Abstract
The aim of this study was to analyze the incidence of fungi in the chapel crypt. The MAS 100 was used to monitor the air pollution. The lowest numbers of fungal colonies were isolated at the entrance to the cemetery (2400 CFU/m3). The outside temperature ranged from 24.5oC to 28.1oC, and relative humidity was between 35.3% and 46.4 %. The highest of fungal colonies from air samples at baseline were isolated inside the crypt when coffin was opened (4820 CFU/m3). The temperature in the crypt at baseline varied between 19.6°C and 25.6°C and humidity was between 50.8% and 60.1%. The number of fungal colonies increased significantly at the end of the study. Ten species of fungi were isolated from air samples inside and outside the chapel, and seven species of fungi were isolated on the surface of the exterior and interior of the chapel. Thirteen types/species of fungus were isolated from air samples collected in the crypt; 15 species of fungi were isolated on the walls, surface of the coffin, bones and other objects. Assessment of fungi in the air samples and different surfaces of the crypt, it revealed very high levels of molds in the air samples.
Implications: Assessment of fungi in the air samples and different surfaces of the crypt revealed high levels of molds in the air samples. Fungal numbers within the crypt exceed recommended limits for occupational exposure. Employees working in the crypts should know about these hazards.
Introduction
Moulds are biological hazards that are ubiquitous both in communal and occupational environments (Samson, Citation1985). Communal objects such as waste dumps, wastewater treatment plants, cemeteries, and ground conditions are serious sources of chemical compounds and bioaerosol emissions that can have negative effects on surrounding areas and human health. These bioaerosols contain many harmful elements such as microorganisms in the form of endospores, spores, conidia, mycelium fragments, or even vegetative forms that can pose serious disease-forming risk to the health of people. The number of microorganisms in bioaerosols depends on various factors, of which the most important are climatic factors and the conditions of storing, way of exploitation, and aeration of chambers (Bernstein et al., Citation2008).
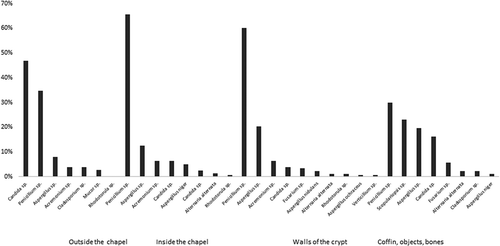
Figure 2. The incidence of fungal colonies isolated from different surfaces of the chapel and crypt.
The state of the air microenvironment is also conditioned by the kind of construction stone and microflora that inhabit it (Cwalina and Zyska, Citation2005; Krajewska-Kułak and Łukaszuk, Citation2008; Polizzi et al., Citation2012). Stone biocoenose consists of phototrophs (cyanobacteria, algae, mosses, higher plants) and chemotrophs (bacteria, fungi, including Aspergillus, Cladosporium, Curvularia, Penicillium, Phoma, Lecythophora, Exophiala, Trichoderma, Alternaria, and Stemphylium).
In 1982, researchers introduced the term “sick building syndrome” (SABS) to define a set of various ailments caused by long-term staying in rooms where construction and equipment can have destructive influences on people’s health (Tsai et al., Citation2012). In 1987, the World Health Organization determined a list of symptoms and disease entities that can be caused by “sick buildings”; it was prepared according to the occurrence frequency of features such as irritation or damage of mucous, drying and irritation of skin, neurotoxic symptoms (headaches, fatigue, annoyance, losing concentration), asthma-like symptoms, air-conditioning fever, and lung tissue damage (Brooks et al., Citation2005).
Within the last few years, the term SBS has been changed into BRI—“building-related illness,” and there were distinguished two groups of diseases: specific (states deriving from allergy, immunity, infections) and nonspecific (irritation of skin and mucous membranes, headaches, fatigue, concentration disorders) (Page and Trout, Citation2001). Other researchers such as Brooks et al. (Citation2005), Rylander and Mégevand (Citation2000), and Afari and Buchwald (Citation2003) extended the range of BRI to infections (legionellosis, flu, rubella, recurrent infections of respiratory tract, chronic inflammation of paranasal sinuses), allergic diseases (asthma, year-round allergic catarrh and conjunctivitis), air-conditioning fever, allergic inflammation of alveolus, dryness and red patches on skin—especially the face, itch, hives, intensification of chronic skin diseases, irritation and chronic conjunctivitis, neurotoxic symptoms, flu-like, and fatigue syndrome.
