ABSTRACT
Understanding nitrogen oxides (NOx = NO + NO2) measurement techniques is important as air-quality standards become more stringent, important sources change, and instrumentation develops. NOx observations are compared in two environments: source testing from the combustion of Southwestern biomass fuels, and urban, ambient NOx. The latter occurred in the urban core of Albuquerque, NM, at an EPA NCORE site during February–March 2017, a relatively clean photochemical environment with ozone (O3) <60 ppb for all but 6 hr. We compare two techniques used to measure NOx in biomass smoke during biomass burning source testing: light absorption at 405 nm and a traditional chemiluminescence monitor. Two additional oxides of nitrogen techniques were added in urban measurements: a cavity attenuated phase shift instrument for direct NO2, and the NOy chemiluminescence instrument (conversion of NOy to NO by molybdenum catalyst). We find agreement similar to laboratory standards for NOx, NO2, and NO comparing all four instruments (R2 > 0.97, slopes between 0.95 and 1.01, intercepts < 2 ppb for 1-hr averages) in the slowly varying ambient setting. Little evidence for significant interferences in NO2 measurements was observed in comparing techniques in late-winter urban Albuquerque. This was also confirmed by negligible NOz contributions as measured with an NOy instrument. For the rapidly varying (1-min) higher NOx concentrations in biomass smoke source testing, larger variability characterized chemiluminescence and absorption instruments. Differences between the two instruments were both positive and negative and occurred for total NOx, NO, and NO2. Nonetheless, integrating the NOx signals over an entire burn experiment and comparing 95 combustion experiments, showed little evidence for large systematic influences of possible interfering species biasing the methods. For concentrations of <2 ppm, a comparison of burn integrated NOx, NO2, and NO yielded slopes of 0.94 to 0.96, R2 of 0.83 to 0.93, and intercepts of 8 to 25 ppb. We attribute the latter, at least in part, to significant noise particularly at low NOx concentrations, resulting from short averaging times during highly dynamic lab burns. Discrepancies between instruments as indicated by the intercepts urge caution with oxides of nitrogen measurements at concentrations <50 ppb for rapidly changing conditions.
Implications: Multiple NOx measurement methods were employed to measure NOx concentrations at an EPA NCORE site in Albuquerque, NM, and in smoke produced by the combustion of Southwestern biomass fuels. Agreement shown during intercomparison of these NOx techniques indicated little evidence of significant interfering species biasing the methods in these two environments. Instrument agreement is important to understand for accurately characterizing ambient NOx conditions in a range of environments.
Introduction
Nitrogen oxides (NOx), taken as the sum of nitrogen dioxide (NO2) and nitric oxide (NO), are produced by both natural and anthropogenic sources. Natural sources include emissions from soils, oceans, and biomass burning, as well as secondary atmospheric production. Anthropogenic NOx sources can be classified as either stationary or mobile combustion sources (Kleinman et al. Citation2007). Stationary sources include power plants and industrial processes. Mobile sources of NOx are primarily vehicles with internal combustion engines. By thermal decomposition of N2 and O2, combustion of fuels produces NO that is subsequently converted to NO2 in the atmosphere by reactions with ambient ozone (O3) and other oxidizing species (Jacob Citation1999). Combustion of fossil fuels from both stationary and mobile sources emits 30 Tg of NOx as nitrogen per year globally (Fowler et al. Citation2013). NOx tends to have elevated concentrations near major roadways and is linked to O3 production via photochemistry (Beckerman et al. Citation2008). Urban NOx levels often correlate to other traffic related pollutants such as CO. Natural sources are smaller and more dispersed, including soil emissions of approximately 5 Tg NO per year globally, oceanic emissions of approximately 5.5 Tg per year, and lightning-produced emissions of approximately 5 Tg per year (Fowler et al. Citation2013).
In 2010, the EPA set the primary 1-hr standard for NO2 at 100 ppb, in addition to the existing annual standard of 53 ppb. The new, more stringent standard is measured as the 3-year average 98th percentile of daily maximum 1-hr values (Code of Federal Regulations [CFR] under 40 CFR parts 50 and 58, U.S. EPA Citation2017). After the revision to the standard in 2010, the requirements for monitoring NO2 were also revised. The goal of the revision was to better characterize NO2 emissions from major roadways and emissions near vulnerable populations. The rule required core-based statistical areas (CBSAs) to establish near-road monitors based on population and daily traffic. In 2011, the EPA established a network of national core (NCORE) monitoring stations to deploy advanced measurement techniques of numerous air pollutants, including various NOx methods. One of the main goals of this network is to track long-term trends of air pollutants to aide in emission control strategies and to establish attainment and nonattainment areas. The ambient measurements described here were at an EPA NCORE site in Albuquerque, NM (AQS # 35–001-0023).
