab Corp., Cleveland, OH, USA) were installed in the chamber to provide UV illumination. The inlet and outlet of the chamber were equipped with relative humidity probes (model HD2XVSX; Veries Industries, Portland, OR, USA) and thermistors (model 44203; YSI, Yellow Springs, OH, USA). A third thermistor was placed in the middle of the reactor, above the concrete sample.
The heat released by the UV lamps provided convective flow leading to well-mixed conditions in the test chamber, which was confirmed by a tracer test using carbon dioxide and ASTM standard D5116-10 (ASTM, Citation2006). Details about the tracer test performed are available in the supplemental file.
Air conditioning and sampling
The experimental system used to condition and sample the air is presented in . An air stream provided by two zero-air generators (model 701; Teledyne, San Diego, CA, USA) was split, and a portion of the air was passed through an impinger in order to adjust the relative humidity. Pollutant species mixed with nitrogen were obtained in gas cylinders (Praxair, Danbury, CT, USA): nitrogen oxide, nitrogen dioxide, propane (as a surrogate for volatile organic compounds), and propylene (as a surrogate for highly reactive volatile organic compounds). Ozone was produced by passing pure oxygen through a UV-based ozone generator (model 97-0067-01; UVP, LLC, Upland, CA, USA). Pollutant species were introduced at a known rate through mass-flow controllers (series FMA 5500; Omega Engineering, Inc., Stamford, CT, USA; series GFC 1700; Aalborg, Orangeburg, NY, USA). The influent air stream then passed through a mixing zone before being introduced in the chamber. The mixing zone consisted of a succession of sections of tubing of two different diameters and a 12-L inlet mixing chamber separated from the main chamber volume by a baffle.
Chamber air was sampled at both the inlet and outlet of the chamber. A system of solenoid valves (models SV123/133; Omega Engineering Inc.) automatically switched sampling between the inlet and outlet. When sampling the outlet, an exhaust pump removed gas at a flow rate equal to the flow rate going to the instruments to maintain a constant gas flow rate through the chamber. Measurements for NO and NO2 concentrations were made by a chemiluminescence analyzer (model 200E; Teledyne). Ozone concentrations were measured by a UV absorbance ozone analyzer (model 1008-AH; Dasibi, Glendale, CA, USA). Volatile organic compound (VOC) concentrations were measured by a methane/nonmethane hydrocarbon analyzer (model 55i; Thermo Fisher Scientific, Waltham, MA, USA). These instruments were calibrated daily using a multigas calibrator (model 700E; Teledyne) and concentration-certified gas standards (Praxair).
Testing conditions deviated from some aspects from the ISO 22197-1 standard for testing of photocatalytic materials. Ultraviolet lamps were placed inside the reactor enclosure. Their heat release participated in producing well-mixed conditions in the reactor and heating the air and concrete to temperatures typically encountered during summer months in southeast Texas. NOx concentrations used for testing are also lower than concentrations prescribed in the ISO 22197-1 standard (1 ppm NO). The goal was to study NOx pollution levels under realistic outdoor conditions.
Full factorial experiments
Environmental parameters tested
To determine which environmental parameters influence NOx removal, the stucco-coated concrete and uncoated concrete underwent two-level factorial testing. Five parameters were varied between a low and a high level, as listed in . Low and high levels represented the bounds of ranges of values commonly observed for the different variables during the ozone season in southeast Texas. Limitations of the experimental system also influenced the range of contact times that could be recreated in the test chamber. Contact times were varied between 1.5 min with a half-size sample (5 L/min inlet flow rate, 2.6 hr−1 air exchange rate) to 15 min with a full-size sample (25 L/min inlet flow rate, 13.1 hr−1) and covered the range of contact times previously reported in the literature. Nitric oxide and nitrogen dioxide removals were tested in the presence of inorganic and organic pollutants to simulate outdoor air conditions. Nitric oxide concentrations are usually highest in the morning, with lower NO2 and ozone concentrations at that time. The situation is reversed in the afternoon, when NO2 and ozone concentrations are highest and NO concentrations are low. However, in the test reactor, immediate titration of NO by ozone was observed (Seinfeld and Pandis, Citation2006). Hence, NO removal was tested in a “morning” condition with a high level of NO (150 ppb) and a low level of NO2 (20 ppb). Nitrogen dioxide removal was tested in an “afternoon” condition with a high level of NO2 (50 ppb) and ozone (150 ppb) and no NO. These concentrations were determined by analyzing Houston air quality data from 2009 (Texas Commission on Environmental Quality [TCEQ], Citation2009). Maximum concentrations of NO, NO2, and ozone for the period between August 15 and September 15, 2009, were analyzed. The maximum concentrations were used to establish experimental values of NO concentration in the morning and NO2 and ozone concentrations in the afternoon. The NO2 concentration used in the morning condition corresponds to twice the value usually observed in morning hours, in order to obtain better detection by the NOx analyzer (lower detection limit: 0.4 ppb). These conditions correspond to a worst-case scenario rather than average conditions. Similarly, low and high levels of VOC concentrations and representative summer temperatures were obtained from historical air monitoring and meteorological data from the Houston, Texas, area for the August 15 to September 15, 2009, period (TCEQ, Citation2009).
