ABSTRACT
The industrial development in Algeria has made a worrying situation for all socioeconomic stakeholders. Indeed, this economic growth is marked in recent years by the establishment of factories and industrial plants that discharge liquid waste in marine shorelines. These releases could destabilize the environmental balance in the coming years, hence the need to support the processing of all sources of pollution. Remediation of such discharges requires several steps of identifying the various pollutants to their treatments. Therefore, the authors conducted this first work of characterization of industrial effluents generated by the mineral fertilizer factory complex Fertial (Arzew), and discussed the pollution load generated by this type of industry. This monitoring would establish a tool for reflection and decision support developed by a management system capable of ensuring effective and sustainable management of effluents from industrial activities of Fertial.
Implications: The authors conducted this first work of characterization of industrial effluents generated by the mineral fertilizer factory complex Fertial (Arzew), and discussed the pollution load generated by this type of industry. This monitoring would establish a tool for reflection and decision support developed by a management system capable of ensuring effective and sustainable management of effluents from industrial activities of Fertial.
Introduction
The pollution caused by the discharge of wastewater is one of the most widespread forms of pollution and the most damaging to all coastal marine ecosystems of the planet. Most often, these waters are released near the coast in subtidal and can affect marine fauna (Espinosa et al., Citation2007). Their release into the natural environment is often associated with disorders at the cellular level and/or physiological level in organisms, and the disruption of habitats caused irreversible changes in community structure. Like all living beings, fauna and flora are exposed to the toxic effects of pollutants released into the wild. They then react by demonstrations that give useful guidance reflecting the degree of pollution of surface waters and marine waters. Today, these effects attract the attention of some regional and national research centers and have as well a big importance to international organizations, which should study the living resources as means of humanity protection. Ammonia is also a building block for the synthesis of many chemicals (nitric acid, ammonium chloride, etc.) and is used in many commercial cleaning products (either as its salts, solutions, or anhydrous). There are several manufacturers worldwide that produce 157 million tons of ammonia per year (International Fertilizer Industry Association [IFA], Citation2012). Choosing industrial unit zone, uncontrolled industrial wastes, and lack of awareness of environmental problems including the harmfulness of certain elements discharged by industries in the marine environment are factors that should be paid a great importance because their negligence can be a serious harm to the population and environment. The discharge of untreated domestic and industrial wastewaters into aquatic bodies is posing a serious eutrophication threat, leading to a slow degradation of the water resources (Renuka et al., Citation2015). These wastes and effluents vary based on the kind of raw materials used in product design and the technology applied during manufacture. Such wastes may have negative impacts at the local, regional, and global levels, inducing adverse or harmful effects to human health and the environment. Thus, both qualitative and quantitative environmental assessment is a must-have, requiring constant monitoring to predict the trend of the receiving environment and for recommending appropriate remedies.
The complex Fertial (Arzew) specialized in the production of ammonia, nitric acid, ammonium nitrate, and urea, is located on the Arzew industrial platform 40 km west of Oran, 3 km east of Arzew, and 4 km west of Bethioua and is close to the sea (). This region is one of the most polluted areas of the Algerian coast. Indeed, the complex pours its unfiltered liquid effluents containing suspended solids, oil, grease, and mineral and organic matter dissolved in the sea, resulting in contaminating and intoxicating the sea. Indeed, a large load of organic matter leads to a growth of aerobic microorganisms and causes a drop of the dissolved oxygen in the acceptor, leading to asphyxia of the species present. High levels of suspended solids can prevent the penetration of light, decrease dissolved oxygen, and then limit the development of aquatic life by creating imbalances leading to the suffocation and death of various aquatic creatures (Rodier, Citation1997).
Also, nitrogen compounds promote proliferation of aquatic plants (including algae), which leads to eutrophication of the receiving environment. A similar phenomenon can be observed when the aquatic environment receives appreciable amounts of oils and fats. The SM (suspended matter), undissolved materials, must also be taken into account in a pollution balance, as they can affect the quality of water through adsorption phenomena, including some toxic elements.
The present study, taken place in the complex Fertial, revolves around a major objective being an environmental assessment of industrial wastewater liquidated into the marine coastline. For this, it is necessary to conduct physicochemical analyses of liquid effluents discharged by the unit. This study focuses on the identification and quantification of pollutants in water downstream of fertilizer manufacturing processes. This is achieved by analyzing various parameters and/or compounds that can cause pollution; further analysis results are compared with the criteria and standards of industrial spill recommended by national legislation.
