ABSTRACT
The process of recovering waste sulfuric acids using H2O2 catalytic oxidation is studied in this paper. Activated carbon was used as catalyst. Main operating parameters, such as temperature, feed rate of H2O2, and catalyst dosage, have effects on the removal of impurities from waste sulfuric acids. The reaction kinetics of H2O2 catalytic oxidation on impurities are discussed. At a temperature of 90°C, H2O2 feeding rate of 50 g (kg waste acid)−1 per hour, and catalyst dosage of 0.2 wt% (waste acid weight), the removal efficiencies of COD and chrominance were both more than 99%, the recovery ratio of sulfuric acid was more than 95%, and the utilization ratio of H2O2 was 88.57%.
Implications: Waste sulfuric acid is a big environmental problem in China. The amount of waste sulfuric acid is huge every year. Many small and medium-sized businesses produced lots of waste acids, but they don’t have an appropriate method to treat and recover them. H2O2 catalytic oxidation has been used to treat and recover waste sulfuric acid and activated carbon is the catalyst here. Main parameters, such as temperature, feed rate of H2O2, and catalyst dosage, have been investigated. The reaction kinetics are discussed. This method can be economical and feasible for most small and medium-sized businesses.
Introduction
Sulfuric acid is a highly important chemical. It has been widely used in pesticides, fertilizers, batteries, and many types of metalworking. Unfortunately, much of it discharges as waste. China consumed 81.92 million tons of sulfuric acid (98 wt%) in 2013, and produced 100 million tons of waste sulfuric acid (Zhang, Citation2015). The waste sulfuric acid contains numerous organic and metallic impurities. Discharge of untreated or improperly treated waste sulfuric acid may cause serious environmental problems such as soil and water acidification and may damage the ecosystem and human health. Treating the waste sulfuric acid not only protects the environment but also allows recovery of valuable components.
Several methods, such as thermal decomposition, distillation, solvent extraction, membrane separation, and oxidative degradation, have been used to treat industrial waste acids and recycle sulfuric acid. Every method has its own suitable range of applications. Membrane separation is widely used to separate the metal ions from waste acids (Thang et al., Citation2005; Xu et al., Citation2009; Wei et al., Citation2010; Marti-Calatayud et al., Citation2014; Feng et al., Citation2016). Because of its low energy requirements and simple operation, membrane separation is considered very suitable for acid recovery. Solvent extraction involves regeneration of the extractant and is not suitable for waste acids with more than one type of complicated impurity (Kesieme et al., Citation2013). Distillation can remove low-boiling-point media from waste acids (Song et al., Citation2013; Park et al., Citation2014). Thermal decomposition can completely decompose organics and sulfuric acid at high temperatures (e.g., 1000–1100°C) and produce highly pure sulfuric acid through the transformation of sulfur dioxide, but the expensive investment restricts its use in small and medium-sized businesses (Bodenbenner et al., Citation1979; Morgenthaler, Citation1983). Unlike thermal decomposition, oxidative degradation selectively destroys organics in waste acids and recycles sulfuric acid with few impurities (Jimenez Jado et al., Citation2004).
H2O2 is an efficient oxidant with standard oxidation–reduction potential of 1.78 V (with a temperature of 25°C, effective concentration of H2O2 and H+ 1 mol/L, and partial pressure of each gaseous reagent 101.235 kPa). It is widely used to decompose organic compounds (Moreno-Castilla et al., Citation2000; Goi and Trapido, Citation2002; Hammedi et al., Citation2015). H2O2 has been used to oxidize organic impurities in waste acid from the refining of crude benzol (Li, Citation2005). Experimental results have indicated that H2O2 can decompose organic impurities in waste acids. Due to the high concentration of chemical oxygen demand (COD, expressing organic content), dilution treatment with water before addition of H2O2 reduces the content of recovered acid (only 35 wt%), whereas it increases the cost-to-benefit ratio of acid reuse. The reaction time of H2O2 oxidation is long and the utilization ratio of H2O2 is low. Previous studies have reported that a catalyst could increase the oxidation rate of H2O2 (Wu et al., Citation2010; Wang et al., Citation2015; Cruz et al., Citation2016; Rey et al., Citation2016). Catalysts such as activated carbon, ferrous iron (Fe2+), and copper and copper oxide supported on medium and solid superacids can catalyze H2O2 oxidation. The scientific research reported that ferrous sulfate enhances the reaction rate and shortens the reaction time of H2O2 oxidation in synthetic waste acid solution (Yang and Zhou, Citation1999). However, it is difficult to separate the catalyst from reaction solution. For the purpose of waste acid oxidation, the catalyst should be easy to recover and should not react with the components in solution. Activated carbon has many catalytic active sites and does not react with sulfuric acid, H2O2, or any other impurities, which makes it a suitable catalyst for H2O2 oxidation of organic impurities in waste acids. Many reports have indicated that activated carbon is selected as the catalyst for H2O2 oxidation to decompose organics in waste water treatment. However, no one uses activated carbon as a catalyst to improve the H2O2 oxidation rate of organic impurities in waste acids.
