ABSTRACT
Response factors (RF) can be used to characterize relative sensitivity of one compound vs. another compound for the same measurement instrument. Use of RF allows the analysts/operators to calibrate the instrument with one compound and make measurement for a large number of compounds. This method is adopted for Flame Ionization Detector (FID) based survey instruments used in the Leak Detection and Repair (LDAR) practice for control of fugitive emissions of volatile organic compounds. Gas detecting Infrared (IR) cameras have been used for leak detection. However, the RF for IR cameras has not been well established despite some attempt to develop a method for IR camera RF. In addition to a method proposed earlier (Method 1), two new methods for IR camera RF are proposed in this paper: Method 2 based on theoretical approach and Method 3 based on experimental approach. All three methods are examined and compared. Both Methods 2 and 3 have shown the ability to characterize the behavior of RF for various compounds and substantially higher accuracy than Method 1. Method 2 provides a mechanism to generate RF for a large number of compounds without conducting experiments, and is recommended for implementation. The RF derived from this method can be used both in the emerging field of Quantitative Optical Gas Imaging (QOGI) and to answer the most common question that IR camera users ask—whether a particular compound can be imaged by a particular IR camera.Implications: Infrared imager is an efficient tool for detecting gas leaks from process equipment and has been used in leak detection and repair (LDAR) programs for control of fugitive emissions. However, the information regarding which chemical compounds can be imaged and how sensitive a given infrared imager is for various compounds is limited. A theoretical method is presented in this paper that can answer these questions without conducting resource-intensive experiment. The results of this theoretical method has good agreement with experimental data. The method has been used to predict relative sensitivity for 398 compounds.
Introduction
In the past decade, infrared (IR) imagers (also commonly called IR cameras) specially designed to image gas plumes have gained wide acceptance as a valuable visual tool for the detection of gas leaks from industrial process equipment (Environ, Citation2004; U.S. Environmental Protection Agency [EPA], Citation2008). This field is generally described as optical gas imaging (OGI) and to date has been primarily a qualitative technology. Recently a method has been developed to utilize these IR cameras to quantify the mass leak rate (e.g., g/hr) or volumetric leak rate (cc/min) (Zeng et al., Citation2015). This technology is an extension of OGI and is referred to as quantitative optical gas imaging (QOGI).
In applications for QOGI, it is desirable to use one compound to calibrate the instrument and use a response factor (RF) to quantify a different compound without having to calibrate the instrument with every compound encountered. The method of using a RF in leak detection and repair (LDAR) practice has been well established when other leak detection instruments such as the flame ionization detector (FID)-based leak survey instruments are used (EPA, Citation1995). However, the RF for FID-based instruments can vary from instrument to instrument and from concentration range to concentration range (EPA, Citation1995, Appendix D), and literature characterizing RF is very limited.
Due to the measurement principle of QOGI and availability of quantitative IR spectral libraries such as the IR library maintained by the Pacific Northwest National Laboratory (PNNL; Citation2015), it appears that the development of RF for QOGI can be more structured and the behavior of RF for QOGI can be better characterized, as compared with the RF for the FID-based method. It is the objective of this paper to explore methodologiesfor developing and characterizing RF for QOGI.
Methods and experimental setup
Three methods for developing RF for QOGI are proposed and discussed in this section. Two of them rely on calculations derived from the quantitative IR spectra for each compound and the design specificationsfor the IR camera. The third method is an experimental method, with RF derived from direct measurements.
Method 1 (effective α method)
The working principle of a gas imaging IR camera is that by using a narrow-bandpass filter in a high-sensitivity IR camera (usually cooled mid-wave IR [MWIR] or long-wave IR [LWIR] camera), the absorption or emission of IR energy by gas molecules between the background and the camera will be significant enough (compared with the background IR radiance) to generate some contrast in the image and therefore the gas plume can be recognized as a plume moving in the atmosphere. If the spectral window of the bandpass filter is too wide, the relatively weak effect of gas absorption or emission is “drowned” in the vast IR radiance that exists everywhere, and the IR camera will not be able to “see” the gas. Also, in order to image a gas, the gas must have an absorption peak or band that coincides with the spectral window of the bandpass filter of the IR camera. Based on these two factors, it is natural to use the “level of overlapping” between the camera bandpass filter spectral window and the IR absorption peak of the compound (gas or vapor) in question to characterize the strength of the IR signal for the gas. For the same IR camera, a compound with an IR spectral peak that has a larger overlap with the bandpass spectral window is expected to have a stronger signal than a compound with a smaller overlapped spectral peak. Mathematically, a RF can be derived using the following equation:
where RF is the response factor for compound T (fraction); αT(λ) is the IR absorption coefficient α of compound T as a function of wavelength λ (ppm−1 m−1); αR(λ) is the IR absorption coefficient α of reference compound R as a function of wavelength λ (ppm−1 m−1); t(λ) is the transmittance of IR camera bandpass filter as a function of λ (%); and λ is the wavelength of IR ray in question (μ).
