ABSTRACT
The performance of ambient temperature anaerobic co-digestion was investigated for mixtures of six substrates: canned tomato and salsa waste, portable toilet waste, septic tank waste, winery waste, beer and cider waste, and fats, oils, and grease (FOG). Laboratory semi-continuous reactor studies and molecular biological analyses revealed that beer/winery, and tomato/FOG/winery/beer mixtures resulted in the best performance in terms of biogas production (515 and 371 mL CH4/g VS, respectively) and methanogenic populations. A portable toilet/septage mixture resulted in the overall poorest performance and inhibition of microbial activity was evident. Average methane content was ~70% for all mixtures tested. The findings of this study reveal that healthy methanogen populations were present, further supporting the feasibility of biogas production via the novel feedstock mixtures in ambient temperature lagoons.
Implications: Disposal of septic tank waste and other high chemical oxygen demand (COD) 10 industrial food processing waste at a small wastewater treatment plant is uncommon, because it can upset the treatment process and requires additional power for treatment. Ambient-temperature covered lagoon digesters can be an alternative low-cost technology for co-digestion of these recalcitrant waste streams while generating bioenergy. The results of this study demonstrated that there is potential for implementation of unheated covered lagoon digester systems 15 for conversion of liquid wastes for production of renewable biomethane while eliminating the need to treat these wastes at a wastewater treatment plant.
Introduction
Potential anaerobic digestion (AD) feedstocks over a range including both municipal and industrial waste sources are often overlooked for bioenergy generation. For example, in California, septic tank waste (septage) and fats, oil, and grease (FOG) amount to 897.1 and 41.6 million L per year, respectively (California Wastewater Training and Research Center Citation2002). These wastes are typically disposed of at large wastewater treatment plants (WWTP) at a fairly low cost (<$0.013/L). However, smaller WWTPs do not have the capability to dispose of these recalcitrant wastes and must transport them some distance, at higher cost ($0.007 to $0.017 per L), to the larger WWTPs (California Wastewater Training and Research Center Citation2002). Additionally, food processing residues are an important part of the available organic waste streams in California, amounting to 544,311 metric tons (Mg) per year of high moisture solids. Also, more than 372.5 billion L of wastewater is generated by food processing industries, equivalent to 158,757 Mg of biochemical oxygen demand (BOD). The handling and treatment of these organic wastes are a significant economic and energy burden (Amon et al. Citation2012).
These waste materials, however, also have the potential to be turned into an energy resource if converted to biogas using AD. For example, in California the total annual potential for energy recovery from converting all of the mentioned substrates to biogas is on the order of 10 million MMBtu, equivalent to about 2% of residential natural-gas usage (U. S. EIA Citation2009). The total potential for energy recovery if used for biogas-fueled combined heat and power (CHP) systems is almost 100 MW of electrical power, along with 3.5 million MMBtu of recovered heat. Further, these waste resources are located primarily in rural areas of the state (Amon et al. Citation2012), and thus, management currently requires costly transport to large WWTPs in more urban areas. Alternative management options are needed, and ideally approaches should harness the inherent energy generation potential.
For management of food processing waste, studies have shown that conventional high-rate AD systems can be more costly than traditional wastewater treatment due to high capital and operating costs and low disposal fees (Moletta Citation2005; Slaughter, Henderson, and Owen Citation2007). However, ambient-temperature covered lagoon digesters have been used successfully in California and other mild climates to treat dilute wastes while producing significant amounts of methane-rich biogas. The appeal of ambient temperature lagoons stems from their low capital and operating costs, features that make this technology appropriate for relatively dilute and recalcitrant waste streams (Miner et al. Citation2003). Despite the benefits of bioenergy generation from municipal and industrial wastes (e.g., canned tomato and salsa waste, portable toilet waste, septage, winery waste, beer and cider waste, and FOG), studies and data are lacking in the lower temperature range because often digesters are heated to mesophilic (30–40°C) or thermophilic (45–65°C) conditions that are presumably more ideal for digestion; most full-scale anaerobic digesters are operated around 35°C (Yenigün and Demirel Citation2013). Thus, studies of ambient-temperature AD for candidate waste feedstocks are needed to support industry consideration.
Anaerobic co-digestion of organic wastes is appealing because of the ability to divert multiple waste streams into the same process with the potential for optimizing gas production. Co-digestion has the potential to be beneficial through the increase in biodegradable organic loads and an enhanced balance of nutrients (i.e., C/N ratio) and microorganisms (Cuetos et al. Citation2008; Khalid et al. Citation2011), while effects of any toxic compounds also have been found to be minimized (Murto, Björnsson, and Mattiasson Citation2004). A C/N ratio of 30 has been generally recommended to avoid ammonia toxicity (Kayhanian and Rich Citation1995); however, this value was developed for mesophilic AD and likely differs for ambient-temperature AD (Wang et al. Citation2014). Also, a BOD5:N:P ratio of 250:5:1 is recommended for anaerobic treatment of wastewaters (Kerri Citation2007; Metcalf and Eddy Citation2003). The chemical oxygen demand (COD)/BOD5 ratio is used as an indicator for biodegradation capacity. It is typically 2:1, and higher values indicate the presence of poorly biodegradable substances (Metcalf, and Eddy Citation2003). Further, avoiding high lipid loads typically is critical for successful co-digestion (Cuetos et al. Citation2008). However, data are lacking on the range of viable co-digestion feedstock compositions for ambient-temperature AD, and studies are needed that investigate mixtures of the aforementioned abundant waste streams.
Thus, the objective of this project was to determine the performance of ambient temperature AD for bioenergy production from mixtures of wastes including tomato waste, portable toilet waste, septage, winery waste, beer and cider waste, and FOG. The approach involved biochemical methane potential (BMP) testing for selection of individual wastes and laboratory semicontinuous digester tests of co-digestion mixtures. To assess the health and activity of the ambient temperature AD microbial communities, DNA- and RNA-targeted molecular biological analyses, including quantitative polymerase chain reaction (qPCR), reverse transcription qPCR (RT-qPCR), and next-generation sequencing, were applied to track methanogenic populations.