Another NOx source, which can be both anthropogenic and natural, is biomass burning, which, on average, adds an additional 5 Tg of NOx (Fowler et al. Citation2013) to the atmosphere. The land area subject to wildfire in the United States has dramatically increased in the 21st century, with a wide range of impacts, including air quality degradation (Rocca et al. Citation2014). In 2016 and 2017, approximately 10 million acres burned annually in the United States, with approximately 10% in New Mexico, Arizona, and western Texas (NIFC Citation2017). Wildfires, a natural source that is subject to growing human perturbations, promote both thermal (high-temperature N2 degradation) and fuel NOx (oxidation of fuel nitrogen) production. The heat produced during biomass combustion dissociates molecular nitrogen and oxygen, leading to NOx formation. Biomass fuels also contain nitrogen cations such as ammonium, anions such as nitrate or nitrite, and organic nitrogen compounds such as amino acids that all can be released as NOx during the oxidation process.
NOx is one product of combustion, and, with other oxidized forms of nitrogen, adds to the population of what is termed NOy. NOy is known as total reactive nitrogen and can be approximated as the sum of NO, NO2, HNO3, HNO4, NO3, N2O5, HONO, alkyl nitrates, and peroxyacyl nitrates (PAN), as well as potentially other gas-phase nitrogen-containing species (and even particulate nitrate in some definitions). In urban source areas, relatively high NOx/NOy ratios are to be expected as NO and NO2 typically are converted into NOy species as the air mass ages. NOx concentrations decrease over time due to oxidation by the hydroxyl (OH) radical. Because of this, the photochemical age of an air mass can be determined using the ratio NOx/NOy (Kleinman et al. Citation2007). Close to biomass burning events, NOx/NOy ratios are typically higher due to proximity to the source (Prenni et al. Citation2014).
The increase of fire size and intensity in the United States over the last several decades (www.nifc.gov) has led to concern over effects of the smoke on air quality in the southwestern U.S. region. Gas-phase NOx measurement methods can be subject to differing interferences, depending on the technique and the measurement environment. This makes it important to characterize these methods under varying conditions. This paper focuses on NOx measurements during the combustion of biomass fuels common in the southwestern United States and ambient measurements taken in an urban area (Albuquerque, NM) impacted by anthropogenic sources and, intermittently, wildland fire.
Methods
Here we operated selected NOx measurement techniques in the same measurement environments—one the urban environment of Albuquerque, NM, and the other source testing with fresh biomass burning emissions—to examine measurement intercomparability for these differing conditions. Description of the methods and quality assurance (QA) efforts follows, prior to discussion of the results.
Measurement techniques
Chemiluminescence (NO/NOx)
The standard analyzer used in air quality monitoring applications is the chemiluminescence monitor (here Thermo Scientific, Inc., model 42C in laboratory combustion experiments, and Teledyne-API, Inc., model T200 in ambient Albuquerque measurements). Chemiluminescence following NO2 conversion to NO via molybdenum converter is designated by the U.S. EPA as a reference method for the measurement of ambient concentrations of NO2 as per the Code of Federal Regulations, Title 40, Part 53. Chemiluminescence is also widely used in Europe for roadside NO2 monitoring (Jiménez et al. Citation2011). Chemiluminescence relies on the emission of infrared light through the reaction of NO with O3 (NO + O3 → NO2 + O2) that produces excited state NO2*. Light is emitted when NO2* decays from an excited state to the ground state NO2 (Tidona, Nizami, and Cernansky Citation1988). The amount of light emitted is linearly related to the concentration of NO reacted.
This method measures NO2 indirectly by first converting it into NO and measuring the sum of NO + NO2 as described already by the addition of excess O3. NO2 is typically transformed to NO via a heated, catalyzed converter using molybdenum, and the NO2 concentration is obtained as the difference between the NO-only measurement and the NO + NO2 (or NOx) measurement (Thermo Electron Corporation Citation2004). Chemiluminescence instruments are typically calibrated with an NO mixture, usually in N2, which is injected directly or converted to NO2 via gas-phase titration (Tidona, Nizami, and Cernansky Citation1988). In order to measure multiple species, NOx instruments cycle through measuring individual species (NOx, NOy, NO, NO2) using pneumatic switching with typical dwell and blank times of ~5 sec each. This slows the instrument response time to individual species; representative response times and other instrument specifications are summarized in .
