ABSTRACT
The formation of PM2.5 (aerosol particulate matter less than 2.5 µm in aerodynamic diameter) in association with SO2 emission during sintering process has been studied by dividing the whole sintering process into six typical sampling stages. A low-pressure cascade impactor was used to collect PM2.5 by automatically segregating particulates into six sizes. It was found that strong correlation existed between the emission properties of PM2.5 and SO2. Wet mixture layer (overwetted layer and raw mixture layer) had the function to simultaneously capture SO2 and PM2.5 during the early sintering stages, and released them back into flue gas mainly in the flue gas temperature-rising period. CaSO4 crystals constituted the main SO2-related PM2.5 during the disappearing process of overwetted layer, which was able to form perfect individual crystals or to form particles with complex chemical compositions. Besides the existence of individual CaSO4 crystals, mixed crystals of K2SO4-CaSO4 in PM2.5 were also found during the first half of the temperature-rising period of flue gas. The interaction between fine-grained Ca-based fluxes, potassium vapors, and SO2 was the potential source of SO2-related PM2.5.
Implications: The emission property of PM2.5 and SO2 throughout the sintering process exhibited well similarity. This phenomenon tightened the relationship between the formation of PM2.5 and the emission of SO2. Through revealing the properties of SO2-related PM2.5 during sintering process, the potential interaction between fine-grained Ca-based fluxes, potassium vapors, and SO2 was found to be the source of SO2-related PM2.5. This information can serve as the guidance to develop efficient techniques to control the formation and emission of PM2.5 in practical sintering plants.
Introduction
Iron ore sintering is an important thermal treatment stage of the whole steel-making chain, the purpose of which is to agglomerate fine iron ores into lumpish iron ore sinter for achieving excellent metallurgical performance in blast furnace (Clout and Manuel, Citation2003; Remus et al., Citation2012). In China, sinter is the major iron-bearing burden used in blast furnace for iron making, which usually takes up a proportion exceeding 75% (Wang and Huang, Citation2007). However, sinter-making process is both pollution-intensive and energy-intensive; 9–12% of the total energy for the whole steel-making process is consumed in this stage. Moreover, a large amount of gaseous pollutants such as carbon oxides (COx), sulfur oxides (SOx), nitrogen oxides (NOx), etc., and aerosol particulate matter, especially PM2.5 (aerosol particulate matter less than 2.5 µm in aerodynamic diameter), are also emitted from this process (Ooi et al., Citation2011; Chen et al., Citation2009; Menad et al., Citation2006).
As PM2.5 characterizes huge specific surface area, it had been proved to act as the natural carriers of dioxins, heavy metals such as Cd and Pb, and alkaline metals such as K and Na (Shih et al., Citation2006, Citation2009; Sammut et al., Citation2010; Hleis et al., Citation2013; Tsai et al., Citation2007). Dioxins and heavy metals are well known as toxic compounds and can probably interfere with biochemical mechanisms (Kyotani and Iwatsuki, Citation2002; Copeland and Kawatra, Citation2011). Reducing the emission of PM2.5 in sintering process is therefore beneficial to environmental protection and human health. However, elucidating the formation mechanism of PM2.5 is the important basis for developing efficient techniques to control PM2.5 emissions. Unfortunately, current research on PM2.5 emitted from sintering stacks mainly focused on its physicochemical characteristics but afforded no information about its formation mechanism (Sammut et al., Citation2010; Hleis et al., Citation2013; Tsai et al., Citation2007).
In China, sintering processes are an important source of SO2, accounting for 7% of its total industrial emissions (Wang et al., Citation2016). In sintering bed, formed SO2 would undergo absorption by sintering mixture layer and then intensive release into the flue gas when flue gas temperature started to rise (Fan, Citation2011; Pan et al., Citation2011; Yu et al., Citation2015). During the absorbing, migrating, and releasing processes, complex physicochemical reactions are accompanied, which has the potential interaction with the formation of PM2.5. Therefore, the detailed information and reaction mechanism about this interaction are still unknown. This investigation aims to build the linkage between the formation of PM2.5 and the emission of SO2. For this purpose, the integrate sintering process has been divided into six typical stages according to the changing curve of flue gas temperature for PM2.5 sampling, and a series of techniques, including X-ray fluorescence (XRF), scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM-EDS), etc., were utilized to examine the characteristics of PM2.5.
