ABSTRACT
Atmospheric mercury in the environment as a result of the consumption of fossil fuels, such as coal used in electricity generation, has gained increased attention worldwide because of its toxicity, atmospheric persistence, and bioaccumulation. Determining or predicting the concentration of this pollutant in ambient air is essential for determining sensitive areas requiring health protection. This study investigated the spatiotemporal variability of gaseous elemental mercury (GEM) concentrations and its dry deposition surrounding the Presidente Plutarco Elías Calles (CETEPEC) coal-fired power plant, located on Mexico’s Pacific coast. The CALPUFF dispersion model was applied on the basis of the daily consumption of coal during 2013 for each generating unit in the power plant and considering the local scale. The established 300-ng/m3 annual average risk factor considered by the U.S. Department of Health and Human Services (U.S. DHHS) and Integrated Risk Information System (IRIS) must not be exceeded to meet satisfactory air quality levels. An area of 65 × 60 km was evaluated, and the results show that the risk level for mercury vapor was not exceeded because the annual average concentration was 2.8 ng/m3. Although the predicted risk level was not exceeded, continuous monitoring studies of GEM and of particulates in the atmosphere, soil, and water may be necessary to identify the concentration of this pollutant, specifically that resulting from coal-fired power plants operated in environmental areas of interest in Mexico. The dry mercury deposition was low in the study area; according to the CALPUFF model, the annual average was 1.40E−2 ng/m2/sec. These results represent a starting point for Mexico’s government to implement the Minamata Convention on Mercury, which Mexico signed in 2013.
Implications: The obtained concentrations of mercury from a bigger coal-fired plant in Mexico, through the application of the CALPUFF dispersion model by the mercury emissions, are below the level recommended according to the US Department of Health and Human Services and Integrated Risk Information System. These results provide evidence of important progress in the planning and installation to the future of monitoring mercury stations in the area of interest.
Introduction
Mercury (Hg) is present in coal that is used as a fuel in three power plants in Mexico. Thus, identifying the concentration and dispersion characteristics of Hg is necessary. The toxic pollutant is persistent and bioaccumulative, according to the U.S. Environmental Protection Agency (EPA, Citation2016a; EPA, Citation2016b). The National Atmospheric Deposition Program (NADP, Citation2016) is a cooperative effort between many different groups, including federal, state, tribal, and local governmental agencies, educational institutions, private companies, and nongovernmental agencies, to measure atmospheric deposition and study its effects on the environment. The Mercury Deposition Network and the Atmospheric Mercury Network are networks of NADP for pollution by mercury on different ecosystems in the United States since 1978.
In Mexico, gaseous elemental mercury (GEM), gaseous oxidized mercury (GOM), and particle bound mercury (PBM) are not monitored, and there are not standards or reference values available for outdoor environments. A reference value of 0.025 mg/m3 was established by the Secretaría del Trabajo y Previsión Social in Mexico (STPS, Citation2016) for indoor environments for 8 hr per day and 40 hr per week; however, this value cannot be applied to outside environments. The STPS performs functions similar to those of the U.S. Occupational Safety and Health Administration.
In accordance with the Minamata Convention (United Nations Environment Programme [UNEP], Citation2016) signed by Mexico in 2013, measures for the prevention, minimization, and control of GEM, GOM, and PBM have been proposed. These measures mandate that the most effective control technology systems be used in coal-fired power plants (CFPPs) to reduce Hg emissions and their impact on the atmosphere. A proposal that has not progressed thus far is regulating Hg content in coal used in coal power plants, in addition to monitoring GEM, GOM, and PBM Hg for regulatory purposes and conducting epidemiological studies on people exposed to mercury to obtain a reference value associated with GEM in Mexico.
Currently, the Sección de Contaminación Ambiental del Centro de Ciencias de la Atmósfera de la Universidad Nacional Autónoma de México (SCA-CCA-UNAM) laboratory is determining the Hg content of coal, ash, and slag from the Presidente Plutarco Elías Calles (CETEPEC) power plant, located on the Pacific coast of Mexico, which is required by Article 8 of the Minamata Convention (UNEP, Citation2016).
According to the U.S. EPA (Citation2011), the profile for GEM, GOM, and PBM is 87.41%, 12.52%, and 0.06%, respectively. They are emitted from a CFPP considering an electrostatic precipitator (ESP) as a control system when used with consumption of subbituminous coal for generating electricity. In 2013, CETEPEC emitted 1,942 kg of mercury, in this case 1,698 kg as GEM, 243 kg as GOM, and 1.2 kg as PBM, using an ESP as a control system for emissions. This is reasonable because the ESP is used for particulate matter control, not gaseous mercury.
According to the U.S. EPA (Citation2002, Citation2011), the mass balance involves only solid matter, which wasn’t combusted into the boiler; then, the composition is 11% bottom ash, and 5% boiler slag, with 84% as fly ash from the ESP.
Workers in the Mexican CETEPEC indicated, for experience in field, that the composition of solid matter that wasn’t combusted into the boiler is 16% bottom ash and slag together, and 84% as fly ash into the ESP. We made the calculations for mass balance as shown in the , considering the coal combusted annually in 2013 by Mexican CETEPEC to be 5,832,660 t, of which 10% corresponds to ash (fly ash, bottom ash, and slag). The mercury concentrations in coal, fly ash, and bottom ash and slag determined by SCA-CCA-UNAM were considered for mass balance too. The consumption of coal in 2013 was 5,832,660 t; then the 10% corresponding to ash was 583,266 t. The mass balance started here from ash types.
Table 1. Mass balance for mercury in Mexican CETEPEC.
