ABSTRACT
A series of porous γ-Al2O3 materials was prepared by solution-combustion and ball-milling processes. The as-prepared powders were physicochemically characterized by x-ray diffraction (XRD), scanning electron microscopy (SEM), and N2 physisorption measurements and their performances in CO2 adsorption at different pressures (0.5 to 1.5 MPa) and temperatures (40 to 60ºC) were investigated. It was found that γ-Al2O3 synthesized by the solution-combustion process and ball milled at 10 hr exhibited the best CO2 adsorption performance at 60ºC and 1.5 MPa, achieving a maximum of 1.94 mmol/g compared to the four studied materials, as a result of their interesting microstructure and surface properties (i.e., nanocrystallinity, specific surface area, narrow pore size distribution, and large total pore volume). Our study shows that the γ-Al2O3 prepared by solution combustion followed by ball milling presents a fairly good potential adsorbent for efficient CO2 capture.
Implications: In this work, γ-Al2O3 materials were successfully obtained by solution combustion and modified via ball milling. These improved materials were systematically investigated as solid adsorbents of accessible surface areas, large pore volumes, and narrow pore size distribution for the CO2 capture. These studied solid adsorbents can provide an additional contribution and effort to develop an efficient CO2 capture method as means of alleviating the serious global warning problem.
Introduction
The processes of carbon dioxide (CO2) adsorption by porous adsorbents are of particular interest around the world. These have been proven to be efficient and economical materials for the reduction of atmospheric emissions of CO2, the gas that contributes the most to global warming (Yamasaky, 2003). In this context, much attention has been devoted to the generation of new and potential solid adsorbents with improved properties that can be used to separate CO2 effectively from flue gases generated by the combustion of fossil fuels (oil, natural gas, and carbon) in various power plants for energy generation. With this emphasis, diverse adsorbents with high specific surface areas, large pore volume, and narrow pore size distribution have been extensively reported for CO2 separation by adsorption (Chen et al., Citation2014; Czaun et al., Citation2013; Wang et al., Citation2014). Currently, metal oxide powders are receiving much attention for CO2 retention because of their important environmental interfaces. Specifically, alumina (Al2O3) and its transition phases such as γ-Al2O3 and α-Al2O3 have been widely used for catalysis and adsorption processes for environmental remediation with good results, due to a favorable combination of their textural properties and acid/base characteristics, which are mainly related to surface chemical composition, local microstructure, and phase composition (Trueba and Trasatti, Citation2005). In terms of the basis of these approaches, alumina materials have been studied and tested as potential supports during gas separation (Chen and Ahn, Citation2011; Ge et al., Citation2013; Mao and Vannice, Citation1994; Rege and Yang, Citation2001: Rosynek, Citation1975); their structural properties and chemical and hydrothermal stabilities above 1000ºC make them very desirables (Yan et al., 2008). In practice, nanosize powders have found a wide range of applications in the decontamination and remediation of water and air, due to their unique physicochemical properties, which make them highly reactive and selective to specific pollutants (Ordoñez-Regil et al., Citation2015). In this regard, alumina compounds obtained by ball milling process can be relevant for CO2 capture. So far, significantly fewer studies have indicated that the ball-milling process as a particle generation method enhances CO2 capture (Granados-Correa et al., Citation2015; Olivares-Marín et al., 2013; Valverde et al., Citation2014). On the other hand, the solution-combustion method is a wet chemical route for the fast synthesis of nanoscale materials and has been widely used to produce diverse, industrially useful materials, which are used in advanced applications, with large surface areas, higher porosity, and with textural properties better than those for similar traditional materials obtained by conventional synthesis procedures (Malkhlouf et al., Citation2013). In this context, porous γ-Al2O3 materials are here systematically investigated as solid adsorbents for the CO2 capture, and these improved materials can provide an additional contribution and effort to develop an efficient CO2 capture method as a means of alleviating the serious global warming problem.
The aim of the present investigation is focused on (1) the synthesis of aluminum oxide by the solution combustion process; (2) reduction of the particle size of the as-prepared metallic oxide by a one-step method carried out by high-energy ball milling; (3) preparation of the respective nanocomposites of γ-Al2O3/Ni and γ-Al2O3/Fe by high-energy ball milling; (4) use of the microstructural, superficial, textural, and morphological characterization of the as-prepared materials; and (5) study and a better understanding of the CO2 adsorption performance on the as-prepared porous γ-Al2O3 compound surfaces.
Experimental
Materials and methods
The starting materials, aluminum nitrate (AR grade: 98.5%) and urea (AR grade: 99.9%), were supplied by Baker and Sigma-Aldrich, respectively. Commercially available nickel powder (Merck, ≥99.5%) and iron powder (Merck, ≥99.5%) were used to fabricate the respective gamma alumina nanocomposites. All of the materials were used without further purification; distilled water was used. All of the gases used during this study were supplied by Praxair gases (México, 99.99% purity helium) and the Infra Company (extra-dry, high-purity-grade CO2, 99.80% purity, and 99.999% ultra-high-purity argon).