Experimental methods
Selection and characterization of individual substrates and inoculum
The feedstocks used in this project were all generated in or near Yolo County, California, and delivered to the Yolo County Central Landfill near Davis, CA. Most substrates were used directly as received by the landfill. Several types of canned tomato waste and salsa (diced tomatoes, whole tomatoes, green chilies, mild salsa, garlic and cilantro salsa, and black bean and corn salsa) were removed from cans and homogenized using a kitchen blender (Ninja Profession-NJ600, USA), and the blended material was used as the tomato waste substrate. Coarser substrates were reduced to less than 2 mm in diameter. All waste substrate samples were collected and stored at 4°C until used later for BMP analysis or as feed for the semicontinuous reactor experiments.
Anaerobically digested cow-manure sludge from a dairy manure digester in Galt, CA (Van Warmerdam Dairy), was used as inoculum. This covered-lagoon anaerobic digester was operated at approximately 25°C when inoculum samples were collected; the temperatures in these unheated digester systems vary somewhat seasonally.
Laboratory chemical analyses of each waste type and the inoculum were performed by a private laboratory (BC Laboratories, Inc., Bakersfield, CA) using the following standard methods for water and wastewater: EPA-6010B for total sodium, EPA-300.0 for nitrate and sulfate, EPA-351.2 for total Kjeldahl nitrogen (TKN), EPA-350.1 for ammonia as NH3, EPA-365.4 for total phosphorous, SM17-5210B for BOD, and EPA-410.4 for COD. Additionally, the substrates were characterized for total solids (TS) and volatile solids (VS) at the Yolo County Division of Integrated Waste Management laboratories.
Biochemical methane potential (BMP) testing of individual substrates
A BMP study was performed to obtain preliminary indications of the biomethane potential from the waste substrates on an individual basis to consider how they could contribute to feeding a semicontinuous digester system. BMP assays were run according to Angelidaki et al. (Citation2009). Briefly, aliquots of each refrigerated sample were warmed to room temperature (25°C) and placed in 150-mL septum bottles with the fresh anaerobic digester sludge inoculum; BMP assay weight compositions are listed in the Supplemental Information, Table S1. The sealed septum bottles were placed in a temperature-controlled water bath at 26.7°C (this temperature was selected because it is the average temperature of a full-scale digester in the northern California region). Bottles were incubated for 30 to 60 days, until detectable gas production had ceased. Each assay was performed in triplicate. Biogas production was monitored once daily using a displacement method; the sealed septum bottles were pierced and the gas was released into a calibrated water displacement gauge with an accuracy of ±0.5 mL. Biogas composition (O2, N2, CH4, CO2, and H2S) was measured only one time on day 16 of this experiment by injecting a syringe sample into a gas chromatograph (GC) (Agilent 2000 Micro GC, Agilent, Inc.) with a thermal conductivity detector (TCD). This was the initial screening of different substrates and it was assumed that the average gas concentration was close to measurement made on day 16 of the experiment. The GC was span calibrated with certified reference gases, including each of the target gasses, prior to use. The TS and VS of the sample from the reactors were determined according to EPA method 1684 at the beginning and at the end of tests.
Determining biogas generation for waste mixtures
Acclimation of anaerobic inoculum
The microbial populations in the ambient-temperature dairy-manure seed were not previously exposed to the wastes targeted in this study. To accelerate digester startup and maximize biogas generation, the seed was acclimated to the wastes. Two sets of acclimated inocula were developed by running semicontinuous digesters for a period of 45 days. One seed was prepared from a mixture of dairy manure sludge and a food-waste mixture (FOG, tomato, and winery); the second was prepared from dairy manure sludge and the human waste mixture (septic and portable toilet waste). The acclimated seeds then were used to start up digesters with all the waste mixtures. Human wastes were separated from other waste sources because of the potential for pathogens and high sulfate content, which would result in production of hydrogen sulfide and require additional treatment before use.
Experimental setup
Ambient-temperature co-digestion tests with waste mixtures were conducted in a climate-controlled box with temperature accuracy of 26.7 ± 0.1°C. Temperature was logged continually using a CN7-A-Process monitor and logger (Version 2.01.00, Omega Engineering, Norwalk, CT).
Six types of organic waste substrates that were readily available in Yolo County were used in co-digestion experiments. Five mixtures of these individual wastes were designed with the goal of optimizing BOD5:N:P and COD/BOD5 ratios and methane production, while also considering available waste stream volumes produced in Yolo County. These waste mixtures were the following (see Supplemental Information, Table S2, for details and relative amounts): tomato/FOG (T/F); tomato/FOG/winery/cider beer (T/F/W/B); cider beer/winery (B/W); FOG/winery (F/W); and portable toilet/septic (P/S). To measure feedstock mixture volumes for each digester, a laboratory scale (PB5001, Mettler Toledo, Columbus, OH) with accuracy of ±0.1 g was used. These waste mixtures were prepared and characterized via chemical analyses to determine their suitability for AD.
Triplicate semicontinuous digesters were set up with 50 mL of inoculum and 50 mL of each liquid waste mixture in 125-mL serum bottles (Supplemental Information, Table S2). Due to limited volume of the acclimated reactors, new reactors were augmented with additional dairy manure sludge as inoculum. Thus, the inocula used were a mixture of dairy manure sludge seed (65% by volume, 32.5 mL) and waste mixture-acclimated inoculum (35% by volume, 17.5 mL). The food waste-acclimated inoculum (45 days) was used for all digesters fed mixtures of food wastes, and the human waste-acclimated inoculum was used for the digester fed a mixture of human waste, as previously described. No additional nutrients or trace elements were added to the digesters to simulate full-scale operation. Control digesters without any feedstock for each inoculum type (food and human waste acclimated) were operated to measure biogas produced from seed alone. Controls were prepared in triplicate using 50 mL of inocula and 50 mL of distilled water. The amount of biogas produced by control digesters was deducted from biogas produced in the fed digesters.