In this case, we obtained the mercury residues (fly ash, bottom ash, and slag) of 74.6 kg, while the mercury amount previously at the combustion system is 1,942 kg. Thus, this indicates that 1,867 kg of mercury is output to the atmosphere. Thus, we can note that the ESP is a control system for particulate matter, not GEM, because around 90% of the GEM is output to the atmosphere. For this reason, in Mexico it is very important to work in the field with the mass balance to obtain real measures on mercury residues, considering the best technologies for this activity.
A U.S. Department of Health and Human Services study (U.S. DHHS, Citation1999) indicates that at an annual average reference value of 300 ng/m3 of Hg, people exhibit adverse health effects; the World Health Organization (WHO, Citation2000; International Programme on Chemical Safety [IPCS], Citation2003) reports that at an annual average value of 100–200 ng/m3, people may exhibit adverse health effects related to GEM exposure.
The U.S. DHHS study examined and interpreted available toxicological information and conducted epidemiological evaluations on hazardous substances to ascertain the levels of significant human exposure for the substance and the associated acute, subacute, and chronic health effects. This study also evaluated whether adequate information on the health effects of each substance is available or in the process of development to determine the levels of exposure that present a significant risk to human health for acute and chronic health effects. Furthermore, and where appropriate, the study recommended performing toxicological testing to identify the types or levels of exposure that may present a substantial risk of adverse health effects in humans.
The principal audiences for the toxicological profiles are health professionals at the federal, state, and local levels; interested private-sector organizations and groups; and members of the public.
Currently, air pollution affects the health of people when GEM concentrations are high. Intake fraction (iF) indicates the inhalation of a substance in a population attributable to a point source; iF depends on several factors, including stack height, temperature, and velocity of the exhaust gases, population, and meteorology: wind speed, stability, and mixing layer height (Li and Hao, Citation2003).
This study considers the spatial and temporal variability of daily GEM concentrations emitted from the CETEPEC power plant by using the CALPUFF dispersion model for the year 2013 considering a local scale. Daily mercury emissions for each generating unit of electricity were used.
Background
Currently, Eulerian models have been applied to identify the transportation, concentration, and interaction of Hg in the atmosphere at regional and global scales (Bergan et al., Citation1999; Xu et al., Citation2000; Lee et al., Citation2001; Peterson et al., 2001; Seigneur et al., Citation2001). Additionally, these models have a complex formulation to account for natural events that occur in the atmosphere.
The use of models to know the concentration of GEM, GOM, and PBM at global and local scale were carried out by Bieser et al. (Citation2014), Cohen et al. (Citation2016), Dastoor et al. (Citation2015), De Simone et al. (Citation2014), Gencarelli et al. (Citation2014), Jung et al. (Citation2009), Selin et al. (Citation2008), and Travnikov and IIyin (Citation2009).
An influential study (Ryaboshapko et al., Citation2002) compared the chemical processes of atmospheric Hg considering five photochemical models: the GKSS tropospheric chemistry model, the community multiscale air quality (CMAQ) model, the AER/EPRI Hg chemistry model, the MSC-E heavy metal model, and the IVL model of atmospheric chemistry model. Of these models, the CMAQ photochemical model is currently the most used for studying the interactions of Hg in the atmosphere. The five models differ in solubility, oxidation, absorption, and adsorption of Hg with other species of the atmosphere and the reaction kinetics of each model.
Ryaboshapko et al. (Citation2007) mentioned several models for identifying chemical reactions and the transformation of the vapor and aqueous phases of Hg in the atmosphere and its dispersion at regional, continental, and global scales: Eulerian (ADOM, CAMx, CMAQ-Hg, CMAQ, CTM-Hg, MSCE-HM, MSCE-HM-Hem, GEOS-Chem, ECHMERIT, MOZART, Dehm, and GLEMOS) and Lagrangian (HYSPLIT and MTCR-Hg) types.
Some studies show the importance of identifying mercury concentrations due to anthropogenic activity and natural sources on a global scale (Arctic Monitoring and Assessment Programme [AMAP]/UNEP, Citation2013a; AMAP/UNEP, Citation2013b; Pirrone et at. Citation2010). In this way we can identify the destination of GEM, GOM, and PBM for a specific region (Zhang et al. Citation2012).
The U.S. EPA (Citation2016c) has certified and approved the application of various models for air quality that are dispersion (AERMOD, CALPUFF, BLP, CALINE3, CTDMPLUS, and OCD) and photochemical (CMAQ, CAMx, REMSAD, and UAM-V) types; for weather study, the U.S. EPA has approved the use of MM5 and WRF models. Depending on research goals, the models described in the preceding paragraphs may be appropriate.
In Mexico, few studies on the measurement of the concentration of GEM, GOM, and PBM have been conducted. De la Rosa et al. (Citation2004) reported that the concentration of GEM at four monitoring sites—an urban area of Mexico City; a rural area in Huejutla, Hidalgo; on the Pacific coast in Puerto Angel, Oaxaca; and a mining area in Zacatecas City—were 9.81 1.32, 1.46, and 71.86 ng/m3, respectively. De la Rosa concluded that the measurements in Mexico City and the Zacatecas City mining area were higher than those reported in studies that used the same instrumentation at locations in Europe, North America, and the polar regions.
Mexico is a country rich in minerals that contain Hg: The average content of Hg in minerals found in Mexican deposits ranges from 0.33% to 10%. The most abundant Hg reserves are located in the states of Zacatecas, Querétaro, San Luis Potosí, Durango, and Guerrero, in addition to the states of Chihuahua, Guanajuato, Hidalgo, Jalisco, Mexico, Michoacán, Morelos, Nayarit, Oaxaca, Puebla, Sonora, and Tlaxcala (Commission for Environmental Cooperation [CEC], Citation2013; Servicio Geológico Mexicano [SGM], Citation2016).