Synthesis and characterization of γ-Al2O3 compounds
The solution-combustion synthesis of γ-Al2O3 powders was performed according to the method of Granados-Correa and Bulbulian (Citation2013) by using an aqueous solution containing a 1:1 molar ratio of the chemical precursors aluminum nitrate [Al(NO3)3.9H2O] as oxidizer and urea [NH2CONH2] as chemical fuel as shown in eq 1. The solids were transferred directly into a 50-mL crucible and mixed with 1 mL of distilled water to obtain a homogeneous solution. The aqueous solution was heated with a hot plate until most of the water had evaporated. Then the resulting solid, together with 1.5 mL of distilled water, was heated at 800°C for 5 min using a muffle furnace at atmospheric pressure in the room. This solution combustion synthesis enables the production of porous γ-Al2O3 powder in a single scale and a short time. Assuming complete combustion of redox mixture, the theoretical equation for the formation of γ-Al2O3 powders with urea, considered to be stoichiometric when the relative fuel-to-oxidizer ratio φc = 1 according to Patil et al. (Citation2008), may be written as follows:
On the other hand, to modify the microstructure, the dry γ-Al2O3 prepared by solution combustion was mechanically milled at 2.5, 5.0, 7.5, and 10 hr in an argon atmosphere by using a 50-mL mechanical high-energy ball mill of the Spex 8000 type designed at Instituto Nacional de Investigaciones Nucleares ().The synthesis of γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites were conducted as follows: 2.94 g of γ-Al2O3 powder obtained by solution combustion and 0.06 g of elemental Ni or Fe powders at a weight percent ratio of 98:2 were placed in a 50-mL stainless-steel container enclosed in an ultra-high-purity argon atmosphere using stainless-steel balls at a weight ratio of ball to powder of 6:1 in the different experiments. show the ball-milling conditions for an experimental setup to make the particles. The samples were also milled in all cases using a Spex 8000 vibrating high-energy ball mill for different milling reaction times (1 to 10 hr), and finally, the structures of the ball-milled powders were characterized at the various stages during milling. The as-prepared γ-Al2O3 samples with better textural and structural characteristics were directly used to evaluate and compare their CO2 capture abilities by using thermal gravimetric analysis (TGA) and mass spectrometry (MS).
Figure 1. Mechanical high-energy ball mill Spex 8000 type for an experimental setup to make the particles.
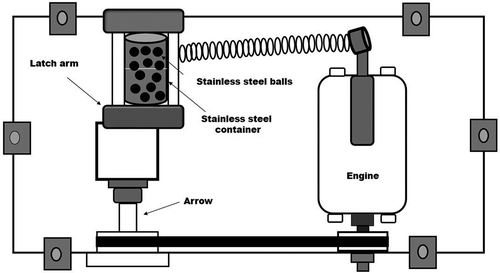
Table 1. Experimental ball milling conditions.
The structural development of all of the as-prepared powders was analyzed by x-ray diffraction analysis (XRD) using a Siemens D-5000 x-ray diffractometer with CuKα (λ = 1.5406 Å) radiation. The XRD peaks were measured in the 2θ region of 15° to 85° at a scan speed of 0.03°/3 sec. The Joint Committee on Powder Diffraction Standard (JCPDS) files and software were used for the analysis of the diffraction patterns. The average crystalline sizes of the alumina samples were calculated in the main XRD peaks at the 2θ values and according to the Debye–Scherrer formula [D = kλ)/βCos θ], where D is the crystalline size of alumina sorbents, nm; k is the Scherrer constant; λ is the x-ray wavelength, 0.15406 nm; β is peak breadth of half height in XRD spectra; and θ is the Bragg angle (degrees). The particle sizes and morphologies of the as-prepared powders were directly imaged using a JEOL-JMS 6610 LV scanning electron microscope at 25 kV. The elemental chemical composition of the samples was performed using an energy-dispersive x-ray spectroscopy technique (EDXS) connected to a JEOL-JMS 6610 detector INCA x-act OXFORD Instrument. The Brunauer–Emmett–Teller (BET) specific surface area, mean pore diameter, total pore volume, and adsorption–desorption isotherms of the synthesized materials were examined by N2 measurements at 77K with a Belsorp-Max (BEL Japan INC instrument). Before this analysis, 0.1 g of each dry sample was degassed at 300°C for 2 hr in the presence of nitrogen.
CO2 capture experiments
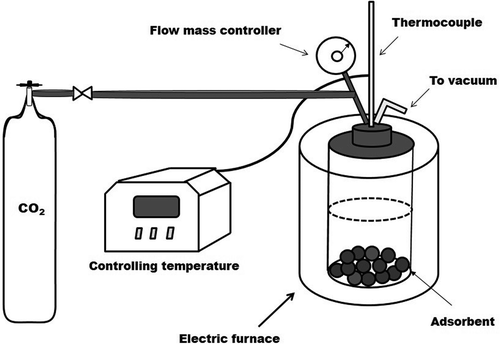
The CO2 adsorption experiments on all of the as-prepared samples were carried out using a simple experimental setup in a 50-mL capacity Parr 4592 stainless-steel pressure reactor and a temperature-controlled oven (). The powder samples were exposed to extra-dry gaseous carbon dioxide (99.8% nominal purity) at different temperatures (40 to 60ºC) under CO2 pressures between 0.5 and 1.5 MPa for 30 min. Approximately 30 mg of each as-prepared sample was used during each experiment. To obtain the highest CO2 gas adsorption capacity and remove any undesired residual molecules, the samples were degassed under vacuum (10−5 MPa) at 450ºC for 30 min. Subsequently, the CO2 adsorption on the adsorbent materials was quantitatively measured at 10 K/min using differential scanning calorimetry and thermogravimetric analysis (DSC-TGA) with a SDT Q600 calorimeter (TA Instrument-Water) that was previously calibrated and coupled to a discovery mass spectrometer instrument (TA Instrument-Waters). The millimoles of CO2 adsorbed per gram of adsorbent were measured.