After waste was introduced to the digesters, headspace of the digester bottles was purged with nitrogen, and bottles were sealed immediately with a rubber stopper and aluminum cap. A B-type polytetrafluoroethylene (PTFE) magnetic stir bar was left inside each serum bottle and reactors were stirred daily 5 days per week. Each digester was opened weekly to remove co-digested waste and inoculum mixture (17.5 mL), and to add the same volume of fresh waste mixture. For the control digesters, distilled water was added. The volume replaced was based on typical covered lagoon hydraulic retention time of 40 days. The pH of each reactor was checked before and after the addition of feedstock and adjusted with sodium bicarbonate to 7 as needed. The volume removed and added was based on a typical covered lagoon hydraulic retention time of less than 40 days (El-Mashad and Zhang Citation2010). A portion of the liquids removed from each digester was used for chemical analyses, and the rest was shipped to Colorado State University for testing with DNA- and RNA-based assays.
Chemical analyses
Liquid waste mixtures for each set of triplicate digesters were tested for various parameters. Due to limited sample volumes, samples from each triplicate digester were combined for chemical analysis. Chemical analyses of COD, volatile fatty acids, sulfate, alkalinity, total phosphorus, and TKN were performed using a Digital Block Reactor (DRB200, Hach Company, Loveland, CO) and a bench-top Spectrophotometer (DR 3900, Hach Company, Loveland, CO). When necessary, samples were diluted to have results within the range for the Hach kits used. The dilutions ranged between 2 and 100, and no dilution was needed for the control reactors. Test methods and kits used are listed in the Supplemental Information, Table S3.
Other aqueous chemistry parameters were measured using a portable meter (HQ40d, Hach Company, Loveland, CO) with various probe attachments. The pH was measured with a pH probe (IntelliCAL PHC10101, Hach Company, Loveland, CO). Conductivity was measured using a four-pole conductivity probe (IntelliCAL CDC40101, Hach Company, Loveland, CO). Total dissolved solids (TDS), salinity, and resistivity were calculated based on conductivity. Nitrate as nitrogen (NO3-N), ammonia as nitrogen (NH3-N), and sodium (Na+) were measured with ion-selective electrodes (ISEs) (IntelliCAL ISENO3181, ISENH318101, and ISENa318101, Hach Company, Loveland, CO). Three-point calibrations were performed for every probe and electrode using standard solutions (Hach Company, Loveland, CO) prior to measurement.
Biogas volume and composition
Biogas was collected from the headspace of each 125-mL serum bottle using 60-mL plastic syringes (accuracy of 0.5 mL). Prior to gas volume measurement and removal of the syringes, the pressure inside each reactor was adjusted to atmospheric pressure. To adjust the pressure, a needle inserted into the reactor headspace was connected to a hand-held gas pressure sensor (model PDM213, Air Neotronics, Oxford, England, with an accuracy of 0.25 mm water), and the syringe volume was adjusted until the pressure reading was equal to atmospheric pressure (zero gauge pressure). The ideal gas law was used to convert the measured gas volumes to standard temperature and pressure. Saturated vapor pressure was assumed as 26.1464 mm Hg for the experiment operated at 26.7°C.
Biogas composition (CH4, CO2, N2, O2, and H2S) was measured using a micro GC (MTI P200, MTI Analytical Instruments, Fremont, CA). The micro GC was equipped with dual TCDs, a 10-m MS-5A capillary column (channel A), and an 8-m Poraplot U capillary column (channel B). Column temperature was independently controlled to allow simultaneous use of both channels. Either two-point (for O2 and H2S) or three-point (for N2, CH4, and CO2) calibration curves were used. H2S was reported as a minimum level, because some amount was presumably lost due to an adsorption to the syringes, septa, and tubes used during the measurements (Nielsen et al. Citation2008).
Determination of methanogenic microbial populations
DNA and RNA extraction
The 5.1-mL or 1.7-mL digester samples were collected for DNA extraction. Week 1 and 3 samples were 5.1 mL, but to accommodate measurement of other parameters, sample volume was reduced to 1.7 mL for subsequent weeks. All samples were centrifuged at 5000 × g for 3 minutes, and supernatant was discarded. DNA was extracted from the pelleted digestate material using the PowerMax Soil DNA Isolation Kit (MoBio Laboratories, Inc., Carlsbad, CA) according to the manufacturer’s protocol. DNA was stored at −20°C. Digester samples (1.7 mL) for RNA analysis were centrifuged at 15,000 × g for 5 min, the supernatant was discarded, and the pellets were frozen with liquid nitrogen upon sampling. Samples were then put on dry ice for shipping, and then stored at −80°C. RNA was extracted using the PowerMicrobiome RNA Isolation Kit (MoBio Laboratories, Inc., Carlsbad, CA). Quantity and purity of DNA and RNA were assessed on a NanoDrop spectrophotometer. RNA integrity was assessed using an Experion RNA Analysis Kit (Bio-Rad Laboratories, Hercules, CA) on an Experion Automated Electrophoresis System (Bio-Rad Laboratories, Hercules, CA).
qPCR and RT-qPCR
Complementary DNA (cDNA) was synthesized by first mixing 8 µl (0.05–1.2 µg) of total RNA and 2 µL of 50 µM random hexamer primers, and incubating at 70°C for 5 min in a PCR machine with the heated lid set at 110°C. Then, 4 µL of Invitrogen 5X First Strand Buffer (Life Technologies, Grand Island, NY), 2 µL of Invitrogen dNTP stock (10 mM of each nucleotide) (Life Technologies, Grand Island, NY), 2 µL nuclease-free water, and 2µL of Superscript Reverse Transcriptase (Life Technologies, Grand Island, NY) were added. These RT reactions were then incubated at 42°C in a PCR machine with heated lid set at 110°C for 1.5 hr. All cDNA was stored at −20°C prior to use in RT-qPCR.