Materials and methods
Fertial has three liquid effluent discharge systems, the first sewer (RL1) and the second one (RL2) are used for the ammonia plants I and II, the third sewer (RL3) is utilized for the acid nitrate unit. The above-mentioned sewers dump their dirt into the sea. The samples taken from various places are representative for the concerned zone Fertial, with, of course, avoiding dead and blocked zones. Qualitative and quantitative assessments of the degree of pollution of effluents produced by the Fertial complex covers the period from 3 April to 29 May. The evaluation process is done by controlling the pollution level of liquid discharges. The samples taken go under several daily and frequently analyses, once or twice per week. This allocation is the set of small samples having a volume of 200 mL every 30 min for 24 hr; it can be considered a representative set of samples of our collection. These analyzes focused on the temperature and pH, SM, total nitrogen and ammonium (NH4+), nitrate (NO3−), oils, and grease. The collected water is taken from deep within the sewer conforming to the International Organization of Standardization (ISO) 5667-10 standard (1979) on the sampling of wastewater.
Sampling method
For the purpose of minimizing possible contamination, the collection and handling of samples must be prudently and carefully done, using polyethylene bottles that are purposefully and suitably designed for the sampling process. These latter should be cleaned and rinsed thoroughly to remove all traces of any detergent. The sample is preserved in cold to be analyzed later. The length of transporting cylinders must not exceed 30 min, and the analyses are made once they arrive at the laboratory except for oils and fats, whose analyses should be made 24 hr after the samples are properly treated.
The various parameters used in the characterization of the liquid pollution generated by the Fertial complex are analyzed with standard methods and usually adopted by this complex. The type of method, the equipment used, and the reference method are summarized in .
Table 1. Methods of analysis used in the characterization of the pollution of liquid discharge.
The measurement of temperature and pH is needed to understand the evolution of other parameters. These parameters may change during transport of samples and are preferably measured in the field. The measurements were made using a multiparameter instrument (Endress Hauser, ASP Station 2000); it contains pH and temperature sensors.
Results and discussion
Temperature and pH
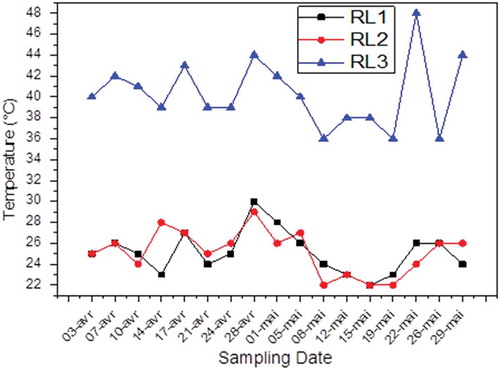
The temperature and pH have a very important role to control an industrial discharge. Excessive changes in both parameters at the receiving environments tend to change the habitat and disrupt aquatic and terrestrial lives. The temperature monitoring of the Fertial unit rejection shows values below 30 °C (permissible norm) in the samples except for RL3. In fact, the temperature is in most cases normal and varies between 22 and 30 °C for RL1 with an average of 25.12 °C, between 22 and 29 °C for RL2 with an average of 25.18 °C, and between 36 and 44 °C for RL3 with an average of 40.29 °C ().
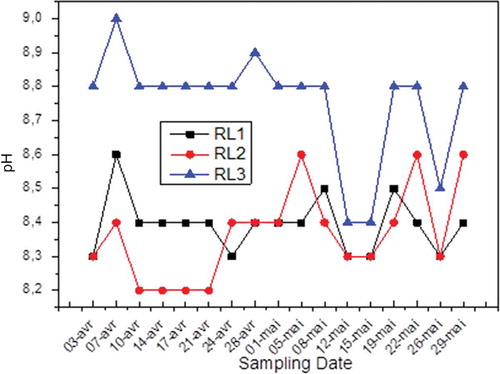
The pH informs us about the acidity of the medium. Therefore, it is noted that for discharges I and II () the pH is between 8.2 and 8.5, and these values are tolerated compared with pH standard accepted (7–8.5). Furthermore, a value of 9 is recorded in April 3 for the discharge and that can be explained by the presence of a high concentration of ammonia in the wash water.