Table 1. Full factorial experimental parameters and their magnitudes.
Experimental procedure
Material samples were placed in the test reactor in the evening. The UV lights were switched on, and the sample was left in the chamber overnight with zero air passing through the chamber. This allowed temperature and relative humidity in the chamber to reach their desired values. Pollutant species were introduced in the inlet stream through mass-flow controllers, and their concentrations were monitored at the inlet and outlet of the test chamber. Concentrations were monitored for 20 min at the inlet, and then the monitoring equipment was switched to the chamber outlet for 20 min. This process was repeated three times for a total of 2 hr for each test with three 20-min inlet measurement periods and three 20-min outlet measurement periods.
Test sample washing
Eight experiments were consecutively run on one test coupon before it was removed from the chamber and the next sample was tested. Before the first experiment and between rounds of experiments, each sample was rinsed with distilled water. The goal was to regenerate the catalyst by washing off nitric acid, the product of the photocatalytic conversion of NO and NO2, that might have remained at the catalyst surface. This was done for consistency between experiments but was not meant to mimic outdoor rinsing by rainwater. For rinsing, full-size samples were placed horizontally first and 4 L of distilled water were poured on the sample in increments of one liter. Between each rinse with 1 L, the sample was left to drip for 30 sec. Finally, the sample was tilted at a 30° angle and 4 L of distilled water were poured on the sample, running off quickly. The sample was then set out to dry in the laboratory for 24 hr. Half-size samples were rinsed following the same methodology, but using half the amount of water.
Data analysis
Oxides of nitrogen removals were calculated using steady-state concentration data obtained during full factorial tests. First, removal by the uncoated concrete was calculated using
where cin,b is the pollutant concentration measured in the chamber inlet for the test run with uncoated concrete (ppb), cout,b is the pollutant concentration measured in the chamber outlet for the test run with uncoated concrete (ppb), and Rb is the pollutant removal by the uncoated concrete (%).
The removals for the photocatalytic coating were calculated in a similar fashion. For each test, the removal by the uncoated concrete was subtracted from that of the coated concrete to determine the removal by the photocatalytic coating over the existing structures made of uncoated concrete. This process allowed an evaluation of the incremental NOx removal that would be obtained by coating existing concrete structures with a photocatalytic coating:
where cin is the pollutant concentration measured in the inlet of the chamber (ppb), cout is the pollutant concentration measured in the outlet of the chamber (ppb), and R is the pollutant removal by the coated concrete (%).
Steady-state inlet and outlet concentrations were taken as the average of concentrations measured during the last 80 min of each test.
The uncertainty associated with calculated removals was determined using standard error propagation techniques. The uncertainty on the concentration measurements was taken as 2 standard deviations of measured values for all calibrations run during the experimental program. Relative uncertainties were determined to be 8.0% for NO measurements and 9.8% for NO2 measurements.
The results from the full factorial experiments were analyzed using JMP software (JMP 9; SAS, Cary, NC, USA) to determine the most important factors affecting NOx removal, using analysis of variance (ANOVA) techniques. ANOVA determines whether the means of different groups are statistically different. Main factors as well as two-factor interactions were taken into account. Higher-level interactions were not considered. Ten percent of the experiments were replicated. The Lenth method was used to provide approximate tests of significance (Tamhane, Citation2009). The Lenth method provides an estimate of the standard error, called the Lenth pseudo standard error (PSE), where PSE = 1.5 × Median(|θi|: θi < 2.5 × τ0) and τ0 = 1.5 × Median(|θi|) with θi the ith contrast.