Generally, tomb workers are at particular health risk for bacterial and fungal contaminations. The microorganisms from air, including moulds, are easily inhaled by the workers. Researchers studied the air in the tomb because of scheduled maintenance in the chapel. Before that, the tomb has not been opened since 1933.
The aim of this study was to analyze the incidence of fungi in the chapel crypt of Buchholt’s at Lutheran Cemetery in Supraśl, Poland.
Materials and Methods
Sampling was performed in cemetery and the burial chapel of the Buchholtz family in Supraśl (Poland) during the month of July (2013). Materials taken for the study were samples of the air at the entrance of the cemetery, the burial chapel, and the tomb. The microbial flora were collected from different surfaces (walls, ceilings, floors, coffins, and skeletal bones). The chapel was built in 1904, and the hatch to the crypt had not been opened since 1933. Humidity and temperature measurements were also taken.
The microorganisms in air were sampled using an MAS-100 (single-stage sieve impactor; Merck, Danmstadt, Germany, Switzerland) for standard 100-mm Petri dishes to collect a 100-L air sample. The number of colony-forming units (CFU) in the samples was calculated by referring to the positive hole conversion table for the MAS-100 per the instructions of the manufacturer. The sampler was placed at a height of 1.5 m from the floor. The microbial flora from the walls were detected using the Count-Tact applicator and the plate Count-Tact (BioMerieux, Warszawa, Poland).
Classification of the isolated fungi was made in accordance with current procedures. The cultured fungi were identified by macroscopic and microscopic characteristics, and biochemical tests were appropriate. Yeast-like fungi were identified by means of the original CHROMagar (Paris, France) Candida-selective and differential media. With the inclusion of chromogenic substrates in the medium, the C. albicans, C. tropicalis, and C. krusei colonies produced different colors, thus allowing the direct detection of these yeast species on the isolation plate. Colonies of C. albicans appear as a light to medium green color, C. tropicalis colonies appear dark blue to metallic blue, and C. krusei colonies appear as a light mauve to mauve color—flat colonies with a whitish border. Other yeasts appear as a light to dark mauve (e.g., C. glabrata and other species).
The incidence of genus/species (indicator F) was calculated according to the formula:
where F is the frequency of species, a is the number of trials in which there was a strain, and n is the number of attempts. For moulds, a microscopical evaluation of the morphological elements used in preparations was performed. Temperature and humidity were measured using a thermo-hygrometer PWT-401 (Elmetron, Zabrze, Poland).
The results were analyzed statistically according to Wilcoxon’s test. For all the analyses, we took the two-tailed significance (P < 0.05).
Results
Mean number of air borne fungi was determined at the entrance of the chapel (2510 ± 210 CFU/m3) and at the entrance of cemetery (2400 ± 220 CFU/m3). The outside temperature ranged from 24.5 to 28.1 °C, relative humidity 35.3% to 46.4%, and airflow was 0.11 to 0.48 m/sec.
Inside the chapel, air samples were isolated from 2670 to 3150 fungal colonies. The temperature in the chapel was 24.2 °C, humidity 42.2%, and the movement of air was 0.02 m/sec. Details are shown in .
Table 1. Number of fungal colonies in air samples collected inside and outside the chapel
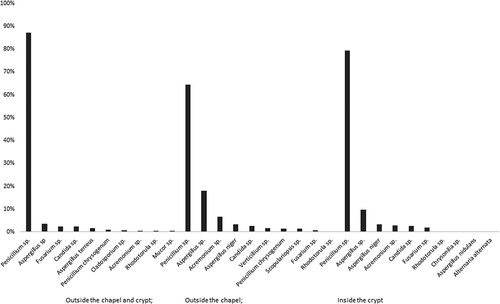
In the air samples, 10 genera/species were collected outside the chapel and 11 genera/species were collected inside the chapel. The most dominant airborne fungi identified were Penicillium sp. and Aspergillus sp., both outside and inside the chapel. Details are shown in and , respectively.