Results and discussion
shows the XRD patterns of γ-Al2O3 samples prepared by solution combustion and solution combustion followed by ball milling in argon for 10 hr, as well as the γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites obtained by ball milling at 7.5 hr. The x-ray diffraction pattern of the alumina prepared by solution combustion at 800ºC for 5 min () shows only Bragg reflections that exhibit a marked broadening of all of the diffraction peaks due to the existence of microstrains or small crystallite sizes in the crystalline phase that were identified, and confirms a porous structure. This structure corresponds to γ-Al2O3, according to JCPDS file 01-074-4629, showing all the corresponding reflections: (111), (220), (311), (222), (400), (511), and (440) respectively, where (311), (400), and (440) are the most intense reflections, whereas (220) and (111) are much weaker and diffuse (Semain et al., Citation2014). These results are consistent with previous report of γ-Al2O3 preparation by a combustion method in comparable conditions (Sathyaseelan et al., Citation2013). The relative synthesis temperature used for γ-alumina preparation by the solution-combustion method may be the main reason for the crystalline phase of this material. On the other hand, it is well known that the solution-combustion method increases the porosity and specific surface area of the materials (Granados-Correa et al., Citation2008). Therefore, the results shown in the preceding indicate that by solution-combustion synthesis was obtained a product easy to fabricate with desirable structure and textural features. Indeed, this method not only yields a nanosized oxide material but also allows a large production ratio of γ-alumina, according to the calculation method of the production rates previously described in experimental section. The microstructural effect of mechanical milling at 10 hr on this γ-Al2O3 sample is shown in ; the results showed the presence of two different alumina phases after approximately 10 hr of milling time, for α-alumina (α-Al2O3), also known as corundum, with low-intensity Bragg peaks and γ-alumina (γ-Al2O3) structures, respectively. Such microstructural change can be explained as follows: It is well known that an important attribute of mechanical milling is to induce phase transitions in solid-state reaction and that these modifications can occur during γ-Al2O3 milling under argon atmosphere to produce a small amount of the α-phase, which corresponds to the digital diffraction pattern (JCPDS file 00-042-1468), showing invariably and exclusively the (012), (104), (113), (024), (116), (214), (0010), and (306) reflections, where (104), (113), and (116) are the most intense reflections. Phase transformations during the ball milling of alumina has been widely reported (Kostic et al., Citation1997), and the results show that the phase transformations are attributed to the high pressure and high temperatures generated locally during the milling process in the ceramic powders. Indeed, other researchers (Bodaghi et al., Citation2006) stated that the nucleation and growth process can account for the γ to α phase transition and that during a phase transition, critical size nuclei are required for transformation to precede. Thus, all of these factors result in the Al2O3 transition that is observed in the present investigation. As a consequence, these modifications could have an effect on the CO2 capture process, as analyzed in the respective CO2 capture experiment results section. The results also show that the γ-Al2O3 that is generated after milling for 10 hr has a high specific surface area, and an important decrease of particle sizes closed to nanoparticles that were observed; thus, the fracture and fragmentation of γ-Al2O3 during ball milling drastically decreases the particle size. This means that further milling resulted in a decrease of the crystallite size because the XRD spectrum peaks show a notable peak broadening that is typical of nanocrystalline material. The main dominant phases and their respective crystallite sizes in each material (, , , and ) were determined by the Scherrer equation. In a γ-Al2O3 dominant phase was observed with crystallite size of 16 nm; shows two phases, γ-Al2O3 and α-Al2O3, with dominant γ-Al2O3 phase with crystallite size of 49 nm and 66 nm respectively; shows one dominant α-Al2O3 phase with crystallite size of 14 nm; and finally, shows one dominant α-Al2O3 phase with crystallite size of 15 nm. Therefore, milling produced nanocrystalline materials. Indeed, it was also observed that the total pore volume of this sample was increased and that the average pore radius was decreased compared to the nonmilled γ-Al2O3 (), which are characteristics that could benefit their CO2 adsorption.
Table 2. Textural properties by nitrogen physisorption of porous γ-Al2O3 samples prepared by solution-combustion and ball-milling processes.
Figure 3. XRD patterns of porous γ-Al2O3 samples prepared by (a) solution combustion, (b) solution combustion after 10 hr of ball milling, (c) γ-Al2O3/Ni, and (d) γ-Al2O3/Fe after 7.5 hr of ball-milling.
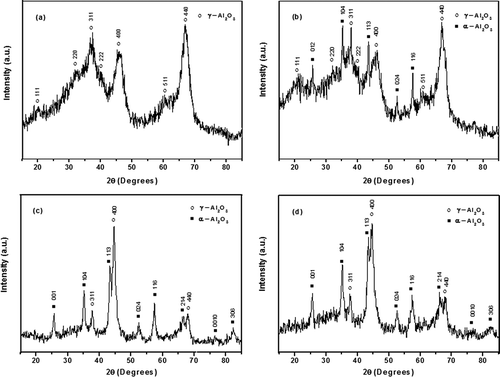
and show the XRD patterns of the γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites, respectively. It was observed that after 7.5 hr of ball milling γ-Al2O3 with Ni and Fe powders, both x-ray patterns showed only characteristic Bragg reflections with a higher intensity, signifying the transformation of the γ-Al2O3 phase to corundo-Al2O3, which was identified as principal phase. The addition of Ni and Fe powders favored the phase transformation of γ-Al2O3 to α-Al2O3 with nanocrystalline structures. A broadening of all of the diffraction reflections was observed for the α-Al2O3 phase and was not observed for the peaks of Ni and Fe elements because the amount of added powders was 2 wt.% (Ni and Fe with 98 wt.% of γ-Al2O3 during the composite preparation). In and , diffractions of the pure transition elements Ni and Fe cannot be seen after 7.5 hr of ball milling by DRX due to their very low amounts; however, the Ni and Fe are successfully dispersed in the γ-Al2O3 phase, respectively, and these dispersions modify the surfaces of the composites via the introduction of those specific metals. Therefore, in our case, the high-energy ball-milling process that facilitated the formation of γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposite phases and a relatively long milling time of 7.5 hr represent the most suitable route for producing their Ni and Fe nanocomposites. This is a consequence of the structural defects (dislocations, vacancies, interstices, and point defects) and the mechanical deformations that occur during high-energy ball milling. Therefore, for the nanocrystalline structures that were observed, their specific surface areas and crystallite sizes can improve the CO2 adsorption capacity in this solid–gas interface (Olivares-Marín et al., 2013). Indeed, the Ni and Fe presence in the alumina can enhance its adsorption activity without prior activation.