According to the information provided by the Secretaría de Economía of Mexico, 314 Hg mines were reported in 2010, whereas the Comisión de Fomento Minero reported the existence of 1,119 mining projects in 1968. These two sources were consulted to determine the physical and legal status of each mine, as well as its current condition (exhausted or productive), and to identify the entity responsible for its operation.
The formal primary production of Hg in Mexico began in the 1840s. According to historical and official information, from 1840 to 1994, Mexico produced 35,555 tons of Hg (an annual average of 229 tons). The highest production years were 1942 and 1955, with 1,117 and 1,030 tons produced, respectively, whereas 1994 was the last year of production when the lowest quantity, estimated at 11 tons (CEC, Citation2013), was produced.
Official information from the CEC (Citation2013) indicates that a reserve of 42,000 tons of Hg could be held in four mines located in Zacatecas, Querétaro, and the State of Mexico. However, considering the existence of more than 300 declared mines, a larger estimate would be required to obtain a total estimate of Mexico’s Hg reserves.
Anne Hansen and David Gay (Citation2013) obtained mercury concentrations in wet deposition particles at two sampling sites of Mexico: 8.2 ng/L at Huejutla, Hidalgo, and 7.9 ng/L at Puerto Angel, Oaxaca, applying the NADP methodologies for this activity (NADP, Citation2016), and these results indicate were much lower than those observed at the US Gulf Coast Mercury Deposition Network sites (Prestbo and Gay, Citation2009).
Study area
CETEPEC is located at a latitude of 17°59’01.14” N and a longitude of 102°06’56” W on the Mexican Pacific Coast, 14 km from the Port of Lázaro Cárdenas. Imported coal reaches the terminal of this port, and is then transported to seven power-generation units via covered conveyors at a rate of 170 t/h. The yard where coal is stored has an area of 54 ha and a capacity of 1.9 million metric tons. Annually, the plant consumes between 6 and 7 million metric tons of imported coal, mainly from the United States and Australia.
According to the technical information provided by CETEPEC administration, the combustion system is pulverized coal accompanied by a system of emission control of low NOx and electrostatic precipitators for each electric generating unit; a control system for SO2 and washers of output gases for the retention of Hg are not applied in Mexico. Finally, CETEPEC does not perform the elemental analysis of coal discharge; thus, this paper is relevant for Mexico for the identification of the concentration of GEM in areas surrounding CETEPEC and can facilitate the development of measures for the prevention, minimization, and control for mercury and other toxic pollutants.
Methodology
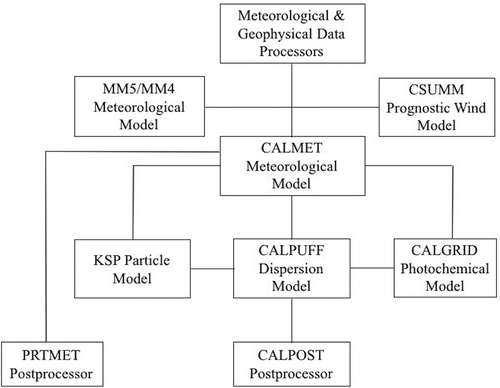
In general, CALPUFF is a nonstationary model-dependent Gaussian puff model that can analyze the dispersion of primary, secondary, and toxic pollutants because it is considered a multispecies and multilayer model for meteorological studies. It also possesses two module-integrated chemical processing for SO2 and NOx (RIVAD and MESOPUFF). The dispersion in CALPUFF is performed in time and space, considering the variation of meteorological parameters; the scale used by the model ranges from hundreds of kilometers to thousands of kilometers. The algorithm CALPUFF includes effects of a terrain, obstacles to air movement, plume effects, wet and dry deposition, chemical transformation, effects of air over the vertical, and effects of visibility (EPA, Citation2005; Scire et al., Citation2000). presents the steps of the CALPUFF model.
We used the CALPUFF modeling system (v.4.0 Lakes Environmental Software) to determine GEM concentrations a local scale. The model consists of three main components: (1) CALMET, the meteorology preprocessor that uses a meteorological data mesoscale to generate wind fields from an MM5 model; (2) CALPUFF, which is the model that simulates air dispersion; and (3) CALPOST, which is a postprocessing package that compiles CALPUFF simulations.
In the simplest terms, CALMET is a meteorological model that develops hourly wind and temperature fields on a three-dimensional gridded modeling domain. Associated two-dimensional fields such as mixing height, surface characteristics, and dispersion properties are also included in the files produced by CALMET. CALPUFF is a transport and dispersion model that advects “puffs” of material emitted from modeled sources, simulating dispersion and transformation processes along the way. In doing so, it typically uses the fields generated by CALMET, or, as an option, it may use simpler nongridded meteorological data much like existing plume models. Temporal and spatial variations in the meteorological fields selected are explicitly incorporated in the resulting distribution of puffs throughout a simulation period. The primary output files from CALPUFF contain either hourly concentrations or hourly deposition fluxes evaluated at selected receptor locations. CALPOST is used to process these files, producing tabulations that summarize the results of the simulation, identifying the highest and second highest 3-hr average concentration at each receptor, for example. When performing visibility-related modeling, CALPOST uses the concentrations from CALPUFF to compute extinction and related measures of visibility, reporting these for selected averaging times and locations (Scire et al., Citation2000).
Domain of simulation
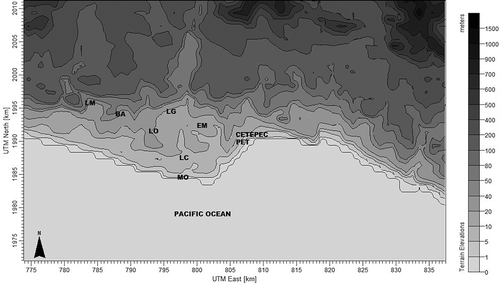
The map used in this study was a Universal Transverse Mercator (UTM) Reference Ellipsoid WGS-84, Global Coverage, UTM Zone 13 North America map with coordinates 17°59’01.14” N and 102°06’56” W. The mesh used was 65 × 60 km and the vertical levels for meteorology of the study area were 0, 20, 40, 80, 160, 320, 640, 1200, 2000, 3000, and 4000 m. The radius of influence was 55 km depending on terrain features, and we considered simulation in a complex terrain to determine the concentration of GEM and its dry deposition. shows the map used for this study.