Because Method 1 is based on the overlap between the IR absorption peak represented by absorption coefficient (commonly denoted as α) and the bandpass window represented by transmittance t, it is referred to as “effective α method.” This method has been used by Zeng et al. (Citation2007) and Footer (Citation2015).
Method 2 (RTE method)
This method is derived based on the radiative transfer theory (Sharkov, Citation2003). The IR gas imaging process can be characterized by the following simplified radiative transfer equation (RTE) at a given wavelength λ:
where IG is the IR energy intensity detected by the IR camera when there is a gas plume between the background and the IR camera (W·m−2·sr−1·Hz−1); ε(λ) is the emissivity of the background as a function of wavelength λ (fraction); B(TB,λ) is the Planck function, a function of λ and background temperature TB (W·m−2·sr−1·Hz−1); B(TG,λ) is the Planck function, a function of λ and gas temperature TG (W·m−2·sr−1·Hz−1); α(λ) is the absorption coefficient of the gas as a function of λ (ppm−1 m−1); C is the concentration of the gas (ppm); and L is the thickness of the gas plume (i.e., the optical path length of the gas; m).
The emissivity term ε(λ) is important in thermography because only the emissive component of the IR energy is predictably related to temperature and therefore of primary interest. In thermography applications, one has to isolate the emissive IR energy from the reflected IR energy to make accurate temperature measurements. In the case of gas imaging, both emissive and reflected energy contribute to the contrast in the image and there is no need to draw a distinction between the two. Therefore, the term ε(λ)B(TB,λ) can be considered as one term B(TB,λ), which could be called apparent radiance in reference to the term “apparent temperature” in the field of thermography. This can be viewed as ε(λ) = 1.
When there is no gas plume between the background and the IR camera, CL = 0 and eq 2 becomes eq 3 below:
where IB is the IR energy intensity detected by the IR camera when there is no gas plume between the background and the IR camera (W·m−2·sr−1·Hz−1).
The reason that the IR camera operator can see the gas plume is due to the difference between IG and IB, i.e., ΔI.
Combine eqs 4, 3, and 2 and rearrange, the following equation is obtained:
Equation 5 is very useful in analyzing a gas imaging application. When the gas temperature is the same as background temperature (TB = TG), B(TB,λ) = B(TG,λ) and ΔI = 0. No contrast is created by the presence of gas plume, and the gas plume cannot be imaged. When α(λ) = 0 (i.e., the gas molecule has no absorption peak at this wavelength), the term = 0, and again no gas image. Same is true when either C (gas concentration) or L (plume depth) equals zero.
The RF of a target compound T with respect to a reference compound R is a relative comparison of magnitude of ΔI of the two compounds integrated over the wavelength range within the spectral window of the camera bandpass filter, i.e.,
Notice that when eq 5 is inserted into eq 6, the term B(TB,λ) − B(TG,λ) for ΔIT and ΔIR is the same and therefore canceled out. The significance of eq 6 is that RF is not affected by gas temperature, background temperature, or the temperature difference of the two, ΔT (= TB − TG), which is generally the most important driving force in gas imaging for any given IR camera and any given gas (Zeng et al., Citation2015). Equation 6 also simplifies analysis of RF to four variables: type of gas (α), concentration (C), optical pathlength (L), and the transmittance curve of the IR camera bandpass filter [t(λ)]. The term t(λ) is fixed for a given IR camera design. The terms C and L are usually evaluated as one parameter, CL, in open air gas imaging because generally the optical pathlength (L) cannot be measured separately.
Method 3 (experimental method)
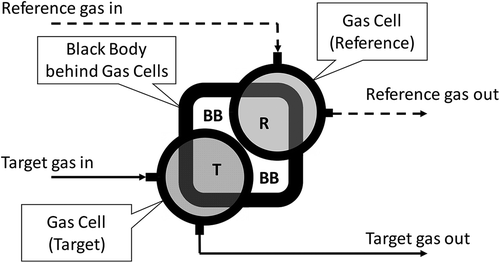
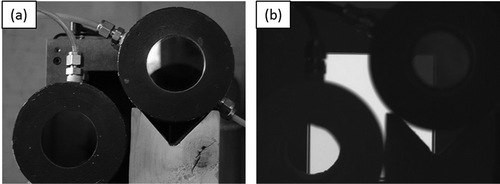
Method 3 is an experimental method. It determines RF experimentally using a laboratory experimental setup designed to eliminate external variables. The experimental setup is illustrated by . The actual experimental setup is shown in . The key components of the experimental setup are a black body and two gas cells. The black body used in this experiment is model DB-04 differential blackbody by Santa Barbara Infrared Inc. (Santa Barbara, CA). The two gas cells are constructed by the authors. They are cylindrical cells with an effective diameter of 5 cm (2 inches) and a length of 10 cm (0.10 m, or 4 inches). IR-transparent silica windows (diameter = 5 cm) are affixed to both ends of the cylindrical gas cells. The gas cells are equipped with fittings for connection to gas tubing. The gas cells are made air tight. One of the two gas cells is used for a reference gas, and the other is used for a target gas. In this RF study, propane is used as the reference gas, and methane and propylene are used as the target gases so that RF for methane and propylene with respect to propane can be determined. The reference gas and target gas flow through their respective gas cells. The gas cells are placed in front of the black body in such a way so that the IR camera (not shown in and ) can see the active portion of the black body surface through the gas cells (see ), as well as directly image the black body itself. The distance from the rear windows of the gas cells to the surface of the black body is 12 inches.
