ABSTRACT
In this study, fine particulate matter (PM2.5) emitted from a municipal solid waste incinerator (MSWI) was collected using dilution sampling method. Chemical compositions of the collected PM2.5 samples, including carbon content, metal elements, and water-soluble ions, were analyzed. Traditional in-stack hot sampling was simultaneously conducted to compare the influences of dilution on PM2.5 emissions and the characteristics of the bonded chemical species. The results, established by a dilution sampling method, show that PM2.5 and total particulate matter (TPM) emission factors were 61.6 ± 4.52 and 66.1 ± 5.27 g ton-waste−1, respectively. The average ratio of PM2.5/TPM is 0.93, indicating that more than 90% of PM emission from the MSWI was fine particulate. The major chemical species in PM2.5 included organic carbon (OC), Cl−, NH4+, elemental carbon (EC) and Si, which account for 69.7% of PM2.5 mass. OC was from the unburned carbon in the exhaust, which adsorbed onto the particulate during the cooling process. High Cl− emission is primarily attributable to wastes containing plastic bags made of polyvinyl chloride, salt in kitchen refuse and waste biomass, and so on. Minor species that account for 0.01–1% of PM2.5 mass included SO42-, K+, Na, K, NO3−, Al, Ca2+, Zn, Ca, Cu, Fe, Pb, and Mg. The mean ratio of dilution method/in-stack hot method was 0.454. The contents of water-soluble ions (Cl−, SO42-, NO3−) were significantly enriched in PM2.5 via gas-to-particle conversion in the dilution process. Results indicate that in-stack hot sampling would underestimate levels of these species in PM2.5.
Implications: PM2.5 samples from a municipal solid waste incinerator (MSWI) were collected simultaneously by a dilution sampling technique and a traditional in-stack method. PM2.5 emission factors and chemical speciation profiles were established. Dilution sampling provides more reliable data than in-stack hot sampling. The results can be applied to estimate the PM2.5 emission inventories of MSWI, and the source profile can be used for contribution estimate of chemical mass balance modeling.
Introduction
Incineration has been a main municipal solid waste treatment method in many countries that lack landfill sites in urban communities. Incineration can effectively reduce the waste volume and recover its energy content to generate electricity and heat. Municipal solid waste is an extremely heterogeneous material that contains manufactured and natural components, including paper, plastics, textiles, food wastes, yard wastes and other organic materials, and inorganic materials such as glass, metals, dirt, and miscellaneous other components (Hasselriis and Licata, Citation1996). The incineration process can generate air pollutants (Yuan et al., Citation2005; Chang et al., Citation2009) and affect ambient air quality near the incinerator (Hu et al., Citation2003; Zhang et al., Citation2014).
Particulate matter (PM) is one of the air pollutants emitted from incinerators. Atmospheric particulates have potentially adverse impacts on human health through inhalation and respiratory deposition. Fine particulate matter (PM2.5, aerodynamic size less than 2.5 μm) is especially harmful as it can be transported deep into the alveolar region of the lungs and the bloodstream (Pope et al., Citation2004; Mills et al., Citation2009). Chemical properties of particulate matter also play an important role affecting human health. It is quite essential to characterize PM2.5 and estimate its contributions to the atmosphere from emission sources.
Characterizing PM emissions directly from emission sources provides reliable emission data for identifying their contributions to ambient air and for designing corresponding control measures to be in compliance with government rules. The traditional in-stack hot filter method has been the most practical way to measure PM2.5 emissions from stationary sources. Most databases, such as EPA AP-42, are based on the in-stack hot filter method. However, in-stack measurement data from emission sources do not allow for normal dilution and cooling that occur in a plume and they may not reflect the variability of actual emissions over time (Yang et al., Citation1998; Lee et al., Citation2008; Yang et al., Citation2015). Additionally, hot exhaust sampling is not appropriate for receptor modeling studies. In contrast, diluted exhaust sampling performs well for collecting particles from combustion sources (Watson et al., Citation2002). The dilution sampling method simulates the cooling and dilution processes of the exhaust flue gas after emission from the stack. The cooling and dilution process allows gases or vapors to nucleate and condense on existing particulates, similar to processes that occur in the atmosphere. Furthermore, ambient air methods can be used to sample and analyze the diluted flue gas, which provide ambient-comparable PM speciation profiles from stationary sources (Li et al., Citation2011). Dilution sampling provides a more representative measurement of PM2.5 for source apportionment and health-risk assessment than traditional in-stack hot filter method (England et al., Citation2007).
