ABSTRACT
In recent years, many air quality monitoring programs have favored measurement of particles less than 2.5 µm (PM2.5) over particles less than 10 µm (PM10) in light of evidence that health impacts are mostly from the fine fraction. However, the coarse fraction (PM10-2.5) may have independent health impacts that support continued measurement of PM10 in some areas, such as those affected by road dust. The objective of this study was to evaluate the associations between different measures of daily PM exposure and two daily indicators of population health in seven communities in British Columbia, Canada, where road dust is an ongoing concern. The measures of exposure were PM10, PM2.5, PM10-2.5, PM2.5 adjusted for PM10-2.5, and PM10-2.5 adjusted for PM2.5. The indicators of population health were dispensations of the respiratory reliever medication salbutamol sulfate and nonaccidental mortality. This study followed a time-series design using Poisson regression over a 2003–2015 study period, with analyses stratified by three seasons: residential woodsmoke in winter; road dust in spring; and wildfire smoke in summer. A random-effects meta-analysis was conducted to establish a pooled estimate. Overall, an interquartile range increase in daily PM10-2.5 was associated with a 3.6% [1.6, 5.6] increase in nonaccidental mortality during the road dust season, which was reduced to 3.1% [0.8, 5.4] after adjustment for PM2.5. The adjusted coarse fraction had no effect on salbutamol dispensations in any season. However, an interquartile range increase in PM2.5 was associated with a 2.7% [2.0, 3.4] increase in dispensations during the wildfire season. These analyses suggest different impacts of different PM fractions by season, with a robust association between the coarse fraction and nonaccidental mortality in communities and periods affected by road dust. We recommend that PM10 monitoring networks be maintained in these communities to provide feedback for future dust mitigation programs.
Implications: There was a significant association between daily concentrations of the coarse fraction and nonaccidental mortality during the road dust season, even after adjustment for the fine fraction. The acute and chronic health effects associated with exposure to the coarse fraction remain unclear, which supports the maintenance of PM10 monitoring networks to allow for further research in communities affected by sources such as road dust.
Introduction
Short- and long-term exposure to particulate matter (PM) pollution is detrimental to cardiopulmonary health. Short-term exposure to ambient PM is associated with increased risk of sudden cardiac arrest and arrhythmia, as well as exacerbations of asthma and other acute respiratory outcomes (World Health Organization Europe, Citation2013). Long-term exposure to PM is associated with increased risk of developing chronic cardiovascular and respiratory diseases, in addition to the onset of lung cancer (Anderson et al., Citation2013; Hoek et al., Citation2013; World Health Organization Europe, Citation2013). As such, monitoring ambient PM concentrations and studying their effects on cardiopulmonary health is a priority for environmental regulators and public health agencies around the world.
Most air quality monitoring programs measure mass concentrations of PM2.5 and/or PM10 (airborne particles with diameters ≤2.5 µm and ≤10 µm, respectively). When these measurements are regressed against health outcomes, studies have typically found that exposure to PM2.5 is more strongly associated with cardiopulmonary health outcomes than PM10 (EPA, Citation2009; World Health Organization Europe, Citation2013). Although PM10 exposure is also correlated with the same health outcomes, these associations may be confounded by the fact that PM10 measurements include particles in the PM2.5 range, also known as the fine fraction. To differentiate between the effects of PM10 and PM2.5, it is necessary to investigate the independent effects of PM10-2.5, also known as the coarse fraction.
Although some instruments can measure the coarse fraction directly (Misra et al., Citation2001; ThermoFisher Scientific, Citation2016), most regulatory air quality monitoring networks measure PM10 and PM2.5 separately. As such, PM10-2.5 is typically estimated as the difference between PM10 and PM2.5, which requires concurrent and co-located PM10 and PM2.5 monitors. It follows that PM10-2.5 data are less available for research when compared with PM10 or PM2.5 alone. Furthermore, most established air quality monitoring programs began to favor monitoring PM2.5 over PM10 when strong evidence emerged around the health impacts of the fine fraction. Indeed, many agencies chose not to measure both PM10 and PM2.5 at all monitoring sites, given the expenses associated with purchasing and maintaining both instruments. This is problematic because there remains uncertainty about the true health impacts of PM10-2.5, as evidenced by conflicting results in the literature (Adar et al., Citation2014; Brunekreef and Forsberg, Citation2005; Health Canada, Citation2016; Hoek et al., Citation2013; EPA, Citation2009; World Health Organization Europe, Citation2013). The evidence is further convoluted by the different constituents of PM10-2.5, which can comprise any combination of sea salt, fugitive agricultural emissions, windblown crustal dust, or road dust (Health Canada, Citation2016; Wilson and Suh, Citation1997; World Health Organization Europe, Citation2013). Similarly, PM2.5 can comprise any combination of biomass smoke, industrial emissions, or vehicular exhaust (Wilson and Suh, Citation1997). The importance of differentiating between PM size fractions and constituent material is being increasingly recognized.
Health Canada published a detailed assessment of the effects of ambient PM10-2.5 on morbidity and mortality in 2016 (Health Canada, Citation2016). In studies of respiratory diseases or medication dispensations, PM2.5 and PM10-2.5 had similar associations (Burnett et al., Citation1997; Chimonas and Gessner, Citation2007; Gordian and Choudhury, Citation2003; Lin et al., Citation2002; McCormack et al., Citation2011; McCormack et al., Citation2009; Millstein et al., Citation2004), with two exceptions (McConnell et al., Citation2003; Slaughter et al., Citation2005). Several studies found that exposure to the coarse fraction was significantly associated with increased mortality (Atkinson et al., Citation2010; Castillejos et al., Citation2000; Chen et al., Citation2011; Cifuentes et al., Citation2000; Mallone et al., Citation2011; Mar et al., Citation2000; Meister et al., Citation2012; Ostro et al., Citation2000; Ostro et al., Citation1999; Perez et al., Citation2008; Staniswalis et al., Citation2005; Zanobetti and Schwartz, Citation2009). Five other studies reported elevated but nonsignificant associations (Burnett et al., Citation2004; Klemm et al., Citation2004; Malig and Ostro, Citation2009; Samoli et al., Citation2013; Villeneuve et al., Citation2003). Studies where PM10-2.5 was directly measured using dichotomous samplers yielded similarly inconsistent results (Bourotte et al., Citation2007; Lagorio et al., Citation2006; Svendsen et al., Citation2007; Yeatts et al., Citation2007). Furthermore, several multipollutant studies demonstrated that the association between PM10-2.5 and health outcomes was attenuated when models were adjusted for PM2.5 concentrations, suggesting residual confounding (Burnett et al., Citation2000; Chen et al., Citation2011; Peng et al., Citation2008). Such multipollutant models are important because they help to differentiate between the effects of PM2.5, PM10-2.5, and gaseous pollutants (Health Canada, Citation2016).
