ABSTRACT
Pollution prevention (P2) assessment was conducted by applying the three R’s, reduce, reuse, and recycle, in a chemical industry for the purpose of reducing the amount of wastewater generated, reusing paint wastewater in the manufacture of cement bricks, recycling cooling water, and improving water usage efficiency. The results of this study showed that the annual wastewater flow generated from the paint manufacturing can be reduced from 1,100 m3 to 488.4 m3 (44.4% reduction) when a high-pressure hose is used. Two mixtures were prepared. The first mixture (A) contains cement, coarse aggregate, fine aggregate, Addicrete BVF, and clean water. The second mixture (B) contains the same components used in the first mixture, except that paint wastewater was used instead of the clean water. The prepared samples were tested for water absorption, toxicity, reactivity, compressive strength, ignitability, and corrosion. The tests results indicated that using paint wastewater in the manufacture of the cement bricks improved the mechanical properties of the bricks. The toxicity test results showed that the metals concentration in the bricks did not exceed the U.S. EPA limits. This company achieved the goal of zero liquid discharge (ZLD), especially after recycling 2,800 m3 of cooling water. The total annual saving could reach $42,570 with a payback period of 41 days.
Implications: This research focused on improving the water usage efficiency, reducing the quantity of wastewater generated, and potentially reusing wastewater in the manufacture of cement bricks. Reusing paint wastewater in the manufacture of the bricks prevents the hazardous pollutants in the wastewater (calcium carbonate, styrene acrylic resins, colored pigments, and titanium dioxide) from entering and polluting the surface water and the environment. We think that this paper will help to find the most efficient and cost-effective way to manage paint wastewater and conserve fresh water resources. We also believe that this paper provides a rich agenda for future research in water conservation and industrial wastewater reuse subjects.
Introduction
The chemical sector represents one of the highly polluting industrial sectors (Gunningham, Citation1998). In the developing countries, industry officials prefer following an end-of-pipe approach rather than considering internalizing amendments to production processes. Several reasons exist for eliminating or reducing the volume of waste, such as increasing cost of end-of-pipe treatment, current legislation, and public pressure (Phillips, Citation2000; García et al., Citation2004; Makwara and Snodia, Citation2013). Recycling and waste prevention represent the best options for preventing pollution caused by this sector (Guyer, Citation1998). The paint industry is one of the most polluting industries in the chemical sector, and it generates large quantities of wastewater during cleaning processes (Ramezani, Citation2015). Paint wastewater is characterized by high concentrations of styrene acrylic resins, chemical oxygen demand (COD), biological oxygen demand (BOD), calcium carbonate, titanium dioxide, suspended solids, and colored materials (El-Shazly et al., Citation2010).
Reducing the volume of the waste produced at the source represents the best way to manage waste (Phiri et al., Citation2012). Pollution prevention (P2) assessment was carried out in one of the chemical industries located in Egypt. P2 is a crucial and positive step aimed at eliminating, avoiding, or reducing the generation of wastes before treatment, recycling, or disposal (Şchiopu et al., Citation2007). Many environmental, health, and economic benefits result from conducting P2 projects. These benefits include increasing public health, reducing regulatory compliance issues, reducing liability, improving employee participation and morale, reducing operating costs, reducing natural resources depletion, and enhancing the company image (Spearing et al., Citation2001). Pollution prevention and wastewater management are directly related to water reuse and conservation. The main purpose of a P2 project involves reducing overall production costs such as expenses for waste treatment and storage, energy, raw materials, management overhead, transportation and disposal, emergency response, and training (Mostafa, Citation2014). Overall production costs can also be reduced by increasing the efficiency of inventory control, production scheduling, material handling, and equipment maintenance (U.S. Environmental Protection Agency [EPA], Citation1995; Wahaab, Citation2010).
During the on-site P2 assessment, the assessment team found that the main products of the targeted company are epoxy and paints materials. Approximately 1,100 m3 of wastewater is generated annually from paint manufacturing, and about 2,800 m3 of wastewater is produced annually during the manufacture of epoxy materials (Mostafa, Citation2014). This facility does not provide a high level of treatment, and the effluent is normally transferred to the nearest industrial wastewater treatment plant by government vacuum trucks. This research aims to reduce the water pollution caused by the chemical sector by improving the water usage efficiency, reducing the quantity of wastewater generated, and potentially reusing wastewater generated by the paint industry in the manufacture of cement bricks.