The dilution sampling method has been used to develop PM2.5 emission factors and speciation profiles from various stationary sources (Hidemann et al., Citation1991; Lee et al., Citation2000; Fine et al., Citation2001; Watson et al., Citation2001; Lipsky et al., Citation2004; Li et al., Citation2011). PM2.5 emission from MSWI by dilution method has never been performed or reported in the literature. In this study, PM2.5 samples from a municipal solid waste incinerator (MSWI) were collected simultaneously by a dilution sampling technique and the traditional in-stack method. PM2.5 emission factors and chemical speciation profiles were established. The influences of dilution on measurement of PM2.5 emission and chemical characteristics were investigated.
Materials and methods
Sampling plan and the MSWI
In this study, PM2.5 emission from a MSWI was measured using a dilution sampling technique. The MSWI selected in this study is located in central Taiwan. Its capacity is 900 tons/d with 2 stoker-type furnaces (450 ton d−1 for each furnace). Solid wastes are fed into the combustion chambers by gravity. The designed combustion temperature is 850–1050ºC and the designed heating value is 2300 kcal kg−1. The fumes leaving the furnaces enter a boiler, allowing the recovery of thermal energy. Air pollution control devices include selective noncatalytic reduction, semidry lime scrubber, activated carbon injection, and baghouse filter. The flue gas, after passing through air pollution control devices, is emitted through a gas stack 100 m in height situated at the end of the system. The sampling port is located >8 stack diameters downstream and >2 stack diameters upstream from any point of flow turbulence. The dimension of the sampling plate is sufficient for dilution and in-stack sampling simultaneously. The physical and chemical properties of the municipal solid waste are listed in .
Table 1. Physical and chemical properties of the municipal solid waste.
Samplings were conducted from January 22 to January 27, 2015, with one sample taken each day. The operative conditions of the main parameters and exhaust gas properties of the MSWI during the sampling campaign are listed in . The parameters and exhaust gas properties were measured online. Stack temperature was 157 ± 1.87ºC. The combustion chamber temperature was 948 ± 8.85ºC and the oxygen content was 10.4 ± 0.22%. The relatively low oxygen content indicates that the wastes were well combusted. The low standard deviations of this incineration plant’s operating parameters imply rather stable operating conditions.
Table 2. Stack temperature and exhaust gas compositions during sampling.
Sampling equipment and method
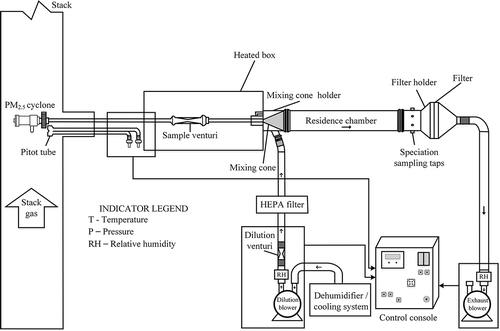
The dilution sampling system used in this study was provided by Environmental Supply Company and meets EPA CTM039 requirements. The dilution sampling system is shown schematically in . The sampling train consists of sample extraction and collection equipment as well as dilution supply air equipment for supplying clean, dry mixing air. The stack gas sample extraction and collection equipment includes PM2.5 cyclone, nozzle, Pitot tube, probe liner, differential pressure transducers, heating system, sample venturi, mixing cone, residence chamber, exhaust air blower, and sensors for relative humidity and temperature. The dilution air supply equipment includes dehumidifier, cooler, dilution air blower, HEPA filter, dilution venturi, and sensors for relative humidity and temperature.
