ABSTRACT
With increasing attention on sulfuric acid emission, investigations on the removal characteristics of sulfuric acid aerosols by the limestone gypsum wet flue gas desulfurization (WFGD) system and the wet electrostatic precipitator (WESP) were carried out in two coal-fired power plants, and the effects of the WFGD scrubber type and the flue gas characteristics were discussed. The results showed that it was necessary to install the WESP device after desulfurization, as the WFGD system was inefficient to remove sulfuric acid aerosols from the flue gas. The removal efficiency of sulfuric acid aerosols in the WFGD system with double scrubbers ranged from 50% to 65%, which was higher than that with a single scrubber, ranging from 30% to 40%. Furthermore, the removal efficiency of WESP on the sulfuric acid aerosols was from 47.9% to 52.4%. With increased concentrations of SO3 and particles in the flue gas, the removal efficiencies of the WFGD and the WESP on the sulfuric acid aerosols were increased.
Implications: Investigations on removal of sulfuric acid aerosols by the WFGD and the WESP in the power plants were aimed at the control of sulfuric acid emission. The results showed that the improvement of the WFGD system was beneficial for the reduction of sulfuric acid emission, while the WESP system was essential to control the final sulfuric acid aerosol concentration.
Introduction
With further understanding of the adverse impacts of air pollution to the environment and human health, sulfuric acid aerosols emitted from coal-fired power plants have attracted increasing attention (Srivastava et al., Citation2004). Approximately 0.5–1.5% of the sulfur in coal was oxidized to be SO3 (Ahn et al., Citation2011) during coal combustion, and the SO3 concentration became significantly higher after selective catalytic reduction (SCR) (Kamata et al., Citation2001). As SO3, in combination with H2O, converted to H2SO4 easily, sulfuric acid aerosols were formed after the flue gas entered into the wet flue gas desulfurization (WFGD) system (Brachert et al., Citation2013, Citation2014) where the temperature rapidly decreased below the acid dew point and supersaturation conditions prevailed, resulting in serious corrosion on the devices and a visible plume (Yang, Citation2010).
The removal of sulfuric acid aerosols across the WFGD scrubbers was inefficient, as they were mainly in the submicrometer range. According to the results from the coal-fired power plants (Cao et al., Citation2010; Teng et al., Citation2008; Moser et al., Citation2006), the SO3 removal efficiency by the WFGD systems ranged from 10% to 60%. Therefore, the SO3 removal efficiency of WFGD system was not verified and it varied with different WFGD systems. Furthermore, sulfuric acid aerosols were proved by the chemical analyses and mass balances to be significant components of fine particles emitted after desulfurization (Gooch et al., Citation1998). In an attempt to reduce sulfuric acid aerosol emission, the inhibition of SO3 formation and enhancement of the removal of the sulfuric acid aerosols were investigated. Investigations (Spörl et al., Citation2014; Fleig et al., Citation2012; Schwaemmle et al., Citation2012) on the effects of combustion conditions and the SCR systems on the SO3 formation characteristics were carried out, while no feasible methods have been proposed without adverse effect on the device performance. In addition, different methods were adopted to reduce the generated SO3 in the flue gas. The concentration could be reduced via the chemical reactions with the agent sprayed into the flue gas (Gray et al., Citation2014; Wang et al., Citation2012) and the installation of a heat exchanger before the electrostatic precipitator (ESP) (Kikkawa et al., Citation2015), but in turn the potential loss of ash sales and adverse impacts on the ESP performance were presented. Among these methods, the wet electrostatic precipitator (WESP) installed after the WFGD system has a significant advantage over others in terms of multipollutant removal and high removal efficiency, as the emissions of fine particles, sulfuric acid aerosols, and visible plume were addressed, leading to increasing full-scale application to coal-fired power plants. For a power plant using the high sulfur content coal in Japan, the concentration of sulfuric acid mist in the flue gas was approximately 60 ppm at the WESP inlet, and less than 1 ppm was achieved at the WESP outlet (Fujishima and Nagata, Citation2001). By using the WESP, the stack opacity, which was closely related to the SO3 concentration, was limited to levels about 10%, as compared to pre-WESP levels of 40% in the Northern States Power/Xcel Energy (Staehle et al., Citation2003). With the stricter national standard on the pollutant emissions, the application of the WESP was promoted in China. Field measurements of the SO3 removal efficiencies of the WESP were achieved in many power plants (Zhao et al., Citation2012; Liu et al., Citation2012, Liao et al., Citation2014; Shen, Citation2014), and generally ranged from 60% to 70%. The current investigations mainly focused on the achievements of the WESP technology, while the effects of flue gas characteristics on the sulfuric acid aerosol removal of the WESP in industry were barely investigated.