In this study, waste sulfuric acid was from the purification process of acetylene gases. After repeated use with distillation, the organic components in waste sulfuric acid were carbide and polymers, which contained vinyl acetylene, divinylacetylene, and 3-butene-1-thioaldehyde. It might contain some phosphoric acids and sulfur dioxide due to the raw materials of producing acetylene gases. H2O2 oxidation with activated carbon as catalyst was used to eliminate impurities and recover sulfuric acid from waste acids. The performance of waste sulfuric acid recovery using H2O2 catalytic oxidation with activated carbon was investigated here. Principal operating parameters such as reaction temperature, feeding rate of H2O2, and catalyst dosage, which affect the removal of impurities from waste sulfuric acid, were also studied. The reaction kinetics of H2O2 catalytic oxidation on impurities are discussed in detail, and these findings might be useful in the application of this technology in waste acid recovery.
Materials and methods
Waste sulfuric acid
Waste sulfuric acid was taken from the purification of acetylene gases in a chemical plant. It was a thick, black liquid with an intense smell and fine particles. The characteristics and components of waste sulfuric acid aere shown in . The main impurities of the waste sulfuric acid were organic compounds and the components were extremely complicated. Therefore, COD was used to evaluate the content of organic compounds in waste sulfuric acid. The COD value in waste sulfuric acid was about 1.7 × 105–2.5 × 105 mg/L and the concentration of sulfuric acid was about 89–93 wt%.
Table 1. Characteristics and components of waste sulfuric acid.
Activated carbon
Coconut shell activated carbon, a popular nut-shell activated carbon among all activated carbons, was cheap, widely sourced, and had good performance. Thus, it was used as the catalyst here. The size of coconut shell activated carbon was 10–24 mesh. The properties of this activated carbon are shown in .
Table 2. Physical and chemical characteristics of activated carbon.
Reagents
H2O2 (30 wt%, analytical reagent) was purchased from Shanghai Linfeng Chemical Reagent Company. Sodium hydroxide (analytical reagent) was purchased from Hangzhou Xiaoshan Chemical Reagent. Potassium dichromate (analytical reagent) was purchased from Wuxi Haishuo Biological Co., Ltd. Activated carbon (analytical reagent) was purchased from Aladdin Chemical Reagent. Potassium acid phthalate (short form KAP, analytical reagent), mercury sulfate (analytical reagent), and silver sulfate (analytical reagent) were purchased from Huadong Medicine Co., Ltd.
Methods of analysis
The COD values in waste sulfuric acid were determined according to Chinese standard HJ/T 399-2007. If needed, the analytic samples were diluted several times with distilled water according to organics content. All standard solutions such as HgSO4, K2Cr2O7, and AgSO4–H2SO4 were prepared in advance. One milliliter of pretreated analytic sample, 2 mL distilled water, 1 mL each of HgSO4 and K2Cr2O7 solution, and 5 mL AgSO4–H2SO4 solution were put into a standard tube. This sample then was digested for 2 hr under a temperature of 150°C by the COD digestion instrument. After digestion, the tube was cooled and 1 mL distilled water was added into the tube. After mixing well and resting, the absorbency of the solution in tube was measured using an ultraviolet–visible (UV-Vis) spectrophotometer (UV-2910, Hitachi, Japan) at a wavelength of 610 nm. The detector of the instrument was a deuterium lamp. The standard curve between COD and absorbency was tested by using KAP standard solutions. Then the COD value of the sample could be calculated.