In the dilution method the sample gas is diluted and cooled prior to collection on a 142-mm Teflon filter. A PM2.5 cyclone is installed after the sampling probe and no in-stack filter is installed. Stack gas is extracted at a predetermined constant sampling rate to achieve near 100% (80–120%) isokinetic sampling ratios through the in-stack PM2.5 cyclone. The cyclone separates particles with nominal aerodynamic diameters of greater than 2.5 μm, and allows particles less than or equal to 2.5 μm, plus stack gases, to continue through the heated sample probe and heated sample venturi to be diluted and cooled in the mixing cone and residence chamber, where nucleation, coagulation, and condensational growth occur, before being captured by a 142-mm filter. Filtered, dehumidified, and temperature-adjusted dilution air is added to the stack gas sample (containing only particle diameters smaller than 2.5 μm) in a mixing cone. After mixing dilution air and stack sample gas to allow for particulate condensation, PM2.5 is captured on a quartz filter. The exposed surfaces of the cyclone, probe, sample venturi, and venturi-cone connector tube are rinsed with acetone. The mixing cone, residence chamber, and filter holder inlet are first rinsed with distilled, deionized (DI) water, then with acetone. The rinses are evaporated until dry and desiccated, then weighed to determine PM2.5 for each sample fraction. The filter is desiccated and weighed. The water, acetone, and filter masses are summed up to determine PM2.5 concentration. Gravimetric mass obtained from the dilution sampling system is measured with a microbalance with a resolution of 1 µg (Sartorius balance, model Cubis 6.6S-DF) inside a humidity chamber maintained at a temperature of 20–23ºC and relative humidity of 30–40% for 24 hr.
In this study, stack parameters including temperature, pressure, volumetric flow rate, and moisture content were monitored for calculating PM2.5 emission concentration. Before each sampling, the dilution sampling system was cleaned with a rinse of distilled, deionized water followed with an acetone rinse to remove residues that might contaminate the sample. Leak checking was performed before and after sampling. A maximum of 2% of the total flow through the sampler was allowed for leakage. A previous study tested various dilution ratios (from 20 to 50) and showed that total PM2.5 mass concentrations were not affected by the dilution ratio (England et al., Citation2007). In this study, the dilution ratio was 30 and sampling time was 3 hr in order to collect sufficient sample for chemical analysis.
Conventional in-stack PM2.5 sampling was performed simultaneously with dilution sampling. An APEX XC-5000 Automated Isokinetic Sampling Console sampling system that meets EPA Methods 201A and 202 requirements was used to measure filterable and condensable PM2.5, respectively. The main equipment for Method 201A includes front nozzle, PM2.5 cyclone, filter holder, Pitot tube and stainless-steel (with glass liner) sampling tube, vacuum pump, and computer control console. Particulates with diameter smaller than 2.5 µm are sucked through the cyclone and are primarily collected on a 47-mm filter. Method 202 equipment includes a condenser, water dropout impinger, modified Greenburg Smith impinge, and condensable PM filter. Condensable PM2.5 is mainly collected in the water dropout impinger and the (backup) modified Greenburg Smith impinger. Condensable PM2.5 is collected by condenser, dry impingers, pipelines, and a backup Teflon filter after filterable PM is removed by a 47-mm filter. A detailed description of this sampling method and materials can be found elsewhere (Yang et al., Citation2014).
Chemical composition analysis
In addition to PM2.5 mass concentration, chemical compositions of the collected PM2.5 samples were analyzed in this study. PM2.5 samples were also collected in three parallel filter packs operated at 16.7 L min−1 located down from the dilution chamber designed for chemical analysis. One filter pack was installed with a 47-mm Teflon-membrane filter used to determine metal element analysis. The second and third filter packs were 47-mm quartz filters for organic carbon (OC), elemental carbon (EC), and ion analyses.
The filter used for OC and EC analysis was baked at 900ºC for 4 hr to remove the background organic carbon of the filter. OC and EC were determined, with a thermal/optical carbon analyzer (DRI, model 2001, Reno, NV), using the Interagency Monitoring of Protected Visual Environments (IMPROVE) protocol. For water-soluble ion analysis, the PM2.5 filter sample was extracted with distilled deionized water in an ultrasonicator (Branson, model 5210) for 120 min. The extracted samples were filtered by a 0.4 μm filter and then analyzed for ions (NH4+, Cl−, NO3−, SO42-) by ion chromatography (IC, Dionex, model DX-120). The eluent was 1.8 mM Na2CO3/1.7 mM NaHCO3 for anion and 20 mM methane sulfonic acid for cation analysis, respectively. Anion standards from High-Purity Standards (1033506 and 1034819) and cation standard from AccuStandard (210125090) were used to make calibration lines of the measured species. The R2 values of the calibrations were all higher than 0.995. Blank and duplicate tests were conducted for quality control.