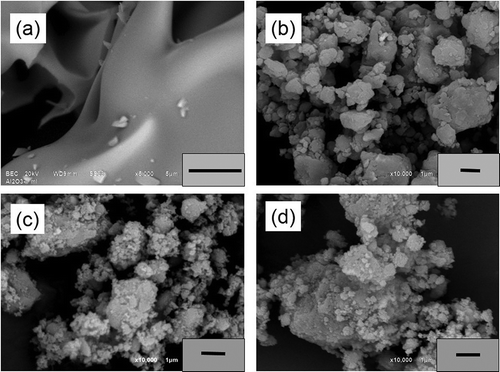
Scanning electron microscopy (SEM) micrographs at the same 10,000× magnification of the porous γ-Al2O3 as-prepared samples are shown in . The γ-Al2O3 sample obtained by solution combustion at 800ºC for 5 min appears to consist of homogeneous particles of smooth and porous surfaces, with particle size greater than 10 μm, as observed in . Confirmation of the morphological changes of the milled γ-Al2O3 powders produced by the ball-milling process after milling for 10 hr can be seen in ; the SEM image shows that the milled particles become smaller and more homogeneous, with particles sizes of less than 3 μm. Such changes of the particle size and appearance may be explained as follows. When the particles expand, there will be fractures on the surface, exposing the interior of the material, and then some of the expanded particles will cold-weld due to the action of pressure and continuing collisions, subsequently forming even smaller porous particles in agglomerated form. These results indicates that the degree of the grain size tends to decrease with a longer milling time of 10 hr, which is an optimal ball-milling condition to obtain a sample with high specific surface area and large pore volume. On the other hand, the magnified micrographs corresponding to γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites obtained after 7.5 hr of milling time ( and , respectively) show aggregates of particles with porous structures due to the breakage of large particles into smaller pieces. We can conclude that the particle diameter of the nanocomposite powders decreases after the longest milling time of 7.5 hr, that there are many smaller particles distributed homogeneously on the surface of these large particles, and that the distribution of pure elemental Ni and Fe on the ceramics is significant. Indeed, SEM showed that Ni and Fe are completely dispersed in their respective aluminas. Therefore, these elements are dispersed homogeneously in the corundo-Al2O3 matrix with particle sizes less than 1.5 μm, indicating the formation of intermetallics during the milling, which also results in a modest decrease of the specific surface areas of the nanocomposites. Therefore, even at 7.5 hr of milling time and using a molar proportion of 98:2 of the chemical precursor, high-energy ball milling produced γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposite materials very efficiently. EDXS analysis revealed that all of the synthesized samples only showed the presence of aluminum, nickel, and iron elements, with oxygen.
Figure 4. SEM micrographs of porous γ-Al2O3 samples prepared by (a) solution combustion, (b) solution combustion after 10 hr of ball-milling, (c) γ-Al2O3/Ni, and (d) γ-Al2O3/Fe after 7.5 hr of ball-milling.
The N2 physisorption measurements results of the as-prepared Al2O3 compounds under study are shown in . It was observed that the γ-Al2O3 obtained by solution combustion exhibits the higher BET specific surface area (189.32 ± 2.21 m2/g) among the four samples. Based on the results, this significant textural property is associated with the solid porous formation involving the solution-combustion reaction as a result of the emission of volatile materials such as gaseous CO2, H2O, N2, and so on. However, this material treated by the ball-milling process over 10 hr slightly decreases their BET specific surface area (from 189.32 ± 2.21 to 120.21 ± 3.67 m2/g). The occurrence of a decreased BET specific surface area during the ball milling is not expected; this surface area decrease can be due to the compression of the alumina particles in the high-energy impacts in the ball mill. It is well known that adsorbents based on high surface area, large pore volume, and narrow pore size distribution are of particular interest for the interaction between CO2 molecules in CO2 capture. According to Heidari et al. (Citation2014), a higher surface area increased adsorption sites for CO2, which can lead to an improved CO2 adsorption performance. In addition, a large pore volume and narrow pore size distribution in adsorbents have also significant influence on the adsorption because they may affect the mass transport and can enable introduction of a large amount of CO2 into its reduced pores. Normally, the gas molecules in the gas–solid interaction are adsorbed by van der Waals forces. Gas adsorption potential is at a maximum in the subnanometer scale when the gas molecules start to overlap with the reducing pore size, resulting in a much higher binding energy emanating from the formation of deep potential wells. According to Xu et al. (2003), the available pore space of adsorbents for CO2 would increase to some extent, leading to faster diffusion of CO2 in the pore microchannels. Thus, absorbents that maintain this higher behavior are crucial for CO2 capture. However, it is remarkable that for the adsorption of gases on solids, there is a suggested linear dependence on CO2 capture with the BET specific surface area, but there is a total dependence of the pore volume and pore size distribution with respect to the adsorption capacity