Most prior coarse fraction studies have not accounted for the primary source of PM10-2.5 by separating sources such as sea salt, agricultural activity, windblown crustal dust, and road dust. All of these sources have different chemical compositions and toxicities (Wilson and Suh, Citation1997), and springtime road dust is of particular interest in colder climates where snow can collect materials over the winter months. One study on road dust in Finland found associations between coughing and each of PM10, PM2.5, and PM10-2.5 (Tiittanen et al., Citation1999), while two others found no evidence of an association between PM10-2.5 and asthma exacerbations (Pekkanen et al., Citation1997; Penttinen et al., Citation2001). A recent study in Stockholm, Sweden, found that PM10-2.5 had stronger associations with daily mortality during months when road dust was a significant problem (Meister et al., Citation2012). However, these associations were no longer statistically significant after adjusting for PM2.5. A similar phenomenon was observed in Saharan windblown dust studies, where stronger effects were observed on days with sandstorms for PM10-2.5 but not PM2.5 (Mallone et al., Citation2011; Perez et al., Citation2008). Overall, the Health Canada risk assessment concluded that the literature was suggestive but insufficient to infer a causal relationship between short-term exposure to the coarse fraction and different health outcomes. This was primarily due to inconsistencies in the database, which can be attributed to a combination of (1) measurement error when estimating PM10-2.5 as the difference between individual PM10 and PM2.5 measurements, (2) not adjusting for co-pollutants such as PM2.5, and (3) not evaluating the coarse fraction in the context of different emission sources (Health Canada, Citation2016).
Road dust is the primary constituent of ambient PM10-2.5 in the province of British Columbia (BC), on the west coast of Canada (Health Canada, Citation2016). Vehicles driving on paved and unpaved roads propel road dust into the air, which can contain mold, pollen, deicing agents, dust suppression agents, traction material, salt, heavy metals from combustion, rubber particles from tires, and asbestos particles from brake linings (BC Ministry of Water, Land,and Air Protection, 2005; Frazer, Citation2003; Gunawardana et al., Citation2012; Rexeis and Hausberger, Citation2009; Thorpe and Harrison, Citation2008). Many BC communities impacted by road dust also experience intermittent high concentrations of ambient PM2.5 from residential woodsmoke in winter and wildfire smoke in summer (Elliott et al., Citation2013; Hong et al., Citation2017). Due to evidence that PM2.5 is more detrimental to cardiopulmonary health than PM10-2.5, the BC Ministry of Environment (MOE) is reviewing the importance of its PM10 monitoring network relative to its PM2.5 monitoring network. To aid in this review, the objective of our study was to investigate the differences in associations between PM10, PM2.5, PM10-2.5, and two indicators of population health in seven communities affected by springtime road dust. The two indicators of population health span the spectrum of potential health outcomes from mild (drug dispensations) to severe (mortality), and the analyses are stratified by mutually exclusive seasons affected by road dust, residential woodsmoke, and wildfire smoke.
Experimental methods
Study area
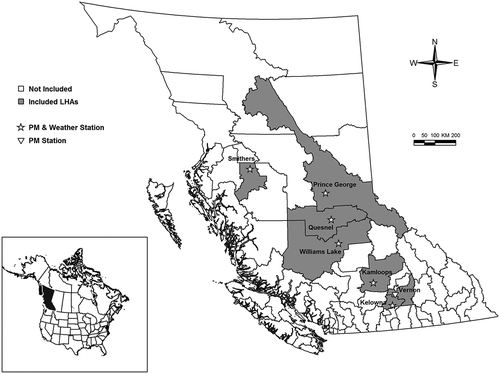
This study was conducted in the province of British Columbia, on the west coast of Canada. The province is geographically divided into 89 local health areas (LHAs) for the purposes of health administration, and we used LHAs as the unit of analyses for this study (). The economy of BC depends on natural resources and the heavy trucks that transport them over 23,710 and 400,000 km of paved and unpaved roads, respectively (Forest Practices Board, Citation2006; KNOWBC, Citation2016). Road dust contributes approximately 72% of total PM10-2.5 emissions in BC, with other major sources being agriculture, construction operations, and quarrying (Environment and Climate Change Canada, Citation2016). Springtime road dust is of particular concern in the colder northern and interior parts of the province, after the snow has melted and particles are resuspended by traveling vehicles (BC Ministry of Water, Citation2005; Environment and Climate Change Canada, Citation2016). Many of the communities that are affected by road dust are also affected by PM2.5 pollution in the form of residential woodsmoke in winter and wildfire smoke in summer (Elliott et al., Citation2013; Environment and Climate Change Canada, Citation2016; Hong et al., Citation2017). Other major sources of PM2.5 include emissions from vehicles and the wood, paper, pulp, and mining industries (Environment and Climate Change Canada, Citation2016).
Environmental data
The BC MOE maintains the air quality monitoring network throughout the study area. A complete set of 1-hr PM10 and PM2.5 data collected using tapered element oscillating microbalances (TEOMs) was obtained from the BC Air Data Archive for the 2003–2015 study period (British Columbia Ministry of Environment, Citation2016). As per the BC MOE data cleaning protocol, all negative or zero concentrations were replaced with the last valid measurement within the past 24 hr, or set to missing if no valid measurement was available. The PM10 and PM2.5 values were then used to calculate daily averages, and daily PM10-2.5 was computed as their difference. When daily PM2.5 measurements exceeded concurrent PM10 measurements, all observations on those days were set to missing and excluded from the analysis. The study was restricted to communities within seven LHAs in the interior of the province with a population of at least 10,000 residents, and at least three consecutive years of overlapping PM10 and PM2.5 measurements ( and ). All monitoring locations were primarily residential within a 500-m radius. We also used daily temperature and relative humidity data from six Environment Canada weather stations that spanned the study period. Each community was matched with its respective weather station, except Vernon, which did not have its own station and was matched with the nearby station in Kelowna ().