For metal analysis, the PM2.5 filter sample was digested with acid mixture (HNO3:HCl = 1:3) on a hot plate for 1 hr. The digested sample was analyzed for metal elements (Al, Ca, Fe, Mg, Mn, Si, Na, K, Pb, Zn, Ni, V, Cu, Cd, Mo, Co, Se, Sr, As, Ba, Sb, Se, Sn) by inductively coupled plasma–optical emission spectrometer (ICP-OES, Thermo Scientific, model iCAP 6000 Series). A standard reference material (SRM 1649) was used for QA/QC. The recoveries of most target elements are within ± 10%. Calibration lines were made according to the Merck standard (1.09492.0100). Blank and duplicate tests were conducted for quality control. Calibration verification was also performed during sample analysis. A new calibration line should be made when the bias is higher than ±10%.
Results and discussion
PM2.5 and TPM concentrations
The in-stack PM2.5 mass concentration was determined as
where CPM2.5 is the concentration of PM2.5 (mg Nm−3), mf is the mass of particulate matter collected on the filter (mg), ma is the mass of particulate matter recovered from the mixing cone, residence chamber, and filter holder inlet with acetone (mg), mw is the mass of particulate matter recovered from the mixing cone, residence chamber, and filter holder inlet with water (mg), and Vs(std) is the volume of stack gas sampled (Nm3, dry),
where Qs(std) is the sample flow rate (Nm3 min−1, dry) and trun is the total actual run time (min).
Flow rates were corrected to normal conditions (1 atm and 293 K) and thus the PM2.5 concentrations reported are for standard condition. PM2.5 concentrations were converted with 11% of O2. PM2.5 concentration is 10.2 ± 0.67 mg Nm−3 (). The low standard deviation indicates stable operation of both incineration and air pollution control devices. Until now, no data for PM2.5 mass concentration collected by dilution sampling method has been found in the literature for comparison with our study. More research is needed in the future.
Table 3. PM2.5 and TPM concentrations and emission factors.
Total particulate matter (TPM) concentration is also listed in . TPM concentration is the concentration of PM2.5 plus the emission of PM higher than 2.5 µm. TPM concentration is 11.0 ± 0.78 mg Nm−3 and the ratio of PM2.5/TPM is 0.93 ± 0.01 (). The results indicate that more than 90% of PM emission from MSWI is fine particulate matter. Both PM2.5 and TPM mean-mass emission factors and uncertainties based on dilution sampling are listed in . The emission factor is reported as PM emission in grams per ton of incinerated waste. PM2.5 and TPM emission factors are 61.6 ± 4.52 and 66.1 ± 5.27 g ton-waste−1, respectively. Many previous studies have investigated the influences of MSWI on ambient air quality. Some showed significant effects of MSWI on the particle and/or the particle-bonded elements of surrounding air (Hu et al., Citation2003; Mao et al., Citation2007), while some other studies indicated that the contribution of particulate from MSWI is negligible (Venturini et al., Citation2013; Buonanno and Morawska, Citation2015). The emission factors estimated by dilution sampling technique in this study provide valuable and reliable data for the assessment of the contribution of MSWI to the atmosphere.
PM2.5 chemical speciation profile
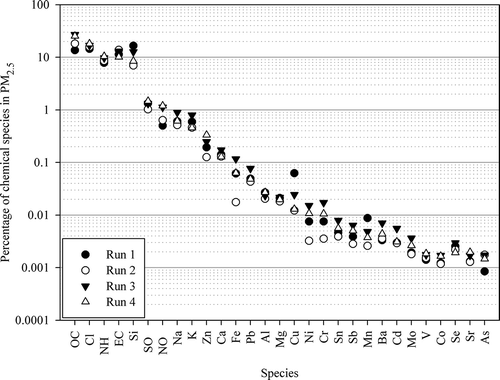
The percentage of analyzed species accounting for PM2.5 is shown in with the y-axis in logarithmic scale. The species analyzed in this study were categorized into three groups: major elements (PM2.5 mass ≥ 1%), minor elements (PM2.5 mass between 0.01 and 1%), and trace elements (PM2.5 mass < 0.01%). The mass closure percentage of the analyzed species for PM2.5 is 73.6 ± 8.41%. About 25% of the PM2.5 mass was not accounted for. The analyzed species in this study are likely present in the particulate as oxidized form, and oxygen would contribute a significant fraction of sample mass. When reconstructed by the same factors as used by Chen et al. (Citation2013), the mass closure was increased 96.2 ± 11.8%. The PM2.5 mass closure percentage for in-stack samples of the analyzed species is 62.6 ± 13.2%, which is lower than the diluted samples.