The chrominance was determined according to Chinese standard GB 11903-89. The sample should be diluted less than 50 times. Then the dilution sample needed to be diluted one times until its color was the same as distilled water. The chrominance of the sample could be calculated based on the number of dilutions.
The concentration of sulfuric acid was measured by titration using 1 mol/L NaOH solution, while phenolphthalein was used as an indicator according to Chinese standard GB/T 534-2002 after dilution. The sample should be properly diluted when red color is easy to identify. The concentration of sulfuric acid was calculated from the volume of NaOH solution used and the weight of the sample. The concentration of sulfuric acid recovered could be used to calculate the recovery ratio of sulfuric acid.
The viscosity was measured by an Ubbelohde viscometer. The time of passing through for a constant volume in the Ubbelohde viscometer was measured and the viscosity of sample was then calculated with the time and viscosity coefficient of the viscometer.
The total organic carbon (TOC) of waste sulfuric acid was measured by TOC automatic analyzer (TOC-VCPN, Shimadzu, Japan). The cations such as Na+, Ca2+, and Fe3+ were measured by inductively coupled plasma–mass spectroscopy (ICP-MS; Elan DRC-e, PerkinElmer, USA). The anions such as F−, Cl−, SO42-, and PO43- were measured by ion chromatography (IC; ICS-2000, Dionex, USA).
Experimental device and procedure
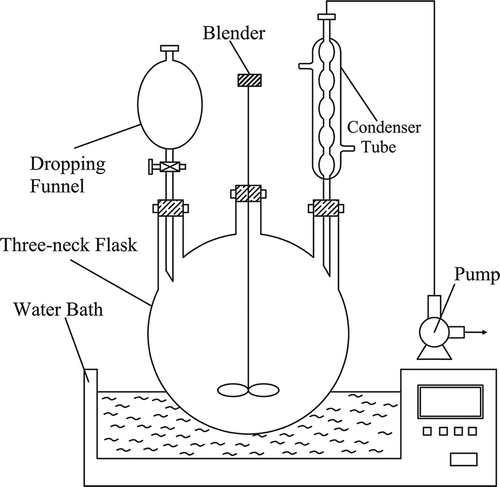
The experimental device was constructed indoors (). It included a flask, blender, funnel, condenser tube, water bath, and pump.
In this study, a 100-mL waste acid sample was placed in a 500-mL three-neck flask. Several preexperiments had been done to determine the maximum catalyst dosage according to the dosage of H2O2 catalytic oxidation in the treatment of wastewater. The maximum catalyst dosage was 1:100 (mass ratio of catalyst and reaction solution) in waste acid treatment. The other dosages were determined to 1:300, 1:500, 1:700, 1:900, and 0, gradually decreased from 1:100. The catalyst dosage of H2O2 catalytic oxidation could be calculated and then fed into the device. A constant temperature was maintained using a water bath where an appropriate temperature could be set. Concentrated sulfuric acid is an oxidant and reacts with organics under different temperatures (Yang, Citation1985; Guo and Chang, Citation1982). In this experiment, it was found that the oxidation reaction took place when the temperature was over 100°C, which causes the consumption of sulfuric acid. Therefore, the maximum temperature was 90°C. Other temperatures were determined as 70°C, 50°C, and 30°C, gradually decreasing from 90°C. H2O2 was fed dropwise using a dropping funnel and the feed rate of H2O2 was controlled by the funnel stopcock. For the safety of reaction, the feed rate of H2O2 should be below 65 g·(kg waste acid)−1 per hour. The appropriate maximum feed rate of H2O2 was 60 g·(kg waste acid)−1 per hour in the experiment. The other feed rates of H2O2 were 50, 40, 30, and 20 g·(kg waste acid)−1 per hour, gradually decreasing from 60 g·(kg waste acid)−1 per hour. The stirring speed of the blender was 120 rpm.