The communities near CETEPEC are La Mira (referred to as LM in the figure), Buenos Aires (BA), La Orilla (LO), Las Guacamayas (LG), El Mirador (EM), Lázaro Cárdenas (LC), Melchor Ocampo (MO), and Petacalco (PET).
Input data, CALPUFF
Terrain and land data
Terrain data (WGS-84, GTOPO30, resolution: 900 m) and land data employed ESR-S, GLCC North America, with a resolution of 1 km, loaded from the webGIS CALPUFF model.
Meteorological data
The weather data used in this study were processed in the Fifth-Generation National Center for Atmospheric Research, NCAR/Penn State Mesoscale Model (MM5), by using the data reanalysis of the U.S. National Centers for Environmental Predictions (NCEP, Citation2016) because Mexico does not collect adequate information related to the meteorological parameters of the study area. The meteorology-laden NCEP continuously collects observational data from the Global Telecommunications System, and other sources, for many analyses. These Operational Global Analysis data are on 1-degree by 1-degree grids prepared operationally every 6 hr. In this study, we have used data file downloads from 2007 to current on 2013 monthly, so we used the meteorological data per day as indicated in the NCEP. These data were subsequently processed in MM5, and they then were input into the CALMET model to facilitate identification of wind vectors in area of interest through CALPUFF model dispersion.
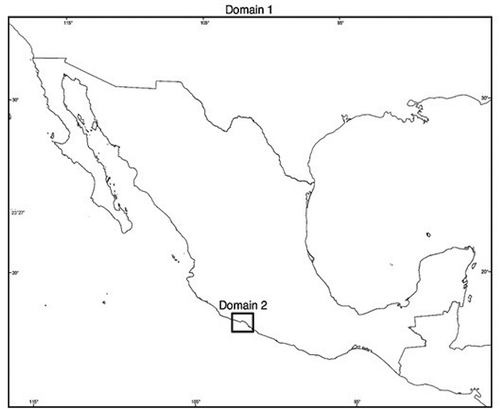
The design of domains in the MM5 model for the processing of the meteorological parameters of the study area consisted of a main domain (Domain 1) of 120 × 170 points with a resolution of 24 km per point and a nested domain (Domain 2) of 31 × 31 points with a resolution of 8 km per point. The CETEPEC power plant was used in Domain 2. shows the coverage of the domains used in this study.
Mercury emission data
Two methods to determine mercury emissions to the atmosphere were developed by the U.S. EPA. The first employs the mercury emission factor in the Compilation of Air Pollutant Emission Factors (AP-42) Volume I, Fifth Edition, Chapter I External Combustion Sources, Section 1.1. Bituminous and Subbituminous Coal Combustion (EPA, Citation2015), and eq 1:
If a coal-fired power plant uses a control system that reduces mercury emissions, the emission factor to be used is then in eq 2:
If a coal-fired power plant does not use a control system for mercury emissions, the emission factor to be used is 16 lb/1012 BTU, corresponding to
The second method developed by the U.S. EPA uses the mercury content in the combusted coal and the modified emission factor (EPA, Citation1997), from which eq 3 is obtained for mercury emissions:
The control systems used by coal-fired power plants in Mexico include electrostatic precipitators with a heating slide of value 1, as recommended in U.S. EPA-developed tables; however, mercury emissions are not retained in the control system because mercury is emitted as vapor.
To determine mercury emissions from CETEPEC for 2013, we used eq 4 according to U.S. EPA methodology and the mercury content in coal of 0.333 mg/kg as determined by Mugica et al. in 2003:
Stacks and building downwash data
The data entry module for CALPUFF consisted of the UTM coordinates, height, and diameter, and the velocity and temperature of exhaust gases for each generating unit; likewise, the pollutant to study entry, Hg, and emissions of this pollutant per day and for each generating unit of the 2013 were input. For building downwash, we used aerial images to identify the dimensions of the structures near each stack that can affect the stacks. Two structures for each stack were identified: one of 40 × 50 m and another of 60 × 80 m. shows the parameters entered into the CALPUFF module.
Table 2. Input parameters used in CALPUFF for each unit of the CETEPEC power plant.
Mercury and deposition data
To determine the GEM, we used mercury emissions per day and per generating unit. To determine dry mercury deposition, we used its diffusivity (0.1509 cm2/sec), Alpha star (1), Reactivity (8), and Henry’s law coefficient (0.04), according to the CALPUFF model’s indications.
Wet mercury deposition was not simulated applying the CALPUFF model because Mexico does not collect completely the meteorological information per day. To determine wet mercury deposition in this study, we have considered the information available from Schroeder et al. (Citation1991) and Seigneur et al. (Citation1994) with respect to the typical concentrations of mercury species in the atmospheric environment. Seigneur et al. (Citation1994) indicate a GEM typical liquid-phase concentration from 6 to 27E-3 ng/L as estimated from gas-phase air concentrations by means of Henry’s law.
Plume rise and dispersion data
We employed partial plume penetration in the simulation because the plumes of each generating unit often frequently interact with the capping inversion at the top of the mixed layer on the coast. For dispersion, we used the puff option because it more accurately represents pollutant emissions.
Finally, the CALPOST module was used to determine the 24-hr average concentrations and dry deposition of mercury. This resulted in 365 concentrations of mercury and dry deposition data.