lists the major compositions and properties of the municipal solid waste. The municipal waste comes from residences, schools, streets, recreational sites, and administrative offices, etc. The composition of waste is important for explaining the chemical characteristics of the emitted PM2.5. The major elements in PM2.5 include OC, Cl−, NH4+, EC, and Si. These 5 species accounted for 69.7% of PM2.5 mass. Total carbon (sum of EC and OC) accounted for 33.1% of PM2.5 mass. The average ratio of OC/EC was 1.73. The loss on ignition of ash residue of this MSWI is around 3.2%, indicating a high combustion efficiency of this plant. Regardless, there is still unburned carbon in the exhaust, which adsorbed onto the particulate during the cooling process. The high Cl− emission is primarily contributed by wastes containing plastic bags made of polyvinyl chloride, the salt in kitchen waste and waste biomass, and so on. Si might come from soil, glass, and ceramics in the waste. NH4+ can be formed from the combustion process of kitchen waste, wood, and garden trimmings. Previous study has also measured high Si and NH4+ emissions from the incinerator (Morawska and Zhang, Citation2002).
The minor elements in PM2.5 include SO42-, K+, Na, K, NO3−, Al, Ca2+, Zn, Ca, Cu, Fe, Pb, and Mg. The K emission is attributable to condiments in kitchen waste. Combustion of wood and grass would also result in K emission. The emission of Ca is caused by the addition of limestone in the air pollution device for removal of acid gas. Although metals are recycled before waste incineration, some metal was frequently present in the solid waste (Hasselriis and Licata, 1996; Singh et al., Citation2002; Hu et al., Citation2003). Other trace elements in PM2.5 are Mn, Ni, Cr, Sn, Sb, Ba, Cd, Se, Mo, V, Sr, Co, and As. These elements accounted for less than 0.05% of PM2.5 mass. Even with low emission of these elements, they were frequently found in the incinerator stack (e.g., Hasselriis and Licata, 1996; Wang et al., Citation2001). In the review article of Morawska and Zhang (Citation2002), the mass percentage accounting for particulate matter higher than 10% included NH4+, Cl, SO42-, and OC. The mass fractions of NO3−, Na, EC, and Si were between 1% and 10%. The mass fractions in the literature were similar to the results in our study. The comparison shows that although the municipal waste compositions might be somewhat different for different countries and areas, the mass fractions of the emitted particulate matter are quite similar.
Comparison with in-stack sampling
PM2.5
The extremely bulky dilution sampling equipment is quite difficult to operate to measure PM2.5 emission for most plants with limited space for sampling plates. Today, the in-stack filter/impinger method is still the most practical way to collect PM2.5 samples from stationary sources. Most published emission factors and profiles are based on traditional in-stack sampling techniques. However, it is important and necessary to compare measurement results between dilution sampling and in-stack sampling methods.
The PM2.5 emission concentrations measured using dilution and in-stack sampling techniques are listed in . Both filterable and condensable PM2.5 concentrations for in-stack sampling method are shown. PM2.5 emission concentration is 28.4 ± 17.0 mg Nm−3 using the in-stack sampling method, with a much higher standard deviation than for the dilution sampling method. The coefficients of variance (COV) of filterable and condensable PM measurement results are 11.3% and 60.3%, respectively (), showing that the high variation of the measurement results using in-stack method comes primarily from condensable PM measurement.
Table 4. PM2.5 emission concentrations for dilution and in-stack sampling methods.