Materials and methods
Samples were collected from the effluent of the plant in polyethylene containers (10 L capacity). Wastewater samples were placed in an ice chest for transport to the laboratory. The collected samples were analyzed for BOD, COD, total suspended solids (TSS), pH, and total dissolved solids (TDS), according to the Standard Methods for Wastewater Analysis (Andrew et al., Citation2005). TDS and pH were measured in the field by using the HM digital TDS meter and WTW multi 340i meter, respectively. Analysis of TSS, BOD, and COD took place in the Housing and Building National Research Center (HBRC) and American University laboratories, where both laboratories were awarded an ISO certificate for quality assurance and management. The 5-day BOD Test 5210B enabled determination of the BOD concentration in the samples, while the closed reflux, titrimetric method 5220C was used for COD determination. Test method 2540D enabled determination of the TSS concentration in the samples. The results were compared with the limits specified in Egyptian law 44/2000.
An important observation made during the site visit was that the total amount of wastewater generated from the paint industrial firm can be significantly reduced by reducing the amount of water used for washing processes. Another important issue observed was that the total amount of wastewater produced in the manufacture of epoxy materials can be significantly reduced through the recycling of cooling water. The effluent from the paint manufacturing can also be reduced by reusing wastewater in the manufacture of cement bricks. Two mixtures were prepared. The first mixture (A) contains cement, coarse aggregate, fine aggregate, Addicrete BVF, and clean water. Addicrete BVF is a liquid material that increases the mixture strength by reducing the water consumption. The second mixture (B) contains the same components used in the first mixture, except that paint wastewater was used instead of clean water. The paint wastewater contains contaminants such as calcium carbonate, colored pigments, titanium dioxide, and styrene acrylic resins (El-Shazly et al., Citation2010). Styrene acrylic resin is considered one of the water-soluble binders. Thus, it can increase the adhesion strength between the mixture components. A concrete mixer was used to homogeneously combine fine aggregate, Portland cement, Addicrete BVF, coarse aggregate, and water or paint wastewater. The mix proportions of the two mixtures are recorded in Coarse aggregate, fine aggregate, and cement were batched by weight, while paint wastewater and water were batched by volume. The cement bricks were produced with dimensions 25 ± 0.5 cm × 12 ± 0.5 cm × 6 ± 0.5 cm using a mobile “egg-laying” machine.
Table 1. Mix proportions of the cement bricks where brick is 25 ± 0.5 cm × 12 ± 0.5 cm × 6 ± 0.5 cm.
The prepared samples were tested for water absorption, reactivity, compressive strength, ignitability, and corrosion. The toxic characteristic leaching procedure test (TCLP) was also conducted to ensure that the concentrations of the following metals and compounds in the solutions did not exceed EPA TCLP limits: arsenic (As), cadmium (Cd), chromium hexavalent (Cr+6), chromium Cr (total), copper (Cu), mercury (Hg), lead (Pb), zinc (Zn), iron (Fe), nickel (Ni), chloroform (CHCl3), chlorobenzene, 1,4-dichlorobenzene, methyl ethyl ketone. These tests were conducted at the Housing and Building National Research Center (HBRC) materials laboratory, located in Cairo, Egypt, awarded an ISO certificate for quality assurance and management. The TCLP test was conducted using U.S. EPA test method 1311 (EPA, Citation1992). The Corrosivity Towards Steel Method was used to determine the corrosivity of the aqueous solution leached from the bricks (EPA, Citation1992). The Pensky–Martens Closed-Cup Method was used to determine the flash point of the bricks (American Society for Testing and Material [ASTM], Citation2003). Using EPA methods 9014 and 9010C enabled determination of cyanides concentration in the aqueous solution (EPA, Citation1996; EPA, Citation2004). The concentration of sulfide in the aqueous solution was determined using EPA method 376.1 (EPA, Citation1978). Three samples from each mixture were tested after 28 days of curing time for compressive strength. Mostafa and Peters (Citation2016) defined the compressive strength as “the ability of a material to resist compressive loads without the occurrence of any collapse” (Mostafa and Peters Citation2016, p. 3). The dimensions of the brick samples are 25 cm length × 12 cm width × 6 cm height. The weight and size of each brick were measured accurately to avoid any error in performing the tests. Using ASTM C 55-06 and Egyptian Code of Practice (ECOP) standards enabled determination of the compressive strength (ASTM, Citation2006a; ECOP, Citation2005). The brick samples were tested for compressive strength using a compression tester machine. Three samples from each mixture were also tested for water absorption. Mostafa and Peters (Citation2016) defined the water absorption as a “test that helps predict the brick performance and durability. Absorption capacity is the weight of water absorbed divided by the dry weight of the sample” (Mostafa and Peters, Citation2016, p. 6). The dimensions of the brick samples are 25 cm length × 12 cm width × 6 cm height. Using ASTM C 140-06 enabled determination of the water absorption (ASTM, Citation2006b).