Suspended matter
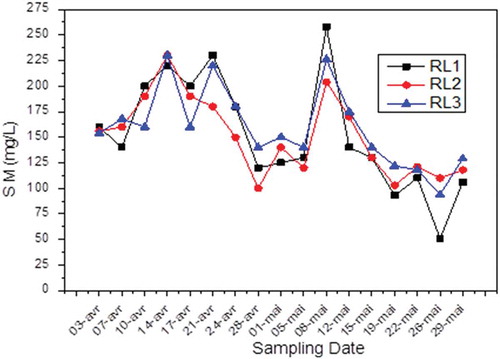
The results of effluent analysis performed for nearly 2 months () show that water discharged from the complex is loaded with SM, exceeding the standard of 35 mg/L. Particularly, to the second week of May, the concentration of these fine particles in the wastewater reaches a value of 258 mg/L, considered a disturbing value. This high concentration may be due to the presence of high levels of fine particles in the system, thereby contaminating the wash water. The presence of the SM in the releases may significantly jeopardize the operation of the sewer system and cause nuisances such as sludge deposits and clogging of the aquatic receptors funds.
These disturbing levels of SM in liquid effluents in Fertial must be considered seriously and the problem properly managed in order to resolve definitively from pollution.
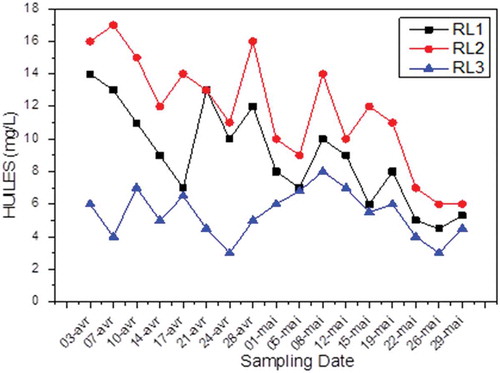
Oil and grease
Results of analysis of oils and fats in wastewater in Fertial show that these substances are present in fat trace () and are therefore much lower than the standard in force in Algeria, namely, 30 mg/L. Under these conditions, no treatment should be considered against this pollution.
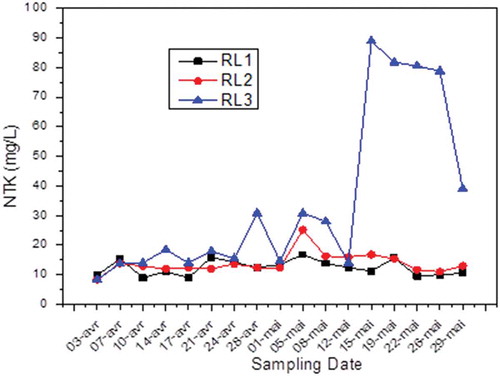
Kjeldahl nitrogen and NH4+
In practice, Total Kjeldhal Nitrogen (TKN) is an indicator of environmental pollution and its control allows monitoring contamination. TKN includes organic nitrogen (proteins, peptides, nucleic acids, etc.) and ammonia nitrogen (Teh et al., Citation2014).
The Kjeldahl nitrogen analysis is performed by distillation in the presence of alloy Dewarda. Results shown in show high values relative to the standard (30 mg/L). This parameter explaining the heavy load of nitrogen compounds in the effluents from the Fertial unit reflects the critical state of discharge and consequently the state of charge of these facilities’ effluents.
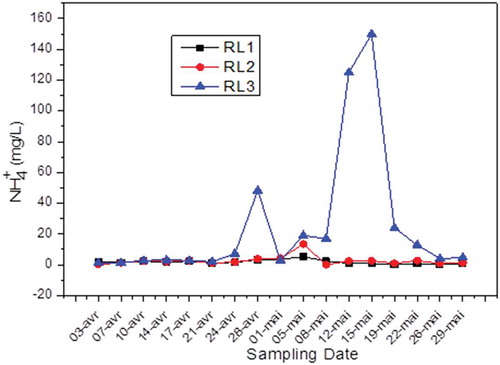
Regarding ammonium ions (NH4+), shows that the values comply with the standard (40 mg/L), with the exception of values corresponding to the last week of April and the second week of May during which the concentration of NH4+ exceeded 40 mg/L at RL3. This anomaly can be explained by a failure that occurred at the resort facilities, which in turn discharged the ammonium nitrate into the sewers.
It can be concluded through such measures that the Fertial complex pours large quantities of water rich in organic nitrogen compounds and sometimes NH4+ ion. These compounds promote proliferation of algae and phytoplankton, which results in eutrophication of the receiving environment and completely unbalances the environment; indeed, light no longer reaches the water layers beneath the surface, thus blocking the photosynthesis of the lower layers.