Materials and methods
Raw materials
For making sinter, iron ore fines, fluxes (limestone, dolomite, and quicklime), return fines (sinter with size range of <5 mm), and solid fuel are necessary raw materials. All of them were directly obtained from an integrated steelwork in China. gives the basic chemical compositions and proportions of raw materials, based on which the final sinter product was characterized by Fe content of 56.38%, SiO2 content of 4.92%, MgO content of 2.00%, and basicity (CaO/SiO2, mass%) of 2.00. As can be seen from , Fe was the main component of mixed iron ores and return fines; CaO and MgO were the main components of limestone, dolomite, and quicklime; and Al2O3 and SiO2 were the major components of coke breeze ash. Particularly, the total content of S in coke breeze was measured, and it was about 0.205%. About 0.16% existed in the form of organics, whereas the rest mainly existed in the form of sulfide. Besides, raw materials also contained some trace elements such as S, K, Na, and Cl.
Table 1. Chemical compositions and proportion of raw materials in mixture (mass %).
Experimental methods
Sinter pot trials
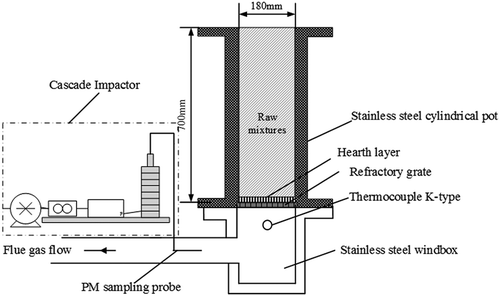
A laboratory-scale sintering pot of depth 700 mm and diameter 180 mm was applied to simulate industrial-scale sinter-making process, which was schematically described in . During the process, raw materials given in were well mixed according to their proportions, with prescribed proportion of water added. The mixture was then charged into a drum for granulating fine-grained materials into granules. After that, the granules were fed into the sintering pot. After feeding, the solid fuel in the surface layer was initially ignited by a natural-gas-fueled ignition hood, and the combustion front moved downward with the support of a downdraught system. The whole sintering process started from the ignition point to the burning through point at which the flue gas had reached its maximum temperature. A K-type thermocouple was used to measure the change of flue gas temperature (FGT). For each individual sampling case, a low-pressure cascade impactor (model WY-1; Beijing Nature Environmental Science and Technology Company, Beijing, China) was used to collect particulate matter from flue gas, as shown in . An infrared flue gas analyzer, model DELTA 65-3 (MRU Corporation, Heilbronn, Germany), was used to analyze the concentration of SO2 in the flue gas.
PM sampling and analytical techniques
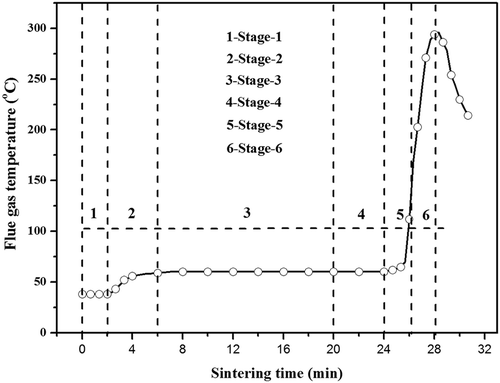
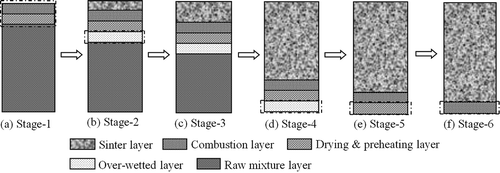
Particulate matter (PM) samples were collected by Whatman QMA quartz fiber filters (HF; Haifan Filter Material Company, Shanghai, China) and using the low-pressure cascade impactor, which was able to segregate PM into 0.7–2.5 μm and 2.5–10 μm according to their aerodynamic diameter. The part of PM with size range 0.7–2.5 μm was selected and analyzed to reflect the properties of PM2.5. During the sampling process, water condensation inside the probe did not occur by using an electrically heated belt covering the surface of the probe. To establish the linkage between PM2.5 formation and SO2 emission during an integrated sintering process, it has been divided into six typical sintering stages according to the FGT curve (). Among them, stage 1 indicated the ignition period, stage 2 showed the gradually stabilizing process of FGT, stage 3 indicated the stable process of FGT, stage 4 was the short period before the rise of FGT, and stage 5 and stage 6 constituted the first half and the second half of FGT-rising process, respectively.