The analytic samples were taken from the three-neck flask within a regular time interval. The COD, chrominance, and concentration of sulfuric acid in the reaction solution were measured. All experiments were done three times and the data were averaged. The effects of catalyst dosage, feeding rate of H2O2, and reaction temperatures on catalytic oxidation were investigated.
When the reaction solution became colorless, the reaction was ended and the acid could be recovered. The activated carbon was separated from recovered acid and reused in the next waste sulfuric acid treatment. The removal efficiencies of COD and chrominance between new catalyst and cyclic catalyst were compared and the cyclic utilization of the catalyst was evaluated.
Results and discussion
Catalyst dosage and oxidation rate
Reaction temperature, catalyst, oxidant, and substrate were the main factors influencing the oxidation rate in catalytic oxidation systems. For the periodic stirring reaction, the primary rate equation based on mass-balance theory could be described as follows:
Here, C is the concentration of reactants; a, b, and n are the corresponding partial orders of reactant concentrations; and t is the reaction time.
According to the empirical kinetic models of H2O2 catalytic oxidation (Lin and Gurol, Citation1998; Lin et al., Citation1999) and the Arrhenius equation, the kinetic model for the catalytic oxidation rate could be described as follows:
Here, C is the concentration of organic compounds, which is described as COD in mg/L; O is the H2O2 concentration in mg/L; H is the catalyst dosage (mg/L); m, n, and L are the corresponding partial orders of reactants concentration; t is the reaction time (hr); T is the reaction temperature (K); R is the gas constant, 8.314 J/(mol·K); Ea is the activation energy in J/mol; and k0 is the frequency factor, which is related to m, n, and L.
Once reaction temperature, feed rate of H2O2, and catalyst dosage were confirmed, the preceding equation could be simplified as follows:
Here, K represents the apparent reaction rate constant (hr−1), which was described using the following equation:
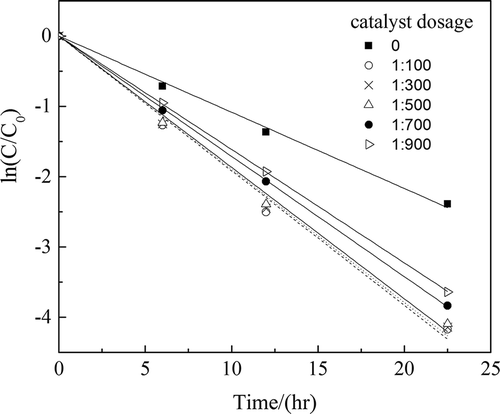
H2O2 catalytic oxidation was carried out when the reaction temperature was 70°C, the feed rate of H2O2 was 50 g·(kg waste acid)−1 per hour, and the catalyst dosage was 0 (no catalyst), 1:100, 1:300, 1:500, 1:700, and 1:900, respectively.
The experimental data were used to perform the curve fitting between and time t. The results are shown in . The correlation coefficients of fitting curves (R2) exceeded 0.99.
For a given operation condition, there was a linear relationship between and time t as shown in . Equation 2 could be simplified as follows:
As shown in , K values at different catalyst dosages could be determined and are shown in .
Table 3. K values at different dosage of catalyst.
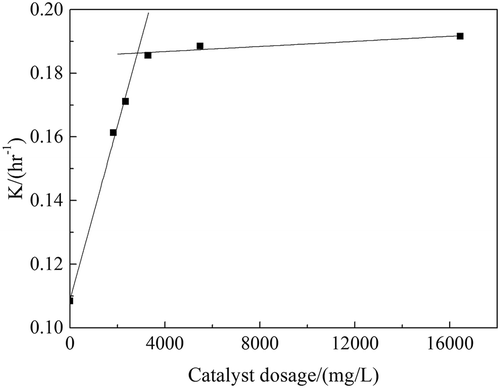
The relation diagram between catalyst dosage and the apparent reaction rate constant is given as , which is based on the data in . The catalyst dosage was mass ratio of catalyst and reaction solution. The concentration of catalyst dosage (mg/L) could be calculated with the weight of catalyst and the volume and density of waste acid. When reaction temperature and feed rate of H2O2 were kept constant, eq 4 could be simplified as follows:
The reaction proceeded significantly more quickly with a catalyst than without one, as shown in and . The apparent reaction rate constant increased by 76.75% and the reaction time was shortened by half. When the catalyst dosage was more than 1:500, the apparent reaction rate constant scarcely increased and peaked at 0.19 hr−1. The value of L could be considered as 0 here. If the catalyst dosage was less than 1:700, the apparent reaction rate constant and catalyst dosage showed a linear relationship, and the value of L here was 1. When the catalyst dosage was between 1:700 and 1:500, the value of L was between 0 and 1.