Results and discussion
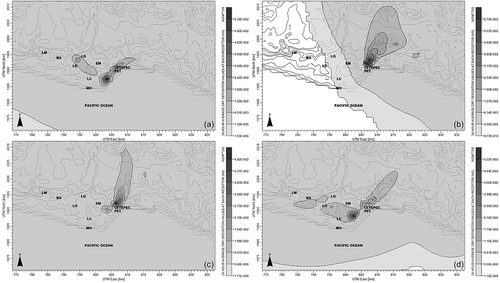
By simulating the CETEPEC power plant by using CALPUFF, 365 concentrations of GEM were obtained as well as in mercury dry deposition. shows the days in April when GEM concentrations were the highest in a 24-hr period; GEM concentrations are directed toward the sea and soil. Furthermore, the GEM concentration was low for the environmental compartments; however, future studies are necessary to determine the effect of this mercury concentration in soil and water on the health of people.
Figure 4. Average of GEM concentration for 24 hr on the days with the highest concentration in 2013: (a) 4/2/2013, (b) 4/18/2013, and (c) 4/19/2013.
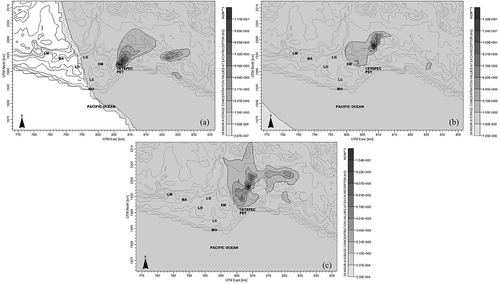
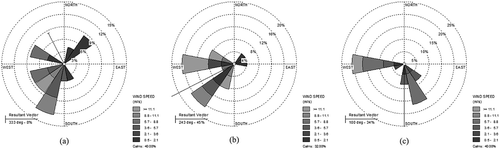
In , GEM concentrations per day obtained from the simulation indicated that the highest concentrations occurred on April 2, 18, and 19 with levels of 11.1, 14.0, and 10.4 ng/m3, respectively. The high GEM levels in April were attributable to the calm weather on those days. shows the wind rose plot for these days, which shows the low wind speeds—the wind speed averages for April 2, 18, and 19 were 2.92, 4.18, and 3.09 m/sec, respectively.
Figure 5. GEM concentrations obtained from the simulation in 2013 from January 1, 2013, to December 31, 2013.
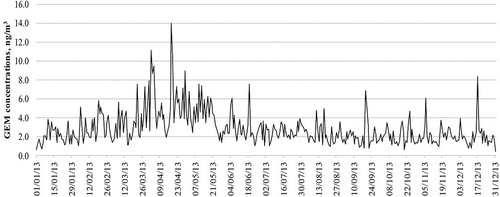
Figure 6. Wind rose plot for days when the mercury concentrations were the highest: (a) 4/2/2013, (b) 4/18/2013, and (c) 4/19/2013.
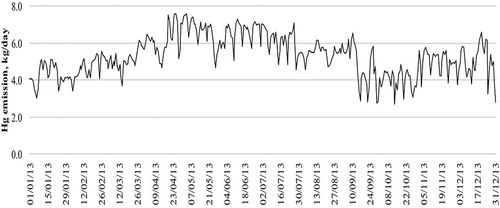
The mercury emissions per day are presented in . The highest mercury emissions were on April 19, 24, and 25 and May 2 and 3 with a level above 7.5 kg/day. Interestingly, the highest GEM concentration does not correspond with the highest mercury emission because of the calmness of the wind; however, on April 19, a higher GEM concentration corresponded with a higher mercury emission.
Another reason for high GEM concentrations is the height of the mixing layer for April 2, 18, and 19. The CALPUFF model indicated that the heights of the mixing layer were 160, 180, and 160 m for these dates, respectively. This indicates that the emissions from each generating unit emanated vertically and that mercury accumulated because of the calmness of the wind on those days. The temperature for those days was 299 K.
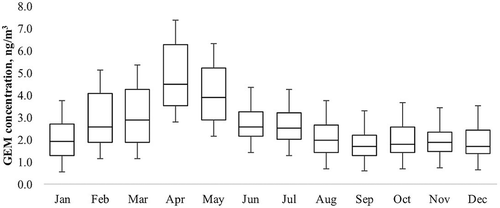
lists the statistical parameters of GEM concentrations from the CETEPEC plant. The most representative cases are those of February, March, April, and May, where 75% of data corresponded to a value of 1.51, 1,36, 1.79, and 1.30 ng/m3, respectively. For consecutive months to December 2013, the data corresponded to a value of an order of 10E−1 ng/m3; for the month of January 2014, the data corresponded to 0.75 ng/m3. provides the trends of 365 concentrations of GEM obtained during 2013 for each month; the concentration increased in the months of February to May because of an increase in the demand for electricity in this period, whereas it decreased from June until the year’s end.
Table 3. Statistical parameters of mercury concentrations from January 1, 2001, to December 31, 2012.
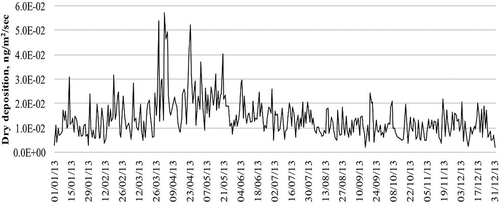
Dry mercury deposition is presented in according to the CALPUFF model. The days having the highest dry mercury deposition were March 28 with 5.4E−2 ng/m2/sec and April 2, 4, and 23 with 5.7E−2, 4.9E−2, and 5.2E−2 ng/m2/sec, respectively. The CALPUFF model includes the following features for determining dry mercury deposition: diffusivity, solubility, toxicity, vegetation type, and atmospheric stability. The velocity of dry mercury deposition was 7.5E−3 m/sec on March 28 and 5.1E−3, 5.2E−3, and 9.4E−3 m/sec on April 2, 4, and 23, respectively. The dispersion of dry mercury deposition is shown in .