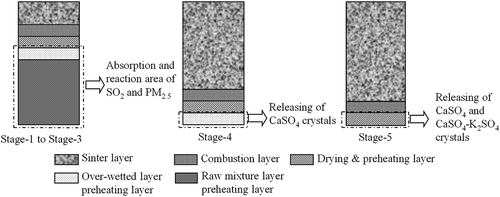
Relevant changing and moving characteristics of each sintering layer during typical sintering stages are schematically shown in ; the division and characteristics of each layer can be found in our previous reports (Yu et al., Citation2016). corresponds to the ignition process, where combustion layer and drying & preheating layer formed gradually in this stage; indicates the gradual forming process of overwetted layer after the ignition process; indicates the typical sintering process with distinguishable five layers; shows the gradual disappearing process of overwetted layer; and respectively describe the gradual disappearing process of drying & preheating layer and combustion layer, where the sintering process is to reach the burning through point. PM samples of each stage were collected respectively by repeating the sintering process with the same condition.
Figure 3. Typical changes of sinter layers during sintering process. (a) Gradual forming process of combustion layer and drying & preheating layer; (b) gradual forming process of overwetted layer; (c) formed overwetted layer; (d) gradual disappearing process of overwetted layer; (e) gradual disappearing process of drying & preheating layer; (f) gradual disappearing process of combustion layer.
For guaranteeing the representatives of PM samples, isokinetic sampling is the prerequisite. To achieve this goal, the real-time linear velocity of flue gas is calculated on the basis of real-time flue gas flow and the dimension of the flue. This information serves as the guidance to adjust the linear velocity of sampled gas, thereby making linear velocity of flue gas and sampled gas equal. After sampling, the upper part of particles with size range 0.7–2.5 μm was removed from the filter for further analysis. The elemental composition of PM2.5 was analyzed by energy-dispersive XRF (X-ray fluorescence; Eagle III; EDAX, Inc., Mahwah, NJ, USA). Field emission scanning electronic microscopy (FESEM; Helios NanoLab 600i) with energy-dispersive X-ray (EDX) was used to analyze particle morphology and major chemical distribution in typical particles.
Results and discussion
Linkage of emission properties between PM2.5 and SO2
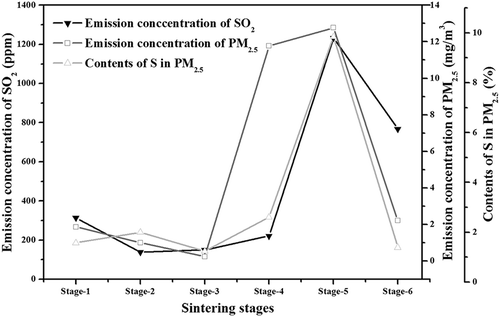
The emission properties of PM2.5 and SO2 during sintering process are given in . Obviously, it was found that the emission concentration of PM2.5 kept at a lower level from stage 1 to stage 3, whereas its emission concentration become considerably high in stage 4 and stage 5. This emission property showed well similarity to that of SO2. As could be observed from , the average emission concentration of SO2 exhibited a lower value from stage 1 to stage 4, whereas it reached the peak value in stage 5. Previous research (Pan et al., Citation2011) had proved that wet mixture layer (raw mixture layer and overwetted layer), especially the overwetted layer, was able to capture SO2 from flue gas via gas-solid and gas-liquid reactions, whereas SO2 was released back into flue gas when the high temperature was approached. In combination with the change of typical sintering layers during stage 1 to stage 4 shown in , the absorption effect of wet layers caused the lower emission concentration of SO2. When the sintering process moved to stage 5, the temperature of sintering bed started to rise, which therefore led to desorption of SO2.