In this experiment, the appropriate catalyst dosage (mass ratio of catalyst and reaction solution) was 1:500. When the H2O2 catalytic oxidation of waste sulfuric acid solution was carried out under the catalyst dosage over 1:500, eq 4 could be simplified as follows:
H2O2 dosage and oxidation rate
As already shown, K (the apparent reaction rate constant) could be described as in eq 7. Similarly, for a specific reaction temperature with over catalyst dosage, k was defined as , and eq 7 could be simplified as follows:
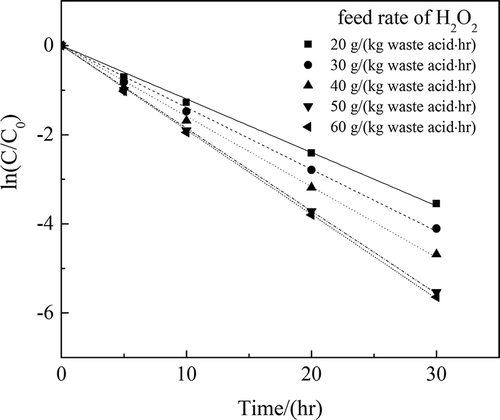
The H2O2 catalytic oxidation was performed under the reaction temperature of 70°C, with catalyst dosage of 1:500, and the feed rate of H2O2 was 20, 30, 40, 50, and 60 g·(kg waste acid)−1 per hour. For the safety of H2O2 oxidation reaction, H2O2 was added dropwise and the feed rate of H2O2 was controlled by the funnel stopcock. When the reaction finished, the amount of H2O2 used was measured according to the total and remaining volume in the funnel. The initial H2O2 concentration (mg/L) was calculated with the weight of H2O2 used and the volume of waste acid solution.
The experimental data were used for curve fitting between and time t. The results are shown in . The correlation coefficients of the fitting curves (R2) were higher than 0.99.
The reaction rate increased constantly as the H2O2 dosage increased, as shown in . When the feed rate of H2O2 was more than 50 g·(kg waste acid)−1 per hour, the reaction rate increased much more slowly and tended to be stable. K values at different feed rates of H2O2 are shown in and . As shown in to and , results indicated that the appropriate feed rate of H2O2 was 50 g·(kg waste acid)−1 per hour.
Table 4. K values at different rates of addition of H2O2.
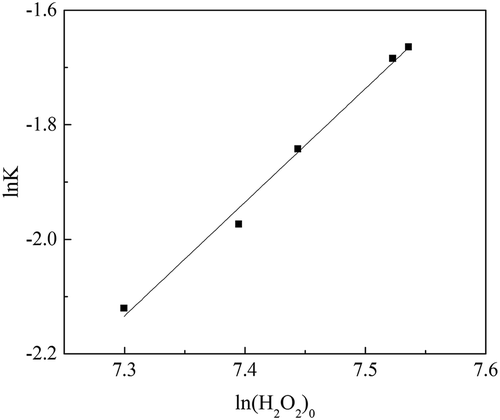
Taking the natural logarithms of both sides of eq 8, the equation became
The data in were used for curve fitting between and
. The curve is shown in . The correlation coefficient of the fitting curve (R2) was greater than 0.99.