PM2.5 emission concentration is higher using in-stack sampling than using the dilution sampling method. The mean ratio of dilution method/in-stack method is 0.454. For the in-stack sampling method, condensable PM is much higher than filterable PM (). Previous studies have indicated that impinger test method for condensable PM might exit potential positive artifacts caused by absorption and reaction of SO2 and organic compounds within the chilled impinger train, which contribute to the sample (Corio and Sherwell, Citation2000; England et al., Citation2007). To reduce the artifact, EPA has made some improvements in the test method (EPA Method 202, implemented in 2011), including using a dry impinger, and has made a nitrogen purge mandatory to remove dissolved SO2, and so on. The comparison of dilution sampling and in-stack sampling method for most previous studies in the literature used the “old” in-stack method. The in-stack measurement method used in this study is the newly amended version with the improvement of artifact reduction. The results of this study show that PM2.5 emission is higher for the in-stack sampling method, even though the potential artifact is reduced in the new method
Chemical composition
The influence of dilution on chemical compositions of PM2.5 can be evaluated using an enrichment factor (EF), which is defined as
where xi,dilute and xi,in-stack are the PM2.5 mass fraction of chemical species i in the diluted and in-stack sample, respectively. An enrichment factor greater than 1.0 implies that the species is enriched in the diluted sample compared to the in-stack sample; an enrichment factor equal to 1.0 means no difference between the dilution and in-stack sampling methods for that species (Lipsky et al., Citation2004).
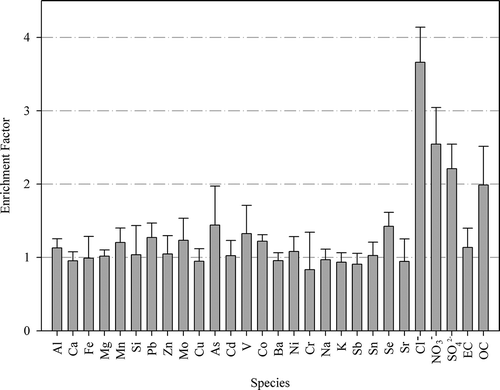
Enrichment factors (EFs) of the analyzed species for the dilution and in-stack PM2.5 samples are shown in . Most species have an EF of around 1.0, indicating that the mass fraction of the species of the diluted sample is the same as that in the in-stack hot sample. These species are primarily low-volatility elements, such as Al, Ca, Fe, Mg and Mn. Some elements with more volatility, such as Pb, As, and Se, have higher EFs than other elements, indicating that these elements are enriched in the dilution process. The EFs of water-soluble ions are significantly higher than 1.0. Note that the EF of NH4+ is also higher than 1.0. The EF of NH4+ is not shown in since its concentration in the hot-stack sample was below the detection limit. The results show that these water-soluble ions are enriched in the dilution process. SO42- and NO3− tend to be formed via gas-to-particle conversion within the dilution sampler. These acidic anions promote the gas-to-particle conversion of gas-phase NH3 to form NH4+ to neutralize the particles. NH3 preferentially reacts with SO42-, then NO3−, and subsequently Cl−. The EF of Cl− is higher than for SO42- and NO3−, indicating that there is sufficient NH3 to react with SO42- and NO3− present in the stack gas and the excess amount of NH3 to react with Cl− to form NH4Cl.
Conclusions
A dilution sampling method was performed to measure PM2.5 emission from an MSWI. Carbon content, metal elements, and water-soluble ions in PM2.5 were analyzed. PM2.5 emission factors and the chemical speciation profile of PM2.5 were established. PM2.5 concentration was 11.1 ± 0.73 mg Nm−3 and PM2.5 emission factor was 61.6 ± 4.52 g ton-waste−1, respectively. The ratio of PM2.5/TPM was 0.93 ± 0.01, showing PM emission from MSWI is mainly fine particulate. OC, Cl−, NH4+, EC, and Si are the major species in PM2.5, and accounted for 69.7% of PM2.5 mass. EC and OC accounted for 33.1% of PM2.5 mass. High Cl− emission is primary contributed by wastes containing plastic bags made of polyvinyl chloride, salt in kitchen waste and waste biomass, and so on. Si might come from soil, glass, and ceramics in the waste. The minor species in PM2.5 accounted for 0.01–1% of PM2.5, and include SO42-, K+, Na, K, NO3−, Al, Ca2+, Zn, Ca, Cu, Fe, Pb, and Mg.
To compare the influences of dilution on PM2.5 emissions and the characteristics of the bounded chemical species, traditional in-stack sampling was simultaneously conducted. PM2.5 emission concentration was 31.0 ± 18.5 mg/Nm3 using the in-stack sampling method, with a much higher standard deviation than for the dilution sampling method. The high variation of the measurement results using the in-stack method comes primarily from condensable PM measurement. PM2.5 emission concentration is higher using in-stack sampling than using the dilution sampling method. The mean ratio of dilution method/in-stack method is 0.454. The content of water-soluble ions (Cl−, SO42-, NO3−) are significantly enriched in PM2.5 via gas-to-particle conversion in the dilution process. The dilution sampling method provides a more reliable estimation of PM2.5 chemical emission to the atmosphere.