Table 2. Airborne fungi identified from the air collected outside the chapel
Table 3. Airborne fungi identified from the air collected inside the chapel
The number of fungal colonies isolated from different surfaces outside the chapel ranged from 41 to 144 colonies. A total of 7 genera/species were collected from the walls outside of the chapel. Details are shown in . The highest number of fungal colonies detected inside the crypt was 3680 ± 440 CFU/m3.
Table 4. Fungi identified from different surfaces from outside and inside the chapel
Air temperature in the crypt was measured to have a baseline range between 19.6 and 25.6 °C, humidity between 50.8% and 60.1%, and the airflow from 0.01 to 0.03m/sec. At the end of the test, the number of fungal colonies isolated from air samples increased significantly (P < 0.05) in the left corner of the crypt from 3680 to 4820 CFU/m3 and in the right corner from 3880 to 4340 CFU/m3. The air temperature dropped from 23.6 to 20.4 °C, humidity increased from 56.5% to 59%, and the airflow was 0.03 m/sec. Similarly, air temperature dropped from 23.4 to 22.1 °C, humidity increased from 51.3% to 56%, and the airflow decreased from 0.02 to 0.01 m/sec in the right corner. Results are shown in .
Table 5. Number of fungal colonies in air samples collected in the crypt
A total of 13 genera/species were isolated in air samples collected inside the crypt. Details are shown in . The number of fungal colonies isolated from different surfaces in the crypt ranged from 16 to 101. A total of 15 genera/species were isolated from the walls, the surface of coffins, bones, and other objects. Details are shown in .
Table 6. Number of fungal colonies in air samples collected in different places of the crypt
Table 7. Number of fungal colonies and species isolated from different surfaces of the crypt
The incidence of each genera/species of fungi (F ratio) was calculated according to the formula described in Materials and Methods.
In the air samples taken outside of the chapel, Penicillium sp. was most frequently (F = 87.2%) isolated, inside the chapel the most frequent was Penicillium sp. (F = 64.5%), and in the crypt also Penicillium sp. (F = 79.4%). Details are presented in .
In the samples taken from the surface outside of the chapel, Candida sp. was most frequently (F = 46.8%) isolated, Penicillium sp. was the most frequently (F = 65.6%) detected inside the chapel, and Penicillium sp. was most frequently (F = 60.1%) isolated from the walls of crypt; Penicillium sp. was isolated most often (F = 29.9%) in the coffins, objects, and bones. Details are presented in .
Discussion
Assessment of fungi in the air samples and different surfaces of the crypt revealed very high levels of molds in the air samples. Unfortunately, in Poland, there is no relevant legislation specifying ranges of concentrations of the harmful biological agents occurring within the Public Service.
In the air samples collected inside the crypt, the fungi isolated included Penicillium sp., Candida sp., Aspergillus sp., Acremonium sp., Scopulariopsis sp., Fusarium sp., Aspergillus niger, Cladosporium sp., Alternaria alternata, Mucor sp., Aspergillus nidulans, Rhodotorula sp., and Verticillium sp. The ambient, humid air of the crypt is appropriate to fungal growth, the temperature being fairly stable at 19–25 °C and the relative humidity being 50–60% further promote it (Sanchez-Moral et al., Citation2005).
In 1923, an English archaeologist, Howard Carter, discovered a tomb of Tutankhamen (Krzysztofik, Citation1992). Almost a year later, Lord Carnavon died, and his death started rumors about the curse that would befall every daredevil who violated eternal peace of the Pharaoh. The lord’s death began a black series: 6 months later a younger brother of Carnavon died. The people related to the works in the Valley of the Kings were terrified of revenge from the other world, although out of 26 people, who were present at the tomb opening, only 6 died within 10 years, and all of them were old.