Salbutamol sulfate dispensations
Every 2 weeks the BC Centre for Disease Control (BCCDC) receives updated data on province-wide dispensations of medications for chronic respiratory and cardiovascular conditions. These data come from the BC PharmaNet database (Province of British Columbia, Citation2016) and are used for the purposes of routine surveillance and public health protection. Salbutamol sulfate is the most commonly administered respiratory drug in BC, used for treating symptoms of asthma, chronic obstructive pulmonary disease (COPD), and other obstructive lung diseases. Our previous work has shown that salbutamol dispensations are a sensitive indicator of population response to ambient changes in PM2.5 concentrations, especially related to forest fire smoke exposures (Elliott et al., Citation2013). For the purposes of this study, we extracted daily counts of salbutamol dispensations for the LHAs containing the seven study communities during 2003–2015.
Nonaccidental mortality
The BCCDC also receives daily data from the BC Vital Statistics Agency for routine surveillance and public health protection. The database includes the following relevant information about each decedent: date of death; underlying cause of death coded according to the 10th Revision of the International Classification of Diseases (ICD-10); and the LHA of residence. We extracted daily counts of nonaccidental mortality (ICD-10 codes starting with the letters A–R) for the LHAs containing the seven study communities for 2003–2015. We also extracted daily counts of respiratory mortality (ICD-10 codes starting with the letter J) for the entire province to systematically identify influenza periods for the subsequent analysis.
Statistical analysis
Poisson regression models were fitted for each community to quantify the relationships between the two health outcomes and same-day concentrations for each of PM10, PM2.5, and PM10-2.5. All models were adjusted for temperature, relative humidity, influenza periods, year and month, day of the week, and holidays (eq 1). Influenza periods were classified as any moving window of 28 days where at least 7 of the 28 days exceeded the 90th percentile in respiratory deaths throughout the province. Two additional models were fitted: one for PM2.5 adjusted for PM10-2.5, and one for PM10-2.5 adjusted for PM2.5 to control for any residual confounding. In other words, both PM2.5 and PM10-2.5 were entered into a single model and their respective coefficients were reported. For each model, we also performed a random-effects meta-analysis across all communities to generate a pooled estimate (Borenstein et al., Citation2007). Sensitivity analyses were conducted for the adjusted PM10-2.5 models by omitting each community from the random-effects meta-analysis to evaluate its effect on the reported pooled estimate. The same-day concentrations were used for all analyses based on model fit statistics, but sensitivity analyses were also conducted with concentrations averaged over 0–1 days and 0–4 days. All effect estimates were expressed per interquartile range (IQR) increase based on the overall distribution of daily exposures contributed by all communities:
where Oij indicates the number of outcomes in community i on date j; PM is the daily mean concentration of one of PM2.5, PM10, or PM10-2.5; T and RH are the daily mean temperature and relative humidity, respectively, fitted as natural cubic splines with three degrees of freedom; I is a binary variable identifying province-wide influenza periods; YM is a categorical variable representing the year and month; DOW is a categorical variable for the day of the week; and H is a binary variable identifying holidays. The multipollutant models had terms for both PM2.5 and PM10-2.5. The PM, T, and RH terms were all averaged over 0–1 days and 0–4 days in the sensitivity analyses.
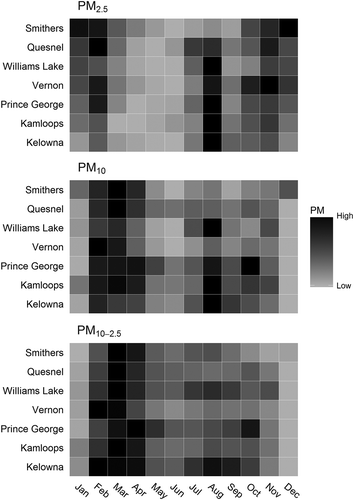
All analyses were stratified by pollutant seasons, which were defined using heat maps to visualize the seasonal patterns in PM2.5, PM10, and PM10-2.5 across all communities (). Based on our previous work we assumed that elevated PM2.5 concentrations from October to February were due to residential woodsmoke (Hong et al., Citation2017), and that elevated PM2.5 concentrations in July and August were due to wildfire smoke (Elliott et al., Citation2013). Based on consultation with the BC MOE we assumed that elevated PM10 and PM10-2.5 from February to April were due to road dust. As such, analyses were stratified into three mutually exclusive seasons: winter residential woodsmoke (October-January); spring road dust (March–April); and summer wildfire smoke (July–August). The month of February was excluded from the analysis due its overlap between the residential woodsmoke and road dust seasons. All analyses were conducted in the R statistical programming environment (R Development Core Team, Citation2015).
Figure 3. These heat maps visualize the seasonal variation in particulate matter (PM) for the seven communities contributing to the analyses. Each of the PM2.5, PM10, and PM10-2.5 categories for each community is normalized to itself, where darker shades represent months of higher PM in that community, and lighter shades represent months of lower PM.
Results
Data summary
In total, seven communities within seven different LHAs were included in the study (). In all cases the named communities were the most populous centers within their respective LHAs, which had 2015 populations ranging from 16,656 to 195,122 (BC Stats, Citation2016) and land areas ranging from 2,911 to 76,104 km2. There was some variation in the age structure and socioeconomic status of the populations, with Vernon having both the lowest median income and the highest population >65 years (). The average daily salbutamol dispensations 10,000 populations across the LHAs ranged from 3.1 to 5.4, and the average daily mortality ranged from 0.1 to 0.2. The median daily concentrations of PM10, PM2.5, and PM10-2.5 had ranges of 13.4–18.2 µg/m3, 4.7–6.5 µg/m3, and 6.3–11.9 µg/m3, respectively (). The overall IQRs for PM10, PM2.5, and PM10-2.5 were 12.0 µg/m3, 5.2 µg/m3, and 8.6 µg/m3, respectively. The correlations between PM2.5 and PM10-2.5 ranged from 0.10 to 0.35.
Table 1. Summary demographic information, mean daily values for the health indicators, and median particulate matter (PM) concentrations (μg/m3) during the study seasons for seven communities located within seven local health areas (LHAs) that were included in the study.
Salbutamol sulfate dispensations
Dispensations of salbutamol sulfate were strongly associated with daily PM concentrations in the wildfire season, but not during the residential woodsmoke or road dust seasons (). During the wildfire season the meta-analysis estimates for the effects [95% confidence interval] of an IQR increase in daily PM10 and PM2.5 on the rate of daily dispensation were 4.9% [3.8, 5.9] and 2.7% [2.0, 3.4], respectively. The estimate for an IQR increase in PM10-2.5 was 6.1% [1.7, 10.5], but this was attenuated to 0.1% [–1.2, 1.5] after adjusting for PM2.5. Conversely, the PM2.5 estimate remained elevated at 2.6% [2.0, 3.2] after adjusting for PM10-2.5. The same pattern was observed across all communities. Estimates for the residential woodsmoke and road dust seasons were null overall and largely null for each community, suggesting that dispensations of salbutamol sulfate are less affected by these exposures. When compared with the same-day concentrations, effect estimates for the average of 0–1 days and 0–4 days were increased with wider confidence intervals (see , shown later), though all models had similar values for the Akaike information criterion (AIC).