According to the fitting curve shown in , results show that n was 2 and ln k was –16.63. Equation 2 was expressed as the following when catalyst dosage was over 1:500:
Reaction temperature and oxidation rate
Taking the natural logarithm of , the equation became the following:
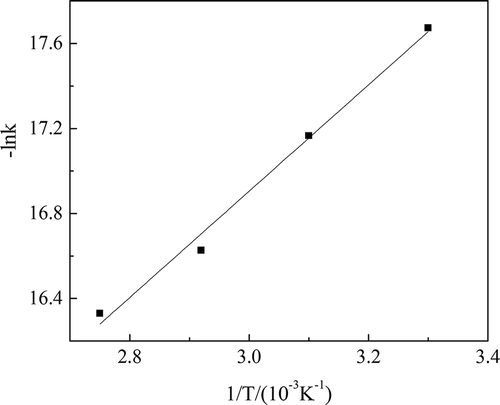
The H2O2 catalytic oxidation took place at a catalyst dosage of 1:500, feed rate of H2O2 of 50 g·(kg waste acid)−1 per hour, and reaction temperatures of 30, 50, 70, and 90°C. According to the experimental data and eq 9, ln k values at different reaction temperatures were calculated and were shown in .
Table 5. Ln k values at different reaction temperatures.
The data in were used for curve fitting between –lnk and 1/T. The curve is shown in . The correlation coefficient of the fitting curve (R2) was over 0.99.
Reaction temperature had an important influence on H2O2 oxidation rate, as shown in and . The apparent reaction rate constant and ln k values increased as the reaction temperature rose. Meanwhile, the reaction time was shortened. However, more energy was consumed to keep a higher reaction temperature. As mentioned in the preceding, the reaction temperature should be controlled under 100°C. Thus, the appropriate reaction temperature was 90°C.
The slope and intercept of the fitting curve are known and are given in . The values of activation energy Ea and frequency factor k0 could be calculated. Ea was 20,801 J/mol and k0 was 8.28 × 10−5. Substituting the values of Ea and k0 into eq 10, the rate model of H2O2 catalytic oxidation could be described by the following equation when catalyst dosage was equal to or greater than 1:500:
Equation 12 then could be simplified further as follows:
H2O2 catalytic oxidation
According to the results of the experiments, the optimized operation parameters were reaction temperature of 90°C, mixer speed of 120 rpm, H2O2 feeding rate of 50 g·(kg waste acid)−1 per hour, and catalyst dosage (mass ratio of catalyst and reaction solution) of 1:500.
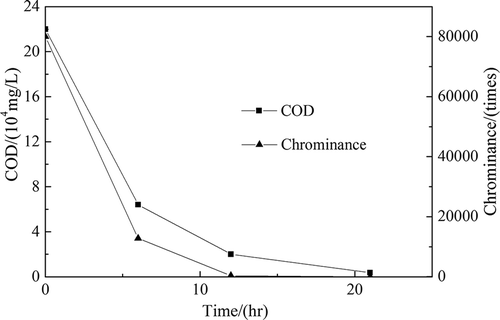
A 100-mL waste sulfuric acid solution was fed in the 500-mL three-neck flask. The H2O2 catalytic oxidation was carried out under the optimized operation parameters until the reaction solution was colorless. The oxidation reaction results are shown in .
During the H2O2 catalytic oxidation, the color of the waste acid solution changed rapidly. The color changed from black to purple, orange, yellow, and finally to light yellow or clear. When the reaction was finished, the COD value in the waste acid solution had decreased from 2.2 × 105 mg/L to 1000 mg/L and the removal efficiency of COD was 99.55%. The chrominance of waste acid solution decreased from 80,000 times to 8 times. The removal efficiency of chrominance was 99.98%. The theoretical and actual dosages of H2O2 were 0.93 and 1.05 g·(g waste acid)−1 as calculated. The utilization ratio of H2O2 was 88.57%.
The content of sulfuric acid in recovered acid was 70 wt%, the density of recovered acid was 1.256 kg/L, and the total volume of recovered acid solution was 160 mL, due to water dilution from H2O2 itself (H2O2 content of 30 wt%) and formation in the oxidation reaction. The recovery rate of sulfuric acid was 95%.
In the experiments, the cyclic utilization of the catalyst was also evaluated. The effects of treating waste sulfuric acid were the same when new and cyclic catalysts were used, respectively, which meant catalyst could be reused. The wear on the catalyst was assessed by gravimetric analysis when oxidation was finished. The wear on the catalyst in every process was 5 wt% of the initial weight of catalyst after several reuses.