qPCR and RT-qPCR assays were used to probe methanogens as a function of time via measuring the quantity of mcrA genes and transcripts (gene encoding α-methyl coenzyme M reductase) (Luton et al. Citation2002). The mcrA gene is involved in methanogenesis and is a specific biomarker for methanogens. The accuracy of specific qPCR assays can depend on the phylotypes present due to mismatches between primers and template genes (Ledeker and De Long Citation2013). Methanogen types can vary and were unknown for the ambient-temperature reactors investigated herein. Thus, two published assays developed for application to methanogenic digesters were used. Primers mcrA_1035F and mcrA_1530R (Pereyra et al. Citation2010) and MFf and MLr (Steinberg and Regan Citation2009) were used. qPCR was conducted with a 7300 real-time PCR system (Applied Biosystems-Life Technologies, Grand Island, NY) as done by Pereyra et al. (Citation2010) with one modification. The qPCR reactions consisted of 12.5 µL of 1 X Power SYBR green PCR master mix (Applied Biosystems-Life Technologies, Grand Island, NY), 0.2 µM of each primer, 2 µL of DNA or cDNA template, and 7.5 µL of PCR water for a total of 25 µL. Additional Mg(OAc)2 was not added (modification to Pereyra et al. Citation2010), because omitting the Mg(OAc)2 was found to increase the amplification efficiency. One nanogram of DNA template was used; this mass was selected to minimize potential for inhibition of amplification and inaccurate qPCR data, based on preliminary tests (data not shown). Also, 0.40 ng of cDNA was used per RT-qPCR reaction. A control without reverse transcriptase was included to verify the absence of DNA contamination; no genomic DNA was detected. All assays were run in triplicate. The qPCR system was run with a temperature program of 10 min at 95°C, followed by 40 cycles of 40 sec at 95°C, 30 sec at 56°C, and 30 sec at 72°C (Pereyra et al. Citation2010). Dissociation curves were run to verify amplicon specificity. Genomic DNA from Methanococcus maripaludis (ATCC 43000D) was used to generate standard curves for quantification as described previously (Pereyra et al. Citation2010); standard concentrations ranged from 0.0005 to 5.0 ng per reaction.
Archaeal 16S rRNA gene amplicon sequencing
To identify methanogens present in each reactor, DNA extracted from samples collected at week 8 was sent to Research and Testing Laboratories, LLC (Lubbock, TX), for amplicon sequencing of the archaeal 16S rRNA gene. Archaeal 16S rRNA genes were amplified with primers Arch519F and Arch1017R and sequenced with an Illumina MiSeq. Sequences were clustered into their respective operational taxonomic units (OTUs) and identified with the RDP Classifier. In the case where OTUs matched multiple phylotypes with the same levels of confidence, the first hit was reported and the identifier modified to include “-related” to indicate the phylotypes were not fully known due to a high level of 16S rRNA gene sequence similarity.
Results and discussion
Selection of municipal and industrial organic waste substrates and mixtures
Substrates were considered useful if they met the following criteria: COD > 5,000 mg/L, TS between 0.3 and 10%, and VS to TS ratio ≥ 0.5 . TS was above 1% for all individual substrates, with the exception of winery and septage, which had TS of 0.3% (see Supplemental Information, Table S4). VS/TS ratios ranged from 0.49 for portable toilet waste to 0.90 for beer and cider waste. Also, BOD5 ranged from 720 mg/L for winery waste to 34,000 mg/L for tomato waste, and COD ranged from 6,400 mg/L for winery waste to 72,000 mg/L for canned tomato and salsa waste (see Supplemental Information, Table S4). Total organic carbon (TOC) was estimated using a relationship with COD developed for wastewater influent () (Dubber and Gray Citation2010). Therefore, C/N ratios (total organic carbon [TOC] divided by the total nitrogen content (NO3-N + TKN) ranged from 1 to 50, and were non-ideal (~30) for most individual substrates (Table S4).
BMP of individual substrates
shows a summary of the final biogas yields and BMP normalized to VS and COD. The methane and hydrogen sulfide composition of accumulated biogas was also analyzed. Based on methane production, all the substrates were viable for co-digestion. All substrates except for portable toilet waste exceeded methane production of 0.25 L/g VS or COD added. Despite the lower BMP results for the human waste substrates, there was still significant gas production.
Table 1. Biogas and methane yield, methane and hydrogen sulfide concentration for BMP of individual waste.
Laboratory digester performance for waste mixtures
Characterization of selected of waste mixtures
Various waste mixtures were designed with the goal of maximizing methane production, and additionally volumetric compositions were selected to mimic available waste streams. Various parameters (e.g., BOD5:N:P ratio) were considered based on the chemical analyses of individual substrates to optimize methane production (Supplemental Information, Table S2). C/N ratios ranged from 3 to 8 due to nitrogen rich waste materials. BOD5:N:P ratios indicated that there was carbon deficiency for all mixtures. COD/BOD5 ratios show that all food waste mixtures (T/F/W/B, T/F, B/W, F/W) had good biodegradability (around 2:1), but poorly biodegradable substances were present in the human waste mixture (P/S) (5.3). The initial organic loading rates were between 1.4 and 2.3 kg VS/m3-day for all waste mixtures, with the exception of the tomato/FOG mixture (loading rate of 3.3 kg VS/m3-day). Final characterization of waste mixtures was also analyzed (Supplemental Information, Table S6). Compared to initial parameters, TS is reduced by 46.7%, VS 60.9%, volatile acids 55.9%, COD 61.3%, total nitrogen 8.4%, nitrite 86.9%, and sulfate 48.9. However, some parameters increased, like sodium concentration by 50%, nitrate as N 113.9%, and phosphorus 38.7%. Further treatment for those parameters may be needed prior to discharge.