Figure 4. Poisson regression and random effect meta-analysis results for the relationship between salbutamol sulfate dispensations and a one interquartile range (IQR) increase in different categories of particulate matter (PM) stratified by season and ordered by population size. The bottom panels show PM2.5 adjusted for PM10-2.5 and PM10-2.5 adjusted for PM2.5, respectively. The y-axis is the percent change in dispensation rates, and is on a different scale for the meta-analysis boxes. Arrows represent confidence intervals extending past the boundaries of the plots.
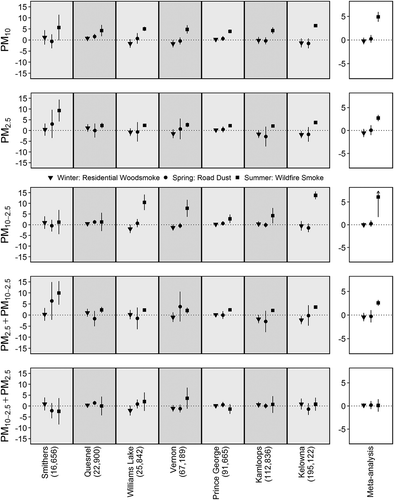
Figure 5. Poisson regression and random effect meta-analysis results for the relationship between nonaccidental mortality and a one interquartile range (IQR) increase in different categories of particulate matter (PM) stratified by season and ordered by population size. The bottom panels show PM2.5 adjusted for PM10-2.5 and PM10-2.5 adjusted for PM2.5, respectively. The y-axis is the percent change in mortality rates, and is on a different scale for the meta-analysis boxes. Arrows represent confidence intervals extending past the boundaries of the plots.
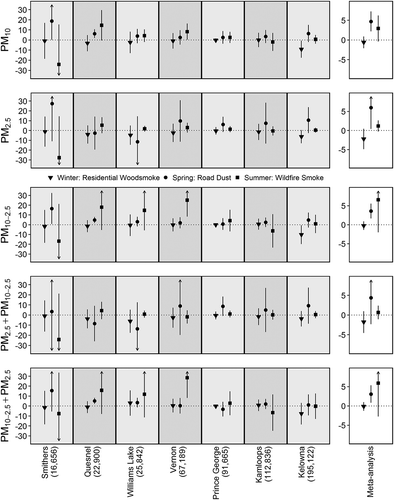
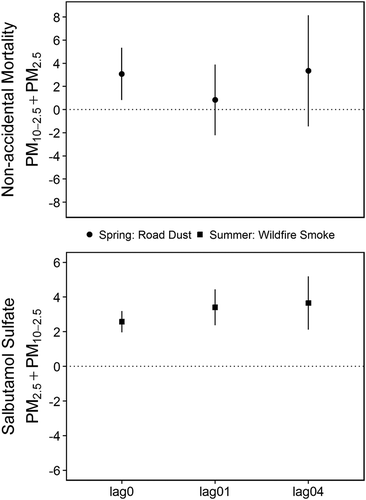
Figure 6. Lag time sensitivity analyses for the random effect meta-analysis results between nonaccidental mortality and PM10-2.5 adjusted for PM2.5 during road dust season (top) and between salbutamol sulfate dispensations and PM2.5 adjusted for PM10-2.5 during the wildfire smoke season (bottom). Plots show the same-day (lag0) reported in the results and the estimates for the exposures averaged over 0–1 days (lag01) and 0–4 days (lag04).
Nonaccidental mortality
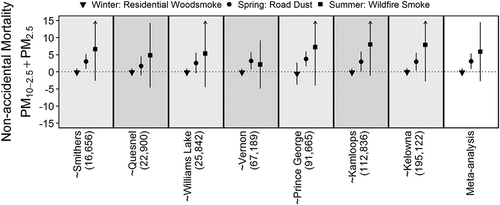
Nonaccidental mortality was strongly associated with daily PM concentrations in the road dust season, weakly associated with daily PM in the wildfire season, and not associated with PM in the residential woodsmoke season (). During the road dust season the meta-analysis estimates for the effects [95% confidence interval] of an IQR increase in daily PM10 and PM2.5 on the rate of nonaccidental mortality were 4.7% [2.2, 7.2] and 6.0% [0.4, 11.5], respectively. The estimate for an IQR increase in PM10-2.5 was 3.6% [1.6, 5.6], which remained elevated at 3.1% [0.8, 5.4] after adjusting for PM2.5. Elevated effects were observed in the communities of Smithers, Quesnel, and Williams Lake, all of which are routinely affected by spring road dust advisories. The PM2.5 estimate was reduced to 4.4% [–2.3, 11.1] after adjusting for PM10-2.5. When compared with the same-day concentrations, effect estimates for the average of 0–1 days and 0–4 days were variable with confidence intervals that crossed zero (). Overall, the AIC values in each community indicated that same-day concentrations were best fitted to the data, and that the average of 0–4 days provided the next best fit.
During the wildfire season the meta-analysis estimates for PM10 and PM2.5 were 2.9% [–0.4, 6.2] and 1.2 [–0.3, 2.6], respectively. The estimate for PM10-2.5 was 6.6% [–2.0, 15.1], which remained elevated at 5.9% [–2.7, 14.5] after adjusting for PM2.5. This could be associated with road dust due to dry conditions in summertime, which likely has different properties when compared with road dust due to the springtime snowmelt. However, sensitivity analyses showed that this result was driven by the very high estimate of 28.4% [8.1, 48.8] for Vernon (). This could be a statistical anomaly, or it could suggest that another summertime source of coarse PM was affecting that community.
Figure 7. Leave-one-out sensitivity analyses for the random effect meta-analysis results between non-accidental mortality and PM10-2.5 adjusted for PM2.5. The tilde identifies the community that was removed from the meta-analysis, ordered by population size. For example, the panel corresponding to ~Vernon indicates that the estimate for the wildfire smoke season was attenuated when data from Vernon were omitted. The y-axis is the percent change in mortality rates, and arrows represent confidence intervals extending past the boundaries of the plots.