for CO2. Some studies have indicated that the surface area is not the decisive factor that affects the gas adsorption capacity on a solid adsorbent. Maroto-Valer et al. (Citation2005) studied the CO2 capture behavior of steam-activated anthracite. They found that the CO2 capture does show a linear relationship with the surface area. This behavior could be explained by certain pore sizes being effective for CO2 adsorption. In this case, the sample with the higher value of the pore volume (0.2009 cm3/g) that means more space for the storage of CO2 and with a narrow pore size distribution of 1.21 nm (microporous) was the ball-milled γ-Al2O3 sample. In consequence, this sample corresponds to the maximum adsorption capacity, such as was seen in the CO2 capture experiments. The variation of CO2 adsorption capacity of the γ-Al2O3 and the ball-milled γ-Al2O3 samples in this study did not result from the differences between their BET surface areas, but from their significant influence of pore volume and pore size distribution on CO2 adsorption. In order to fully prove the argument, the textural properties of the materials were analyzed in order to observe the influences with respect to adsorption capacity enhancement on the prolonged or reduced ball milling times of γ-Al2O3 prepared by solution combustion, and the results are shown in . It can be seen that the BET specific surface areas of alumina samples are significantly decreased as the ball milling time increases from 0 to 15 hr (189.32 to 84.37 m2/g). Therefore, increasing ball milling time involves a decrease in the effective adsorption sites, making CO2 adsorption on porous materials less favorable. Therefore, these samples cannot provide abundant adsorption sites, but facilitated the mass transport due to their increased pore volume and reduced pore size distribution. As shown in , it was also interesting to note that the pore volume of γ-Al2O3 ball milled during 10 hr was larger than that of other samples. This observation implied that higher pore volume of the solid favored the gas–solid diffusion reaction, which resulted in a higher CO2 capture. Indeed, this sample presented a narrow pore size distribution, in the micropore region; consequently, this sample yielded high CO2 adsorption capacity. On the other hand, also shows that γ-Al2O3 ball milling during 15 hr presented better CO2 adsorption capacity with respect to γ-Al2O3 ball milling during 5 hr. Thus, the maximum allowable amount of CO2 was introduced to the pores of γ-Al2O3 ball milled at 10 hr principally based on the large pore volume and the narrow pore size distribution.
Table 3. γ-Al2O3 prepared by solution combustion; textural properties and adsorption capacity (qads) as a function of ball milling time.
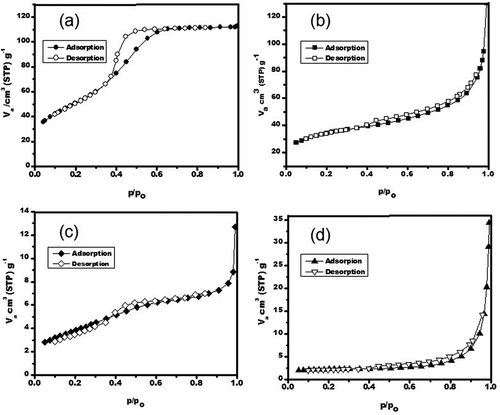
The nitrogen adsorption/desorption isotherms of the as-prepared γ-Al2O3 compounds are shown in .The γ-Al2O3 sample prepared by solution combustion () showed a type IV isotherm, with a very large hysteresis loop at p/po between 0.4 and 0.65, denoting the porous characteristics of this sample; this hysteresis in the physical adsorption isotherms is normally associated with the capillary condensation step related to a structure of small mesopores (Bodaghi et al., Citation2006). The other samples (, , and ) exhibited type II isotherms and have a well-defined plateau, exhibiting the characteristic of microporous materials; indeed, the curves present a very small hysteresis loop. According to the International Union of Pure and Applied Chemistry (IUPAC), all these obtained isotherms correspond to microporous and mesoporous materials, in which unrestricted monolayer-multilayer retention can occur (Sing, Citation1995); in the case of a micro-mesopore, CO2 molecules are adsorbed, forming a monolayer.
Figure 5. Nitrogen adsorption–desorption isotherms curves for the porous γ-Al2O3 samples prepared by (a) solution combustion, (b) solution combustion after 10 hr of ball milling, (c) γ-Al2O3/Ni, and (d) γ-Al2O3/Fe after 7.5 hr of ball milling.
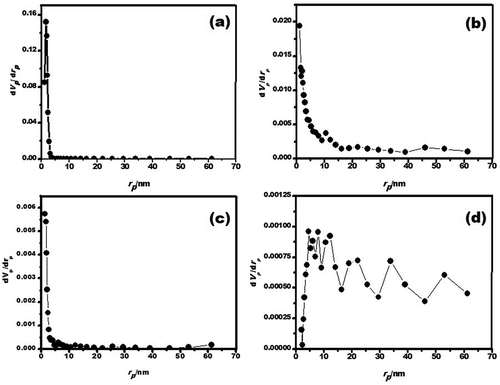
The pore size distributions of the porous γ-Al2O3 samples prepared by solution-combustion and ball-milling processes, were calculated by the Barrett–Joyner–Halenda (BJH) method ().The BJH pore size distribution results show good agreement and are reported in . Average micropore radius values of 1.64, 1.21, 1.21, and 4.61 nm have been found for the γ-Al2O3 solution combustion, γ-Al2O3 solution combustion and ball milling, γ-Al2O3/Ni, and γ-Al2O3/Fe samples, respectively. In general narrow pore size distribution in the micropore (<2 nm) and mesopore region (2–50 nm) was obtained for all as-prepared aluminas; this important characteristic and a large surface area are the principal properties for potential CO2 adsorbent, which are expected to increase adsorption sites for CO2 and lead to an improved CO2 adsorption performance. In addition, the large pore volume of aluminas also would enable introduction of a large amount of CO2 into the micropore structure in a well-dispersed manner, so they can function as effective CO2 adsorbents.