The IR camera used for this study is model GF300 camera made by FLIR Systems, Inc. (Wilsonville, OR), and the lens used is a 38 mm fixed focal length lens. The camera is positioned 10 feet away from the gas cells, and looking into the gas cells. The experimental setup shown in and is fully captured by the camera. For each test condition, the back body is set at a specific temperature; both reference and target gases are flowing through gas cells without back pressure; environmental temperature is recorded; and the image of gas cells and black body is captured by the FLIR GF300 camera. The raw 16-bit pixel intensity data for the image is captured. The ratio of average ΔI, as defined in eq 4, in the pixels representing the target gas cell (the area denoted by “T” in ) and that of the reference gas cell (the area denoted by “R” in ) is calculated, which represents the RF value. In addition, the windows of the gas cells are not 100% IR transparent, and the effect of the windows needs to be taken into account. A test is done with pure air flowing through both gas cells to determine the intensity difference caused by the windows of the gas cells. Such a “blank sample” intensity is subtracted from the intensities of the reference and target gas cells before the ratio (i.e., the RF value) is determined. Multiple series of tests have been conducted generating a multidimensional matrix of test conditions, including gases, concentrations, background temperatures, and ΔTs.
Results and discussions
Evaluation of the three methods
Seven hydrocarbon compounds are evaluated in this study using Methods 1 and 2. Three of them (propane, methane, and propylene) are also evaluated using the experimental method (Method 3). Using propane as the reference compound, RF normalized to propane is derived for the remaining six compounds. The results from Method 1 are presented in . Also included in for comparison are the RF values for five of the six compounds reported by Footer (Citation2015). The two sets of the results are essentially same. As indicated by eq 1, the RF determined by Method 1 is a static value for a given compound [α(λ)] and a given IR camera [t(λ)], and it does not change with concentration-pathlength product CL. As discussed later in this paper, the concentration-pathlength does have an effect on RF, which is not accounted for with Method 1.
Table 1. RFs determined by Method 1.
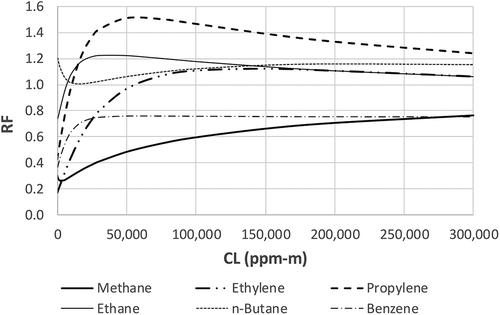
Results from Method 2 are summarized in –. As shown in , RF of all six compounds changes with CL. The changes are more drastic when CL is less than about 50,000 ppm-m. Once CL is greater than about 100,000 ppm-m, the RF values become more constant and they all tend to converge toward 1, but are not equal to 1 for these six compounds. These two aspects, i.e., RF varying with CL and the tendency to converge toward 1, represent the significant differences between RF determined by Method 1 and Method 2. Recall that according to Method 1, RF is static and does not change with CL.
Figure 4. RF of methane and propylene determined by Methods 1, 2, and 3. (a) methane, CL = 0–300,000 ppm-m; (b) methane, CL = 0–20,000 ppm-m; (c) propylene, CL = 0–300,000 ppm-m; (d) propylene, CL = 0–20,000 ppm-m.
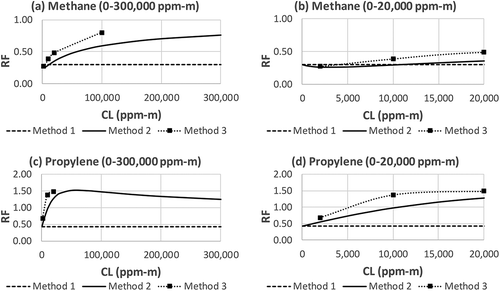
Figure 5. RF of ethylene, ethane, n-butane, and benzene determined by Methods 1 and 2. (a) ethylene, CL = 0–300,000 ppm-m; (b) ethylene, CL = 0–20,000 ppm-m; (c) ethane, CL = 0–300,000 ppm-m; (d) ethane, CL = 0–20,000 ppm-m; (e) n-butane, CL = 0–300,000 ppm-m; (f) n-butane, CL = 0–20,000 ppm-m; (g) benzene, CL = 0–300,000 ppm-m; (h) benzene, CL = 0–20,000 ppm-m.