The Lenth t-ratio was calculated as the ratio of the contrast for a factor over the PSE (Mee, Citation2009). The t-statistic for each factor was compared with critical values provided by Mee (Citation2009). Factors with a Lenth t-ratio larger than the critical value were deemed significant. A significance level α = 0.15 was used to determine the critical value. Outliers were determined by using the studentized residuals test described by Mee (Citation2009).
Results
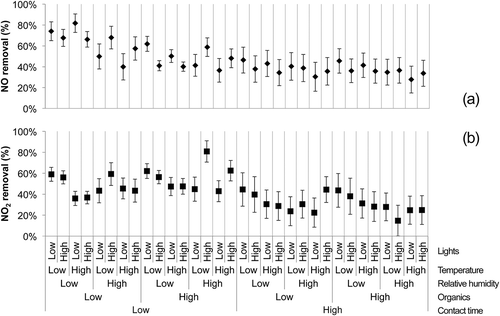
Results for NO and NO2 removals observed during full factorial testing are presented in . Nitric oxide removals averaged 46% (standard deviation: 13%), whereas NO2 removals averaged 41% (standard deviation: 14%). The pattern of the removal data was different for NO and NO2 removals, suggesting that different factors or two-factor interactions affected the removal of each gas. In this figure, it is possible to observe the range of changes that occur with changing environmental conditions, but an in-depth statistical analysis is necessary to parse out the individual and combined effects of environmental parameters.
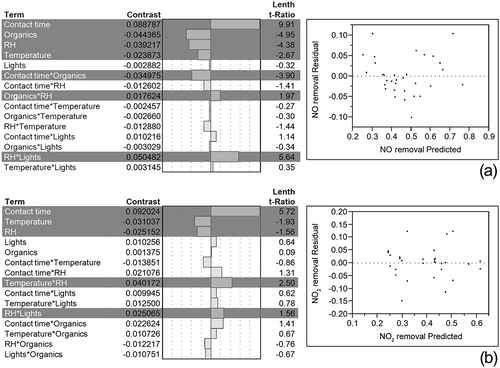
Results from the analysis of data using JMP software are presented in . The PSE were 0.896 and 1.609 for NO and NO2 removal, respectively. The critical value to which Lenth t-ratios were compared is 1.446 according to Mee (Citation2009) for a significance level α = 0.15. Four main factors (contact time, organic pollutants concentrations, RH, temperature) and three two-factor interactions (contact time × organic pollutants concentrations, RH × organic pollutants concentrations, RH × UV light intensity) were deemed significant for NO removal and three main factors (contact time, temperature, RH) and two two-factor interactions (temperature × RH, RH × UV light intensity) were deemed significant for NO2 removal.
The linear models associated with NO and NO2 removals and determined using JMP had R2 of 0.89 and 0.79, respectively. Residuals calculated with respect to the linear model determined using JMP were fairly consistent across the range of predicted values, suggesting that the hypothesis of equal variance was met. Residuals were also normally distributed as verified using a Shapiro-Wilk test, with W = 0.97, P = 0.41 for NO removal residuals and W = 0.94 and P = 0.08 for NO2 removal residuals.
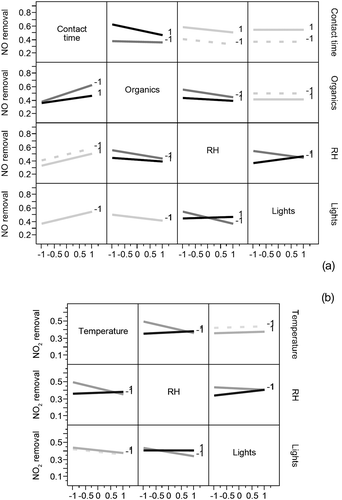
Interaction plots for the three two-factor interactions for NO and the two two-factor interactions for NO2 removal are presented in . It is interesting that for both gases, the interaction plots exhibit crossing lines for the plots involving interactions between relative humidity and light intensity. This suggests that when RH was at its high level, an increase in light intensity also increased removal, but when RH was at its low level, removal decreased with increasing light intensity. Other interactions had different effects on each NOx species.