Figure 6. Pore size distribution curves obtained using the BJH method for the porous γ-Al2O3 samples prepared by (a) solution combustion, (b) solution combustion after 10 hr of ball milling, (c) γ-Al2O3/Ni, and (d) γ-Al2O3/Fe after 7.5 hr of ball milling.
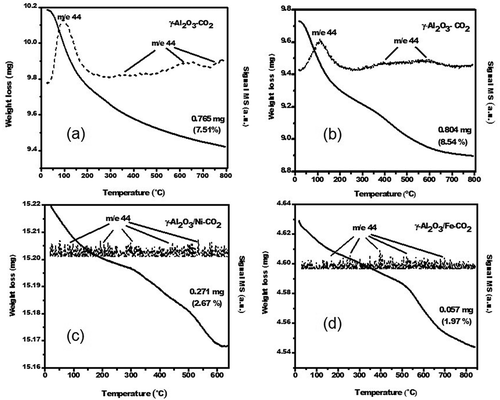
Different CO2 capture experiments were performed on the studied as-prepared materials as a function of different temperatures (40–60ºC) and pressures (1 to 1.5 MPa) of CO2 flow. The results showed that the optimal conditions for the maximum CO2 capture efficiencies were at 60ºC and 1.5 MPa of CO2 pressure for the alumina prepared by solution combustion followed by ball milling and at 60ºC and 1.0 MPa for the Al2O3/Ni and Al2O3/Fe composites, respectively. In relation to the temperature of CO2 capture system, our study coincides with the optimal temperature used and reported by several researchers using micro- and mesoporous materials; considering that the adsorption process is exothermic, moderate temperatures favor the CO2 adsorption process (Aaron and Tsouris, Citation2005). In this context, shows the TGA curves and MS profiles of the evolved gas: CO2 (m/e = 44) during the decomposition of the porous γ-Al2O3 samples prepared by solution combustion () and solution combustion and ball milling (), as well as γ-Al2O3/Ni-CO2 () and γ-Al2O3/Fe-CO2 () in helium.
Figure 7. TGA curves and its MS profiles of the evolved gas: CO2 (m/e = 44) during the decomposition of porous γ-Al2O3 samples prepared by (a) solution combustion, (b) solution combustion and ball milling, (c) γ-Al2O3/Ni-CO2, and (d) γ-Al2O3/Fe-CO2 in helium.
The results determined by TGA indicated that the best CO2 adsorption capacity (1.94 mmol/g) was exhibited by the γ-Al2O3 sample prepared by solution combustion followed by ball milling for 10 hr. In comparison with the other three different γ-Al2O3 adsorbents studied here, the CO2 adsorption capacity of the ball-milling sample was slightly larger than for the γ-Al2O3 sample without ball milling (1.70 mmol/g) and much higher than for the composite adsorbents. In order to explain the experimental results and to examine the factors influencing the adsorption difference of CO2, this adsorption behavior is associated with the structural changes produced during the high-energy ball-milling process. One can consider that when a porous alumina is in contact with CO2 a suitable porous size is recommended for increasing the CO2 physical adsorption. As was noted in our results, all the studied samples have a significant contribution of microporous and mesoporous (rp < 5nm) structure with kinetic radius enough for CO2 molecule diffusion inside the alumina porous structure (molecular size of CO2 is 0.33 nm); in this way, intraparticle diffusion improvement occurs in the CO2 capture process in all studied aluminas, as observed by Yunes et al. (2013). Additionally, for microporous and mesoporous materials, the increase of adsorption amount and selectivity also is ascribed to a large pore volume that also would enable us to introduce a large amount of CO2 capturing agent into its pores in a well-dispersed manner. As expected, the γ-Al2O3 synthesized by the solution combustion method and milled at 10 hr with the highest micropore volume of 0.2009 cm3/g exhibited the best CO2 adsorption, achieving a maximum of 1.94 mmol/g.
The total relative weight loss of all of the samples in the TGA results is associated with full CO2 desorption, which was verified by mass spectrometry. All of the materials show different types of weight loss in the thermogravimetric CO2 profiles. As mentioned earlier, this desorption behavior is associated their specific structural characteristics. For the γ-Al2O3 solution combustion sample (), two stages are shown in the TGA profiles when weight loss is observed from low to high-temperature. This weight loss is associated with the complete CO2 desorption from the γ-Al2O3 phase with a total weight loss of 7.5 wt.%. For the γ-Al2O3 solution-combustion and ball-milling sample, in the presence of the γ-Al2O3 and α-Al2O3 phases detected simultaneously by XRD, two weight losses were observed in the TGA curve profile (). The first weight loss corresponds to the complete decarbonation of γ-Al2O3 and the partial decarbonation of α-Al2O3, at a high temperature. The second weight loss corresponds to carbon dioxide desorption from α-Al2O3. For both samples, γ-Al2O3 and γ-Al2O3 ball milling, an m/e = 44 signal of high intensity is observed at approximately 125ºC. At higher temperatures, an additional broad m/e = 44 signal appears, which can be explained by residual carbonate decomposition. In general, the higher efficiency CO2 capture on this sample can be because the γ-Al2O3 and α-Al2O3 mixture shows a synergistic effect during CO2 adsorption–desorption. This improvement of the CO2 desorption properties becomes more significant as the milling time is increased over 10 hr as a consequence of a better phase intermatrix of γ- and α-Al2O3. The TGA curve for γ-Al2O3/Ni-CO2 () shows two weight losses. The first is observed from room temperature to 300ºC, which corresponds to the CO2 physisorbed on the composite surface. The second weight loss is observed in the range of 300 to 600ºC, which corresponds to residual carbonate decomposition. This sample shows a maximum of CO2 capture of 2.67 wt.% due to total weight loss. For γ-Al2O3/Fe-CO2 (), the maximum capacity of CO2 desorption for this composite was 1.97 wt.%, according to the TGA and MS m/e = 44 signal profile curve of CO2 desorption, indicating that it has the same CO2 desorption behavior as that for the γ-Al2O3/Ni–CO2 system in which two endothermic peaks were observed with a total weight loss of 1.97 wt.%, which was the minimum CO2 capacity observed in these studies of porous γ-Al2O3 compounds. As was seen, only the γ-Al2O3 sample presents with higher efficient CO2 capture, but the effect of ball milling for γ-Al2O3 enhances its CO2 capture capacity. However, the obtained metal matrix nanocomposites with Ni and Fe with outstanding microstructural properties, such as specific area, size grain, dislocations, and pore diameter, did not improve their CO2 adsorption capacity. Finally, we can conclude that the synthesized γ-Al2O3 by solution combustion followed by ball milling for 10 hr has been shown to have better CO2 adsorption properties in comparison with the other studied porous γ-Al2O3 compounds. In view of the satisfactory CO2 capture scheme and taking the preparation cost into consideration for practical use, in order to dispose of materials more economically acceptable, this new material adsorbent can be successfully prepared by a simple solution combustion method using cheap chemical precursors and by a low-cost ball-milling process. Therefore, this can significantly can reduce the capture cost as the most important issue for the CO2 capture processes, which is still a challenge to be resolved in a short time frame.