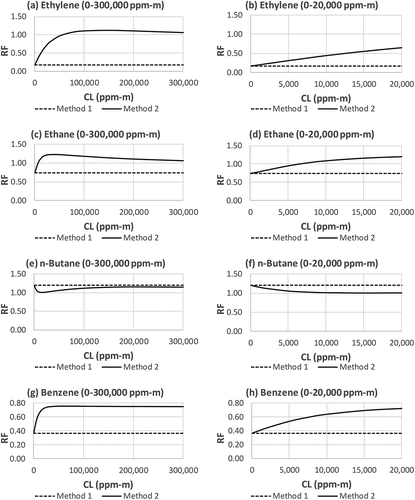
and provide more details of the difference between Method 1 and Method 2 by showing the results of the two methods compound by compound, and for a large CL range (the left chart) and a “zoomed-in” small CL range (the right chart). and show that RF varies with CL and that the RF values predicted by Method 2 are generally much closer to 1 than the RF values determined by Method 1, especially as CL increases. In the very low CL region, the two methods converge. This can be proven mathematically. Based on Taylor expansion exp(x) = 1 + x + x2/2! + x3/3! …, when x is very small, exp(x) ≈ 1 + x. For very low CL values, eq 6 can be approximated as eq 7 below:
which is identical to eq 1, i.e., Method 1. This means that Method 1 is valid only when CL is very low. As shown in the right charts in and , with exception of methane (), Methods 1 and 2 begin to diverge at a very low CL values (e.g., 2000 ppm-m). For most IR camera gas imaging applications, such a low CL value (e.g., 2000 ppm-m) is approaching the camera’s ability to “see” the gas under low ΔT conditions. The implication is that if quantitative measurements rely on the RF derived from Method 1, the results could be significantly biased high due to the significantly underestimated RF (it is opposite for n-butane; see ). This bias is less severe for methane where the RF values from Methods 1 and 2 are still relatively close for values of CL of up to 20,000 ppm-m ().
Method 3 is used to examine the RF for methane and propylene. Because Method 3 is an experimental method, it is considered the most reliable method and the RF determined by it is most authoritative. Also because it is an experimental method, the conditions under which the RF is determined are limited by resources. In this study, the RF for methane is determined at CL values of 2000, 10,000, 20,000, and 100,000 ppm-m. The RF for propylene is determined at CL values of 2000, 10,000, and 20,000 ppm-m. The results of these RF values determined by Method 3 are included in along with Methods 1 and 2.
As shown in , generally there is a better agreement between Methods 2 and 3 than between Methods 1 and 3. This is validation of Method 2, which can be utilized to derive RF for a large number of compounds without using the resource intensive Method 3. As shown in eq 6, Method 2 is a mathematic model that derives RF based on two input data sources, the IR spectrum α(λ) and an imager bandpass filter transmittance curve t(λ). Although there may be errors associated with the measurement of α(λ) and t(λ), such errors are expected to be very small due to the maturity and accuracy of these methods used to measure these properties. The deviation of Method 2 from Method 3 (the true RF) is likely caused by another factor. Method 2 is derived from a simplified radiative transfer equation, or RTE (eq 2). Comprehensive RTE takes various forms for different applications, and they are very complex (Sharkov, Citation2003). Without certain assumptions and simplifications, the RTE is not very meaningful to OGI practitioners. The simplified RTE, eq 6, makes very good sense. It relates all key factors in OGI applications: the relationship between the IR spectrum α(λ) and the imager bandpass filter spectral window t(λ), the requirement of sufficient ΔT, and the presence of a gas plume CL. These three prerequisite factors make OGI possible, i.e., make sufficient contrast (ΔI) for gas imaging. This simplified RTE can be used as a theoretical foundation to provide tangible support for variety of OGI applications. However, this powerful theoretic tool comes with a compromise. Some degree of imperfection is introduced by the necessity for simplification of the much more comprehensive and complex RTE, which in many cases cannot be analytically solved. Such imperfections are the most significant contributor to the deviation of Method 2 from the experiment-based Method 3.
Treating RF from Method 3 as the true RF, the errors in RF derived by Method 2 have been evaluated for methane and propylene for which the experimental data are available. These errors are presented in . The errors are in a range of −2.9% to −29.1%. The choice between Method 2 and Method 3 is a choice between accuracy and cost. In the context of OGI applications where measurements are made in uncontrollable atmospheric conditions and inherent errors are typically higher than laboratory measurements, the level of errors introduced by Method 2 may be acceptable when compared with the high cost of using Method 3 to develop RF for a large number of compounds. A later discussion on the circular reference between RF and CL also supports this opinion.