Volatile fatty acids (VFA) and pH
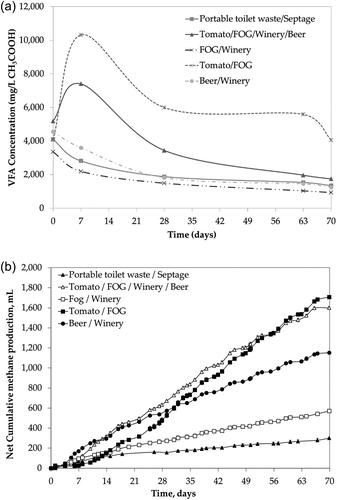
For optimal performance, VFA concentrations ≤ 5,000 mg/L and a neutral pH are desired (Huber et al. Citation1982; Liu et al. Citation2008). Initial VFA concentrations for all reactors were 5,191 mg/L or lower, and VFA concentrations remained well below this threshold over the course of the study for most mixtures ( and Supplemental Information, Table S5). The initial pH of all substrate and inoculum mixtures used in the digesters was between 6.5 and 7.5, and stable pH generally was maintained (see Supplemental Data, Figure S1). However, during the first week for reactors with tomato waste (T/F/W/B and T/F waste mixtures), the VFA concentrations increased to 7,402 and 10,319 mg/L, respectively. This VFA increase indicated that bacteria hydrolyzing tomato waste outpaced methanogens, leading to acid accumulation. To avoid reactor failure, the pH was adjusted using sodium bicarbonate (NaHCO3). After the second week, VFAs dropped and pH increased, indicating active methanogenesis and reactor recovery. The temporary spike in VFAs and pH drop suggested that the organic loading rate for the tomato waste mixtures was too high; indeed, the initial organic loading rate for the T/F mixture was the highest (3.3 kg VS/m3-day). Reducing the tomato waste loading rates and increasing the buffering capacity of the reactor improved the pH stability. After the third week, the pH of all reactors was within an acceptable range for methanogenesis of 6.5–8.2 (Lee et al. Citation2009).
Biogas and methane production
Biogas yields ranged from 195 mL/ g VS to 851 mL/ g VS, and the highest yields were for the B/W (851 mL/g VS) and T/F/W/B (637 mL/g VS) mixtures ( and ). Biogas volumes produced from the other food waste mixtures (T/F and F/W) were 39.6% and 45.4% lower than for the B/W mixture, respectively. The lowest biogas yield was from the P/S waste mixture (195 mL/g VS), which was 77.1% lower than the best biogas yield. The methane concentration in the digesters fluctuated weekly as the reactors were opened to add new feedstock and remove digested waste for testing (see Supplemental Information, Figure S2–Figure S6). All digesters had an average methane content greater than 67%, and some had methane contents as high as 73% (B/W and F/W) (). Biogas from P/S waste had the lowest average methane concentration (67.2 ± 8.3%), and the highest concentration of hydrogen sulfide (890.3 ± 273.9 ppm). Low methane generation could due to the lower biodegradability of COD/BOD5, 5.3. It also could be attributed to potential inhibitory chemicals such as detergents and fragrance mixtures that are added to minimize portable toilet odor (Jimenez-Gonzalez et al. Citation2001). Also, portable toilet waste was found to contain high levels of sulfate (340 mg/L, see Table S4, Supplemental Information). Sulfate likely was microbially reduced to hydrogen sulfide, thus leading to the high measured sulfide levels (Peu et al. Citation2012).
Table 2. Average biogas and methane yield, methane concentration, and hydrogen sulfide concentration for waste mixtures.
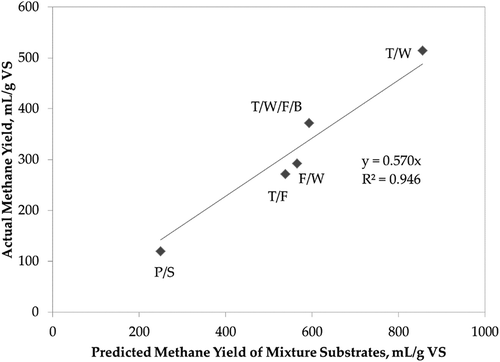
Actual methane yields from the digesters fed the mixed substrates were compared to methane yields predicted using the measured BMPs for the individual substrates. Actual and predicted methane yields were compared in terms of volatile solids conversion () and in terms of COD conversion (see Supplemental Information, Figure S7). For each mixture, the average methane production in the semicontinuous batch reactors was ~50–60% of the yields predicted based on measured methane potential. The correlation is strongest with methane per mass of volatile solids (R2 = 0.946), showing a factor of 0.57 between actual methane production and potential of the substrates in the mixture. On the other hand, correlation per mass of COD was not strong (R2 = 0.573). COD is a measure of the capacity of water to consume oxygen during the decomposition of organic matter and the oxidation of inorganic chemicals. Concentrations of inorganic chemicals such as ammonia and nitrite vary among waste mixtures, which leads to poor correlation. These results indicate that using individual BMP assay results can be useful to predict performance for a mixture. Results also show that some amount of the ideal potential conversion may not be realized in a continuously fed system, but the correlation developed could be used to predict performance in such systems.
Quantification of methanogenic microbial populations
Methanogen populations were quantified via qPCR targeting mcrA, which is specific to methane-producing organisms (Dubey et al. Citation2014). For the laboratory digesters, concentrations ranged from 106 to 108 mcrA genes/mL of reactor volume (see Supplemental Information, , and Figure S8), which is similar to population sizes reported for mesophilic digesters (Steinberg and Regan Citation2009; Citation2011). Control digesters (control human and control food) contained low methanogen populations throughout the study period, as expected because they were not provided a carbon source (waste mixture feedstock). All waste mixtures tested, with the exception of P/S, led to healthy methanogenic populations, with the quantity of methanogens clearly increasing over time. Further, digesters with the highest methanogen populations (B/W and T/F/W/B) also produced the highest amount of biogas (851 mL/g VS and 637 mL/g VS), providing multiple lines of evidence that these were some of the best mixed waste feedstocks tested. Digesters with mid-range methanogen population sizes (T/F and F/W) produced biogas, but at a relatively lower level. The P/S digester’s methanogen population size was clearly the lowest, and these digesters produced little biogas. Low biogas production in the P/S digesters suggests that microbial processes in these digesters were inhibited. Inhibition may have affected hydrolysis, acido/acetogenesis, methanogenesis, or multiple stages; these effects could not be distinguished by available data.