Using a paired-samples t-test enabled determination of the difference between the means of the brick samples made with paint wastewater and clean water. The null hypothesis (H0) is an assumption of no differences between the means of the two samples. The alternate hypothesis (H1) refers to a differences between the means of the two samples.
Results and discussion
Paint wastewater analysis
Analysis of wastewater samples collected from the effluent of the plant showed that the concentrations of BOD, TDS, TSS, and COD exceeded the permissible limits specified in Egyptian law no 44/2000 (see ). Therefore, this kind of waste must be handled in an efficient manner, and sufficient treatment must be applied before the waste is discharged to surface waterways.
Table 2. Physicochemical characterization of industry effluent.
This company has 10 mixing tanks and a low-pressure hose was used for washing and cleaning purposes. A comparison was conducted between the low- and high-pressure hoses in terms of water consumption. The lowest water consumption (0.89 m3/tank) was observed when using a high-pressure hose and the highest water consumption (2.0 m3/tank) was observed when using a low-pressure hose. The water consumption decreased by about 44.40% with the use of a high-pressure hose instead of a low-pressure hose in the washing process, due to decreasing the cleaning time. The preliminary results showed that the annual wastewater flow can be reduced from 1,100 m3 to 488.4 m3 when a high-pressure hose is used. The use of such a hose improves the rinse efficiency by reducing the amount of water needed for the washing process, in addition to reducing the amount of wastewater generated.
This company has four epoxy mills that require about 2,800 m3 of water every year for cooling purposes. The amount of water needed to operate the mills is withdrawn from an underground storage tank. A closed-loop system has been implemented to enable recycling of cooling water. The hot water is transferred from the mills to an underground storage tank by means of underground pipes. The capacity of the storage tank is approximately 700 m3, and the amount of returned hot water is about 10.5 m3/day. Consequently, there is no need to install a cooling system to cool the hot water. Recycling cooling water has resulted in a reduction of about 2,800 m3 of water per year that was previously discharged to treatment facility. The remaining wastewater was used in the manufacture of the cement bricks.
Tests for bricks
Toxicity Characteristic Leaching Procedure (TCLP) test
The TCLP test was conducted in one brick from each mixture; the test results are presented in . The results indicated that the concentrations of Zn and Cr (total) were lower than the permissible limits specified in U.S. EPA standards. No hazardous components were reported in the brick made of paint wastewater. Consequently, the broken bricks can be reused or dumped into regular landfills for nonhazardous wastes without causing any environmental hazards.
Table 3. TCLP test results.
Compressive strength
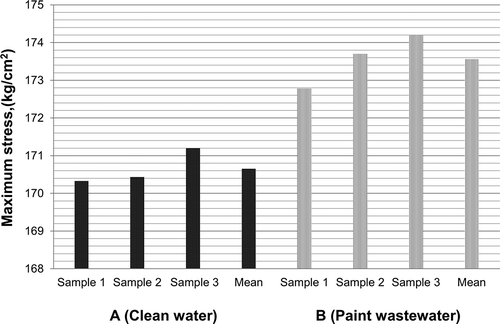
Three bricks from each mixture were tested for compressive strength; the test results are presented in . ASTM C55-06 and the Egyptian Code of Practice 204-2005 for a concrete masonry unit stated that the average compressive strength of a concrete masonry unit must exceed 140 kg/cm2 and 80 kg/cm2, respectively (ASTM, Citation2006a; ECOP, Citation2005). As presented in , the average compressive strength for bricks from mixture A and mixture B was 170.65 kg/cm2 and 173.56 kg/cm2, respectively. These values clearly exceeded the permissible limits specified in ASTM C55-06 and the Egyptian Code of Practice 204-2005. The results also showed that the bricks from mixture A containing clean water showed compressive strength values lower than those bricks from mixture B containing paint wastewater (). Consequently, replacing clean water with paint wastewater will increase the compressive strength due to increasing the adhesion and cohesion strength between the bricks components.