Table 2. Error rates from Method 2 in comparison with Method 3.
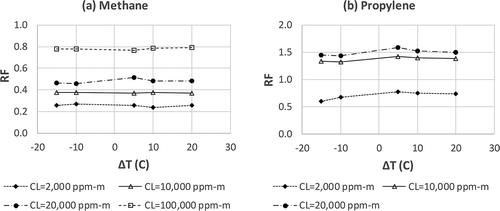
Method 3 is also used to prove or disprove the theoretical prediction that RF is not affected by the ΔT between the gas temperature and the background temperature (refer to the discussion following eq 6). In this experimental study, different concentrations of methane and propylene gases (balance with nitrogen) flow through the gas cell labeled as “T” in . At same time, propane gas with the matching concentration flows through the gas cell labeled as “R” in . Because the depth of the gas cells is 0.1 m, the concentration of methane and propane at 2% will result in a CL of 2000 ppm-m [(2/100) × 1,000,000 × 0.1 m = 2000 ppm-m]. For each gas concentration level (e.g., 2%, corresponding to 2000 ppm-m), the black body temperature is set at 15 °C below the environmental temperature (the gas temperature), i.e., ΔT = −15 °C. The image is taken, and the average intensities in the areas represented by “T” and “R” in (after subtracting the intensity contribution by the gas cell windows) are used to determine the RF. The experiment progresses with the next ΔT, at 5 °C increments, and the corresponding RF is determined. The results of this experimental study are represented in . shows that the RF essentially remains constant within respective CL levels, thus confirming the theoretical prediction that RF is not affected by ΔT.
Figure 6. RF of methane and propylene determined by Method 3 at different CL levels with varying ΔT. (a) methane; (b) propylene.
The RF values discussed in this paper are for pure gas or one gas mixed with nitrogen, which is transparent to the IR camera, i.e., the α(λ) for nitrogen in the IR camera’s spectral window is virtually zero. For an industrial process stream that contains more than one gas, the stream RF (in contrast to a single compound RF) can be represented by a weighted-average RF calculated based on the RF values of the individual components in the mixture and the volume percentage of the components. This approach was also used by Zeng et al. (Citation2007).
Further discussion on RF’s dependency on CL
RF’s dependency on CL can be examined from multiple perspectives. First, as eq 6 shows, the CL terms in the numerator (target compound) and denominator (reference compound) are multiplied by the absorption coefficient α(λ) of their respective compounds. Because the α(λ) values are different for the two compounds and the term 1 − exp(−α(λ)CL) is not linear, the numerator and denominator in the RF equation, eq 6, diverge as CL changes. is constructed using propane, propylene, and methane as examples to further explain the relationship between the RF and CL. In , charts on the left column, a, c, and e, are plots of the term 1 − exp(−α(λ)CL) versus λ for propane, propylene, and methane, respectively. Under the same ΔT, the term 1 − exp(−α(λ)CL) drives the gas plume intensity (see eq 5). Unless noted otherwise, each chart in has three curves representing CL = 1000, 10,000, and 300,000 ppm-m, respectively. The term 1 − exp(−α(λ)CL) is a function of λ, and the area under each curve represents the value of 1 − exp(−α(λ)CL) integrated over the wavelength covered by the bandpass filter of the OGI camera. A larger area under the curve represents a larger value of the integrated 1 − exp(−α(λ)CL) term, which in turn represents a stronger gas image. The area difference between the CL = 1000 ppm-m curve and the CL = 10,000 ppm-m curve represents the incremental change of image intensity when CL changes from 1000 to 10,000 ppm-m. As illustrated in , for the same CL = 1000 ppm-m, the area covered by the propylene curve c is significantly smaller than that of propane curve a. Therefore, the RF is <1. As the CL increases to 10,000 ppm-m, the rise of the curve for propylene is faster than the rise for propane, resulting in a RF >1 (refer to both and ). This is caused by the difference in α(λ) as it multiplies CL in the 1 − exp(−α(λ)CL) term.
Figure 7. Gas image intensity (intensity expressed as 1 − exp(−α(λ)CL) under a constant ΔT) as a function of wavelength (λ) at three levels of CL. (a) propane without filter; (b) propane with filter; (c) propylene without filter; (d) propylene with filter; (e) methane without filter (CL = 300,000 only); (f) methane with filter (CL = 300,000 only).