Discussion
Nitrogen oxide removal main effects
The NO removals observed in this study (~46% on average; ) were in the same range as removals previously observed by researchers testing photocatalytic concrete specimens or pavers. It should be noted, however, that the other studies used higher inlet concentrations (300 ppb and over) than this study (150 ppb). For example, Dylla et al. (Citation2010) observed NO removals in the 20–60% range for contact time varying from 2.4 to 7.1 min. Similarly, Ballari et al. (Citation2011) observed NO removals in the 43–83% range for UV light intensity, temperature, and RH conditions similar to the ones used here and contact times less than 1 min. Both researchers also noted a decrease in removal with increasing relative humidity as observed in this study (). It has been assumed that at higher relative humidity, water molecules compete with pollutant molecules for adsorption sites at the material surface, which would explain the decrease in pollutant removal (Ao et al., Citation2003; Maggos et al., Citation2007b; Hüsken et al., Citation2009; Laufs et al., Citation2010). Dylla et al. (Citation2010) also observed an increase in removal with higher contact times as observed in this study (). Increasing contact time increases the probability of pollutants coming in contact with the material surface and being adsorbed and reacted, hence the positive contrast for that factor.
Other researchers have studied the effect of the presence of VOCs on photocatalytic removal of NOx. Ao et al. (Citation2003) studied the degradation of NO at realistic outdoor levels (200 ppb) in the presence of benzene, toluene, ethylbenzene, and xylenes (BTEX; 0–70 ppb) and observed a reduction in NO removal when BTEX was present versus when it was absent. However, for BTEX concentrations between 3 and 70 ppb, NO conversion was almost constant. In this study, we found NO removal to decrease with increasing VOC concentration (). However, the change in concentration spanned an order of magnitude (0.3–3 ppm). It is possible that VOC molecules competed with NO molecules for adsorption sites at the surface of the photocatalytic material, which would explain the decrease in removal with increased VOC concentration.
Finally, Bengtsson and Castellote (Citation2010) also observed a reduction in NO removal with increasing temperature as observed in this study (). Changes in adsorption kinetics with temperature could explain the decrease observed.
Nitrogen dioxide removal main effects
Nitrogen dioxide removals observed in this study under multigas conditions (~41% average; ) are similar to removals observed by Ballari et al. (Citation2011) when studying removal of NO2 alone on photocatalytic paving stones. Ballari et al. (Citation2011) observed removals between 20% and 75% for UV light intensity, temperature, and RH conditions similar to the ones used here and contact times less than 1 min. Similar to NO removal, an increase in contact time led to an increase in NO2 removal, whereas increases in relative humidity or temperature led to decreases in removal. It is interesting to note that, unlike NO removal, levels of VOCs did not influence NO2 removal. Nitrogen dioxide is an oxidant, and it is possible that it reacted with organics in the gas phase or at the material surface, hence counteracting the reduced availability of photocatalytic reaction sites at the material surface.
Interactions involving two environmental parameters
Interaction plots presented in give more insight into how different environmental parameters interact to affect pollutant removal by the photocatalytic coating. These plots exhibit how one parameter might have a different effect depending on the level of one of the other parameters.
Light intensity and relative humidity interaction
For both NO and NO2 removal, the effect of light intensity was different when the relative humidity was at its high level (coded 1 on the plots) or at its low level (coded −1). When UV light intensity was at its high level, an increase in RH led to no or very small increase in removal, but when UV light intensity was at its low level, increasing RH decreased removal. It is possible that when UV light was at its low level, some photocatalytic sites did not receive enough energy to be activated and increasing RH only led to more water molecules competing with pollutants for adsorption sites. Without enough sites activated, these water molecules were not oxidized to form hydroxyl radicals. When the UV light intensity was at its high level, more photocatalytic sites were active, and by increasing the RH, more water molecules were made available to form hydroxyl radicals that could then react with pollutant molecules, balancing the reduced availability of reaction sites.
Interactions involving organic pollutants
For NO removal, organic pollutant concentrations were involved in two significant interactions, with RH and contact time (). When RH increased from its low level to its high level, NO removal decreased. But the decrease was larger when the organic pollutant concentrations were low versus high. Competition between water, organic pollutant, and NO molecules for adsorption sites might explain this interaction. At the low organic pollutant concentrations condition, few adsorption sites were occupied by VOC molecules, but the change in RH made a large difference in how many sites were available for NO adsorption. On the other hand, under the high organic concentrations condition, a large number of sites were already occupied by VOC molecules, and the addition of water did not change dramatically the number of sites available for NO adsorption, which might explain the interaction observed.