Table 4. Flash point, corrosivity, reactive cyanides, and sulfide tests results.
Flash point and corrosivity tests
The EPA regulations state that the flash point of a concrete masonry unit must exceed 60°C (ASTM, Citation2003). As presented in , the flash point of the brick from mixture A and from mixture B was 97°C and 100°C. According to the ignitability characteristic, these bricks were considered to be nonflammable.
The corrosivity test was conducted in one brick from each mixture; the test results are presented in . According to EPA standards, an aqueous waste is considered corrosive if pH is below 2.0 or above 12.50. As shown in , the aqueous solutions extracted from bricks samples made of paint wastewater and from those made of clean water have a pH of 10.0 and 12.04, respectively. According to the corrosivity characteristic, these bricks were classified as noncorrosive.
Reactive cyanides and sulfide tests
The reactive sulfide and cyanides tests were conducted in one brick from each mixture; the tests results are presented in . The results indicated that the concentrations of sulfide and cyanides in the aqueous solutions were lower than the permissible limits specified U.S. EPA standards (EPA, Citation1992). No hazardous components were reported in the brick made with paint wastewater.
Water absorption
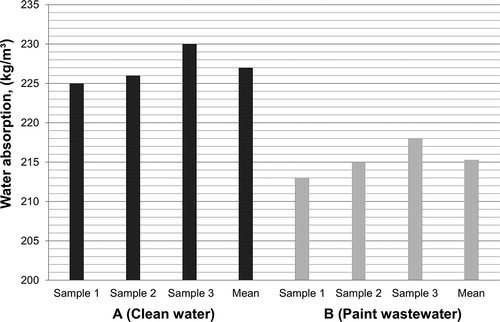
Three bricks from each mixture were tested for water absorption; the test results are presented in .ASTM C140-06 for a concrete masonry unit states that the average water absorption of a concrete masonry unit must not exceed 240 kg/m3 (ASTM, Citation2006b). As presented in , the average water absorption for bricks from mixture A and mixture B were 227.0 kg/m3 and 215.30 kg/m3, respectively. These values did not exceed the permissible limits specified in ASTM C140-06. The results also showed that the bricks from mixture A containing clean water showed water absorption values higher than those bricks from mixture B containing paint wastewater. Consequently, replacing clean water with paint wastewater will decrease the water absorption due to increasing the adhesion and cohesion strength between the bricks components.
A paired-samples t-test for compressive strength and water absorption tests
The t-test was performed for compressive strength and water absorption tests. For both tests, the results showed that there are a significant difference between the brick samples made with paint wastewater and clean water. For compressive strength test, the t-statistic (12.05) was greater than the t-critical (+4.302), and the p value (0.0068) was less than 0.05. For water absorption test, the t-statistic (–35) was smaller than the t-critical (-4.302), and the p-value (0.000815) was significantly less than 0.05. In these two cases, the t-statistic falls in the rejection region, so the null hypothesis (H0) was rejected in favor of the alternative hypothesis (H1). Consequently, replacing clean water with paint wastewater in the manufacture of the cement bricks will increase adhesion and cohesion strength between the bricks components, thus increasing compressive strength and reducing the water absorption.