Table 1. Summary of measurement methods used in this study. All instruments were operated with manufacturer default settings unless otherwise specified.
A known limitation of the chemiluminescence method is the use of the nonselective catalytic converter for measurement of NO2, allowing potential interferences. Other reactive nitrogen-containing atmospheric gaseous species are known to interfere with the NO2 measurement, such as HNO3 and PAN (e.g., Dunlea et al. Citation2007; Ge et al. Citation2013). Acetonitrile is a nitrogen compound often found in biomass smoke that could potentially interfere with chemiluminescent NH3 instruments. For example, a study performed with a chemiluminescence instrument with a higher temperature conversion found that there was significant sensitivity (70 ± 10%) with regard to acetonitrile for ammonia measurements using chemiluminescence, underscoring the nonselective nature of chemiluminescence (Benedict et al. Citation2017). A negative interference with the chemiluminescence method can result from the collision of third-body species with NO2*, quenching the excited state instead of producing light (Tidona, Nizami, and Cernansky Citation1988). This is a concern for water vapor, which has a high efficiency for quenching and varies greatly within the atmosphere. Other considerations for possible interferences with any method are changes in sampling and monitoring conditions such as temperature and pressure. For example, another study found changes in ambient pressure greater than ±10 kPa can impact the measurements of a chemiluminescence monitor up to 7% due to quenching of NO2* (Miñarro et al. Citation2011).
Direct absorption (NO2/NO)
For both laboratory combustion experiments and ambient measurements in Albuquerque, we measured NOx with a direct absorption method. The direct light absorption method (2B Technologies, Inc., model 405) directly measures NO2 concentrations based on light absorption at λ = 405 nm (Birks et al. Citation2018). The absorption instrument here uses a light-emitting diode (LED) with a wavelength of 405 nm. The direct absorption method relies on a wavelength where NO2 absorbs but other relevant species have negligible absorption. The method was designated by the U.S. EPA as an equivalent method for NO2 measurements under equivalency EQNA-0217–243 in May 2017. This method provides a direct measurement of NO2, the regulated species, and an indirect measurement of NO by conversion to NO2 using ozone.
To measure NO2, the sample alternately passes through or bypasses a heated (110°C) NO2 scrubber. To minimize interference from changing humidity, the model 405 equilibrates the humidity of the gas entering the cell with the surrounding humidity using a Nafion humidity equilibrator. The light intensity is then measured with and without NO2 present. The concentration of NO2 is calculated using the Beer–Lambert relationship, which describes the exponential decay of light intensity as a function of path length through an absorbing medium. The measurement of NO is made by adding excess O3 and quantitatively converting the NO to NO2. The NO concentration is then calculated from the Beer–Lambert relationship using the light intensity measured with only sample air as the reference. NO and NO2 are alternately measured every 5 sec, and the concentration of total NOx is taken as the sum of NO and NO2. The instrument corrects for temperature and pressure to adjust measured concentrations in molecules/cm3 to parts per billion by volume (ppb). Instrumental comparisons are limited to the range of 0 to 2000 ppb as given by manufacturer testing and specification for a linear response. Further study, focusing on deviations in linearity over this broad range, would help further quantify uncertainties for all instruments.
Cavity attenuated phase shift instrument (NO2)
At the Albuquerque ambient sampling site, we deployed a cavity attenuated phase shift instrument (CAPS) instrument (Teledyne-API, Inc., model T500U) (). The instrument is a “direct” measurement of NO2 only and avoids any use of converters. The CAPS method uses a light-emitting diode (LED) (450 nm) emitting into a long-path-length measurement cell with high-reflectivity mirrors and a vacuum photodiode detector. Rather than measuring a change in intensity, the shift in phase of light with and without sample is measured and correlates to concentration. Due to the wavelength band, the instrument is considered free of interference from other reactive nitrogen species of atmospheric significance, though sensitivity to glyoxal compounds is possible (Ge et al. Citation2013; Kebabian, Herndon, and Freedman Citation2005; Kebabian et al. Citation2008).
NOy instrument (NO/NOx/NOy)
Also at the Albuquerque ambient sampling site, we deployed an NOy analyzer. To address interferences associated with the traditional chemiluminescence analyzer, the NOy instrument has been developed to intentionally measure all odd nitrogen species by placing the converter at the inlet to avoid variable losses of sticky compounds like HNO3. Traditional NO/NOx chemiluminescent instruments identify only some fraction of these compounds due to compound reactivity and loss. The Teledyne model T200U-NOy measures NOx, NO, and the sum of oxidized nitrogen species (or NOy). NO2 is quantified by the difference between NOx and NO, while an estimate of “NOz” is obtained from NOy – NOx.