Methods of treatment
Treatments that can be applied to liquid effluents depend on the characteristics of the effluent (Hachicha, Citation2008) and are as follows: type of pollution, concentration, load (mass of pollutant released per unit of time), and the sensitivity of the receiving environment (natural environment, and biological or physicochemical treatment). The main goal of these treatments is to purify and transform the wastewater into a state and quality conforming to the criteria announced and provided by the environmental convention.
Physical treatments
They consist of physical or mechanical operations.
Screening that eliminates the coarse elements through grids
Grit that decanted from the heavier elements
Oiling laceration
Chemical treatments
They consist in adding to the effluent a chemical reagent for converting an unfavorable substance into a harmless compound or trap in an easily removable compound. The reagents used are appropriate to the nature of each toxic substance to neutralize (neutralization, coagulation, flocculation, dechromatation, cyanide removal, metal precipitation, and ion exchange resins).
Biological treatments
These processes use living organisms (bacteria) to degrade organic matter (Hajji et al., Citation2012). Impurities must be biodegradable and contain no toxic. The biological purification reproduced in specific reactors a phenomenon that would naturally be in place in rivers. At the end of this process, bacteria are the “sludge” that must be separated from the purified water. Depending on the technology used, these bacterial cultures can be free (activated sludge) or fixed (organic and trickling filters).
Conclusion
The main objective of this study is to evaluate the degree of pollution in liquid effluents from the complex Fertial (Arzew), which is known for its diversity in the manufacture processes of mineral fertilizers. The results of the various analyses carried out over a 2-month period revealed the presence of a large pollution in liquid waste poured directly in the sea caused by SM and nitrogen compounds.
To address these industrial pollution problems, cleansing and filtering the liquid wastes before being poured into the sea must be inked in the policy of this industrial unit. In addition, some existing wastewater-treating machines are overexploited or amortized and have to be renovated. Furthermore, warning and alerting devices must be implemented in the environmental management program of the Fertial complex to inform and alert for any pollution peaks (ammonia and fine particles).
Additional information
Notes on contributors
Fares Redouane
Fares Redouane and Lounis Mourad both work in the Laboratory for Analysis and Application of Radiation at the University of Science and Technology–Mohamed Boudiaf, Oran, Algeria.
Lounis Mourad
Fares Redouane and Lounis Mourad both work in the Laboratory for Analysis and Application of Radiation at the University of Science and Technology–Mohamed Boudiaf, Oran, Algeria.
References
- AFNOR [French Standards Organization]. 1979. Collection of French Standards. Waters: Test Methods. Paris: Author.
- Espinosa, F., J.M. Guerra-Garcia, and J.C. Garcia-Gomez. 2007. Sewage pollution and exinction risk: An endangered limpet as bioindicator. Biodivers. Conserv. 16:377–397. doi:10.1007/s10531-005-3014-3
- Hachicha, R. 2008. Determination of priority actions for pollution of the coastal area south of Grand Sfax except Siape, scenarios & remediation environmental regulations. In French language SMAP III Tunisia project (2006–2008). Strategy Management Integrated Coastal Zone South Grand Sfax.
- Hajji, C., A. Bendou, H. Mouhanni, and M. Hassou. 2012. Qualité physico-chimique des effluents de la station de traitement des eaux de cale des navires au port d’Agadir-Maroc. Rev. Int. Héliotech. 44:14–22.
- International Fertilizer Industry Association. 2012. Annual production and international trade statistics: Production and trade statistics Covering nitrogen, phosphate, potash and sulphur products. Production, exports, imports from 1999 to 2010 (calendar year). http://www.fertilizer.org/ifa/content/download/7832/121893/version/6/file/2010_ammonia_public.xls ( accessed July 3, 2012).
- Renuka, N., A. Sood, R. Prasanna, and A.S. Ahluwalia. 2015. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 12:1443–1460. doi:10.1007/s13762-014-0700-2
- Rodier, J. 1997. L’analyse de l’eau (eaux naturelles, eaux résiduaires et eaux de mer), 8ème édition; Paris: Dunod.
- Rodier, J., B. Legube, N. Merlet, R. Brunet. 2009. Analysis of Water: Natural Water, Waste-water, Sea Water, 9th Edition; Paris: Dunod.
- Teh, C.Y., T.Y. Wu, and J.C. Juan. 2014. Potential use of rice starch in coagulation-flocculation process of agro-industrial wastewater: Treatment performance and flocs characterization. Ecol. Eng. 71:509–519. doi:10.1016/j.ecoleng.2014.07.005