Economical study
This industry has eliminated the treatment and disposal costs by (1) recycling cooling water, (2) reusing paint wastewater in the manufacture of the bricks, and (3) using a high-pressure pump for the washing purposes. Wastewater treatment and disposal costs are approximately $10.5 per cubic meter. Epoxy mills need about 2,800 m3 of water a year for cooling purposes. The cost of water per cubic meter was $0.414. This company could save up to $30,748 a year by recycling cooling water. The total annual wastewater generated from this industry is about 1,100 m3, and the annual treatment and disposal cost was estimated at about $11,623. Employing a high-pressure pump for the washing process can reduce the annual wastewater flow to 488.4 m3 and thus save about $6,462/year. The company’s average annual production rate of bricks (solid and hollow) is roughly 2.1 million bricks and consumes about 584 m3 of water. As a result, this company could annually save nearly $5,160 by reusing the remainder of the wastewater (488.40 m3) for the manufacture of the cement bricks. The cost of water per cubic meter was $0.414. This company can also save approximately $202.5/year by reducing clean water consumption. The manufacture units for the bricks are 30 m away from the wastewater settling tank. The wastewater is normally transferred from the settling tank to the manufacture units through an underground sewer pipe. shows the supplies needed and the total costs of modifications. The payback period is computed by dividing the total costs of modifications by the annual savings. The payback period calculations are shown in . The total annual savings could reach $42,570, and the payback period would only be 41 days. Applying the three R’s in the paint industry sector in Egypt is expected to reduce the yearly wastewater going for treatment by about 200,000 m3.
Table 5. Supplies needed and payback period estimation.
Environmental benefits
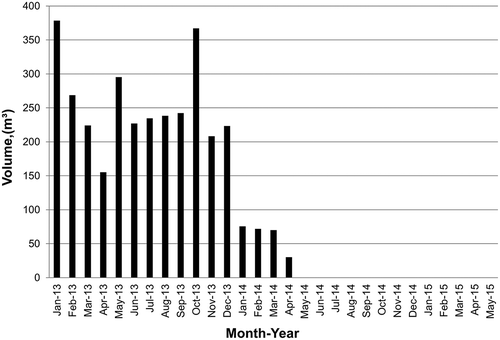
Reusing paint wastewater in the manufacture of the bricks prevents the hazardous pollutants in the wastewater (calcium carbonate, styrene acrylic resins, colored pigments, and titanium dioxide) from entering and polluting the surface water and the environment. Additional environmental benefits have been achieved, such as recycling cooling water and reducing the volume of wastewater generated during the washing process. The monthly volumes of wastewater going for treatment have significantly decreased from the beginning of January 2014 after the amendment processes were instituted, as shown in . This company achieved the goal of zero liquid discharge (ZLD) from the beginning of May 2014 after instituting the amendment processes and beginning to reuse wastewater in the manufacture of the cement bricks.
Conclusion
The preliminary results showed that the annual wastewater flow can be reduced from 1,100 m3 to 488.4 m3 when a high-pressure hose is used. In addition, the total amount of wastewater produced in the manufacture of epoxy materials can be significantly reduced through the recycling of cooling water. The compressive strength and water absorption tests results indicated that using paint wastewater in the manufacture of the cement bricks has improved the mechanical properties of the bricks. The TCLP test results showed that the metals concentration in the bricks did not exceed the U.S. EPA limits. Wastewater generated from the paint industry can also be used in many production applications, such as roof tiles, pavers, concrete retaining wall units, concrete blocks, and masonry mortar. The other benefits of reusing the paint wastewater include reducing clean water consumption, eliminating disposal and treatment costs, and reducing environmental pollution.
Acknowledgment
The authors thank Khaled El-Sayed for standing behind this research and supporting them with the necessary resources. The authors also thank the Egyptian Housing Building Research Center and the American University laboratory for their help in performing tests.
Funding
This research was supported by the Department of Civil, Construction, and Environmental Engineering at the University of Alabama at Birmingham and National Science Foundation under NSF grant number 1104027.
Additional information
Notes on contributors
Mohamed K. Mostafa
Mohamed K. Mostafa is a doctor of environmental engineering in the Environmental Engineering Program, Zewail City of Science and Technology, Giza, Egypt.
Robert W. Peters
Robert W. Peters is a professor of environmental engineering in the Department of Civil, Construction and Environmental Engineering, University of Alabama at Birmingham, Birmingham, AL.
References
- American Society for Testing and Materials. 2003. Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM Volume 05.01 Petroleum Products, Liquid Fuels, and Lubricants (I): C1234 D3710. West Conshohocken, PA: ASTM International.
- American Society for Testing and Materials. 2006a. Standard Specification for Concrete Building Brick. ASTM C 55-06. Masonry Standards for the Building Industry. West Conshohocken, PA: ASTM International.