The analyzer splits the sample into two separate gas streams. One stream goes through an external heated molybdenum converter at the inlet (typically at 10 m height) and total NOy is measured (i.e., any unstable species such as HNO3 are converted immediately at the inlet). The other stream bypasses the converter and the NO and NOx concentrations are measured as with the traditional chemiluminescence monitor.
All instruments described in the preceding were operated in manufacturer default settings unless otherwise specified. A summary of relevant specifications and instrumental details is provided in .
Laboratory and field measurements
Biomass burning source experiment
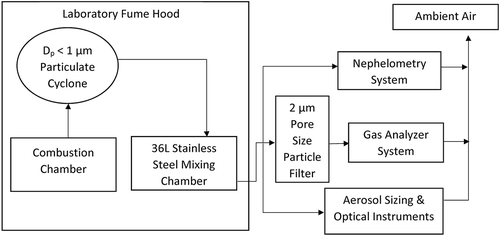
In the laboratory portion of the study, the direct absorption and chemiluminescence methods were used to measure NO and NO2 concentrations in fresh smoke generated from combustion of Southwestern biomass fuels. The fuel quantities combusted ranged from 0.2 to 5 g of fuel (Gomez et al. Citation2018). All burns were performed in a small combustion chamber at Los Alamos National Laboratory (LANL) as shown in . Based on high modified combustion efficiency, most of these small combustion experiments represented flaming conditions due to dry fuels, large surface area to volume, and large flow of combustion air provided by the laboratory fume hood (Gomez et al. Citation2018). The gas measurements were conducted in parallel with a suite of aerosol measurements to examine fresh aerosols from biomass combustion (Carrico et al. Citation2018; Gomez et al. Citation2018). The smoke from the combustion chamber passed through a particulate cyclone (URG, Inc., model URG-2000-30EHB cyclone with Dp < 1 µm at 16.7 lpm) for removing large particles before entering the stainless-steel distribution plenum. Here a subset of burns is illustrated as representative of the range of burn types and behavior across the experiments. These case studies were also chosen as both instruments reported continuous data within instrument range throughout the entire experiment. An overall summary comparison of all burns is also presented. In the summary comparison, NOx signals during each burn are integrated over each individual experiment, resulting in a single data point for each parameter during each of 126 burn experiments. The integration for each burn occurred during the time frame when the species concentrations were above the background level (0.2 ppb) of NO for the chemiluminescence instrument. As discussed later, 95 out of 126 burns were retained in the data set (those eliminated were mostly above the 2 ppm upper limit). Data not representing valid measurements (e.g., housekeeping periods) were removed manually from the data set referencing field notes and processed by spreadsheet (MS Excel), all verified by a second observer.
Urban ambient measurements in Albuquerque, NM
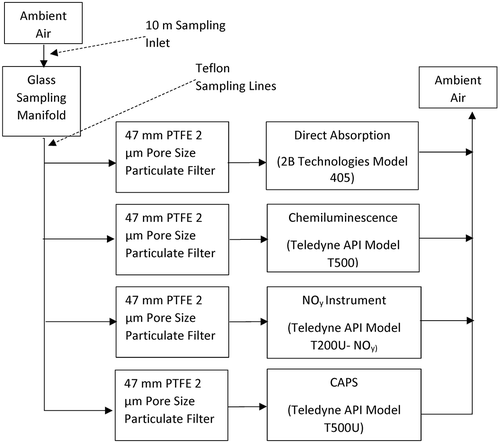
To compare measurement techniques for ambient air, four instruments were deployed at an EPA NCORE sampling site in Albuquerque, NM, during February and March 2017. The monitoring location is located at Del Norte High School, near the corner of San Mateo and Montgomery (35°08’03.4” N, 106°35’06.7” W). The area is in the urban core of Albuquerque and is heavily trafficked. The Mid-Region Council of Governments of New Mexico (MRCOG) reports the average annual work day traffic (Monday through Friday) from 2000 to 2014 for this intersection as more than 26,000 vehicles per day according to the Mid-Region Council of Governments of New Mexico (https://www.mrcog-nm.gov/). This site is part of the New Mexico Environment Department Air Quality Bureau Air Monitoring Network (https://www.env.nm.gov). shows the instrument configuration used when all instruments were sampling in parallel through a glass manifold flow splitter. The ambient sampling resulted in 96% capture for the oxides of nitrogen instruments over the 2-month sampling period.