Discussion
Our objective was to better understand the health impacts of the fine and coarse PM fractions in BC communities affected by road dust, and to provide evidence that will inform upcoming decisions about potential changes to the provincial PM10 monitoring network. Using Poisson regression and meta-analyses we were able to estimate the effects of PM10, PM2.5, and PM10-2.5 on population health during seasons dominated by residential woodsmoke, road dust, and wildfire smoke. We found that an IQR increase in the coarse fraction was associated with a 3.1% [0.8, 5.4] increase in nonaccidental mortality during the road dust season, even after adjusting for the fine fraction. The adjusted coarse fraction was also associated with a 5.9% [–2.7, 14.5] increase in nonaccidental mortality during the wildfire season, though this estimate was largely driven by a high estimate in a single community. We also found that an IQR increase in the fine fraction was associated with a 2.6% [2.0, 3.2] increase in dispensations of respiratory relief medication during the wildfire season, after adjusting for the coarse fraction. Neither fraction was associated with either health indicator during the residential woodsmoke season.
Our coarse fraction results for mortality during the road dust season were consistent with a recent study in Stockholm, Sweden (Meister et al., Citation2012). Those authors reported that a 10-µg/m3 increase in unadjusted PM10-2.5 lagged from 0–1 days was associated with a 1.7% [0.2, 3.2] increase in nonaccidental mortality during the road dust season, compared with the same-day unadjusted value of 4.2% [1.9, 6.5] increase reported here. However, when these models were adjusted for PM2.5 the effect in Stockholm was attenuated to 1.4% [–0.2, 3.0] while ours remained significant at 3.6% [0.9, 6.3]. Our null results for the respiratory indicator during the road dust season are also relatively consistent with other studies. Here we report no observed association between PM10-2.5 and dispensations of salbutamol sulfate, while other work has reported no association between PM10-2.5 and asthma exacerbations in asthmatic children and adults (Pekkanen et al., Citation1997; Penttinen et al., Citation2001). Another study in asthmatic children did report a weak increase in risk of cough during the road dust season when exposures were averaged over 4 days (Tiittanen et al., Citation1999). Although the literature specific to road dust is very limited, the exposure may be more strongly associated with mortality than with respiratory outcomes.
Our results for the wildfire season are quite consistent with the wider literature on forest fire smoke exposures. In previous work we identified all seven communities as being routinely affected by wildfire smoke (Elliott et al., Citation2013), and the unadjusted 5.5% [3.8, 7.2] increase in dispensations per 10-µg/m3 increase in PM2.5 we reported there was almost identical to the 5.2% [3.8, 6.5] increase we reported here. One other study on wildfire smoke and pharmaceutical dispensations has reported similar results (Caamano-Isorna et al., Citation2011). A recent systematic review identified six methodologically comparable studies on wildfire smoke and nonaccidental mortality, and concluded that the combined evidence was suggestive of increased risk (Reid et al., Citation2016). Here we report that an unadjusted 10-µg/m3 increase in PM2.5 during the wildfire season was associated with a 2.3% [–0.6, 5.0] increase in the rate of nonaccidental mortality, which is generally consistent with studies that were specifically designed to evaluate smoke exposures. The difference between our results for the wildfire and residential woodsmoke seasons may be due to key differences between the two exposures. Wildfire smoke causes intermittently extreme concentrations of PM2.5, while residential woodsmoke has more persistent but milder air quality impacts. As such, long-term exposures are more relevant than short-term exposures when considering the population health effects attributable to residential woodsmoke (Brook et al., Citation2004).
Finally, our results for the residential woodsmoke season are not consistent with the wider literature. Although PM10 had little or no effect on salbutamol sulfate dispensations or nonaccidental mortality, PM2.5 had a negative effect on both indicators ( and ). The literature suggests that short-term exposure to residential woodsmoke has adverse effects on a range of population health indicators, from increased respiratory symptoms to increased rates of hospital admissions (Naeher et al., Citation2007). In previous work we identified the community of Smithers as ranking 8 of 23 for residential woodsmoke impacts in BC, while Quesnel, Prince George, Williams Lake, Kelowna, Vernon, and Kamloops ranked 13, 15, 16, 17, 19, and 23, respectively (Hong et al., Citation2017). In other words, none of the communities included in this study are among those most impacted by residential woodsmoke. We reiterate that this study was designed to evaluate the effects of PM in communities affected by road dust, and that the analyses were stratified by season to better separate potential sources of exposure. The results for the residential woodsmoke season might have been different if communities more affected by the exposure had been included.
There are three common problems when assessing the effects of the coarse fraction on population health: (1) not adjusting PM10-2.5 by PM2.5 and vice versa to account for residual confounding; (2) not considering the primary source and toxicity of the PM10-2.5 in question; and (3) obtaining an indirect measurement of PM10-2.5 by taking the difference between PM10 and PM2.5. Our study has addressed the first two problems, but could not address the third with the available data. In almost all cases, the effects of PM2.5 and PM10-2.5 were attenuated in the two pollutant models. One extreme case was observed in the analysis between PM10-2.5 and salbutamol sulfate dispensations in the wildfire smoke season (), where the positive effect of PM10-2.5 was attenuated to the null after adjustment for PM2.5. On the contrary, we found that PM10-2.5 remained strongly associated with nonaccidental mortality during the road dust season after adjusting for PM2.5. Similarly, PM2.5 remained strongly associated with salbutamol sulfate dispensations during the wildfire smoke season after adjusting for PM10-2.5. These results suggest that road dust has an independent effect on nonaccidental mortality, and that wildfire smoke has an independent effect on salbutamol sulfate dispensations. However, these analyses were not adjusted for other potentially important co-pollutants such as carbon monoxide, ozone, nitrogen dioxide, and sulfate (Health Canada, Citation2016).
Another limitation was our inability to measure PM10-2.5 directly, meaning that two sources of measurement error were introduced into the calculations because the coarse fraction was estimated as the difference between concurrent PM10 and PM2.5 measurements. We know this method produced some error because it introduced negative coarse fraction values when PM2.5 measurements exceeded PM10 measurements, in which case the entire day was excluded from the community-level analysis. Furthermore, we have used data from a central monitoring site to represent exposure over large geographic areas for a pollutant that can rapidly settle out of the ambient air (Day, Citation1965). While most people within the areas live near to the monitoring sites, this type of ecologic exposure assessment limits our power to detect significant effects (Armstrong, Citation1998).