Figure 10. Average dry mercury deposition for 24 hr on the days with the highest dry deposition in 2013: (a) 3/28/2013, (b) 4/2/2013, (c) 4/4/2013, and (d) 4/23/2013.
Wet mercury deposition was determined by month applying the GEM typical concentration in liquid-phase of 27E-3 ng/L according to Schroeder et al. (Citation1991) and Seigneur et al. (Citation1994). The results obtained are shown in ; we are not applying the CALPUFF model for this activity because Mexico does not collect meteorological data completely per day. According to , we note that in September there is a higher wet mercury deposition of 23.19 ng/L, and in this month is the maximum precipitation of 859 mm. Wet deposition mercury for the year was 47.11 ng/L with a precipitation level of 1745 mm.
Table 4. Wet mercury deposition by month in 2013 on the study region, considering a GEM typical concentration in liquid phase of 27 ng/L.
Conclusion
This study reports the temporal and spatial variability of GEM concentrations from the CETEPEC plant, as well as dry mercury deposition over a period of 365 days in 2013, by applying the CALPUFF dispersion model and employing the actual daily consumption of fuel for each electricity generating unit.
The annual average concentration of GEM obtained in 2013 was 2.8 ng/m3, which does not exceed the risk level (300 ng/m3) established by the U.S. Department of Health and Human Services and the EPA’s Integrated Risk Information System. This indicates that the air quality is satisfactory, according to the CALPUFF model.
The annual average dry mercury deposition was low—1.4E−2 ng/m2/sec—and its deposition velocity was low, of an order of 10−3 m/sec, indicating no concern attributed to Hg deposition in the study area, according to the CALPUFF model; however, monitoring this parameter in water, sediments, and soil is essential, as well as the GOM and PBM in the study region.
The annual precipitation in the study region was 1,745 mm, and its wet mercury deposition was 47.11 ng/L; the maximum level of wet mercury is present in September at 23.19 ng/L with a precipitation level of 859 mm. Our expectation in running the CALPUFF model to determine wet deposition mercury is that it will be similar to this study, but it is necessary to monitor wet mercury deposition in the study region applying the NADP methodologies.
Epidemiological studies are relevant for the health of people and the environmental compartments in Mexico to identify whether GEM concentrations reported in this study cause any effects.
GEM, GOM, and PBM monitoring in the study region is necessary in areas where the highest GEM concentrations were determined according to the model. Verifying mercury concentrations and dry deposition according to this study is recommended.
The results obtained in this study contribute to information regarding the toxic, persistent, and bioaccumulative pollutant mercury in Mexico and are relevant to the Minamata Convention. Furthermore, the results reflect an increase in Hg concentrations in Mexico because of the operations of the CETEPEC plant, which is the cause of some of the highest levels of emissions in Latin America.
Acknowledgment
The authors acknowledge the collaboration of Dr. Elías Granados Hernández, Ana Luisa Alarcón Jiménez, María del Carmen Torres Barrera, Pablo Sánchez Álvarez, María de los Ángeles López Portillo Guzmán of Sección de Contaminación Ambiental del Centro de Ciencias de la Atmósfera de la UNAM; Norma Popoca Méndez of the Comisión Federal de Electricidad; and Dr. Sergio A. Guerra, Senior Environmental Engineer of Wind Engineering & Air Quality Consultants in Colorado. We thank CONACyT for providing me a scholarship support for the doctoral study, and Dr. Enrique Cesar Valdez, Dr. Armando Aguilar Marquez, Dr. Agustín García Reynoso, and Dr. Víctor Hugo Páramo Figueroa for the revisions and suggestions for this manuscript.
Additional information
Notes on contributors
Gilberto Fuentes García
Gilberto Fuentes García is a doctoral student at Universidad Nacional Autónoma de México (UNAM) of Environmental Pollution Area in México City.
Humberto Bravo Álvarez
Humberto Bravo Álvarez and Rodolfo Sosa Echeverría are research professors at Centro de Ciencias de la Atmósfera of the UNAM, and Sergio Rosas de Alba was responsible in the area of environmental control at Comisión Federal de Electricidad in México City.
Rodolfo Sosa Echeverría
Humberto Bravo Álvarez and Rodolfo Sosa Echeverría are research professors at Centro de Ciencias de la Atmósfera of the UNAM, and Sergio Rosas de Alba was responsible in the area of environmental control at Comisión Federal de Electricidad in México City.
Sergio Rosas de Alba
Humberto Bravo Álvarez and Rodolfo Sosa Echeverría are research professors at Centro de Ciencias de la Atmósfera of the UNAM, and Sergio Rosas de Alba was responsible in the area of environmental control at Comisión Federal de Electricidad in México City.
Víctor Magaña Rueda
Víctor Magaña Rueda and Ernesto Caetano Dosantos are research professors at Instituto de Geografía of the UNAM in México City, and Gustavo Vázquez Cruz is a professor of meteorology in the same institution.
Ernesto Caetano Dosantos
Víctor Magaña Rueda and Ernesto Caetano Dosantos are research professors at Instituto de Geografía of the UNAM in México City, and Gustavo Vázquez Cruz is a professor of meteorology in the same institution.
Gustavo Vázquez Cruz
Víctor Magaña Rueda and Ernesto Caetano Dosantos are research professors at Instituto de Geografía of the UNAM in México City, and Gustavo Vázquez Cruz is a professor of meteorology in the same institution.