In 1962, a biologist from Cairo University, Ezzedin Taha (Krzysztofik, Citation1992), had found poisonous fungi in ancient tombs and mummies, which were able to stay active for thousands of years. The researcher emphasized that in cases where fungal spores got to human lungs, they caused high fever, infections, and inflammations of respiratory tract.
In Poland, at the beginning of the 1970s, there was an opening of a tomb of King Casimir Jagiellon (Gąska-Jedruch and Dudzinska, Citation2009). Many researchers of the Wawel crypt had serious problems with health, and afterwards, there began a black series, just like in Egypt. All of them were middle-aged and had never complained about their health before. In 1974, three researches died and in 1975, one.
Furthermore, the members of the archaeological team, working in the church of St. Peter and Paul in Tworków (Poland), were complaining about health problems (Trojanowska, et al., Citation2012). During their work, they felt fatigue, dizziness, and consciousness disturbances. The archaeologists opened 10 copper sarcophagi of the Reiswitz family. In this crypt, which has not been opened for about 300 years, 449 colonies of moulds were collected in the air samples.
According to Krzystofik (Citation1992), permitted number of fungi per m3 of the internal room should be in the range of 100–300 cells and in the outdoor air, 1000 cells. By the European Union (EU) requirements, the number of microorganisms in 1 m3 of indoor air should not exceed 500 cells (Directive Citation2000/54/EC; EC-European Union, Citation2000). In the present study, the numbers of colonies in both outdoor air samples as well as internal samples considerably exceeded the EU limit.
Szczepanowska and Cavaliere (Citation2004) assessed fungi in the air samples and different surfaces of the crypts from the World War I. They found that number of fungal spores in 1 m3 ranged from 1719 to 5244 CFU/m3. They found a few species of fungi, such as Aspergillus, Penicillium, Cladosporium, Saccharomyces, and Rhodotorula. In the present study, the number of fungal colonies isolated from air samples of the crypt ranged from 3680 to 4820 CFU/m3.
Trojanowska et al. (Citation2012) fount that in the examined crypts, the largest percentage (50%) was constituted by Penicillium. Fungi belonging to Aspergillus genus constituted 4.5% of the fungi grown. The rest of the isolated moulds belonged to the genera Paecilomyces, Fusarium, Stachybotrys, Cladosporium, Chrysosporium, and Acremonium. From swabs and scraps collected from walls, ceilings, coffins, and remains, besides isolated moulds that were identified in the air, there were also A. fumigatus, Mucor spp., Absidia spp., and Scopulariopsis spp.
Conclusion
In the air samples collected inside the crypt, the fungi isolated included Penicillium sp., Candida sp., Aspergillus sp., Acremonium sp., Scopulariopsis sp., Fusarium sp., Aspergillus niger, Cladosporium sp., Alternaria alternata, Mucor sp., Aspergillus nidulans, Rhodotorula sp., and Verticillium sp. Assessment of fungi in the air samples and different surfaces of the crypt revealed very high levels of molds in the air samples that may be dangerous for tomb workers health.
Additional information
Notes on contributors
Cecylia Łukaszuk
Cecylia Łukaszuk, PhD, is the Assistant of Department of Integrated Medical Care at the Medical University of Białystok.
Elżbieta Krajewska-Kułak
Elżbieta Krajewska-Kułak, MD, PhD, is a Professor and Head of Department of Integrated Medical Care at the Medical University of Białystok.
Andrzej Guzowski
Andrzej Guzowski, PhD, is the Assistant of Department of Integrated Medical Care at the Medical University of Białystok.
Bogumiła Kraszyńska
Bogumiła Kraszyńska, is a Laboratory technician at the Medical University of Białystok.
Magdalena Grassmann
Magdalena Grassmann, PhD, is the Head of Department of History of Medicine and Pharmacy at the Medical University of Białystok.
Radosław Dobrowolski
Radosław Dobrowolski, is a Master of history at the Town Hall Supraśl.