Overall, there was substantial variation in effect estimates between different pollutant seasons, suggesting that it is important to consider the primary source of PM in future analyses. Because PM2.5 and PM10-2.5 have a range of anthropogenic and natural sources, their toxicology may vary. Distinguishing between PM10-2.5 in the form of sea salt, agricultural activity, windblown crustal dust, and road dust has important policy implications. The same is true for distinguishing between PM2.5 in the form of residential woodsmoke, wildfire smoke, vehicular emissions, and industrial emissions. Here, we aimed to better evaluate the effects of residential woodsmoke, road dust, and wildfire smoke by stratifying the analyses by the months when these sources likely dominated the PM exposures (). This method is not perfect, however, because most PM10-2.5 from May to November is also likely to be road dust (Environment and Climate Change Canada, Citation2016) caused by dry conditions rather than by springtime snowmelt. Additionally, nonseasonal PM pollutants such as emissions from agriculture, construction, industry, and vehicles could not be eliminated using this simple approach.
Conclusion
We found positive and statistically significant associations between the coarse fraction and nonaccidental mortality during the spring road dust season, and between the fine fraction and dispensations of salbutamol sulfate during the summer wildfire season. There was also some evidence of the coarse fraction being associated with nonaccidental mortality during the summer months, though the pooled meta-analysis effect estimate was largely driven by a single community. We believe that continuation of PM10 monitoring in communities most affected by the coarse fraction will help inform future management strategies. Additionally, this would allow use of the data to issue air quality advisories and enable future studies using more refined methods.
Acknowledgment
The authors thank the peer reviewers, whose excellent insight helped strengthen this work.
Funding
The authors acknowledge the British Columbia Ministry of Environment in providing funding for this project.
Additional information
Funding
Notes on contributors
Kris Y. Hong
Kris Y. Hong is an undergraduate researcher at the BC Centre for Disease Control.
Gavin H. King
Gavin H. King is an Air Quality Meteorologist in the Air Quality Section (Assessments) with the Regional Operations Branch of the BC Ministry of Environment.
Arvind Saraswat
Arvind Saraswat is the Head of Air Quality Section (Assessments) with the Regional Operations Branch of the BC Ministry of Environment and is an Adjunct Professor at the Institute for Resources, Environment and Sustainability at the University of British Columbia (UBC).
Sarah B. Henderson
Sarah B. Henderson is the Senior Environmental Health Scientist at the BC Centre for Disease Control, and Assistant Professor at the UBC School of Population and Public Health.
References
- Adar, S.D., P.A. Filigrana, N. Clements, and J.L. Peel. 2014. Ambient coarse particulate matter and human health: A systematic review and meta-analysis. Curr. Environ. Health Rep. 1:258–74. doi:10.1007/s40572-014-0022-z.
- Anderson, H.R., G. Favarato, and R.W. Atkinson. 2013. Long-term exposure to air pollution and the incidence of asthma: Meta-analysis of cohort studies. Air Qual. Atmos. Health 6(1):47–56. doi:10.1007/s11869-011-0144-5.
- Armstrong, B.G. 1998. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup. Environ. Med. 55(10):651–56. doi:10.1136/oem.55.10.651
- Atkinson, R.W., G.W. Fuller, H.R. Anderson, R.M. Harrison, and B. Armstrong. 2010. Urban ambient particle metrics and health: A time-series analysis. Epidemiology 21(4):501–11. doi:10.1097/EDE.0b013e3181debc88.
- BC Ministry of Water, Land, and Air Protection. 2005. Best management practices to mitigate road dust from winter traction materials. https://www.for.gov.bc.ca/hfd/library/documents/bib95657.pdf (accessed May 22, 2017).
- BC Stats. 2016. Population Extrapolation for Organizational Planning with Less Error (P.E.O.P.L.E). https://www.bcstats.gov.bc.ca/apps/PopulationProjections.aspx (accessed May 22, 2017).
- Borenstein, M., L. Hedges, and H. Rothstein. 2007. Meta-analysis fixed effect vs. random effects. https://www.meta-analysis.com/downloads/Meta-analysis%20fixed%20effect%20vs%20random%20effects.pdf. (accessed May 22, 2017)
- Bourotte, C., A.-P. Curi-Amarante, M.-C. Forti, L.A.A. Pereira, A.L. Braga, and P.A. Lotufo. 2007. Association between ionic composition of fine and coarse aerosol soluble fraction and peak expiratory flow of asthmatic patients in São Paulo City (Brazil). Atmos. Environ. 41(10):2036–48. doi:10.1016/j.atmosenv.2006.11.004.
- British Columbia Ministry of Environment. 2016. BC air data archive. https://envistaweb.env.gov.bc.ca/ (accessed May 22, 2017).
- Brook, R.D., B. Franklin, W. Cascio, Y. Hong, G. Howard, M. Lipsett, R. Luepker, M. Mittleman, J. Samet, and S.C. Smith. 2004. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109(21):2655–71. doi:10.1161/01.CIR.0000128587.30041.C8
- Brunekreef, B., and B. Forsberg. 2005. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 26(2):309–18. doi:10.1183/09031936.05.00001805.
- Burnett, R.T., J. Brook, T. Dann, C. Delocla, O. Philips, S. Cakmak, R. Vincent, M.S. Goldberg, and D. Krewski. 2000. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal. Toxicol. 12(Suppl. 4):15–39. doi:10.1080/08958370050164851.
- Burnett, R.T., S. Cakmak, J.R. Brook, and D. Krewski. 1997. The role of particulate size and chemistry in the association between summertime ambient air pollution and hospitalization for cardiorespiratory diseases. Environ. Health Perspect. 105(6):614–20. doi:10.2307/3433607
- Burnett, R.T., D. Stieb, J.R. Brook, S. Cakmak, R. Dales, M. Raizenne, R. Vincent, and T. Dann. 2004. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch. Environ. Health 59(5):228–36. doi:10.3200/AEOH.59.5.228–36.
- Caamano-Isorna, F., A. Figueiras, I. Sastre, A. Montes-Martínez, M. Taracido, and M. Piñeiro-Lamas. 2011. Respiratory and mental health effects of wildfires: An ecological study in Galician municipalities (north-west Spain). Environ. Health 10(1):1. doi:10.1186/1476-069X-10-48
- Castillejos, M., V.H. Borja-Aburto, D.W. Dockery, D.R. Gold, and D. Loomis. 2000. Airborne coarse particles and mortality. Inhal. Toxicol. 12(suppl. 1):61–72. doi:10.1080/0895-8378.1987.11463182.
- Chen, R., Y. Li, Y. Ma, G. Pan, G. Zeng, X. Xu, B. Chen, and H. Kan. 2011. Coarse particles and mortality in three Chinese cities: The China Air Pollution and Health Effects Study (CAPES). Sci. Total Environ. 409(23):4934–8. doi:10.1016/j.scitotenv.2011.08.058.