References
- Arctic Monitoring and Assessment Programme/United Nations Environment Programme (AMAP/UNEP). 2013a. Technical background report for the global mercury assessment. Arctic Monitoring and Assessment Programme, Oslo, Norway, and UNEP Chemicals Branch, Geneva, Switzerland. http://www.amap.no/documents/doc/technical-background-report-for-the-global-mercury-assessment-2013/848 (accessed November 2016).
- Arctic Monitoring and Assessment Programme/United Nations Environment Programme (AMAP/UNEP). 2013b. Geospatially distributed mercury emissions dataset 2010v1. http://www.amap.no/mercury-emissions/datasets ( accessed December 2016).
- Bennett, D.H., T.E. McKone, J.S. Evans, W.W. Nazaroff, M.D. Margni, O. Jolliet, and K.R. Smith. 2002. Defining intake fraction. Environ. Sci. Technol. 36:207A–11A. doi:10.1021/es0222770.
- Bergan T., L. Gallardo, and H. Rodhe. 1999. Mercury in the global troposphere: A three-dimensional model study. Atmos. Environ. 33:1575–85. doi:10.1016/S1352-2310(98)00370-7.
- Bieser, J., F. De Simone, C. Gencarelli, B. Geyer, I. Hedgecock, V. Matthias, O. Travnikov, and A. Weigelt. 2014 A diagnostic evaluation of modeled mercury wet depositions in Europe using atmospheric speciated highresolution observations, Environ. Sci. Pollut. Res. 21:9995–10012. doi:10.1007/s11356-014-2863-2, 2014.
- Cohen, M.D., R.R. Draxler, R.S. Artz, M. Gustin, Y.-J. Han, T.M. Holsen, D. Jaffe, P. Kelley, H. Lei, C. Loughner, et al. 2016. Modeling the global atmospheric transport and deposition of mercury to the Great Lakes. Elementa Sci. Anthropocene 4:000118. doi:10.12952journal.elementa.999118.
- Commission for Environmental Cooperation (CEC). 2013. An assessment of primary and secondary mercury supplies in Mexico. Montreal, QC, Canada: Commission for Environmental Cooperation. http://www.cec.org (accessed November 2016).
- Dastoor, A., A. Ryzhkov, D. Durnford, I. Lehnherr, A. Steffen, and H. Morrison. 2015. Atmospheric mercury in the Canadian Arctic. Part II. Insight from modeling. Sci. Total Environ. 509–510:16–27. doi:10.1016/j.scitotenv.2014.10.112.
- De la Rosa, D.A., T. Volke-Sepúlveda, G. Solórzano, C. Green, R. Tordon, and S. Beachamp. 2004. Survey of atmospheric total gaseous mercury in Mexico. Atmos Environ. 38:4839–46. doi:10.1016/j.atmosenv.2004.06.013
- De Simone, F., C.N. Gencarelli, I.M. Hedgecock, and N. Pirrone. 2014. Global atmospheric cycle of mercury: a model study on the impact of oxidation mechanisms. Environ. Sci. Pollut. Res. 21:4110–23. doi:10.1007/s11356-013-2451-x
- Gencarelli, C.N., F. De Simone, I.M. Hedgecock, F. Sprovieri, and N. Pirrone. 2014. Development and application of a regional-scale atmospheric mercury model based on WRF/Chem: A Mediterranean area investigation. Environ. Sci. Pollut. Res. 21:4095–109. doi:10.1007/s11356-013-2162-3
- Hansen, A.M., and D.A. Gay. 2013. Observations of mercury wet deposition in Mexico. Environ. Sci. Pollut. Res. 20:8316–25. doi:10.1007/s11356-013-2012-3.
- International Programme on Chemical Safety (IPCS). 2003. Concise international chemical assessment document 50. Elemental mercury and inorganic mercury compounds: human health aspects, Geneva. http://www.who.int/ipcs/publications/cicad/en/cicad50.pdf (accessed December 2015).
- Jung, G., I.M. Hedgecock, and N. Pirrone. 2009. ECHMERIT V1.0—A new global fully coupled mercury-chemistry and transport model. Geosci. Model Dev. 2:175–95. doi:10.5194/gmd-2-175-2009.
- Lee, D.S., E. Nemitz, D. Fowler, and R.D. Kingdon. 2001. Modeling atmospheric mercury transport and deposition across Europe and the UK. Atmos. Environ. 35:5455–66. doi:10.1016/S1352-2310(01)00284-9.
- Li, J., and J. Hao. 2003. Application of intake fraction to population exposure estimates in Hunan Province of China, J. Environ. Sci. Health Part A 38(6):1041–54. doi:10.1081/ESE-120019862.
- Mugica, V., M.A. Amador, M. Torres, and J. Figueroa. 2003. Mercurio y metales pesados tóxicos en cenizas provenientes de procesos de combustión e incineración. Rev. Int. Contam. Ambient 19(2):93–100. http://www.revistascca.unam.mx/rica/index.php/rica/article/viewFile/25141/23637 ( accessed August 2014).
- National Atmospheric Deposition Program (NADP). 2016. http://nadp.sws.uiuc.edu (accessed January 2016).
- National Centers for Environmental Prediction. 2016. NCEP FNL Operational Model Global Tropospheric Analyses, continuing from July 1999. http://rda.ucar.edu/datasets/ds083.2 ( accessed August 2013).
- Petersen, G., R. Bloxam, S. Wong, J. Munthe, O. Krüger, S.R. Schmolke, and A.V. Kumar. 2001. A comprehensive Eulerian modeling framework for airborne mercury species: Model development and applications in Europe. Atmos. Environ. 35:3063–74. doi:10.1016/S1352-2310(01)00110-8.
- Pirrone, N., S. Cinnirella, X. Feng, R.B. Finkelman, H.R. Friedli, J. Leaner, R. Mason, A.B. Mukherjee, G.B. Stracher, D.G. Streets, and K. Telmer. 2010. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 10:5951–64. doi:10.5194/acp-10-5951-2010.