Conclusions
In this study, reaction temperature, feed rate of H2O2, and catalyst dosage were investigated during H2O2 catalytic oxidation of waste sulfuric acid while activated carbon was selected as catalyst. The kinetic model for H2O2 catalytic oxidation of waste sulfuric acid could be described as follows when catalyst dosage was equal or great than 1:500:
The optimized operation parameters for H2O2 catalytic oxidation on organic impurities in waste sulfuric acid were the temperature of 90°C, mixer speed of 120 rpm, feed rate of H2O2 of 50 g·(kg waste acid)−1 per hour, and catalyst dosage (mass ratio of catalyst and reaction solution) of 1:500.
The performance of H2O2 catalytic oxidation on waste sulfuric acid was satisfactory. The removal efficiencies of COD and chrominance were both more than 99% and the utilization ratio of H2O2 was 88.57%. The content of sulfuric acid in recovered acids was 70 wt% and the impurity content in recovered acids was less than 0.05 wt%. The recovery ratio of sulfuric acid was 95%. The recovered acids could be reused in the original process directly or after distillation.
This process of H2O2 catalytic oxidation could be used to eliminate organic impurities in waste sulfuric acid and to recover sulfuric acid. It was economical and feasible for most small and medium-sized waste sulfuric acid recovery factories.
Funding
This research was supported by the Chinese National Science and Technology Support Program (number 2014BAC03B00).
Additional information
Funding
Notes on contributors
Jiade Wang
Jiade Wang is a professor in the College of Environment, Zhejiang University of Technology.
Binxun Hong
Binxun Hong is a master’s student in the College of Environment, Zhejiang University of Technology, currently working with the recovery of waste sulfuric acid.
Xinyang Tong
Xinyang Tong and Shufeng Qiu are engineers with Hangzhou Zhonghao Technology Company Limited.
References
- Bodenbenner, K., G. Muller, and H. Muller. 1979. Process for regeneration of sulfuric acid. U.S. Patent US4157381, issued June 5, 1979. doi: 10.1126/science.482931
- Cruz, P., Y. Perez, I.D. Hierro, and M. Fajardo. 2016. Copper, copper oxide nanoparticles and copper complexes supported on mesoporous SBA-15 as catalysts in the selective oxidation of benzyl alcohol in aqueous phase. Microporous Mesoporous Mater. 220:136–47. doi:10.1016/j.micromeso.2015.08.029
- Feng, X., L.Y. Jiang, and Y. Song. 2016. Titanium white sulfuric acid concentration by direct contact membrane distillation. Chem. Eng. J. 285:101–11. doi:10.1016/j.cej.2015.09.064
- Goi, A., M. Trapido. 2002. Hydrogen peroxide photolysis, Fenton reagent and photo-Fenton for the degradation of nitrophenols: A comparative study. Chemosphere 46:913–22. doi:10.1016/S0045-6535(01)00203-X
- Guo, C.T., and B. Chang. 1982. Recover method of waste sulfuric acid containing organic impurities. Dyestuffs Colorat. 4:50–55.
- Hammedi, T., M. Triki, Z. Ksibi, A. Ghorbel, and F. Medina. 2015. Catalytic wet hydrogen peroxide oxidation of p-hydroxybenzoic acid over Fe/TiO2 and 0.5Ru-3Fe/TiO2. J. Sol-Gel Sci. Technol. 76(3):679–85. doi:10.1007/s10971-015-3820-3
- Jimenez Jado, N.E., C. Fernandez Sanchez, and J.R. Ochoa Gomez. 2004. Electrochemical degradation of nitroaromatic wastes in sulfuric acid solutions: Part I. J. Appl. Electrochem. 34(5):551–56. doi:10.1023/B:JACH.0000021833.92966.9f
- Kesieme, U.K., H. Aral, M. Duke, N. Milne, and C.Y. Cheng. 2013. Recovery of sulphuric acid from waste and process solutions using solvent extraction. Hydrometallurgy 138:14–20. doi:10.1016/j.hydromet.2013.06.005
- Li, M.X. 2005. Recover research of waste acid from crude benzol refining. Master’s thesis, Shandong University, Shandong, China.