- Chimonas, M.A., and B.D. Gessner. 2007. Airborne particulate matter from primarily geologic, non-industrial sources at levels below National Ambient Air Quality Standards is associated with outpatient visits for asthma and quick-relief medication prescriptions among children less than 20 years old enrolled in Medicaid in Anchorage, Alaska. Environ. Res. 103(3):397–404. doi:10.1016/j.envres.2006.08.013.
- Cifuentes, L.A., J. Vega, K. Kopfer, and L.B. Lave. 2000. Effect of the fine fraction of particulate matter versus the coarse mass and other pollutants on daily mortality in Santiago, Chile. J. Air Waste Manage. Assoc. 50(8):1287–98. doi:10.1080/10473289.2000.10464167
- Day, P.R. 1965. Particle fractionation and particle size analysis. In Methods of Soil Analysis, C. A. Black (ed.), vol. 1, p. 545–67. Madison, WI: American Society of Agronomy.
- Elliott, C.T., S.B. Henderson, and V. Wan. 2013. Time series analysis of fine particulate matter and asthma reliever dispensations in populations affected by forest fires. Environ. Health 12:11. doi:10.1186/1476-069X-12-11.
- Environment and Climate Change Canada. 2016. Air pollutant emission inventory (APEI), Canada, 2014. http://www.ec.gc.ca/inrp-npri/donnees-data/ap/index.cfm?lang=En (accessed May 22, 2017).
- Forest Practices Board. 2006. Forest Practices Board 2005–06 annual report. Forest Practices Board. https://www.bcfpb.ca/wp-content/uploads/2016/03/FPB-2005-2006-Annual-Report_0.pdf (accessed May 22, 2017).
- Frazer, L. 2003. Down with road dust. Environ. Health Perspect. 111(16):A892–95. doi:10.1289/ehp.111-a892
- Gordian, M.E., and A.H. Choudhury. 2003. PM10 and asthma medication in schoolchildren. Arch. Environ. Health 58(1):42–7. doi:10.3200/AEOH.58.1.42-47.
- Gunawardana, C., A. Goonetilleke, P. Egodawatta, L. Dawes, and S. Kokot. 2012. Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere 87(2):163–70. doi:10.1016/j.chemosphere.2011.12.012.
- Health Canada. 2016. Human health risk assessment for coarse particulate matter. Ottawa, ON, Canada: Health Canada. http://publications.gc.ca/collections/collection_2016/sc-hc/H144-30-2016-eng.pdf (accessed May 22, 2017).
- Hoek, G., R.M. Krishnan, R. Beelen, A. Peters, B. Ostro, B. Brunekreef, and J.D. Kaufman. 2013. Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ. Health 12(1):43. doi:10.1186/1476-069x-12-43.
- Hong, K.Y., S. Weichenthal, A. Saraswat, G.H. King, S.B. Henderson, and M. Brauer. 2017. Systematic identification and prioritization of communities impacted by residential woodsmoke in British Columbia, Canada. Environ. Pollut. 220(Pt B):797–806. doi:10.1016/j.envpol.2016.10.056.
- Klemm, R.J., F.W. Lipfert, R.E. Wyzga, and C. Gust. 2004. Daily mortality and air pollution in Atlanta: Two years of data from ARIES. Inhal. Toxicol. 16 Suppl 1:131–41. doi:10.1080/08958370490443213.
- KNOWBC. 2016. Roads and highways. KNOWBC 2016. http://knowbc.com/ebc/Books/Encyclopedia-of-BC/R/Roads-and-Highways (accessed March 22, 2016).
- Lagorio, S., F. Forastiere, R. Pistelli, I. Iavarone, P. Michelozzi, V. Fano, A. Marconi, G. Ziemacki, and B.D. Ostro. 2006. Air pollution and lung function among susceptible adult subjects: A panel study. Environ. Health 5:11. doi:10.1186/1476-069X-5-11.
- Lin, M., Y. Chen, R.T. Burnett, P.J. Villeneuve, and D. Krewski. 2002. The influence of ambient coarse particulate matter on asthma hospitalization in children: Case-crossover and time-series analyses. Environ. Health Perspect. 110(6):575–81. doi:10.1289/ehp.02110575
- Malig, B.J., and B.D. Ostro. 2009. Coarse particles and mortality: Evidence from a multi-city study in California. Occup. Environ. Med. 66(12):832–9. doi:10.1136/oem.2008.045393.
- Mallone, S., M. Stafoggia, A. Faustini, G.P. Gobbi, A. Marconi, and F. Forastiere. 2011. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ. Health Perspect. 119(10):1409–14. doi:10.12989/ehp.1003026.
- Mar, T.F., G.A. Norris, J.Q. Koenig, and T.V. Larson. 2000. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ. Health Perspect. 108(4):347–53. doi: 10.2307/3454354
- McConnell, R., K. Berhane, F. Gilliland, J. Molitor, D. Thomas, F. Lurmann, E. Avol, W.J. Gauderman, and J.M. Peters. 2003. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am. J. Respir. Crit. Care Med. 168(7):790–7. doi:10.1164/rccm.200304-466OC.
- McCormack, M.C., P.N. Breysse, E.C. Matsui, N.N. Hansel, R.D. Peng, J. Curtin-Brosnan, D.L. Williams, M. Wills-Karp, G.B. Diette, and Center for Childhood Asthma in the Urban Environment. 2011. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann. Allergy Asthma Immunol. 106(4):308–15. doi:10.1016/j.anai.2011.01.015.
- McCormack, M.C., P.N. Breysse, E.C. Matsui, N.N. Hansel, D. Williams, J. Curtin-Brosnan, P. Eggleston, G.B. Diette, and Center for Childhood Asthma in the Urban Environment. 2009. In-home particle concentrations and childhood asthma morbidity. Environ. Health Perspect. 117(2):294–8. doi:10.1289/ehp.11770.
- Meister, K., C. Johansson, and B. Forsberg. 2012. Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden. Environ. Health Perspect. 120(3):431–6. doi:10.1289/ehp.1103995.
- Millstein, J., F. Gilliland, K. Berhane, W.J. Gauderman, R. McConnell, E. Avol, E.B. Rappaport, and J.M. Peters. 2004. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 Southern California communities enrolled in The Children’s Health Study. Arch. Environ. Health 59(10):505–14. doi:10.1080/00039890409605166.