As for the emission property of PM2.5, it was believed that wet layer has the ability to store particulate matter temporarily. Indeed, previous research (Khosa et al., Citation2003; Nakano and Okazaki, Citation2007; Debrincat and Loo, Citation2007) had verified that wet mixture layer, especially the overwetted layer, played an important role in scrubbing dust from flue gas. However, their research gave no information about the function of wet mixture layer on scrubbing finer particulate matter such as PM2.5. From sintering stage 1 to stage 3, the wet mixture layer was relevantly thicker compared with that in stage 4. More PM2.5 could be captured by the wet mixture layer due to strong capture function, which therefore showed lower emission concentration in flue gas. Sintering stage 4 referred to the gradual disappearing process of wet mixture layer. This function was correspondingly weakened in this stage. Therefore, the captured PM2.5 was able to escape from the sintering bed, and the newly formed PM2.5 could also easily pass through the sintering bed due to the weak function. This was the main reason for the low emission concentration of PM2.5 from stage 1 to stage 3. More information about the function mechanism of wet mixture can also be found in our previous research. (Gan et al., Citation2015)
Additionally, the content of S was relatively lower in PM2.5 from stage 1 to stage 3, whereas its content reached the highest level in PM2.5 from stage 5, which showed well relativity to the emission concentration of SO2 during different sintering stages. This interesting phenomenon potentially indicated that SO2 served as an important participant in PM2.5 formation, especially in the formation of PM2.5 emitted from stage 4 and stage 5, where absorbed SO2 started to release back into the flue gas.
Characteristics of SO2-related PM2.5 from stage 4 and stage 5
SO2-related PM2.5 from stage 4
During sintering stage 4, overwetted layer began to disappear gradually, and scrubbed SO2 and PM2.5 started to transfer from sintering bed to flue gas. The morphology and major inorganic composition of SO2-derived PM2.5 are given in and .
Figure 5. SEM-EDS of perfect crystallized and nonperfect crystallized CaSO4 crystals. (a) Morphology of perfect crystallized CaSO4 crystals; (b) EDX spectrum of perfect crystallized CaSO4 crystals; (c) morphology of nonperfect crystallized CaSO4 crystals; (d) EDX spectrum of nonperfect crystallized CaSO4 crystals.
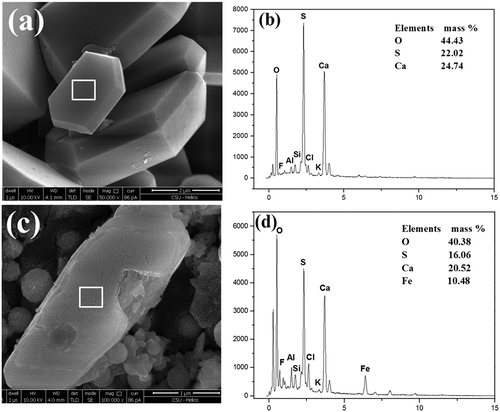
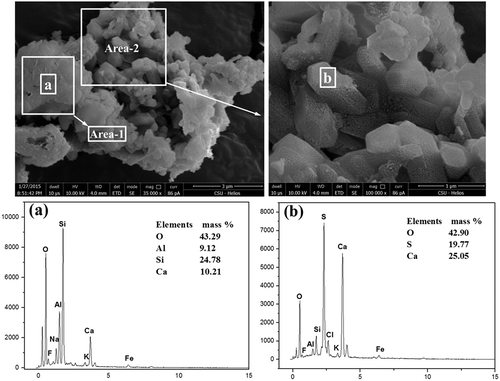
Figure 6. SEM-EDS of CaSO4-assembled particles. (a) EDX spectrum of selected area 1; (b) EDX spectrum of selected area 2.
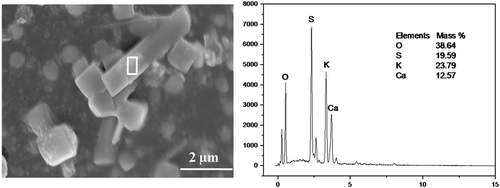
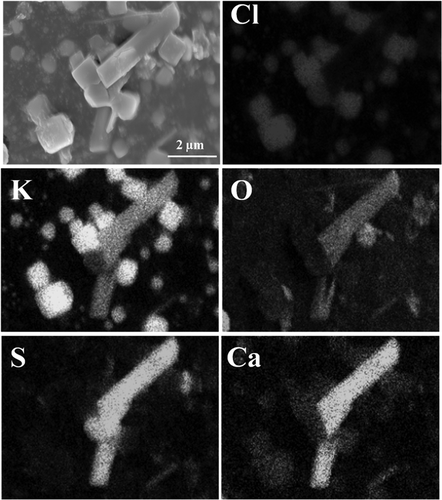
As could be seen from and , the particles with smooth surface and standard polyhedral structure mainly consisted of Ca, S, and O, which accounted for 24.74%, 22.02%, and 44.43% of the total weight, respectively. It could be found that the molar ratio of Ca, S, and O was about 1:1:4. Therefore, this kind of particles was the typical CaSO4 crystals. Additionally, the rod-like particle shown in also mainly comprised Ca, S, and O. However, Fe took up about 10% of the total mass in this particle, as shown in . Taking the molar ratio of Ca and S, which is about 1:1, into consideration, this particle was a nonperfect crystallized CaSO4 crystal. Moreover, taking the main chemical composition of this particle into account, Fe was probably existed in the form of oxides or sulfates.