References
- Afari, N., and D. Buchwald D. 2003. Chronic fatigue syndrome: A review. Am. J. Psychiary 160:221–236. doi:10.1176/appi.ajp.160.2.221
- Bernstein, J.A., N. Alexis, H. Bacchus, I.L. Bernstein, P. Fritz, E. Horner, N. Li, S. Mason, A. Nel, J. Oullette, K. Reijula, T. Reponen, J. Seltzer, A. Smith, and S.M. Tarlo. 2008. The health effects of non-industrial indoor air pollution. J. Allergy Clin. Immunol. 121:585–591. doi:10.1016/j.jaci.2007.10.045
- Brooks, S.M., W. Spaul, and J.D. McCluskey. 2005. The spectrum of building-related airway disorders: Difficulty in retrospectively diagnosing building-related asthma. Chest 128:1720–1727. doi:10.1378/chest.128.3.1720
- Cwalina, B., and B. Zyska. 2005. Mineral building materials—Stone, concrete, brick, mortar construction, glass [in Polish]. In Mikrobiologia materiałów, ed. B. Zyska and Z. Żakowska, 377–406. Łódź: Wydawnictwo Politechniki Łódzkiej.
- EC-European Union. 2000. Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16 (1) of Directive 89/391/EEC. Official Journal of the European Communities 17 October 2000.
- Gąska-Jedruch, U., and M.R. Dudzinska. 2009. Microbial contaminants in indoor air [in Polish]. In Materiały naukowe III Ogólnopolskiego Kongresu Inżynierii Środowiska, Materiały Zjazdowe, tom 2, 31–40. Lublin: 13-17.09.2009.
- Krajewska-Kułak, E., and C. Łukaszuk. 2008. Fungal infections in the human environment [in Polish]. In Mikologia—co nowego, ed. E. Baran, 158–173. Wrocław: Cornetis.
- Krzysztofik, B. 1992. Microbiology of Air [in Polish]. Warszawa: Wydawnictwo Politechniki Warszawskiej.
- Page, E., and D.B. Trout. 2001. The role of Stachybotrys mycotoxins in building-related Illness. Am. Ind. Hyg. Assoc. J. 62:644–648. doi:10.1202/0002-8894(2001)062%3C0644:TROSMI%3E2.0.CO;2
- Polizzi, V., A. Adams, S. De Saeger, C. Van Peteghem, A. Moretti, and N. De Kimpe. 2012. Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. Sci. Total Environ. 414:277–286. doi:10.1016/j.scitotenv.2011.10.035
- Rylander, R., and Y. Mégevand. 2000. Environmental risk factors for respiratory infections. Arch. Environ. Health 55:300–303. doi:10.1080/00039890009604021
- Samson, R.A. 1985. Occurrence of moulds in modern living and working environments. Eur. J. Epidemiol. 1:54–61. doi:10.1007/BF00162313
- Sanchez-Moral, S., L. Luque, S. Cuezva, V. Soler, D. Benavente, L. Laiz, J.M. Gonzalez, and C. Saiz-Jimenez. 2005. Deterioration of building materials in Roman catacombs: The influence of visitors. Sci. Total Environ. 349:260–276. doi:10.1016/j.scitotenv.2004.12.080
- Sharma, K., and A. Nafis. 2012. Microbiological impacts on the Qutub Khan’s tomb (Gurgaon), India. World J. Sci. Technol. 2:63–65.
- Szczepanowska, H.M., and A.R. Cavaliere. 2004. Tutankhamun tomb: A closer look at biodeterioration—Preliminary report [in German]. In München Schimmel-Gefahr für Mensch und Kulturgut durch Mikroorganismen, VDR-Schriftenreihe zur Restaurierung, ed. A. Rauch, S. Miklin-Kniefacz, and A. Harmssen, 42–47. München: VDR-Schriftenreihezur Restaurierung.
- Trojanowska, D., M. Tokarczyk, B. Bogusz, and A. Budak. 2012. Analysis of the occurrence of moulds in crypts of the Saints Peter and Paul Church in Krakow, performed in connection with the building/construction of the National Pantheon [in Polish]. Mikol. Lek. 19:69–73.
- Tsai, D.H., J.S. Lin, and C.C. Chan. 2012. Office workers’ sick building syndrome and indoor carbon dioxide concentrations. J. Occup. Environ. Hyg. 9:345–351. doi:10.1080/15459624.2012.675291