- Misra, C., M.D. Geller, P. Shah, C. Sioutas, and P.A. Solomon. 2001. Development and evaluation of a continuous coarse (PM10–PM2.5) particle monitor. J. Air Waste Manage. Assoc. 51(9):1309–17. doi: 10.1080/10473289.2001.10464360
- Naeher, L.P., M. Brauer, M. Lipsett, J.T. Zelikoff, C.D. Simpson, J.Q. Koenig, and K.R. Smith. 2007. Woodsmoke health effects: A review. Inhal. Toxicol. 19(1):67–106. doi:10.1080/08958370600985875.
- Ostro, B.D., R. Broadwin, and M.J. Lipsett. 2000. Coarse and fine particles and daily mortality in the Coachella Valley, California: A follow-up study. J. Expos. Anal. Environ. Epidemiol. 10(5):412–9. doi:10.1038/sj.jea.7500094
- Ostro, B.D., S. Hurley, and M.J. Lipsett. 1999. Air pollution and daily mortality in the Coachella Valley, California: A study of PM10 dominated by coarse particles. Environ. Res. 81(3):231–8. doi:10.1006/enrs.1999.3978.
- Pekkanen, J., K.L. Timonen, J. Ruuskanen, A. Reponen, and A. Mirme. 1997. Effects of ultrafine and fine particles in urban air on peak expiratory flow among children with asthmatic symptoms. Environ. Res. 74(1):24–33. doi:10.1006/enrs.1997.3750.
- Peng, R.D., H.H. Chang, M.L. Bell, A. McDermott, S.L. Zeger, J.M. Samet, and F. Dominici. 2008. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. J. Am. Med. Assoc. 299(18):2172–9. doi:10.1001/jama.299.18.2172.
- Penttinen, P., K.L. Timonen, P. Tiittanen, A. Mirme, J. Ruuskanen, and J. Pekkanen. 2001. Ultrafine particles in urban air and respiratory health among adult asthmatics. Eur. Respir. J. 17(3):428–35. doi:10.1183/09031936.01.17304280
- Perez, L., A. Tobias, X. Querol, N. Kunzli, J. Pey, A. Alastuey, M. Viana, N. Valero, M. Gonzalez-Cabre, and J. Sunyer. 2008. Coarse particles from Saharan dust and daily mortality. Epidemiology 19(6):800–7. doi:10.1097/EDE.0b013e31818131cf
- Province of British Columbia. 2016. PharmaNet 2016. http://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/pharmanet (accessed July 11, 2016).
- R Development Core Team. 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Reid, C.E., M. Brauer, F. Johnston, M. Jerrett, J.R. Balmes, and C.T. Elliott. 2016. Critical review of health impacts of wildfire smoke exposure. Environ. Health Perspect. 124(9):1334–43. doi:10.1289/ehp.1409277
- Rexeis, M., and S. Hausberger. 2009. Trend of vehicle emission levels until 2020—Prognosis based on current vehicle measurements and future emission legislation. Atmos. Environ. 43(31):4689–98. doi:10.1016/j.atmosenv.2008.09.034.
- Samoli, E., M. Stafoggia, S. Rodopoulou, B. Ostro, C. Declercq, E. Alessandrini, J. Diaz, A. Karanasiou, A.G. Kelessis, A. Le Tertre, P. Pandolfi, G. Randi, C. Scarinzi, S. Zauli-Sajani, K. Katsouyanni, F. Forastiere, and MED-PARTICLES Study Group. 2013. Associations between fine and coarse particles and mortality in Mediterranean cities: Results from the MED-PARTICLES project. Environ. Health Perspect. 121(8):932–8. doi:10.1289/ehp.1206124.
- Slaughter, J.C., E. Kim, L. Sheppard, J.H. Sullivan, T.V. Larson, and C. Claiborn. 2005. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J. Expos. Anal. Environ. Epidemiol. 15(2):153–9. doi:10.1038/sj.jea.7500382.
- Staniswalis, J.G., N.J. Parks, J.O. Bader, and Y.M. Maldonado. 2005. Temporal analysis of airborne particulate matter reveals a dose-rate effect on mortality in El Paso: Indications of differential toxicity for different particle mixtures. J. Air Waste Manage. Assoc. 55(7):893–902. doi:10.1080/10473289.2005.10464696
- Svendsen, E.R., K.B. Yeatts, D. Peden, S. Orton, N.E. Alexis, J. Creason, R. Williams, and L. Neas. 2007. Circulating neutrophil CD14 expression and the inverse association of ambient particulate matter on lung function in asthmatic children. Ann. Allergy Asthma Immunol. 99(3):244–53. doi:10.1016/S1081-1206(10)60660-6.
- ThermoFisher Scientific. 2016. ParitsolTM 2025i-D Dichotomous sequential air sampler. https://www.thermofisher.com/order/catalog/product/2025ID.
- Thorpe, A., and R.M. Harrison. 2008. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 400(1–3):270–82. doi:10.1016/j.scitotenv.2008.06.007.
- Tiittanen, P., K.L. Timonen, J. Ruuskanen, A. Mirme, and J. Pekkanen. 1999. Fine particulate air pollution, resuspended road dust and respiratory health among symptomatic children. Eur. Respir. J. 13(2):266–73.
- U.S. EPA. 2009. Final report: Integrated science assessment for particulate matter. Washington, DC: U.S. EPA.
- Villeneuve, P.J., R.T. Burnett, Y. Shi, D. Krewski, M.S. Goldberg, C. Hertzman, Y. Chen, and J. Brook. 2003. A time-series study of air pollution, socioeconomic status, and mortality in Vancouver, Canada. J. Expos. Anal. Environ. Epidemiol. 13(6):427–35. doi:10.1038/sj.jea.7500292.
- Wilson, W.E., and H.H. Suh. 1997. Fine particles and coarse particles: Concentration relationships relevant to epidemiologic studies. J. Air Waste Manage. Assoc. 47(12):1238–49. doi:10.1080/10473289.1997.10464074.
- World Health Organization Europe. 2013. Review of evidence on health aspects of air pollution—REVIHAAP project: Final technical report. Copenhagen, Denmark: WHO.
- Yeatts, K., E. Svendsen, J. Creason, N. Alexis, M. Herbst, J. Scott, L. Kupper, R. Williams, L. Neas, W. Cascio, R.B. Devlin, and D.B. Peden. 2007. Coarse particulate matter (PM2.5-10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ. Health Perspect. 115(5):709–14. doi:10.1289/ehp.9499.
- Zanobetti, A., and J. Schwartz. 2009. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ. Health Perspect. 117(6):898–903. doi:10.1289/ehp.0800108.