gives the morphology of an irregularly shaped particle assembled with numerous finer-grained particles. As for the area 1, the main chemical composition was O, Si, Al, and Ca, which accounted for about 43.29%, 24.78%, 9.12%, and 10.21% of the total mass, respectively. It could be speculated that this part of particle was the mixture of SiO2, Al2O3, and CaO. Area 2 consisted of many particles less than 1.0 μm in diameter, the major chemical composition of which was Ca, S, and O, with mass proportion of 25.05%, 19.77%, and 42.90%, respectively. Also, it was found that the particle was a CaSO4 crystal according to the molar ratio of Ca, S, and O. Consequently, CaSO4 was able to form submicron particles and assemble together to form larger particles.
SO2-related PM2.5 from stage 5
During sintering stage 5, FGT started to rise, which was accompanied by the large-scale release of SO2 and PM2.5 into flue gas. The properties of SO2-related particles from this stage are given in , , and .
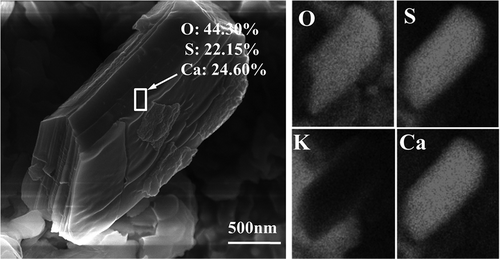
As could be observed in , Ca, S, O, K, and Cl took up the major part of the total mass in the rod-like particle. The high K content was in great agreement with the high K contents of PM2.5 in this stage, as reported in our previous research (Gan et al., Citation2015). According to the mass ratio of S and O, it was observed that their molar ratio was about 1:4. Therefore, S and O existed in this particle in the form of sulfate. Taking the mass proportion of K and Ca into consideration, it could be found that the molar ratio of K, Ca, and S was about 2:1:2, which indicated that the rod-like particle was the hybrid crystals of K2SO4 and CaSO4, with molar ratio 1:1. The SEM-EDS mapping of the rod-like particle was described in , where it could be seen clearly that the distribution areas of K, Ca, S, and O were well overlapped, which further verified that K2SO4 and CaSO4 participated in the formation of PM2.5 simultaneously. From , it was also found that K could participate in the formation of PM2.5 by forming KCl. Consequently, the contribution of K2SO4 and KCl during PM2.5 formation possibly existed some competitiveness.
With the exception of forming mixed crystal with K2SO4, CaSO4 could also participate in forming PM2.5 individually. gives the SEM-EDS mapping of a polyhedral particle. On the basis of the contents and distribution property of Ca, S, and O in this particle, it could be observed that this particle was also CaSO4.