Figure 3. (a) Quantity of methanogens in digesters: mcrA genes per reactor volume as a function of time. (b) Activity of methanogens in digesters: mcrA transcripts per reactor volume at week 8. (Error bars represent standard deviations).
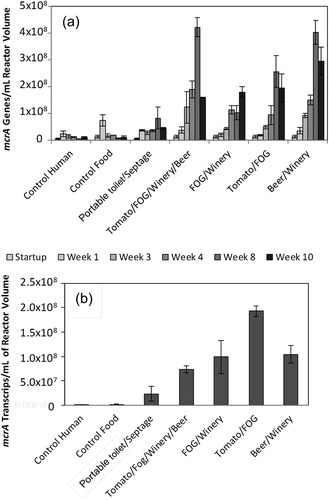
A moderate decrease in methanogen population size was observed at week 10, with the exception of the F/W reactor. Decreases also were observed at week 10 with primer set mcrA_1035F/mcrA_1530R (see Supplemental Information, Figure S8), but the fractional decreases measured with this primer set were larger. This finding indicates that around week 10, there was a shift in the types of methanogens present, with emerging methanogenic groups being less well quantified by the mcrA_1035F/mcrA_1530R primer set than the MLf/MLr primer set. Such shifts in microbial populations have been shown to impact quantification accuracy (Ledeker and De Long Citation2013) because the sequences of target genes vary between phylotypes. Thus, it is possible that overall methanogen populations did not decline significantly, but rather appeared to decline due to limitations with currently available targeted methods. None of the bulk parameters measured offered an underlying explanation for a decline or a population change, and additional tests would be required to determine causes. For example, high-resolution metagenomic sequencing through time could clarify how methanogenic populations changed, because omics data are nontargeted. These data could be coupled with detailed characterization of aqueous phase substrates, metabolites, and hydrogen levels to determine causes. However, given that population sizes during week 10 were still generally as high as during week 4, it is unlikely that the decreased population size at week 10 indicates a longer term issue.
Methanogen concentration is a useful indicator of digester health; however, methanogen population sizes can be influenced by the feedstock concentration provided (Yi et al. Citation2014). Feedstock was provided to all digesters in the same volume; however, the concentration of substrates varied between digesters. Thus, methanogen populations were normalized to g of VS or COD (see Supplemental Information, Figure S9 and data not shown, respectively). Similar trends were observed. Although VS and COD concentrations are imperfect surrogates for feedstock concentrations, because microorganisms are also measured as VS or as COD, these findings indicate that the differences in methanogen populations were likely influenced by the quality of the substrate (e.g., biodegradability and presence of inhibitory substances).
A more direct indicator of the methanogenic population activity is the concentration of mcrA transcripts, which are only produced by active microorganisms. Analysis of mcrA transcript concentrations indicated that the T/F/W/B, F/W, T/F, and B/W digesters all contained active methanogens, while methanogens present in the control digesters were inactive ( and see Supplemental Information, Figure S10). Interestingly, transcript levels in the P/S digester were significantly higher than in control reactors and were only ~3-fold lower than levels in the T/F/W/B. Given this finding, it is possible that portable toilet and septage waste could be used as digester feedstock if mixed with other feedstocks to dilute out inhibiting compounds. Further studies would be required to test this concept, and economics and removal cost of hydrogen sulfide in the biogas would need to be considered.
Actual methane production was compared with mcrA gene quantities measured via qPCR (see Supplemental Information, Figure S11). The results show a considerable amount of scatter; however, a general trend toward higher gas production with larger predicted methanogen population (i.e., more mcrA genes) was observed. While the qPCR data are not likely to be useful as a quantitative predictor of biogas production rate, qPCR data may be a useful indicator of methanogenic population health and thus an indicator for process stability.
Characterization of archaeal community composition
Next-generation sequencing of archaeal 16S rRNA genes led to identification of phylotypes previously associated with ambient temperature AD (). Three orders of Archaea dominated all of the digesters that produced high levels of methane: Methanomicrobiales, Methanobacteriales, and Methanosarcinales (see Supplemental Information, Figure S12). These orders of methanogens also all were present in the P/S digester; however, they did not dominate. Rather, these digesters were dominated by Thermoplasmatales-related Archaea, which are poorly characterized and their ability to generate methane has yet to be fully established (Paul et al. Citation2012). Thus, few known methanogens were able to survive and grow in the P/S digester. This finding further suggests that substances present were inhibitory for methanogens in the inoculum.
Figure 4. Histogram of genus-level archaeal phylotypes based on archaeal 16S rRNA gene amplicon sequencing. Archaea that made up ≤1% of the total identified phylogenetic groups were categorized as “Other”.