- American Society for Testing and Materials. 2006b. Standard Specification for Concrete Building Brick. ASTM C 140-06, Masonry Standards for the Building Industry. West Conshohocken, PA: ASTM International.
- Andrew, D.E., S.C. Lenore, W.R. Eugene, and E.G. Arnold. 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. Washington, DC: American Public Health Association.
- Egyptian Code of Practice. 2005. Cairo, Egypt: Ministry of Housing and Urban Communities.
- El-Shazly, M.A., A.H. Ezzat, and K.M. Kamel. 2010. Appropriate technology for industrial wastewater treatment of paint industry. Am.-Eurasian J. Agric. Environ. Sci. 8(5):597–601.
- García, V., E. Pongrácz, and R. Keiski. 2004. Waste minimization in the chemical industry: From theory to practice. Proc. Waste Minimization and Resources Use Optimization Conference, pp. 93–106.
- Gunningham, N. 1998. Environmental management systems and community participation: Rethinking chemical industry regulation. J. Environ. Law Policy 16(2):319–439.
- Guyer, H.H. 1998. Industrial Processes and Waste Stream Management. New York, NY: John Wiley & Sons.
- Makwara, E.C., and M. Snodia. 2013. Confronting the reckless gambling with people’s health and lives: Urban solid waste management in Zimbabwe. Eur. J. Sustain. Dev. 2(1):67–98.
- Mostafa, M. 2014. Modeling of Pollutant Transport in the Nile Delta Egypt. Ph.D. dissertation, University of Alabama at Birmingham, Birmingham, AL.
- Mostafa, M.K., and R.W. Peters. 2016. Reuse Paint Wastewater in the Manufacture of Cement Bricks and Tiles. doi:10.1007/s10163-016-0485-0, 1–11.
- Phillips, P.S. 2000. Guide for waste minimization. In Lecture Material Presented on the GSCE Industrial Ecology Seminar, pp. 22–26. University College Northampton, UK.
- Ramezani, L. 2015. Waste minimization in solvent-based paint industries. Nova J. Eng. Appl. Sci. 4(1):1–5. doi: 10.20286/jeas.v4i1.32
- Phiri, A., L. Godfrey, and D.R. Snyman. 2012. Modeling the generation of domestic waste for supporting the planning of municipal waste services. Int. J. Water Resources Environ. Eng. 4(6):171–191. doi: 10.5897/IJWREE11.030
- Şchiopu, A., I. Apostol, M. Hodoreanu, and M. Gavrilescu 2007. Solid waste in Romania: Management, treatment, and pollution prevention practices. Environ. Eng. Manage. J. 6(5):451–465.
- Spearing, D.R., L.S. Gray, and L.P. Robert. 2001. Environmental Issues and Waste Management Technologies in the Ceramic and Nuclear Industries VI (Ceramic Transactions). Westerville, OH: American Ceramic Society, Westerville, OH, USA.
- U.S. Environmental Protection Agency. 1978. Sulfide (titrimetric, iodine). Method 376.1, Toxicity characteristic leaching procedure. http://www.caslab.com/EPA-Methods/PDF/EPA-Method-3761.pdf (accessed November 2, 2016).
- U.S. Environmental Protection Agency. 1992. Test methods for evaluationg solid waste: Physical/chemical methods (SW-846). Method 1311, Toxicity Characteristic Leaching Procedure. https:///www.epa.gov/sites/production/files/2015-12/documents/1311.pdf (accessed November 2, 2016).
- U.S. Environmental Protection Agency. 1995. Federal Facility Pollution Prevention Project Analysis: A Primer for Applying Life Cycle and Total Cost Assessment Concepts. Washington, DC: Office of Enforcement and Compliance Assurance Planning, Prevention, and Compliance Division, Federal Facilities Enforcement Office, U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 1996. Titrimetric and Manual Spectrophotometric Determinative Methods for Cyanide. Method 9014. Washington, DC: U.S. EPA.
- U.S. Environmental Protection Agency. 2004. Total and amenable cyanide: Distillation. Method 9010C. Washington, DC: U.S. EPA.
- Wahaab, A.B. 2010. Stress management among artisans in construction industry in Nigeria. Global J. Res. Eng. 10(1):93–103.