Additional information
Notes on contributors
Hsi-Hsien Yang
Hsi-Hsien Yang is a research professor at the Department of Environmental Engineering and Management, Chaoyang University of Technology, Taichung, Taiwan.
Shao-Wei Luo
Shao-Wei Luo, Kuei-Ting Lee, and Jhin-Yan Wu are atmospheric scientists at the Department of Environmental Engineering and Management, Chaoyang University of Technology, Taichung, Taiwan.
Chun Wei Chang
Chun Wei Chang is the Principal Engineer at Plant Affairs Department, Sino Environmental Services Corporation, Taipei, Taiwan.
Pei Feng Chu
Pei Feng Chu is the Vice-President at the Plant Affairs Department, Sino Environmental Services Corporation, Taipei, Taiwan.
References
- Buonanno, G., and L. Morawska. 2015. Ultrafine particle emission of waste incinerators and comparison to the exposure of urban citizens. Waste Manage. 37:75–81. doi:10.1016/j.wasman.2014.03.008
- Chang, C.Y., C.F. Wang, D.T. Mui, M.T. Cheng, and H.L. Chiang. 2009. Characteristics of elements in waste ashes from a solid waste incinerator in Taiwan. J. Hazard. Mater. 165:766–73. doi:10.1016/j.jhazmat.2008.10.059
- Chen, S.C., S.C. Hsu, C.J. Tsai, C.C.K. Chou, N.H. Lin, C.T. Lee, G.D. Roam, and D.Y.H. Pui. 2013. Dynamic variations of ultrafine, fine and coarse particles at the Lu-Lin background site in East Asia. Atmos. Environ. 78:154–62. doi:10.1016/j.atmosenv.2012.05.029
- Corio, L.A., and J. Sherwell. 2000. In-stack condensable particulate matter measurements and issues. J. Air Waste Manage. Assoc. 50:207–18. doi:10.1080/10473289.2000.10464002
- England, G.C., J.G. Watson, J.C. Chow, B. Zielinska, M.C.O. Chang, K.R. Loos, and G.M. Hidy. 2007. Dilution-Based emissions sampling from stationary sources: part 1-compact sampler methodology and performance. J. Air Waste Manage. Assoc. 57:65–78. doi:10.1080/10473289.2007.10465291
- EPA. 2004. Conditional test method (CTM) 039. Measurement of PM2.5 and PM10 emissions by dilution sampling (constant sampling rate procedures). https://www3.epa.gov/ttnemc01/ctm/ctm-039.pdf (accessed July 1, 2016).
- Fine, P.M., G.R. Cass, and B.R.T. Simoneit. 2001. Chemical characterization of fine particle emissions from fireplace combustion of woods grown in the northeastern united states. Environ. Sci. Technol. 35:2665–75. doi:10.1021/es001466k
- Hartenstein, H.U., and M. Horvay. 1996. Overview of municipal waste incineration industry in west Europe (based on the German experience). J. Hazard. Mater. 47:19–30. doi:10.1016/0304-3894(95)00124-7
- Hidemann, L.M., G.R. Markowski, and G.R. Cass. 1991. Chemical composition of emissions from urban sources of fine organic aerosol. Environ. Sci. Technol. 25:744–59.