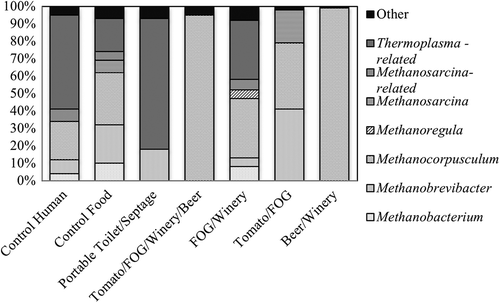
Microbial community compositions varied among digesters fed the other four waste mixtures, but some common phylotypes emerged. The three families of methanogens common among all reactors included Methanocorpusculaceae, Methanobacteriaceae, and Methanosarcinaceae (see Supplemental Information, Figure S12). Interestingly, Methanosarcinaceae were most abundant (19%) in the T/F reactors. Methanosarcinaceae have been found to dominate under high levels of NH3 and VFAs (Demirel and Scherer Citation2008). T/F reactors maintained the highest levels of VFAs throughout the study, leading to selection of these VFA-tolerant methanogens. Interestingly, one genus (Methanocorpusculum) overwhelmingly dominated the two digesters with the highest biogas production: B/W (99% Methanocorpusculum) and T/F/W/B (95% Methanocorpusculum) (). This genus also was present at high levels in the T/F digester (38%) and the F/W digester (34%). Methanocorpusculum-like methanogens previously have been reported to dominate psychrophilic digesters (McKeown et al. Citation2009). An AD study of VFA- and sucrose-based wastewaters that lowered temperature from 37°C to 16°C observed a shift from acetoclastic to hydrogenotrophic methanogens; specifically, Methanosarcina and Methanosaeta were replaced by Methanomicrobiales, in particular Methanocorpusculum parvum (McHugh et al. Citation2004). At lower temperatures, the threshold H2 partial pressure for hydrogenotrophic methanogenesis is lower (Conrad and Wetter Citation1990), likely explaining the dominance of hydrogenotrophic methanogenes in the ambient temperature digesters studied herein. The T/F digester also contained high levels of Methanobrevibacter (41%) and Methanosarcina (19%), and the F/W digester contained low levels of these genera. Findings suggest that suitable methanogenic communities for low-temperature AD were successfully developed over the course of the study.
Conclusion
This study demonstrated that six underutilized high-moisture organic wastes potentially can be feedstocks for biogas production in ambient temperature digestion conditions. Combinations of tomato waste, winery waste, beer and cider waste, and FOG lead to production of substantial biogas, providing clear indication that these are viable ambient-temperature AD substrates. By contrast, combining portable toilet waste and septage led to low biogas generation. Molecular biology assays and next-generation sequencing data indicated that healthy low-temperature methanogen communities were developed. Thus, results demonstrated that there is potential for implementation of unheated covered lagoon digester systems for conversion of liquid wastes to produce renewable biomethane.
Supplemental Material
Download MS Word (1.2 MB)Supplemental data
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
Notes on contributors
Ramin Yazdani
Ramin Yazdani is an Assistant Research Professor at the Air Quality Research Center, University of California, Davis, CA, USA and the Director of Integrated Waste Management, Yolo County, Woodland, CA, USA.
Kyuhwan Shim
Kyuhwan Shim is a Junior Engineer (Civil) Yolo County, Division of Integrated Waste Management, Woodland, CA, USA.
Zhi Chen
Zhi Chen is an Assistant Distiller at the E. & J. Gallo Winery, Modesto, CA, USA.
Christy Cheung
Christy Cheung is an Assistant Engineer, Natural Resources Consulting Engineers, Oakland, CA, USA.
Matthew D. Summers
Matthew D. Summers is a consultant with Summers Consulting, Auburn, CA, USA.
Douglas W. Williams
Douglas W. Williams is a consultant with Williams Engineering Associates, Woodland, CA, USA.
Reinhard Seiser
Reinhard Seiser is an Associate Project Scientist at University of California at San Diego, Department of Mechanical and Aerospace Engineering, La Jolla, CA, USA.
Susan K. De Long
Susan K. De Long is an Assistant Professor at the Colorado State University, Department of Civil & Environmental Engineering, Fort Collins, CO, USA.
References
- Amon, R., M. Jenner, R.B. Williams, H. El-Mashad, and S.R. Kaffka, (California Biomass Collaborative). 2012. California Food Process. Ind. Org. Residue Assess. California Energy Commission.
- Angelidaki, I., M. Alves, D. Bolzonella, L. Borzacconi, J.L. Campos, A.J. Guwy, S. Kalyuzhnyi, P. Jenicek, and J.B. Van Lier. 2009. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 59 (5):927–934. doi:10.2166/wst.2009.040.
- California Wastewater Training and Research Center. 2002. Surv. septage treat. handling disposal pract. California. Chico: California State University.
- Conrad, R., and B. Wetter. 1990. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch. Microbiol. 155(1):94–98. doi:10.1007/bf00291281.
- Cuetos, M.J., X. Gómez, M. Otero, and M. Antonio. 2008. Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: Influence of co-digestion with the organic fraction of municipal solid waste (OFMSW). Biochem. Eng. J. 40(1):99–106. doi:10.1016/j.bej.2007.11.019.
- Demirel, B., and P. Scherer. 2008. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Reviews in Environmental Science and Bio/Technology 7:173–190. doi: 10.1007/s11157-008-9131-1
- Dubber, D., and N.F. Gray. 2010. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health. Part A Environ. Sci. Health Part A Environ. Sci. Eng 45(12):1595–1600. doi:10.1080/10934529.2010.506116.
- Dubey, S.K., A. Singh, T. Watanabe, S. Asakawa, A. Singla, H. Arai, and K. Inubushi. 2014. Methane production potential and methanogenic archaeal community structure in tropical irrigated indian paddy soils. Biol. Fertil. Soils 50(2):369-379. doi: 10.1007/s00374-013-0858-7.
- El-Mashad, H.M., and R. Zhang. 2010. Biogas production from co-digestion of dairy manure and food waste. Bioresour. Technol. 101(11):4021–4028. doi:10.1016/j.biortech.2010.01.027.
- Huber, H., M. Thomm, H. König, G. Thies, and K.O. Stetter. 1982. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch. Microbiol. 132(1):47–50. doi:10.1007/bf00690816.
- Jimenez-Gonzalez, A., M. Salazar-Gonzalez, M. Gutierrez-Rojas, and O. Monroy. 2001. Anaerobic digestion of a nonionic surfactant: Inhibition effect and biodegradation. Water Sci. Technol. 44(4):175–181. doi:10.2166/wst.2001.0214.