The increase in NO removal from a low to a high contact time condition was larger when organic pollutant concentrations were at the low level. This might be related to the adsorption rate of organic pollutants to the material surface. If the time scale for adsorption of organics to the material surface was longer than the 1.5 min contact time, few organics adsorbed and the change between low and high concentrations of organics did not significantly affect removal. But if the time scale for adsorption was shorter than the 15 min contact time tested, a larger portion of adsorption sites may be occupied when the level of organics was high versus low. This might explain why the NO removal did not increase as much with increasing contact time when the concentration of VOCs was high versus low.
Interaction between temperature and relative humidity
An interaction between RH and temperature was also determined to be significant for NO2 removal (). An increase from the low to the high temperature condition led to a decrease in NO2 removal when RH was at its low level, but not when it was at its high level. It is possible that this was again related to adsorption kinetics. At low RH, there was little competition from water for adsorption of NO2 molecules to the surface. An increase in temperature may change adsorption kinetics for NO2, leading to reduced removal at the stucco surface. However, at high RH, a large portion of adsorption sites may be occupied by water molecules, reducing the effect of the change in temperature on NO2 adsorption and removal.
Implications for roadside removal of NOx
Overall, NOx removal increased with increased contact time but was negatively impacted by high relative humidity and, to a lesser extent, temperature. Organic pollutants negatively affected removal of NO but did not affect removal of NO2. Although NOx emissions from motor vehicles are mostly in the form of NO, the NO is rapidly transformed to NO2 in the presence of ozone (Yao et al., Citation2005). Moreover, new diesel technologies have led to increased emissions of primary NO2 from road vehicles. As such, NO2 accounts for about 20% of NOx emitted on roadways in European cities (Carslaw, Citation2005; Hueglin et al., Citation2006; Kessler et al., Citation2006; Grice et al., Citation2009). This makes photocatalytic coatings particularly attractive for removal of NO2 near roadways, where it is present at elevated levels in mixed pollutant gases. The case for NO removal near roadways is more complex. Removals observed in this study indicate that on average NO is removed more efficiently than NO2 (average removals of 46% and 41%, respectively). However, peak NO concentrations occur in the morning when temperatures are low and RH high. Although low temperature is beneficial to NO removal, high RH and VOC levels are detrimental, making it difficult to evaluate what the overall impact on removal might be. Finally, NO removal is affected by more two-factor interactions than NO2 removal. It is possible that, because NO2 is more reactive than NO with cementitious materials (Judeikis and Wren, Citation1978), some removal occurs through deposition and reaction to nonphotocatalytic sites on the material surface. This might explain why its removal was less affected by environmental parameters. Nitric oxide dry deposition, on the other hand, has been found to be low compared with NO2 dry deposition (Judeikis and Wren, Citation1978). The overall higher removals observed for NO than NO2 in this study suggest that the photocatalytic process removes NO particularly efficiently.
Conclusions
Results of this study suggest that stucco photocatalytic coatings may be appropriate for removing NOx in some realistic outdoor conditions. Placement of the photocatalytic coating materials will matter, as locations with longer contact time will lead to greater removals. Areas experiencing lower relative humidity will also be more suitable for use of photocatalytic materials similar to the one tested here. The results also suggest that the presence of organic pollutants does not affect NO2 removal by the material. Hence, photocatalytic coatings applied on new or existing concrete structures near roadways could be suitable as a control strategy for roadside NO2, because elevated NO2 levels in combination with VOCs are observed near roadways.
Funding
This work was funded by the Texas Department of Transportation (TxDOT) under project 0-6636. The findings are part of an ongoing research project and the final results of the study will be considered by TxDOT before full release of the project final report.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Supplemental Material
Download PDF (2.2 MB)Additional information
Funding
Notes on contributors
Clement J. Cros
Clement J. Cros and Alexandra L. Terpeluk are graduates from the Department of Civil, Architectural and Environmental Engineering at the University of Texas at Austin.
Neil E. Crain
Neil E. Crain is a research scientist at the Center for Energy and Environmental Resources of the University of Texas at Austin.
Richard L. Corsi
Maria C.G. Juenger and Richard L. Corsi are professors in the Department of Civil, Architectural and Environmental Engineering at the University of Texas at Austin.
References
- Allen, N.S., M. Edge, G. Sandoval, J. Verran, J. Stratton, and J. Maltby. 2005. Photocatalytic coatings for environmental applications. Photochem. Photobiol. 2:279–290. doi:10.1111/(ISSN)1751-1097
- American Society for Testing and Materials (ASTM). 2006. Standard D5116-10: Standard Guide for Small-Scale Environmental Chamber Determinations of Organic Emissions From Indoor Materials/Products, Vol. 11. West Conshohocken, PA: ASTM International.