- Prestbo, E.M., and D.A. Gay. 2009. Wet deposition of mercury in the US and Canada, 1996–2005: Results and analysis of the NADP mercury deposition network (MDN). Atmos. Environ. 43(27):4223–33. doi:10.1016/j.atmosenv.2009.05.028
- Ryaboshapko, A., R. Bullock, J. Christensen, M. Cohen, A. Dastoor, I. Ilyin, G. Petersen, D. Syrakov, R. Artz, D. Davignon, R. Draxer, and J. Munthe. 2007. Intercomparison study of atmospheric mercury models, 1. Comparison of models with short-term measurements. Sci. Total Environ. 376:228–40. doi:10.1016/j.scitotenv.2007.01.072.
- Ryaboshapko A., R. Bullock, R. Ebinghaus, I. Ilyin, K. Lohman, J. Munthe, G. Petersen, C. Seigneur, and I. Wängberg. 2002. Comparison of mercury chemistry models. Atmos. Environ. 36:3881–98. doi:10.1016/S1352-2310(02)00351-5.
- Schroeder, W.H., G. Yarwood, and H. Nikki. 1991. Transformation processes involving mercury species in the atmosphere. Water Air Soil Pollut. 56:653–66. doi:10.1007/BF00342307.
- Scire, J.S., D.G. Strimaitis, and R.J. Yamartino. 2000. A user’s guide for the CALPUFF dispersion model (version 5.0). Earth Tech, Inc., Concord, MA. http://www.src.com/calpuff/download/CALPUFF_UsersGuide.pdf (accessed February 2014).
- Seigneur, C., J. Wrobel, and E. Constantinou. 1994. A chemical kinetic mechanism for atmospheric inorganic mercury. Environ. Sci. Technol. 28:1589–97. doi:10.1021/es00058a009
- Seigneur, C., P. Karamchandani, K. Lohman, and K. Vijayaraghavan. 2001. Multiscale modeling of the atmospheric fate and transport of mercury. J. Geophys. Res. 106:27795–809. doi:10.1029/2000JD000273.
- Selin, N.E., D.J. Jacob, R.M. Yantosca, S. Strode, L. Jaeglé, and E.M. Sunderland. 2008. Global 3-D land–ocean–atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochem. Cycles 22. doi:10.1029/2007GB003040.
- Servicio Geológico Mexicano (SGM). 2016. http://www.gob.mx/sgm ( accessed November 2016).
- Secretaría del Trabajo y Previsión Social (STPS). 2016. NOM-010-STPS-2014: Agentes Químicos Contaminantes del Ambiente Laboral. Ciudad de México. http://asinom.stps.gob.mx:8145/upload/nom/.pdf ( accessed January 2016).
- Travnikov, O., and L. Ilyin. 2009. The EMEP/MSC-E mercury modeling system. In Mercury fate and transport in the global atmosphere: Emissions, measurements, and models, ed. N. Pirrone and R.P. Mason, 571–87. New York, NY: Springer. doi:10.1007/978-0-387-93958-2_20.
- United Nations Environment Programme (UNEP). 2016. Minamata Convention. www.unep.org and www.mercuryconvention.org (accessed January 2016).
- U.S. Department of Health and Human Services (U.S. DHHS). 1999. Toxicological profile for mercury. Public Health Service, Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf ( accessed September 2015).
- U.S. Environmental Protection Agency (EPA). 1997. Environmental Protection Agency. Locating and estimating air emissions from sources of mercury and mercury compounds. December. http://www.epa.gov/ttnchie1/le/mercury.pdf (accessed March 1, 2015).
- U.S. Environmental Protection Agency (EPA). 2002. Control of mercury emissions from coal-fired electric utility boilers: Interim report. EPA-600/R-01-109.
- U.S. Environmental Protection Agency (EPA). 2005. Rules and regulations. Revision to the guideline on air quality models. 40 CFR, Federal Register part 51, vol. 70, no. 216. http://www3.epa.gov/scram001/guidance/guide/appw_05.pdf ( accessed August 2014).
- U.S. Environmental Protection Agency (EPA). 2011. Electric generating utility mercury speciation profiles for the Clean Air Mercury Rule. EPA-454/R11-010.
- U.S. Environmental Protection Agency (EPA). 2015. Bituminous and subbituminous coal combustion. In Compilation of air pollutant emission factors (AP-42), Vol. I, 5th ed., chap. I. www.epa.gov/ttnchie1/ap42 (accessed January 2015).
- U.S. Environmental Protection Agency (EPA). 2016a. Mercury. www.epa.gov/mercury (accessed January 2016).
- U.S. Environmental Protection Agency (EPA). 2016b. National emissions standards for hazardous air pollutants compliance monitoring. http://www.epa.gov/ttn/atw/188polls.html (accessed January 2016).
- U.S. Environmental Protection Agency (EPA). 2016c. Technology transfer network support center for regulatory atmospheric modeling. http://www.epa.gov/scram001 (accessed January 2016)
- World Health Organization (WHO). 2000. Air quality guidelines for Europe. Copenhagen, Denmark: WHO.
- Xu X., X. Yang, D.R. Miller, J.J. Helble, and R.J. Carley. 2000. A regional scale modeling study of atmospheric transport and transformation of mercury. I. Model development and evaluation. Atmos. Environ. 34:4933–44. doi:10.1016/S1352-2310(00)00228-4.
- Zhang L., P. Blanchard, D.A. Gay, E.M. Prestbo, M.R. Risch, D. Johnson, J. Narayan, R. Zsolway, T.M. Holsen, E.K. Miller, et al. 2012. Estimation of speciated and total mercury dry deposition at monitoring locations in eastern and central North America. Atmos. Chem. Phys. 12:4327–40. doi:10.5194/acp-12-4327-2012.