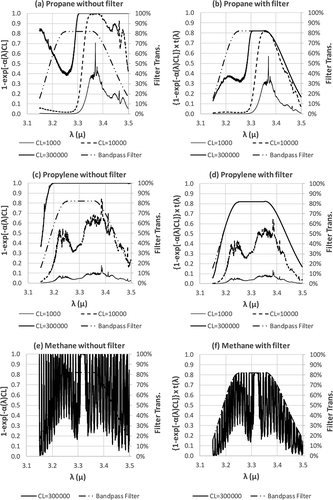
Secondly, when CL becomes very large (e.g., CL = 300,000 ppm-m, the top curves in and , mathematically the term exp(−α(λ)CL) becomes very small, approaching zero, and the term 1 − exp(−α(λ)CL) approaches 1 and is capped at 1. The physical phenomenon corresponding to this mathematic relationship is that when the CL is very high, there are too many molecules in the path of the measurement that the signal becomes saturated. Due to the shape of the Infrared spectrum of the compound, i.e., α(λ), this saturation phenomenon does not happen uniformly across the wavelength λ. In the case of propane, the saturation occurs in a longer wavelength (λ > 3.3 μm) (the right side of ) before it reaches saturation in a shorter wavelength (the left side of ). At CL = 300,000 ppm-m, propylene will be completely saturated in the entire spectral region of 3.2–3.5 μm (see ). When a portion of either propane or propylene curve has reached saturation, the rate of change in RF will slow, and eventually become a constant (refer to and ).
Thirdly, these changes discussed above will be confined by the spectral window of the bandpass filter of the OGI imager. The effect of the bandpass filter is that it truncates a portion of the curve. This effect can be seen by comparing the left column with the right column in , i.e., a versus b, c versus d, and e versus f. At CL = 300,000 ppm-m, propylene has reached complete saturation within the spectral window of the bandpass filter, whereas propane has reached saturation only in the right side of the window, leaving some area on the left “unfilled.” This is the reason for the propylene RF approaching the value of 1, but is greater than 1 (the cumulative ΔI for propane is smaller than that of propylene due to the “unfilled” portion on the left of chart b. For methane, the opposite is true. There is more “untilled” area in chart f than in chart b, resulting RF smaller than 1 for methane. If two compounds are completely saturated across the bandpass filter spectral window, the RF will be equal to 1.
Implication of the findings
Although RF is developed for the purpose of quantitative analyses (i.e., QOGI applications), it can be used to answer a qualitative question commonly raised in the gas imaging field (i.e., OGI), which is whether a particular gas (or vapor) can be imaged by a particular IR camera. The concept of “chemical detectability” proposed by Zeng et al. (Citation2007) was an attempt to answer this question. The current study provides a more comprehensive and more accurate framework toward this goal. If it is known that the IR camera can image one gas (e.g., propane), the question of whether or not it can image another gas can be answered by comparing the RF of the second gas with respect to the first gas without an experiment to demonstrate the technical feasibility of being able to image the second gas, provided that the IR spectra of both gases are available and the transmittance curve of the bandpass filter of the IR camera is also available so that the RF can be determined using Method 2 (although Method 1 could be used, it is not recommended for reasons discussed in the previous section). If the RF is 0.20, the image of the gas in question is expected to be approximately only 20% as strong as the reference gas (propane in this example). When the RF is too small (e.g., 0.05 or 5% of the strength of the reference gas), the image of the gas in question may be too weak to be visible by the IR camera. It will be somewhat subjective to establish an exact RF cutoff value that divide detectable and undetectable gases. It is authors’ opinion that if the RF of a gas is less than 0.10, the detectability of the gas becomes questionable or infeasible. It should be noted that RF could be used to assess detectability of a compound with respect to a known reference compound (e.g., propane) under the same condition, i.e., the comparison of one compound against the reference compound (propane) is in relative sense. The reference compound itself has certain sensitivity/detectability. Previous effort was made to study the minimum detectability (Environ, Citation2004). However, a crucial factor, the ΔT between the background and the gas, was not documented for these studies. It has been demonstrated in recent research that the ΔT will significantly affect the OGI imager sensitivity and detectability (Zeng et al., Citation2015). The importance of the ΔT can be seen in eq 5. Without a reference to the ΔT, an OGI camera’s sensitivity to detect the minimum mass or volumetric leak rates is not sufficiently meaningful. If a new set of minimum detection limits are established with a controlled and documented ΔT (preferably a standard ΔT for ease of comparison across different studies), a method could be developed to use the RF to assess the detectability of a compound in terms of leak rate. Until then, the use of RF to assess the detectability is only in a relative sense (relative to the reference compound under the same environmental conditions).
Based on quantitative IR spectra maintained by PNNL (PNNL, Citation2015), RF values for nearly 398 gaseous chemicals have been determined using Method 2. These RF values have been made available to OGI practitioners at the Web site: http://rfcalc.providencephotonics.com/.