- Ao, C.H., S.C. Lee, C.L. Mak, and L.Y. Chan. 2003. Photodegradation of volatile organic compounds (VOCs) and NO for indoor air purification using TiO2: Promotion versus inhibition effect of NO. Appl. Catal. B Environ. 2:119–129. doi:10.1016/S0926-3373(02)00219-9
- Ballari, M.M., M. Hunger, G. Hüsken, and H.J.H Brouwers. 2010a. NOx photocatalytic degradation employing concrete pavement containing titanium dioxide. Applied Catalysis B: Environmental. 95:245–254.
- Ballari, M.M., M. Hunger, G. Hüsken, and H.J.H Brouwers. 2010b. Modeling and experimental study of the NOx photocatalytic degradation employing concrete pavement with titanium dioxide. Catal. Today 151:71–76. doi:10.1016/j.cattod.2010.03.042
- Ballari, M.M., Q.L. Yu, and H.J.H Brouwers. 2011. Experimental study of the NO and NO2 degradation by photocatalytically active concrete. Catal. Today 1:175–180.
- Bauer, M.A., M.J. Utell, P.E. Morrow, D.M. Speers, and F.R. Gibb. 1986. Inhalation of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm in asthmatics. Am. Rev. Respir. Dis. 6:1203–1208.
- Beeldens, A. 2006. An environmental friendly solution for air purification and self-cleaning effect: The application of TiO2 as photocatalyst in concrete. In Proceedings of Transport Research Arena Europe, Göteborg, Sweden, June 12–15, 2006. Linköping, Sweden: Swedish National Road and Transport Research Institute (VTI).
- Bengtsson, N., and M. Castellote. 2010. Photocatalytic activity for NO degradation by construction materials: Parametric study and multivariable correlations. J. Adv. Oxid. Technol. 3:341–349.
- Brunekreef, B., N.A.H. Janssen, J. de Hartog, H. Harssema, M. Knape, and P. van Vliet. 1997. Pollution from truck traffic and lung function in children living near motorways. Epidemiology 3:298–303. doi:10.1097/00001648-199705000-00012
- Carslaw, D.C. 2005. Evidence of an increasing NO2/NOx emissions ratio from road traffic emissions. Atmos. Environ. 39:4793–4802. doi:10.1016/j.atmosenv.2005.06.023
- Chen, J., and C.-s. Poon. 2009. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 9:1899–1906. doi:10.1016/j.buildenv.2009.01.002
- Clark, N.A., P.A. Demers, C.J. Karr, M. Koehoorn, C. Lencar, L. Tamburic, and M. Brauer. 2009. Effect of early life exposure to air pollution on development of childhood asthma. Environ. Health Perspect. 2:284–290. doi:10.1289/ehp.0900916
- Dalton, J., P. Janes, N. Jones, J. Nicholson, K.R. Hallam, and G.C. Allen. 2002. Photocatalytic oxidation of NOx gases using TiO2: A surface spectroscopic approach. Environ. Pollut. 120:415–422. doi:10.1016/S0269-7491(02)00107-0
- Devahasdin, S., C. Fan, K. Li, and D.H. Chen. 2003. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A 156:161–170. doi:10.1016/S1010-6030(03)00005-4
- Dylla, H., M.M. Hassan, L.N. Mohammad, T. Rupnow, and E. Wright. 2010. Evaluation of environmental effectiveness of titanium dioxide photocatalyst coating for concrete pavement. Trans. Res. Rec. 2164:46–51. doi:10.3141/2164-06
- Dylla, H., M.M. Hassan, M. Schmitt, T. Rupnow, and L.N. Mohammad. 2011. Laboratory investigation of the effect of mixed nitrogen dioxide and nitrogen oxide gases on titanium dioxide photocatalytic efficiency in concrete pavements. J. Mater. Civ. Eng. 7:1087–1093. doi:10.1061/(ASCE)MT.1943-5533.0000248
- Ente Nazionale Italiano di Unificazione (UNI). 2010. Standard 11247: Determination of the Degradation of Nitrogen Oxides in the Air by Inorganic Photocatalytic Materials: Continuous Flow Test Method. Milan, Italy: UNI. https://www.ihs.com/products/uni-standards.html
- Finlayson-Pitts, B.J., L.M. Wingen, A.L. Sumner, D. Syomin, and K.A. Ramazan. 2002. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism. Phys. Chem. Chem. Phys. 2:223–242. doi:10.1039/b208564j