Conclusions
The solution combustion process provides a safe and easy route to prepare porous γ-Al2O3 powders. The as-prepared material is quite homogeneous in terms of pore type and size; it also has a higher porosity and larger specific surface area, which is highly beneficial during CO2 capture. However, the γ-Al2O3 prepared by solution combustion followed by the ball-milling process during 10 hr increased its CO2 adsorption capacity due to the increased pore volume, and decreased pore size distribution in the micropore region as compared with the other studied adsorbents. However, when this sample was ball milled during 15 hr its CO2 adsorption capacity decreased considerably. The more suitable route for producing the γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites with a 98:2 wt.% composition in a corundo-Al2O3 phase doped with Ni and Fe powders was by the high-energy ball-milling process after a 7.5 hr milling time. CO2 capture experiments in the as-prepared materials studied by thermogravimetric analysis and mass spectrometry (TGA-MS) at different CO2 temperatures and pressure flows showed that the best CO2 capture efficiencies were at 60ºC and 1.5 MPa of CO2 pressure for the γ-Al2O3 prepared by combustion and treated by high-energy ball milling (1.94 mmol/g) and at 60ºC and 1 MPa for the γ-Al2O3/Ni and γ-Al2O3/Fe nanocomposites with 0.60 mmol/g and 0.44 mmol/g, respectively. The results here confirm that γ-Al2O3 that has a reduced particle size is important for CO2-related processes and that its large pore volume and narrow pore size distribution are critical during CO2 capture. In general, the differences in the CO2 capture between these materials was principally due to their interesting microstructures and surface properties (i.e., nanocrystallinity, total pore volume, and narrow pore size distribution).
Funding
The authors thank the ININ (Instituto Nacional de Investigaciones Nucleares) for financially supporting this research under project number CB-406, stages I, II, and III.
Additional information
Funding
Notes on contributors
F. Granados-Correa
F. Granados-Correa is a researcher at ININ (Instituto Nacional de Investigaciones Nucleares) México
J. Bonifacio-Martínez
J. Bonifacio-Martínez is a associate researcher at the National Institute of Nuclear Research, México.
H. Hernández-Mendoza
H. Hernández-Mendoza is researcher that work in National Research Laboratory in Nuclear Forensic from ININ México, and S. Bulbulian is a invited researcher of CCADET-UNAM (Centro de Ciencias Aplicadas y Desarrollo Tecnológico-Universidad Nacional Autónoma de México), México.
S. Bulbulian
H. Hernández-Mendoza is researcher that work in National Research Laboratory in Nuclear Forensic from ININ México, and S. Bulbulian is a invited researcher of CCADET-UNAM (Centro de Ciencias Aplicadas y Desarrollo Tecnológico-Universidad Nacional Autónoma de México), México.