Compared with the current brief introduction on the removal of sulfuric acid aerosols, investigations on the removal characteristics of sulfuric acid aerosols by the limestone gypsum WFGD system and the WESP device were carried out in two coal-fired power plants, and the effect of the WFGD scrubber type and the flue gas characteristics were discussed in this paper.
Experimental details
Experimental conditions
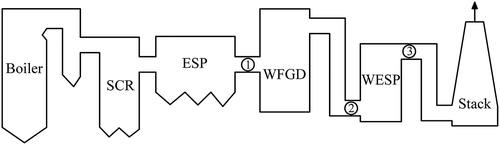
Sulfuric acid emissions were measured in two coal-fired power plants, which were equipped with similar devices for the flue gas treatment, as shown in . Testing points were located at both the inlet and outlet of the WFGD system and the outlet of the WESP equipment. In the two coal-fired power plants, both of the WFGD systems adopted the technology of limestone gypsum desulfurization where the flue gas and the desulfurization slurry maintained a countercurrent flow. Compared with one spray scrubber for desulfurization in power plant A, a prescrubber was installed before the absorption scrubber in power plant B. The detailed configuration and the main parameters of the WFGD systems under normal conditions are summarized in .
Table 1. Summary of experimental conditions.
Sulfuric acid measurements
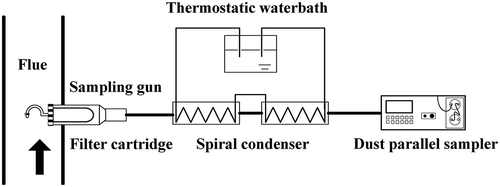
As the controlled condensation method (CCM) is recognized as the most reliable measurement method (Cao, Citation2010; GB/T 21508-2008), the schematic diagram of sulfuric acid aerosol sampling is shown in . The flue gas was sampled by a heated sampling probe with setting temperature at 180ºC. Then the temperature in the glass spiral condenser was kept below the acid dew point and gaseous sulfuric acid was condensed to droplets, which were collected by the condenser. The SO3 concentration in the flue gas was calculated via the SO42- content in the condensed fluid collected.
Measurement technique
The SO2 concentration in the flue gas was measured by a flue gas analyzer (J2KN Pro, Ecom Ltd., Germany). Particles were collected in the filter cartridge with a heated sampling probe and the WJ-60B automatic smoke sampler for particle concentration measurement and sulfuric acid aerosol sample. The SO42- concentration in the condensed droplets was measured by the ion chromatograph (ICS-2100, Dionex Ltd., USA).