- Frampton, M.W., P.E. Morrow, C. Cox, F.R. Gibb, D.M. Speers, and M.J. Utell. 1991. Effects of nitrogen dioxide exposure on pulmonary function and airway reactivity in normal humans. Am. J. Respir. Crit. Care Med. 3:522–527. doi:10.1164/ajrccm/143.3.522
- Fujishima, A., and K. Honda. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38. doi:10.1038/238037a0
- Gauderman, W.J., E. Avol, F.W. Lurmann, N. Kuenzli, F. Gilliland, J. Peters, and R. McConnell. 2005. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology 6:737–743. doi:10.1097/01.ede.0000181308.51440.75
- Grice, S., J. Stedman, A. Kent, M. Hobson, J. Norris, J. Abbott, and S. Cooke. 2009. Recent trends and projections of primary NO2 emissions in Europe. Atmos. Environ. 43:2154–2167. doi:10.1016/j.atmosenv.2009.01.019
- Hoffmann, M.R., S.T. Martin, W. Choi, and D.W. Bahnemann. 1995. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1:69–96. doi:10.1021/cr00033a004
- Hueglin, C., B. Buchmann, and R.O. Weber. 2006. Long-term observation of real-world road traffic emission factors on a motorway in Switzerland. Atmos. Environ. 40:3696–3709. doi:10.1016/j.atmosenv.2006.03.020
- Hunger, M., G. Hüsken, and H.J.H. Brouwers. 2010. Photocatalytic degradation of air pollutants—From modeling to large scale application. Cem. Concr. Res. 2:313–320. doi:10.1016/j.cemconres.2009.09.013
- Hüsken, G., M. Hunger, and H.J.H Brouwers. 2009. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 12:2463–2474. doi:10.1016/j.buildenv.2009.04.010
- International Organization for Standardization. 2007. Standard 22197-1:2007: Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. Geneva, Switzerland: International Organization for Standardization.
- Janssen, N.A.H., B. Brunekreef, P. van Vliet, F. Aarts, K. Meliefste, H. Harssema, and P. Fischer. 2003. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ. Health Perspect. 111:1512–1518. doi:10.1289/ehp.6243
- Japanese Industrial Standards. 2010. Standard R1701-1:2010: Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air Purification Performance of Photocatalytic Materials—Part 1: Removal of Nitric Oxide. Tokyo, Japan: Japanese Industrial Standards.
- Jenkin, M.E., R.A. Cox, and D.J. Williams. 1988. Laboratory studies of the kinetics of formation of nitrous acid from the thermal reaction of nitrogen dioxide and water vapour. Atmos. Environ. 3:487–498. doi:10.1016/0004-6981(88)90194-1
- Judeikis, H.S., and A.G. Wren. 1978. Laboratory measurements of NO and NO2 depositions onto soil and cement surfaces. Atmos. Envron. 12:2315–2319. doi:10.1016/0004-6981(78)90272-X
- Kessler, C., A. Niederau, and W. Scholz. 2006. Estimation of NO2/NOx relations of traffic emissions in Baden-Württemberg from 1995 to 2005. In 2nd Conference Environment & Transport, Reims, France, June 12, 2006, 101–105. Arcueil, France: Inrets Editions.
- Krämer, U., T. Koch, U. Ranft, J. Ring, and H. Behrendt. 2000. Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology 1:64–70. doi:10.1097/00001648-200001000-00014
- Laufs, S., G. Burgeth, W. Duttlinger, R. Kurtenbach, M. Maban, C.E. Sandy Thomas, C. Thomas, P. Wiesen, and J. Kleffman. 2010. Conversion of nitrogen oxides on commercial photocatalytic dispersion paints. Atmos. Environ. 19:2341–2349. doi:10.1016/j.atmosenv.2010.03.038
- Lim, T., S. Jeong, S. Kim, and J. Gyenis. 2000. Photocatalytic decomposition of NO by TiO2 particles. J. Photochem. Photobiol. A 134:209–217. doi:10.1016/S1010-6030(00)00265-3
- Maggos, Th., J.G. Bartzis, M. Liakou, and C. Gobin. 2007a. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 3:668–673. doi:10.1016/j.jhazmat.2007.04.079
- Maggos, Th., J.G. Bartzis, P. Leva, and D. Kotzias. 2007b. Application of photocatalytic technology for NOx removal. Appl. Phys. A Mater. Sci. Process. 1:81–84. doi:10.1007/s00339-007-4033-6
- Mee, R.W. 2009. A Comprehensive Guide to Factorial Two-Level Experimentation. New York: Springer.