This study has demonstrated that RF is not static, but rather it varies with the concentration-pathlength product, CL. In the leak detection and quantification applications, the CL at the pixel level changes depending on multiple factors such as dispersion of the gas and orientation of the gas plume in relation to the imager’s line of sight. In general, the concentration C is higher near the origin of the leak source and lower downwind from the leak site. If a pure gas (100% or C = 1,000,000 ppm) is leaking out from a hole with 10 cm diameter (L = 0.1 m), the center region of the gas plume near the release point will have CL = 100,000 ppm-m (1,000,000 ppm × 0.1 m). Somewhere downwind, there could be a region where the plume is dispersed to 1 m deep but the concentration is diluted to 100,000 ppm due to dispersion, the CL value will still be 100,000 ppm. In terms of plume orientation, the pathlength L is larger when the plume is flowing toward or away from the imager and smaller when the plume is flowing horizontally or vertically across the imager’s field of view. If the gas is leaking out with considerable exit velocity, in the region near the leak site the pathlength (L) will be well defined and smaller but the concentration (C) will be high (as high as the concentration inside of the equipment). In a region farther away from the leak site, concentration C will decrease and pathlength L will increase. In an ideal scenario, the effect in C and L has potential to be canceled out (mass conservation) if all plume regions are accounted for. This ideal scenario may help conceptualize the physical process, but is unrealistic due to the stochastic nature of the plume behavior in the atmosphere.
The dependency of RF on CL presents a practical challenge in application of RF in QOGI. The primary objective of QOGI is to quantify leak rates or the concentration of a gas plume. The underlying methodology of QOGI directly or indirectly relates to some forms of measurement of CL in the open air environment. The practical challenge is that there is a circular reference between the unknown CL and unknown RF. One cannot determine CL without RF. On the other hand, one cannot determine what RF value to use without knowing the CL. A possible compromised solution could be standardizing RF at an agreed CL level. The results may not be as accurate as desired, but they would be comparable across the industry. As discussed in the above paragraph, the variability of RF with respect to CL should be less severe when a large number of pixels in the plume image are evaluated as a whole instead of individual pixels being evaluated in isolation. For these two reasons, the approach of standardizing RF at a fixed CL is worth considering in the context of environmental regulatory control of fugitive emissions. In this case, a set of standardized RF (e.g., RF at CL = 10,000 ppm-m) can be determined by either Method 2 or Method 3 depending on resource constraints. Such candidate standardized RF values for the seven compounds are listed in . Once the standard CL is selected, a set of RF values similar to the ones listed in can be easily generated using Method 2 for hundreds of compounds for which the IR spectral data are available.
Table 3. Possible candidate values for standardized RF determined by Method 2.
Conclusion
As optical gas imaging (OGI) technology transforms from a visual, qualitative tool to a quantitative measurement technology (QOGI), it is becoming more and more important to develop a set of response factor (RF) values for various commonly encountered gaseous compounds so that the QOGI imaging instruments do not need to be calibrated for each of these compounds.
In this study, three methods for development of RF are examined. Method 1 has been used before (Zeng et al., Citation2007; Footer, Citation2015), and it derives RF based on the IR absorption coefficient, α(λ), as it overlaps with the bandpass filter transmittance curve of the IR camera. Both Method 2 and Method 3 are newly proposed concepts. Method 2 is analytically derived from the well-established radiative transfer equation (RTE). Method 3 is an experimental method. The experimental setup proposed to implement Method 3 eliminates external factors that are not inherently associated with RF so that the true RF can be measured. Because it is based on actual measurement, Method 3 is the most reliable and authoritative method. It can be applied to a desired number of compounds (either individually or in a mixture) to determine their RF values. The disadvantage of Method 3 is that it requires significant resources.
The advantage of Method 2 is that it can be used to derive RF for a large number of compounds without conducting any experiments. The only required information is a quantitative IR spectrum of the compound in question and the transmittance curve of the bandpass filter of the IR camera in question (eq 6). Quantitative IR spectra for a large number of compounds are available from various sources such as PNNL (PNNL, Citation2015). This study suggests that although the RF determined by Method 2 does not exactly match the true RF determined by Method 3, it tracks the true RF well in a wide CL range and it is substantially closer to the true RF than the RF determined by Method 1. The deviation of Method 2 from Method 3 is attributed to the necessary simplifications used in the RTE. In addition, the accuracy of Method 2 will be affected by the accuracy of the quantitative IR spectral data and the IR camera’s filter curve function.
Both Method 1 and Method 2 can be considered as models for predicting RF. However, RF values predicted by the two models are drastically different except in the very low CL regions where the two methods converge. According to Method 1, RF is static and does not change with CL. This has been demonstrated to be false. For those RF values that have been measured by Method 3 in this study, the results of Method 2 are much closer to the measured RF than those of Method 1. For all compounds included in this study (with the exception of methane), Method 1 and Method 2 diverge drastically as CL increases above approximately 2000 ppm-m. For methane, the results from Methods 1 and 2 are reasonably close as long as CL is less than approximately 20,000 ppm-m. When CL is greater than approximately 20,000 ppm-m, the similar drastic divergence between Methods 1 and 2 is observed.