- Hu, C.W., M.R. Chao, K.Y. Wu, G.P. Chang-Chien, W.J. Lee, L.W. Chang, and W.S. Lee. 2003. Characterization of multiple airborne particulate metals in the surroundings of a municipal waste incinerator in Taiwan. Atmos Environ. 37:2845–52. doi:10.1016/S1352-2310(03)00208-5
- Lee, S.W., T. Herage, I. He, and B. Young. 2008. Particulate characteristics data for the management of PM2.5 emissions from stationary combustion sources. Powder Technol. 180:145–50. doi:10.1016/j.powtec.2007.03.025
- Lee, S.W., R. Pomalis, and B. Kan. 2000. A New methodology for source characterization of oil combustion particulate matter. Fuel Process. Technol. 65–66:189–202. doi:10.1016/S0378-3820(99)00086-7
- Li, X., S. Wang, L. Duan, J. Hao, and Z. Long. 2011. Design of a compact dilution sampler for stationary combustion sources. J. Air Waste Manage. Assoc. 61:1124–30. doi:10.1080/10473289.2011.604556
- Lipsky, E.M., N.J. Pekney, G.F. Walbert, W.J. O’Dowd, M.C. Freeman, and A.L. Robinson. 2004. Effects of dilution sampling on fine particle emissions from pulverized coal combustion. Aerosol Sci. Technol. 38:574–87. doi:10.1080/02786820490479851
- Mao, I.F., C.N. Chen, Y.C. Lin, and M.L. Chen. 2007. Airborne particle PM2.5/PM10 mass distribution and particle-bound PAH concentrations near a medical waste incinerator. Atmos Environ. 41:2467–75. doi:10.1016/j.atmosenv.2006.04.064
- Morawska, L., and J. Zhang. 2002. Combustion sources of particles. 1. Health relevance and source signatures. Chemosphere. 49:1045–58. doi:10.1016/S0045-6535(02)00241-2
- Mills, N.L., K. Donaldson, P.W. Hadoke, N.A. Boon, W. MacNee, F.R. Cassee, T. Sandström, A. Blomberg, and D.E. Newby. 2009. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 6:36–44. doi:10.1038/ncpcardio1399
- Pope, III.C.A., R.T. Burnett, G.D. Thurston, M.J. Thun, E.E. Calle, D. Krewski, and J.J. Godleski. 2004. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 109:71–77. doi:10.1161/01.CIR.0000108927.80044.7F
- Singh, M., P.A. Jaques, and C. Sioutas. 2002. Size distribution and diurnal characteristics of particle-bound metals in source and receptor sites of the Los Angeles basin. Atmos. Environ. 36:1675–89. doi:10.1016/S1352-2310(02)00166-8
- Venturini, E., I. Vassura, L. Ferroni, S. Raffo, F. Passarini, D.C.S. Beddows, and R.M. Harrison. 2013. Bulk deposition close to a municipal solid waste incinerator: one source among many. Sci. Total Environ. 456-57:392–403. doi:10.1016/j.scitotenv.2013.03.097
- Wang, K.S., K.Y. Chiang, C.C. Tsai, and C.J. Sun. 2001. The effects of FeCl3 on the distribution of the heavy metals Cd, Cu, Cr and Zn in a simulated multimetal incineration system. Environ Int. 26:257–63. doi:10.1016/S0160-4120(00)00115-X
- Waston, J.G., J.C. Chow, and J.E. Houck. 2001. PM2.5 chemical source profiles for vehicle exhaust, vegetative burning, geological material, and coal burning in northwestern Colorado during 1995. Chemosphere. 43:1141–51.
- Watson, J.G., T. Zhu, J.C. Chow, J. Engelbrecht, E.M. Fujita, and W.E. Wilson. 2002. Receptor modeling application framework for particle source apportionment. Chemosphere. 49:1093–36. doi:10.1016/S0045-6535(02)00243-6
- Yang, H.H., K.T. Lee, Y.S. Hsieh., S.W. Luo, and R.J. Huang. 2015. Emission characteristics and chemical compositions of both filterable and condensable fine particulate from steel plants. Aerosol Air Qual. Res. 15:1672–80. doi:10.4209/aaqr.2015.06.0398
- Yang, H.H., K.T. Lee, Y.S. Hsieh, S.W. Luo, and M.S. Li. 2014. Filterable and condensable fine particulate emissions from stationary sources. Aerosol Air Qual. Res. 14:2010–16. doi:10.4209/aaqr.2014.08.0078
- Yang, H.H., W.J. Lee, S.J. Chen, and S.O. Lai. 1998. PAH emission from various industrial stacks. J. Hazard. Mater. 60:159–74. doi:10.1016/S0304-3894(98)00089-2
- Yuan, C.S., H.Y. Lin, C.H. Wu, and M.H. Liu. 2005. Partition and size distribution of heavy metals in the flue gas from municipal solid waste incinerators in Taiwan. Chemosphere 59:135–45. doi:10.1016/j.chemosphere.2004.09.095
- Zhang, M., S. Zhang, Z. Zhang, Z. Xu, G. Feng, and M. Ren. 2014. Influence of a municipal solid waste incinerator on ambient air PCDD/F levels: A comparison of running and non-running periods. Sci. Total Environ. 491–92:34–41. doi:10.1016/j.scitotenv.2014.03.100