- Mohsenin, V. 1987. Airway responses to nitrogen dioxide in asthmatic subjects. J. Toxicol. Environ. Health 4:371–380. doi:10.1080/15287398709531080
- Nafstad, P., L. Lund Håheim, T. Wisløff, F. Gram, B. Oftedal, I. Holme, I. Hjermann, and P. Leren. 2004. Urban air pollution and mortality in a cohort of Norwegian men. Environ. Health Perspect. 5:610–615. doi:10.1289/ehp.6684
- Ohko, Y., Y. Nakamura, N. Negishi, S. Matsuzawa, and K. Takeuchi. 2009. Photocatalytic oxidation of nitrogen monoxide using TiO2 thin films under continuous UV light illumination. J. Photochem. Photobiol. A 1:28–33. doi:10.1016/j.jphotochem.2009.04.005
- Ollis, D.F., E. Pelizzetti, and N. Serpone. 1991. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 9:1522–1529. doi:10.1021/es00021a001
- Peters, A., E. Liu, R.L. Verrier, J. Schwartz, D.R. Gold, M.A. Mittleman, J. Baliff, J.A. Oh, G. Allen, K. Monahan, and D.W. Dockery. 2000. Air pollution and incidence of cardiac arrhythmia. Epidemiology 11:11–17. doi:10.1097/00001648-200001000-00005
- Raaschou-Nielsen, O., and P. Reynolds. 2006. Air pollution and childhood cancer: A review of the epidemiological literature. Int. J. Cancer 12:2920–2929. doi:10.1002/(ISSN)1097-0215
- Saliba, N.A., H. Yang, and B.J. Finlayson-Pitts. 2001. Reaction of gaseous nitric oxide with nitric acid on silica surfaces in the presence of water at room temperature. J. Phys. Chem. A 45:10339–10346. doi:10.1021/jp012330r
- Scanlon, D.O., C.W. Dunnill, J. Buckeridge, S.A. Shevlin, A.J. Logsdail, S.M. Woodley, C. Richard, A. Catlow, M.J. Powell, R.G. Palgrave, I.P. Parkin, G.W. Watson, T.W. Keal, P. Sherwood, A. Walsh, and A.A. Sokol. 2013. Band alignment of rutile and anatase TiO2. Nature Materials 9:798–801. doi:10.1038/nmat3697
- Seinfeld, J.H., and S.N. Pandis. 2006. Atmospheric Chemistry and Physics, 2nd ed. Hoboken, NJ: Wiley-Interscience.
- Svensson, R., E. Ljungström, and O. Lindqvist. 1987. Kinetics of the reaction between nitrogen dioxide and water vapour. Atmos. Environ. 7:1529–1539. doi:10.1016/0004-6981(87)90315-5
- Tamhane, A.C. 2009. Statistical Analysis of Designed Experiments. New York: John Wiley & Sons.
- Texas Commission on Environmental Quality (TCEQ). 2009. Hourly air pollution data. http://www.tceq.texas.gov/airquality/monops/hourly_data.html ( accessed October 27, 2010).
- Thoma, E.D., R.C. Shores, V. Isakov, and R.W. Baldauf. 2008. Characterization of near-road pollutant gradients using path-integrated optical remote sensing. J. Air Waste Manage. Assoc. 58:879–890. doi:10.3155/1047-3289.58.7.879
- U.S. Environmental Protection Agency (EPA). 2010. Final Revisions to the Primary National Ambient Air Quality Standard for Nitrogen Dioxide (NO2). Washington, DC: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards.
- U.S. Environmental Protection Agency (EPA). 2011. National Emissions Inventory (NEI) Air Pollutant Emissions Trends Data. Washington, DC: U.S. Environmental Protection Agency Office of Air Quality Planning and Standards.
- Yao, X., N.T. Lau, C.K. Khan, and M. Fang. 2005. The use of tunnel concentration profile data to determine the ratio of NO2/NOx directly emitted from vehicles. Atm. Chem. Phys. Discuss. 5:12723–12740. doi:10.5194/acpd-5-12723-2005