In summary, this study has demonstrated that Method 1 is not suitable for determining RF. For the same prerequisite information and comparable level of effort, RF can be significantly better predicted with Method 2. The most authoritative RF should be produced using Method 3. Between Methods 2 and 3, it is a trade-off between accuracy and resources.
In addition to the above conclusions regarding the RF methods, the following conclusions regarding the characteristics of RF can be drawn based on this study.
RF is not affected by ΔT between background temperature TB and gas temperature TG. This is significant considering that other QOGI measurements, such as IR intensity, vary significantly as ΔT changes.
RF is not static and it varies with concentration-pathlength product CL.
RF can vary significantly with CL when CL is low. The variability of RF decreases as the CL increases. After CL reaches a value higher than approximately the 100,000–200,000 ppm-m range, the RF generally becomes constant and RF for all compounds tends to converge towards, but not equal to, the value of 1. This implies that the difference among different compounds becomes smaller and use of RF becomes less important as CL becomes very large.
Because RF is a function of CL, one cannot be determined without the other. CL (or its derivative) is typically the target of the measurement and therefore unknown, so it is recommended that a set of agreed RF values to be established as “standard RF” under one agreed reference CL level (e.g., 10,000 ppm-m). This approach should be practical and desirable for regulatory applications where all regulated communities will be subject to the same standardized RF. In this scenario, Method 2 is recommended for generating the standardized RF values for a wide range of compounds. It should be noted that although RF varies with CL at the pixel level, if the subject of a measurement is the entire gas plume or a portion of the plume represented by a large number of pixels, the effect will be reduced. Due to the principle of mass conservation, CL can vary throughout the plume but the total response will remain relatively constant, i.e., a 1-m plume of concentration 10,000 ppm is equivalent to a 10-m plume of concentration 1000 ppm. If the RF is applied to a distribution of pixels with varying CL to obtain a quantitative result, the difference in RF due to variations in CL will be averaged and the use of a “standardized RF” will work well.
Additional information
Notes on contributors
Yousheng Zeng
Yousheng Zeng, Ph.D., is the chief executive officer of Providence Photonics based in Baton Rouge, Louisiana.
Jon Morris
Jon Morris, M.S., is the chief technology officer of Providence Photonics based in Baton Rouge, Louisiana.
Albert Sanders
Albert Sanders, Srikanth Mutyala, and Cory Zeng are staff members of Providence Photonics based in Baton Rouge, Louisiana.
References
- Environ. 2004. Development of Emission Factors and/or Correlation Equations for Gas Leak Detection, and Development of an EPA Protocol for the Use of a Gas-imaging Device as an Alternative or Supplement to Current Leak Detection and Evaluation Methods. Final report prepared for the Texas Council on Environmental Technology and the Texas Commission on Environmental Quality. http://tceq.state.tx.us/assets/public/implementation/air/terp/ntrd/prog_rpts/02-R04-01G%20Environ%20Final%20Report.pdf ( accessed August 3, 2016).
- Footer, T.L. 2015. Draft Technical Support Document Appendices; Optical Gas Imaging Protocol (40 CFR Part 60, Appendix K), prepared for Jason DeWees, U.S. Environmental Protection Agency (EPA). EPA Contract No. EP-D-11-006 Work Assignment 5-09. http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OAR-2010-0505-4949 (accessed October 27, 2015).
- Pacific Northwest National Laboratory. 2015. Pacific Northwest National Laboratory (PNNL) Infrared Spectral Database. https://secure2.pnl.gov/nsd/nsd.nsf/Welcome (accessed October 27, 2015).
- Sharkov, E.A. 2003. Passive microwave remote sensing of the earth: Physical foundations. New York: Springer Praxis Books. Springer.
- U.S. Environmental Protection Agency. 1995. Protocol for Equipment Leak Emission Estimates. EPA-453/R-95-17. November 1995. Washington, DC: U.S. Environmental Protection Agency.
- U.S. Environmental Protection Agency. 2008. Alternative work practice to detect leaks from equipment. Fed. Regist. 73:78199–219.
- Zeng, Y., J. Morris, A. Sanders, D. McGregor, P. Kangas, and H. Abdel-Moati. 2015. New optical gas imaging technology for quantifying fugitive emission rates. Paper presented at the Air & Waste Management Association 108th Annual Conference & Exhibition, Raleigh, NC, June 22–25, 2015.
- Zeng, Y., L. Zhou, and V. Matherne. 2007. A method to rank detectability of compounds and process streams using an infrared optical imaging device. Paper presented at the Symposium on Air Quality Measurement Methods and Technology, San Francisco, CA, April 30–May 3, 2007.