Discussion on the formation of SO2-related PM2.5
The source of CaO for the formation of CaSO4 in PM2.5
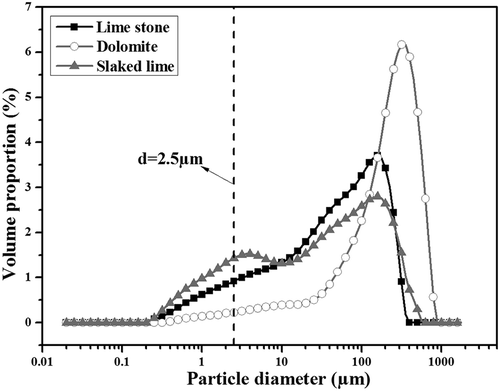
According to the results shown in “Characteristics of SO2-related PM2.5 from stage 4 and stage 5,” CaSO4 crystals were the important SO2-related PM2.5 formed from both stage 4 and stage 5. Except for the participating of SO2, Ca-based compounds were also necessary in the formation of CaSO4 crystals. As introduced in , three kinds of Ca-based fluxes were used as the necessary materials for producing sinter, which could provide abundant CaO to capture SO2 from flue gas. However, the size distribution of Ca-based fluxes served as another important factor to decide the dimension of the SO2-related particulate matter formed. For elucidating the possibility of the transformation of gaseous SO2 into PM2.5 in the form of CaSO4 crystals, the size distributions of slaked lime, dolomite, and lime stone were measured by laser diffraction using a Malvern Mastersizer 3000, as shown in . Obviously, all of Ca-based fluxes contained a part of particles less than 2.5 µm in diameter, which therefore guaranteed the appearance of CaSO4 in PM2.5 via the main reactions given in eqs 1–7, as reported previously (Pan et al., Citation2011; Yu et al., Citation2015; Li et al., Citation2014):
The source of K for the formation of K2SO4 in PM2.5
K was the trace elements in raw materials as introduced in , and it was able to volatilize partially in the sintering process, where the highest temperature easily reached above 1300 °C, as has been reported (Remus et al., Citation2012; Yu et al., Citation2012). Actually, the removal ratio of K during the sintering process with increasing ratio of coke breeze had been investigated. From the results shown in , it could be observed that K achieved an increasing removal ratio with enhancing the ratio of coke breeze. Reaction 8 could serve as the explanation to the increased removal ratio of K (Zhang et al., Citation2010), which showed that K can enter into the flue gas in the form of its vapors. As has been widely known, K is of extremely high chemical activity and could react with O2, CO2, SO2, etc., in the flue gas. Among these, the reactions 10 and 11 potentially constituted the main routes of the formation of K2SO4.
Table 2. Removal ratio of K with increasing the ratio of coke breeze.
Formation mechanism of SO2-related PM2.5
During the sintering process, water vapor generated from the drying & preheating layer would condense on the surface of granules in the lower material layer, thereby forming the overwetted layer. Results introduced in “Linkage of emission properties between PM2.5 and SO2” had clarified that wet mixture layer, especially the overwetted layer, had the ability to capture PM2.5 and SO2 from flue gas, which occurred from sintering stage 1 to stage 3, as shown in . During the absorption process, Ca-based basic fluxes with size range less than 2.5 µm could serve as the efficient absorbent to absorb SO2, thereby forming PM2.5 in the form of CaSO4 crystals. Besides, in combination with the distinguishingly high K content in PM2.5 from stage 5 (Gan et al., Citation2015), K-bearing compounds were first captured in the wet mixture layer and then intensively released into the flue gas, as also found elsewhere (Kobayashi et al., Citation1979). Therefore, the potassium vapor generated from the reduction process of its compounds also participated in the formation of PM2.5, which could be transformed into new compounds such as KCl and K2SO4, the existence of which had been verified, with SO2, O2, CaCl2, etc., and were temporarily captured in the wet mixture layer. As the absorption reactions occurred on the surface of the granules, generated K2SO4 had the convenience to collide with PM2.5 in the form of CaSO3 or CaSO4 crystals, and finally forming CaSO4-K2SO4 mixed crystals through particle aggregation. When the sintering process moved to stage 4 and stage 5, wet mixture layer started to disappear. PM2.5 was correspondingly rereleased into the flue gas. SO2-related PM2.5 such as CaSO4 and CaSO4-K2SO4 crystals were also released into flue gas ().
Conclusion
The formation of PM2.5 in association with SO2 during sintering process has been studied by dividing the whole sintering process into six typical sampling stages. On the basis of the properties of the SO2-related PM2.5, conclusions can be drawn as follows.
Great similarity is existed between the emission properties of PM2.5 and SO2. Wet mixture layer has the same function to capture SO2 and PM2.5 during the early sintering stages, and to release them back into flue gas mainly in the temperature-rising period of flue gas.
CaSO4 crystals are the main SO2-related PM2.5 during the disappearing process of overwetted layer, which is able to form perfect individual crystals or particles with complex chemical composition. Besides the existence of individual CaSO4 crystals, mixed crystal of K2SO4-CaSO4 is also observed during the first half of the rising period of flue gas temperature.