Results and discussion
Removal of sulfuric acid in the flue gas
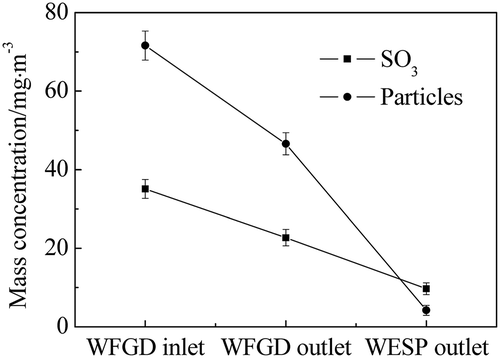
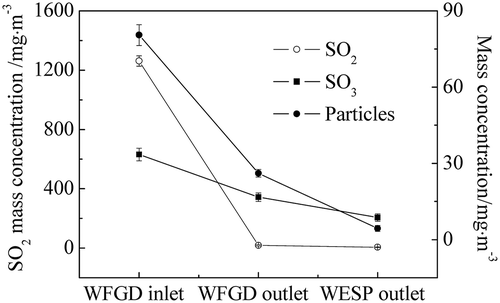
In order to clarify the removal effect of the WFGD system and WESP equipment on the sulfuric acid aerosols, the transformation of SO3 and particles in the power plant A is presented in . At the inlet of the WFGD system, the SO3 concentration was 35.1mg m−3, and it decreased to 22.7 mg m−3 at the outlet, indicating that 35.3% of sulfuric acid aerosols was removed by the WFGD system. During the desulfurization process, the absorption of gaseous sulfuric acid by the desulfurization slurry was much slower than the condensation rate, and thus the sulfuric acid in the flue gas mainly turned into the droplets. Meanwhile, sulfuric acid aerosols were partly formed by homogeneous nucleation with sizes that were mainly less than 0.1 μm. For these submicrometer particles, Brownian diffusion was recognized to be the primary force of the mass transfer between the scrubbing slurry and sulfuric acid aerosols, which was not adequate to result in high-efficiency capture under the relative velocity in the scrubber. In addition, for particles with size near 1 μm, the removal efficiency of the ESP reached the lowest value, indicating that fine particles were the major ones in the flue gas before desulfurization. As these particles remained in the flue gas, heterogeneous nucleation and condensation occurred between the particles and sulfuric acid during the desulfurization process. The sulfuric acid could be eliminated along with the removal of these particles via slurry scrubbing, while the removal efficiency of particles during the desulfurization process was 34.9%. Therefore, high removal efficiency of SO3 was not achieved by the WFGD system. In addition, as the SO3 concentration was 22.7mg m−3 after desulfurization, this would lead to a highly visible plume without following treatment. Hence, WESP equipment after the WFGD system was installed in the power plant. Compared with the removal effect of ESP, particles collected by the WESP on the precipitating electrode plates were cleared by the liquid scrubbing instead of rapping, avoiding the re-entrainment of particles, especially for fine particles. As can be seen in , the concentration decreased to 4.2 mg·m−3 at the outlet of the WESP, with the removal efficiency of particles 91.0%. Furthermore, as the sulfuric acid aerosols were formed via homogeneous nucleation and heterogeneous nucleation, the emission of sulfuric acid aerosols was efficiently controlled by the WESP and the concentration decreased to 9.7 mg m−3. As a consequence, the application of the WESP was essential and effective for the control of sulfuric acid aerosol emission.
Effect of WFGD system on sulfuric acid removal
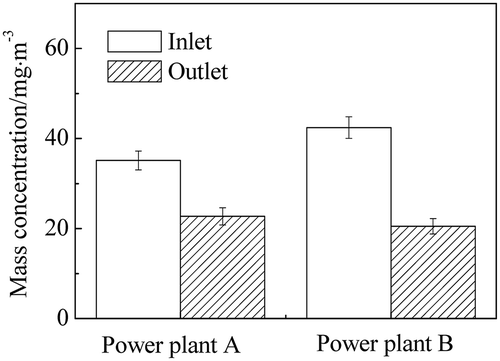
As can be seen in , the SO2 concentration in the flue gas before desulfurization displayed a distinct difference in the two power plants, which was mainly attributed to the sulfur content in the coal burned. In an attempt to reach higher removal efficiency of SO2, a prescrubber was installed in power plant B. Considering that different WFGD systems would in return influence the removal of other pollutants, the SO3 removal efficiency of WFGD system in the two power plants was investigated, as demonstrated in . The WFGD system with double scrubbers showed higher removal efficiency, and in spite of higher concentration at the inlet of the WFGD system, fewer sulfuric acid aerosols were emitted out of the scrubber in power plant B. As mentioned earlier, the removal of sulfuric acid aerosols mainly depended on the slurry scrubbing during desulfurization. With the extended resident time of the slurry scrubbing in the double scrubbers, collision odds between the slurry droplets and the components in the flue gas were increased. Correspondingly, the capture of sulfuric acid aerosols and particles during desulfurization was enhanced, resulting in higher SO3 removal efficiency. Also, the pH values varied in the two scrubbers on account of the gypsum generation. As lower pH value was beneficial for the oxidation of gypsum, the pH value of the desulfurization slurry in the prescrubber was 5.2 ± 0.2 and the absorption scrubber adopted pH value at 6.2 ± 0.3 for high-efficiency SO2 removal. After the scrubbing by the first scrubber, lower SO2 concentration and higher pH value in the next scrubber were beneficial for the further removal of sulfuric acid aerosols. As a consequence, except for the high removal efficiency of SO2 in the flue gas, the removal efficiency of SO3 in the double scrubbers was higher than that in the single scrubber, which reduced the load of the downstream WESP equipment at the same time.