References
- Aaron, D., and C. Tsouris. 2005. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 40:321–48. doi:10.1081/SS-200042244
- Bodaghi, M., A.R. Mirhabibi, H. Zolfonum, M. Tahriri, and M. Karimi. 2006. Investigations of phase transition of γ-alumina to α-alumina via mechanical milling method. Phase Trans. 81:571–80. doi:10.1080/01411590802008012
- Chen, C., and W. Ahn. 2011. CO2 capture using mesoporous alumina prepared by a sol-gel process. Chem. Eng. J. 166:646–51. doi:10.1016/j.cej.2010.11.038
- Chen, C., D.W. Park, and W.S. Ahn. 2014. CO2 capture using Zeolite 13X prepared from bentonite. Appl. Surf. Sci. 292:63–67. doi:10.1016/j.apsusc.2013.11.064
- Czaun, M., A. Goeppert, R.B. May, D. Peltier, H. Zhang, and S. Prakash. 2013. Organoamines-grafted and nano-sized silica for carbon dioxide capture. J. CO2 Utilization. 1:1–7. doi:10.1016/j.jcou.2013.03.007
- Ge, J., K. Deng, W. Cai, J. Yu, X. Liu, and J. Zhou. 2013. Effect of structure during agents on facile hydrothermal preparation of hierarchical γ-Al2O3 and their adsorption performance toward Cr(VI) and CO2. J. Colloid. Interface Sci. 401:34–39. doi:10.1016/j.jcis.2013.03.028
- Granados-Correa, F., and S. Bulbulian. 2013. Surface characterization of γ-Al2O3 powders and their Co2+ adsorption properties. Int. J. Appl. Ceram. Technol. 19:E295. doi:10.1111/j.1744-7402.2012.02827.x
- Granados-Correa, F., J. Bonifacio-Martínez, H. Hernández-Mendoza, and S. Bulbulian. 2015. CO2 capture on metallic powders prepared through chemical combustion and calcination methods. Water Air Soil Pollut. 226:281. doi:10.1007/s11270-015-2552-x
- Granados-Correa, F., J. Bonifacio-Martínez, V.H. Lara, P. Bosch, and S. Bulbulian. 2008. Cobalt sorption properties of MgO prepared by solution combustion. Appl. Surf. Sci. 254:4688–94. doi:10.1016/j.apsusc.2008.01.074
- Heidari, A., H. Younesi, A. Rashidi, and A. Ghoreyshi. 2014. Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: Effect of chemical activation. J. Taiwan Inst. Chem. Eng. 45:579–88. doi:10.1016/j.jtice.2013.06.007
- Kostic, E., S. Kiss, S. Boskovic, and S. Zec. 1997. Mechanical activation of the gamma to alpha transition in Al2O3, Powder Technol. 91:49–54. doi:10.1016/S0032-5910(96)03244-5
- Malkhlouf, M.T., B.M. Abu-Zied, and T.H. Mansoure. 2013. Effect of calcination temperature on the H2O2 decomposition activity of nano-crystalline Co3O4 prepared by combustion method. Appl. Surf. Sci. 274:45–52.
- Mao, C.F., and M.A. Vannice. 1994. Adsorption properties and heats of adsorption of carbon monoxide, carbon dioxide, and ethylene. Appl. Catal. A Gen. 111:151–73.
- Maroto-Valer, M.M., Z. Tang, and Y. Zhang. 2005. CO2 capture by activated and impregnated anthracites. Fuel Process. Technol. 86: 1487–502. doi:10.1016/j.fuproc.2005.01.003
- Olivares-Marín M., E.M. Cuerda-Correa, A. Nieto-Sánchez, S. García, C. Pevida, and S. Román. 2014. Influence of morphology, porosity and crystal structure of CaCO3 precursors on the CO2 capture performance of CaO-derived sorbents. Chem. Eng. J. 217:71–81. doi:10.1016/j.cej.2012.11.083
- Ordoñez-Regil, E., F. Granados-Correa, E. Ordoñez-Regil, and M.G. Almazán-Torres. 2015. Nanoparticles of KFeP2O7 implanted on silica gel beads for Cd2+ ion adsorption. Environ. Technol. 36:188–97. doi:10.1080/09593330.2014.941942
- Patil, K.C., M.S. Hegde, T. Rattan, and S.T. Aruna. 2008. Chemistry of nanocrystalline oxide materials. In Combustion Synthesis, Properties and Applications, 42–45. Singapore: World Scientific.
- Rege, S.U., and R.T. Yang. 2001. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina. Chem. Eng. Sci. 56:3781–96. doi:10.1016/S0009-2509(01)00095-1
- Rosynek, M.P. 1975. Isotherms and energetics of carbon dioxide adsorption on at 100ºC–300ºC. J. Phys. Chem. 78:1280–84. doi:10.1021/j100580a011
- Sathyaseelan, B., I. Baskaran, and K. Sivakumar. 2013. Phase transition behavior of nanocrystalline Al2O3 powders. Soft Nanosci. Lett. 3:69–74. doi:10.4236/snl.2013.34012
- Semain, L., A. Jaworski, M. Edén, D. M. Ladd, D. Seo, F.J. García-García, and U. Häussermann. 2014. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 217:1–8. doi:10.1016/j.jssc.2014.05.004
- Sing, K.S.W. 1995. Physisorption of nitrogen by porous materials. J. Porous Mater. 2:5–8. doi:10.1007/BF00486564
- Trueba, M., and S.P. Trasatti. 2005. γ-alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 17:3393–403. doi:10.1002/(ISSN)1099-0682
- Valverde, J.M., P.E. Sanchez-Jimenez, and L.A. Perez-Maqueda. 2014. Relevant influence of limestone crystallinity on CO2 capture in the Ca-looping technology at realistic calcination conditions. Environ. Sci. Technol. 48:9882–89. doi:10.1021/es5014505
- Wang, K., X. Wang, P. Zhao, and X. Guo. 2014. High-temperature capture of CO2 on lithium-based sorbents prepared by a water-based sol-gel technique. Chem. Eng. Technol. 37:1552–58. doi:10.1002/ceat.201300584
- Yamasaki, A. 2003. An overview of CO2 mitigation options for global warming emphasizing CO2 sequestration options. J. Chem. Eng. Jpn. 36:361–75. doi:10.1252/jcej.36.361
- Yuan, Q., A.X. Yin, C. Luo, L.D. Sun, Y.W. Zhang, W.T. Duan, H.C. Liu, and C.H. Yan. 2008. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J. Am. Chem. Soc. 130:3465–72. doi:10.1021/ja0764308
- Yunes, S., P. Wommack, M. Still, J. Kenvin, and J. Exley. 2014. Physical characterization of microporous materials using various adsorbates. Correlation between their micropore volume and their capacity to adsorb H2 and CO2. Appl. Catal. A. Gen. 474:250–56. doi:10.1016/j.apcata.2013.07.049