The interactions between fine-grained basic fluxes, potassium vapors, and SO2 in the wet mixture layer serve as the potential sources of SO2-related PM2.5.
Funding
The authors are grateful to the National Natural Science Foundation of China (51304245), the Postdoctoral Science Foundation (2013M540639), Hunan Provincial Co-Innovation Center for Clean, and Efficient Utilization of Strategic Metal Mineral Resources, and the Fundamental Research Funds for the Central Universities of Central South University (2015zzts086) for supporting this research.
Additional information
Funding
Notes on contributors
Zhiyun Ji
Zhiyun Ji is a Ph.D. at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Xiaohui Fan
Xiaohui Fan is a research professor at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Min Gan
Min Gan and Xuling Chen are research professors at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Xuling Chen
Min Gan and Xuling Chen are research professors at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Wei Lv
Wei Lv, Qiang Li, Yang Zhou, and Ye Tian are postgraduate fellows at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Qiang Li
Wei Lv, Qiang Li, Yang Zhou, and Ye Tian are postgraduate fellows at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Yang Zhou
Wei Lv, Qiang Li, Yang Zhou, and Ye Tian are postgraduate fellows at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Ye Tian
Wei Lv, Qiang Li, Yang Zhou, and Ye Tian are postgraduate fellows at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
Tao Jiang
Tao Jiang is a research professor at the Department of Iron and Steel Metallurgy, Central South University, in Changsha, China.
References
- Chen, Y.G., Z.C. Guo, and Z. Wang. 2009. Influence of CeO2 on NOx emission during iron ore sintering. Fuel Process. Technol. 90:933–938. doi: 10.1016/j.fuproc.2009.03.021.
- Clout, J.M.F., and J.R. Manuel. 2003. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores. Powder Technol. 130:393–399. doi: S0032-5910(02)00241-3.
- Copeland, C.R., and S.K. Kawatra. 2011. Design of a dust tower for suppression of airborne particulates for ironmaking. Miner. Eng. 24:1459–1466. doi: 10.1016/j.mineng.2011.07.008.
- Debrincat, D., and C.E. Loo, 2007. Factors influencing particulate emissions during iron ore sintering. ISIJ Int. 47:652–658. doi: 10.2355/isijinternational.47.652.
- Fan, Z.Y. 2011. A fundamental investigation on flue gas circulation sintering [in Chinese with English abstract]. Engineering Master’s thesis, Central South University, Changsha, China. doi: 10.7666/d.y1914450.pdf.
- Gan, M., Z.Y. Ji, X.H. Fan, X.L. Chen, Q. Li, L. Yin, X.N. He, Y. Zhou, and T. Jiang. 2015. Emission behavior and physicochemical properties of aerosol particulate matter (PM10/2.5) from iron ore sintering process. ISIJ Int. 55:2582–2588. doi: 10.2355/isijinternational.ISIJINT-2015-412.
- Hleis, D., I. Fernandez-Olmo, F. Ledoux, A. Kfoury, L. Courcot, T. Desmonts, and D. Courcot. 2013. Chemical profile identification of fugitive and confined particle emissions from an integrated iron and steelmaking plant. J. Hazard. Mater. 250–251:246–255. doi: 10.1016/j.jhazmat.2013.01.080.
- Khosa, J., J. Manuel, and A. Trudu. 2003. Results from preliminary investigation of particulate emission during sintering of iron ore. Trans. Inst. Min. Metall. C. 112:25–32. doi: 10.1179/037195503225011367.
- Kobayashi, K., Y. Miura, and A. Okamoto. 1979. Behavior of alkali compounds in the sintering process. Tetsu-to-Hagané 65:1355–1364.
- Kyotani, T., and M. Iwatsuki. 2002. Characterization of soluble and insoluble components in PM2.5 and PM10 fractions of airborne particulate matter in Kofu city, Japan. Atmos. Environ. 36:639–649. doi: S1352-2310(01)00494-0.
- Li, G.H., L. Chen, M.J. Rao, Z.Y. Fan, Z.X. You, Y.B. Zhang, and T. Jiang. 2014. Behavior of SO2 in the process of flue gas circulation sintering (FGCS) for iron ores, ISIJ Int. 54:37–42. doi: 10.2355/isijinternational.54.37.