- Kayhanian, M., and D. Rich. 1995. Pilot-scale high solids thermophilic anaerobic digestion of municipal solid waste with an emphasis on nutrient requirements. Biomass Bioenergy 8(6):433–444. doi:10.1016/0961-9534(95)00043-7.
- Kerri, K.D. 2007. Oper. wastewater treat plants. Sacramento: Office of Water Programs, California State University.
- Khalid, A., M. Arshad, M. Anjum, T. Mahmood, and L. Dawson. 2011. The anaerobic digestion of solid organic waste. Waste Manage. (Oxford) 31(8):1737–1744. doi:10.1016/j.wasman.2011.03.021.
- Ledeker, B.M., and S.K. De Long. 2013. The effect of multiple primer-template mismatches on quantitative PCR accuracy and development of a multi-primer set assay for accurate quantification of pcrA gene sequence variants. J. Microbiol. Methods 94(3):224–231. doi:10.1016/j.mimet.2013.06.013.
- Lee, D. H., S.K. Behera, J.W. Kim, and H.S. Park. 2009. Methane production potential of leachate generated from Korean food waste recycling facilities: A lab-scale study. Waste Manage. (Oxford) 29(2):876–882. doi:10.1016/j.wasman.2008.06.033.
- Liu, C., X. Yuan, G. Zeng, W. Li, and J. Li. 2008. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 99:882–888. doi:10.1016/j.biortech.2007.01.013.
- Luton, P.E., J.M. Wayne, R.J. Sharp, and P.W. Riley. 2002. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology. 148(Pt 11):3521–3530. doi:10.1099/00221287-148-11-3521.
- McHugh, S., M. Carton, G. Collins, and V. O’Flaherty. 2004. Reactor performance and microbial community dynamics during anaerobic biological treatment of wastewaters at 16–37°C. FEMS Microbiol. Ecol. 48(3):369–378. doi:10.1016/j.femsec.2004.02.012.
- McKeown, R.M., C. Scully, A.M. Enright, F. A. Chinalia, C. Lee, T. Mahony, G. Collins, and V. O’Flaherty. 2009. Psychrophilic methanogenic community development during long-term cultivation of anaerobic granular biofilms. The ISME J. 3(11):1231–1242. doi:10.1038/ismej.2009.67.
- Metcalf, and Eddy. 2003. Wastewater engineering : Treatment and reuse, 4th ed. revised by George Tchobanoglous, Franklin L. Burton, H. David Stensel. Boston: McGraw-Hill.
- Miner, J.R., F.J. Humenik, J.M. Rice, D.M.C. Rashash, C.M. Williams, W. Robarge, D.B. Harris, and R. Sheffield. 2003. Evaluation of a permeable, 5 cm thick, polyethylene foam lagoon cover. Trans. ASAE. 46(5):1421. doi:10.13031/2013.15442.
- Moletta, R. 2005. Winery and distillery wastewater treatment by anaerobic digestion. Wastewater Eng. 51(1):137–144. doi:10.2166/wst.2005.0017.
- Murto, M., L. Björnsson, and B. Mattiasson. 2004. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manage. 70(2):101–107. doi:10.1016/j.jenvman.2003.11.001.
- Nielsen, A.H., J. Vollertsen, H.S. Jensen, T. Wium-Andersen, and T. Hvitved-Jacobsen. 2008. Influence of pipe material and surfaces on sulfide related odor and corrosion in sewers. Water Res. 42(15):4206–4214. doi:10.1016/j.watres.2008.07.013.
- Paul, K., J.O. Nonoh, L. Mikulski, and A. Brune. 2012. “Methanoplasmatales,” Thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl. Environ. Microbiol. 78(23):8245–8253. doi:10.1128/AEM.02193-12.
- Pereyra, L.P., S.R. Hiibel, M.V. Prieto Riquelme, K.F. Reardon, and A. Pruden. 2010. Detection and quantification of functional genes of cellulose- degrading, fermentative, and sulfate-reducing bacteria and methanogenic archaea. Appl. Environ. Microbiol. 76(7):2192–2202. doi:10.1128/aem.01285-09.
- Peu, P., S. Picard, A. Diara, R. Girault, F. Béline, G. Bridoux, and P. Dabert. 2012. Prediction of hydrogen sulphide production during anaerobic digestion of organic substrates. Bioresour. Technol. 121:419–424. doi:10.1016/j.biortech.2012.06.112.
- Slaughter, R., P. Henderson, and S. Owen. 2007. Anaerobic treatment of septage/biosolids to produce biogas, electrical power and treated biosolids, Growing the Margins Energy Conference, Canada, ON.
- Steinberg, L.M., and J.M. Regan. 2009. mcrA-targeted real-time quantitative PCR method to examine methanogen communities. Appl. Environ. Microbiol. 75(13):4435–4442. doi:10.1128/aem.02858-08.
- Steinberg, L.M., and J.M. Regan. 2011. Response of lab-scale methanogenic reactors inoculated from different sources to organic loading rate shocks. Bioresour. Technol. 102(19):8790–8798. doi:10.1016/j.biortech.2011.07.017.
- U.S. EIA. 2009. Residential Energy Consumption Survey (RECS) Survey Data, Tables HC6.11, HC7.11, HC8.11. U.S. Energy Information Administration. www.eia.gov/consumption/residential (accessed June 13, 2018).
- Wang, X., L. Xingang, L. Fang, and G. Yang. 2014. Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PLoS One 9(5):e97265. doi:10.1371/journal.pone.0097265.
- Yenigün, O., and B. Demirel. 2013. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 48(5–6):901–911. doi:10.1016/j.procbio.2013.04.012.
- Yi, J., B. Dong, J. Jin, and X. Dai. 2014. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS One 9(7):e102548. doi:10.1371/journal.pone.0102548.