Table 2. Analysis of coal quality.
Effect of inlet flue gas characteristics on sulfuric acid removal
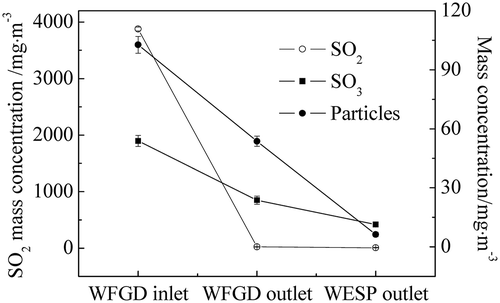
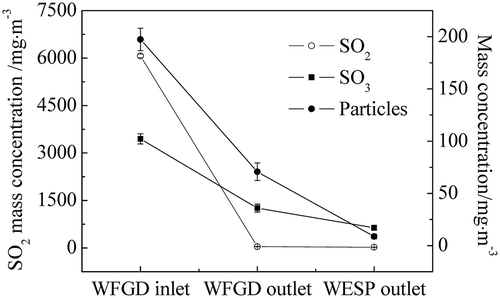
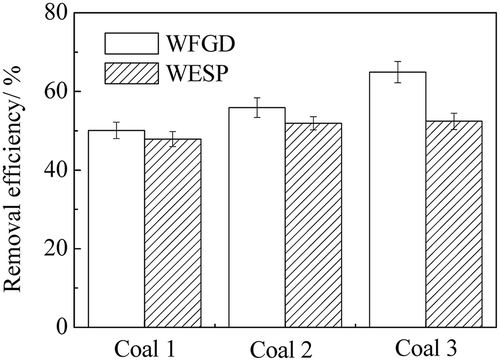
As the pollutant concentrations in the flue gas varied with different operation conditions, investigations on the removal of the WFGD system and the WESP on sulfuric acid aerosols with different flue gases were carried out in power plant B. The characteristics of the flue gas were changed as three kinds of mixed coals were burned and the coal qualities were analyzed as listed in . The concentrations of SO2, SO3, and particles at both the inlet and outlet of the WFGD system and the outlet of the WESP equipment were measured as illustrated in –. The coal quality had an important effect on the pollutant concentrations in the flue gas. From coal 1 to coal 3, with the increase of the ash content and the sulfur content, the concentrations in the flue gas increased correspondingly. As can be seen in –, the SO2 concentration at the inlet of WFGD system was significantly increased from 1262 mg m−3 to 6067 mg m−3. As the SO3 was generated via the oxidation of SO2 during the combustion and the SCR process, the SO3 concentration at the inlet of WFGD system was increased correspondingly from 33.5 mg m−3 to 102.2 mg m−3. Meanwhile, the particle concentration was related to the ash content in the coal and the concentration at the WFGD inlet was increased from 80.5 mg m−3 to 197.4 mg m−3. High removal efficiency of SO2 by the WFGD system is depicted in – and the emission met the national standard with concentrations below 30 mg m−3. For the particles in the flue gas, the scrubbing of the desulfurization slurry mainly captured large ones and most fine particles were removed by the WESP, leading to emission concentrations below 10 mg m−3. When coal 3 with high sulfur content was used in the power plant, the emission of SO3 reached 17.1 mg m−3 and notable blue flume was observed at the outlet of the stack.