- Menad, N., H. Tayibi, F.G. Carcedo, and A. Hernandez, 2006. Minimization methods for emissions generated from sinter strands: A review. J. Cleaner Prod. 14:740–747. doi: 10.1016/j.jclepro.2004.03.005.
- Nakano, M., and J. Okazaki. 2007. Influence of operational conditions on dust emission from sintering bed. ISIJ Int. 47:240–244, doi: 10.2355/isijinternational.47.240.
- Ooi, T.C., D. Thompson, D.R. Anderson, R. Fisher, T. Fray, and M. Zandi. 2011. The Effect of charcoal combustion on iron-ore sintering performance and emission of persistent organic pollutants. Combust. Flame 158:979–987. doi: 10.1016/j.combustflame.2011.01.020.
- Pan, J., D.Q. Zhu, Y. Cui, D. Chen, and X.L. Zhou. 2011. Emission rule of SO2 in flue gas during sintering [in Chinese]. J. Central South Univ. 42:1495–1500. doi: 1672-7207(2011)06-1495-06.
- Remus, R., M.A.A. Monsonet, S. Roudier, and L.D. Sancho. 2012. Best Available Techniques (BAT) Reference Document for Iron and Steel Production. Luxembourg: Publications Office of the European Union.
- Sammut, M.L., Y. Noack, J. Rose, J.L. Hazemann, O. Proux, M. Depoux, A. Ziebel, and E. Fiani. 2010. Speciation of Cd and Pb in dust emitted from sinter plant. Chemosphere 78:445–450. doi: 10.1016/j.chemosphere.2009.10.039.
- Shih, M.L., W.J. Lee, T.S. Shih, S.L. Huang, L.C. Wang, G.P. Chang-Chien, and P.J. Tsai. 2006. Characterisation of dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in the atmosphere of different workplaces of a sinter plant. Sci. Total Environ. 366:197–205. doi: 10.1016/j.scitotenv.2005.07.006.
- Shih, T.S., M.L. Shih, W.J. Lee, S.L. Huang, L.C. Wang, Y.C. Chen, and P.J. Tsai. 2009. Particle size distributions and health-related exposures of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) of sinter plant workers. Chemosphere 74:1463–1470. doi: 10.1016/j.chemosphere.2008.11.053.
- Tsai, J.H., K.H. Lin, C.Y. Chen, J.Y. Ding, C.G. Choa, and H.L. Chiang. 2007. Chemical constituents in particulate emissions from an integrated iron and steel facility. J. Hazard. Mater. 147:111–119. doi: 10.1016/j.jhazmat.2006.12.054.
- Wang, M.J., M.F. Hou, K. Zhao, H.F. Li, Y. Han, X. Liao, X.B. Chen, and W.B. Liu. 2016. Removal of polychlorinated biphenyls by desulfurization and emissions of polychlorinated biphenyls from sintering plants. Environ. Sci. Pollut. Res. 23:7369–7375. doi: 10.1007/s11356-015-5903-7.
- Wang, W.X., and J. Huang. 2007. Review of technological development of blast furnace ironmaking in China [in Chinese]. Iron Steel 42:1–4. doi: 0449-749X(2007)03-0001-04.
- Yu, H., C.X. Zhang, and H.F. Wang. 2015. SO2 absorption in the sinter bed during the sintering process. ISIJ Int. 55:1876–1881. doi: 10.2355/isijinternational.ISIJINT-2014-790.
- Yu, Y.M., M.H. Zheng, X.W. Li, and X.L. He. 2012. Operating condition influences on PCDD/Fs emissions from sinter pot tests with hot flue gas recycling. J. Environ. Sci. 24:875–881. doi: 10.1016/S1001-0742(11)60869-3.
- Yu, Z.Y., X.H. Fan, M. Gan, X.L. Chen, Q. Chen, and Y.S. Huang. 2016. Reaction behavior of SO2 in the sintering process with flue gas recirculation. J. Air Waste Manage. Assoc. 66:687–697. doi: 10.1080/10962247.2016.1167790.
- Zhang, S.Z., F. Zhang, and G.P. Luo. 2010. Influence of alkali metal on the process of sintering [in Chinese with English abstract]. J. Inner Mongolia Univ. Sci. Technol. 29:108–111. doi:1004-9762(2010)02-0108-04.