- Lin, S.H., C.M. Lin, and H.C. Leu. 1999. Operating characteristics and kinetic studies of surfactant wastewater treatment by fenton oxidation. Water Res. 33(7):1735–41. doi:10.1016/S0043-1354(98)00403-5
- Lin, S.S., and M.D. Gurol. 1998. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 32(10):1417–23. doi:10.1021/es970648k
- Marti-Calatayud, M.C., D.C. Buzzi, M. Garcia-Gabaldon, E. Ortega, A.M. Bernardes, J.A.S. Tenorio, and V. Perez-Herranz. 2014. Sulfuric acid recovery from acid mine drainage by means of electrodialysis. Desalination 343(SI):120–27. doi:10.1016/j.desal.2013.11.031
- Moreno-Castilla, C., M.V. Lopez-Ramon, and F. Carrasco-Marin. 2000. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 38(14):1995–2001. doi:10.1016/S0008-6223(00)00048-8
- Morgenthaler, J.H. 1983. Process for the regeneration of spent sulfuric acid. U.S. Patent US4376107, issued August 3, 1983.
- Park, S.H., J.H. Jung, K.W. Song, K.S. Kshetrimayum, C.H. Jeong, and C.H. Han. 2014. Systematic regeneration of waste sulfuric acid in semiconductor manufacturing using batch vacuum distillation. Ind. Eng. Chem. Res. 53(20):8543–52. doi:10.1021/ie500016v
- Rey, A., A.B. Hungria, C.J. Duran-Valle, M. Faraldos, A. Bahamonde, J.A. Casas, and J.J. Rodriguez. 2016. On the optimization of activated carbon-supported iron catalysts in catalytic wet peroxide oxidation process. Appl. Catal. B 181:249–59. doi:10.1016/j.apcatb.2015.07.051
- Song, K., Q.Q. Meng, F. Shu, and Z.F. Ye. 2013. Recovery of high purity sulfuric acid from the waste acid in toluene nitration process by rectification. Chemosphere 90:1158–62. doi:10.1016/j.chemosphere.2012.09.043
- Thang, V.H., W. Koschuh, K.D. Kulbe, and S. Novalin. 2005. Detailed investigation of an electrodialytic process during the separation of lactic acid from a complex mixture. J. Membr. Sci. 249(1–2):173–82. doi:10.1016/j.memsci.2004.08.033
- Wang, Y.M., H.Z. Wei, P.J. Liu, Y. Yu, Y. Zhao, X.N. Li, W.T. Jiang, J.H. Wang, X. Yang, and C.L. Sun. 2015. Effect of structural defects on activated carbon catalysts in catalytic wet peroxide oxidation of m-cresol. Catal. Today 258(1):120–31. doi:10.1016/j.cattod.2015.04.016
- Wei, C., X.B. Li, Z.G. Deng, G. Fan, M.T. Li, and C.X. Li. 2010. Recovery of H2SO4 from an acid leach solution by diffusion dialysis. J. Hazard. Mater. 176(1–3):226–30. doi:10.1016/j.jhazmat.2009.11.017
- Wu, D.L., D. Duan, and L.M. Ma. 2010. Fenton-like oxidation of refractory organic contaminants in wastewater using pyrite cinder at neutral pH. J. Chem. Ind. Eng. 4:1001–8.
- Xu, J., D. Fu, and S.G. Lu. 2009. The recovery of sulphuric acid from the waste anodic aluminum oxidation solution by diffusion dialysis. Sep. Purif. Technol. 69(2):168–73. doi:10.1016/j.seppur.2009.07.015
- Yang, D.W. 1985. Purification and recovery of waste sulfuric acid in petrochemical industry. Environ. Protect. Chem. Ind. 5:78–84.
- Yang, R.C., and S.T. Zhou. 1999. Catalytic oxidation of waste sulfuric acid in organic chemical industry. Nat. Sci. J. Xiangtan Univ. 21(4):74–77.
- Zhang, R. 2015. Waste sulfuric acid drained away one hundred million tons per year. China Environment News, September 10. http://news.cenews.com.cn/html/2015-09/10/content_33660.htm (accessed September 10, 2015).