Furthermore, the removal efficiency of the WFGD system and the WESP equipment with different flue gases is illustrated in . With the increase of the concentrations of SO3 and particles in the flue gas before desulfurization, the SO3 removal efficiency of the WFGD system was increased from 50.1% to 64.9%, which had correlation with the size of droplets and particles in the flue gas. For the homogeneous nucleation of sulfuric acid in the scrubber, higher SO3 concentration resulted in larger droplets. Also, more sulfuric acid was condensed on the particles and removed via the capture of these particles. Therefore, an increase tendency of the removal efficiency of the WFGD system is depicted in . Meanwhile, as the majority of sulfuric acid aerosols and particles after desulfurization were in the submicrometer range, it was more difficult to eliminate them from the flue gas and a slight increase of the removal efficiency of the WESP from 47.9% to 52.4% was showed. Hence, further optimization of the device design and operation parameters was required for higher removal efficiency.
Summary
Investigations on the removal characteristics of the WFGD and the WESP on sulfuric acid aerosols were carried out in the coal-fired power plants, and the conclusions can be drawn from the results as follows:
The WFGD system was inefficient to remove sulfuric acid aerosols from the flue gas and the WESP equipment needed to be adopted after desulfurization for the control of sulfuric acid aerosol emission.
The SO3 removal efficiency of the WFGD system with double scrubbers ranged from 50% to 65%, which was higher than that with a single scrubber ranging from 30% to 40%.
The SO3 removal efficiency of WESP was from 47.9% to 52.4%. With the increases concentrations of SO3 and particles in the flue gas, the removal by the WFGD and the WESP of the sulfuric acid aerosols was enhanced and notable blue flume was observed at the outlet of the stack when coals with high sulfur was burned.
Funding
The authors thank the National Natural Science Foundation of China (No. 21276049), the National Basic Research Program of China (973 Program No. 2013CB228505), the Jiangsu Science and Technology Support Program (No. BE2014856), and the Jiangsu Environmental Monitoring Research Program (1412) for their financial support.
Additional information
Funding
Notes on contributors
Danping Pan
Danping Pan is a Ph.D. student at Southeast University in Nanjing, PRC.
Linjun Yang
Linjun Yang is a professor at Southeast University in Nanjing, PRC.
Hao Wu
Hao Wu and Rongting Huang are Ph.D. students at Southeast University in Nanjing, PRC.
Rongting Huang
Hao Wu and Rongting Huang are Ph.D. students at Southeast University in Nanjing, PRC.
References
- Ahn, J., R. Okerlund, A. Fry, and E.G. Eddings. 2011. Sulfur trioxide formation during oxy-coal combustion. Int. J. Greenhouse Gas Control 5:S127–35. doi:10.1016/j.ijggc.2011.05.009
- Brachert, L., T. Kochenburger, and K. Schaber. 2013. Facing the sulfuric acid aerosol problem in flue gas cleaning: Pilot plant experiments and simulation. Aerosol Sci. Technol. 47(10): 1083–91. doi:10.1080/02786826.2013.824549
- Brachert, L., J. Mertens, P. Khakharia, and K. Schaber. 2014. The challenge of measuring sulfuric acid aerosols: Number concentration and size evaluation using a condensation particle counter (CPC) and an electrical low pressure impactor (ELPI+). J. Aerosol Sci. 67:21–27. doi:10.1016/j.jaerosci.2013.09.006
- Cao, Y., H. Zhou, W. Jiang, C.W. Chen, and W.P. Pan. 2010. Studies of the fate of sulfur trioxide in coal-fired utility boilers based on modified selected condensation methods. Environ. Sci. Technol. 44(9): 3429–34. doi: 10.1021/es903661b
- Fleig, D., K. Andersson, and F. Johnsson. 2012. Influence of operating conditions on SO3 formation during air and oxy-fuel combustion. Ind. Eng. Chem. Res. 51(28):9483–9491. doi: 10.1021/ie301303c
- Fujishima, H., and C. Nagata. 2001. Experiences of wet type electrostatic precipitator successfully applied for SO3 mist removal in boilers using high sulfur content fuel. http://www.epscointernational.com/isesp/ICESP%20IX%20PAPERS/ICESP%2009%20B02.pdf
- Gooch, J.P., and E.B. Dismukes. 1998. Formation of sulfate aerosol in a SO2 scrubbing system. Paper presented at the Formation, Distribution, Impact, and Fate of Sulfur Trioxide in Utility Flue Gas Streams Conference, Pittsburgh, PA.
- Gray, S.M., J.B. Jarvis, and S.W. Kosler. 2014. Combined mercury and SO3 removal using SBS injection. Power 158(7):22–26.
- GB/T 21508-2008. Performance test method for coal-fired flue gas desulphurization equipment. http://www.doc88.com/p-2095553367355.html
- Kamata, H., H. Ohara, K. Takahashi, A. Yukimura, and Y. Seo. 2001. SO2 oxidation over the V2O5/TiO2 SCR catalyst. Catal Lett. 73(1):79–83. doi:10.1023/A:1009065030750
- Kikkawa, H., W. Shimohira, T. Nagayasu, M. Kiyosawa, Y. Nagai, and S. Kagawa. 2015. Highly-efficient removal of toxic trace elements and particulate matter in flue gas emitted from coal-fired power plants by air quality control system (AQCS). Mitsubishi Heavy Industries Tech. Rev. 52(2):88.
- Liao, D.B. 2014. The development of wet electrostatic precipitator and its application in thermal power plant. Guangzhou: South China University of Technology.
- Liu, H., and Q. Tao. 2012. Exploration application of wet electric dust catcher to engineering. Electr. Power Survey Des. 3:43–47. doi:10.3969/j.issn.1671-9913.2012.03.010
- Moser, R.E. 2006. SO3’s impacts on plant O&M: Part I. Power 150(8): 40–40.
- Shen, H. 2014. Comprehensive evaluation for wet electrostatic precipitator used to control the coal-fired flue gas pollutant. Power Energy 35(1):54–58. doi:10.3969/j.issn.2095-1256.2014.01.014
- Srivastava, R.K., C.A. Miller, C. Erickson, and R. Jambhekarb. 2004. Emissions of sulfur trioxide from coal-fired power plants. J. Air Waste Manage. Assoc. 54(6):750–62. doi:10.1080/10473289.2004.10470943
- Spörl, R., J. Walker, L. Belo, K. Shah, R. Stanger, J. Maier, T. Wall, and G. Scheffknecht. 2014. SO3 emissions and removal by ash in coal-fired oxy-fuel combustion. Energy Fuel 28(8):5296–306. doi: 10.1021/ef500806p
- Schwaemmle, T., B. Heidel, K. Brechtel, and G. Scheffknecht. 2012. Study of the effect of newly developed mercury oxidation catalysts on the DeNOx-activity and SO2-SO3 conversion. Fuel 101:179–86. doi:10.1016/j.fuel.2010.11.043
- Staehle, R.C., R.J. Triscori, K.S. Kumar, G. Ross, and R. Cothron. 2003. Wet electrostatic precipitators for high efficiency control of fine particulates and sulfuric acid mist. Institute of Clean Air Companies (ICAC) Forum, April. https://www.adeq.state.ar.us/downloads/commission/p/closed%20permit%20dockets%202006-2016/08-006-p%20aep%20service%20corp.%20&%20swepco-sierra%20club%20&%20audubon(consolidated)/2009-03-06_hc_sc-bp_ex_22.pdf
- Teng, N., Y.Y, Zhang, H. Wei, and W.J. Zhang. 2008. Discussion on ash removal efficiency and sulfur trioxide removal efficiency of WFGD system. Electr. Power Environ. Protect. 24(4):27–28. doi: 10.3969/j.issn.1674-8069.2008.04.008
- Wang, Z., Q. Huan, C. Qi, L. Zhang, L. Cui, X. Xu, and C. Ma. 2012. Study on the Removal of Coal Smoke SO3 with CaO. Energy Procedia 14:1911–17. doi:10.1016/j.egypro.2011.12.1187
- Yang, Y. 2010. Corrosive and anticorrosive research on reinforced concrete chimney after wet flue gas desulfurization in power plant. Beijing, China: Beijing Jiaotong University.
- Zhao, Q., Z. Chen, C. Zhou, and D. Yin. 2012. Discussion on wet ESP technology and its application prospect in coal-fired power plants. Electr. Power Environ. Protect. 28(4):24–26. doi: 10.3969/j.issn.1674-8069.2012.04.008