Calibration and data quality control
Intercomparison at the Albuquerque NCORE ambient monitoring site using EPA protocols and standards was employed for ongoing NOx measurements at this site. The Albuquerque NCORE station was operated as per standard EPA QA requirements as specified in the Code of Federal Regulations 40 CFR Part 51 and EPA guidance documents (U.S. EPA Citation2017). Calibrations including zero, span, and linearity checks were conducted before, during, and after the measurement periods with multiple gas standards (e.g., Scott Specialty Gas, Inc., Linde Specialty Gas, Inc., or other National Insitute of Standards and Technology [NIST] traceable EPA protocol mixture) and three dilution calibration systems. Known concentrations of NO and NO2 were generated and supplied to the instruments and the responses were recorded. Spans and zeroes were conducted weekly at approximately 450 ppb, while precision points at 100 ppb were done every 2 weeks. No meaningful change occurred (within 5% of expected for span and 7% for all points in multipoint calibrations) in instrument response during the intercomparison periods, and the checks resulted in no changes to the instrument span coefficients. Small changes (a few parts per billion) were made in zero offsets during the intercomparison periods. Dilution calibrators (e.g., Teledyne-API, Inc., model 700E) were verified with external flow standards (e.g., Mesa Labs, Inc., Defender Series). Flow standards were NIST traceable and certified within the past year. Zero air generation with particle filtration, activated charcoal, and a Purafil scrubber (Teledyne-API, Inc., model 701 or similar) were used to test zero response. The response of the chemiluminescence instrument was tested via typical gas-phase titration used to generate NO2. The converted response was tested to maintain the standard 95+% conversion efficiency. The instrument flow rates were also measured and verified approximately weekly (Mesa Labs, Inc., Defender Series), along with weekly changing of the Teflon particle prefilter (and daily during combustion tests due to high concentrations). Periods of maintenance, calibration, and known instrumental problems (including off-scale, communication lapses with one or more instruments, flow disruptions, and other typical operational malfunctions) were discarded from the measurement intercomparisons. With the biomass source testing, to compensate for differing transit times and instrument response times, instrument data sets were aligned based on the leading edge of the signals recorded during burn experiments. Due to rapid changes during the first 30 sec of combustion experiments, the data points during this period were excluded from the regression comparisons. Measurements were compared at time resolutions as fine as 1 min for all instruments.
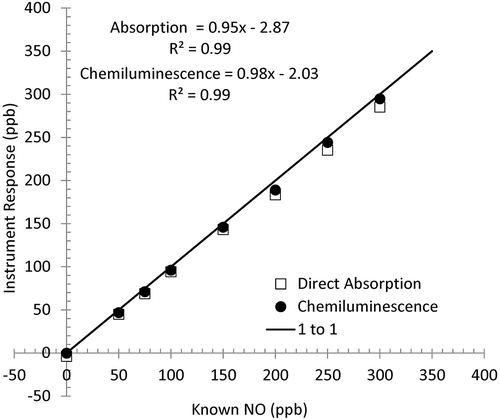
Shown in are representative calibration data for the direct absorption and chemiluminescence instruments used in the laboratory biomass combustion tests. The instruments were supplied with known concentrations of NO ranging from 0 to 450 ppb using dilution calibrators. Both instruments showed strong agreement (R2 > 0.99) with expected values, slopes within 5% of 1:1 agreement, and offsets < 3 ppb.
As a summary comparison of the biomass burning experiments, the NOx emission profiles were integrated over each of the 126 experiments, giving a single value for the concentration produced for each chemical species during each combustion experiment. This mitigated differences in time response and transit time in the instruments during the highly dynamic lab experiments, as well as internal instrument data filtering routine differences. Furthermore, we restricted the analysis to NO concentrations of <2 ppm as specified by manufacturer instrument ranges given (). Beyond this concentration the instrument comparison became nonlinear. Excluding the high-concentration data eliminated approximately one-quarter of the burn data. Furthermore, based on field observation and subsequent manufacturer testing of the direction absorption instrument, ozone generation in the absorption instrument was not sufficient for complete reaction with NO at high concentration. This was upgraded by the manufacturer in this early revision of the instrument prior to redeployment at the ambient sampling site in Albuquerque (though NO concentrations above 2 ppm were not observed during the ambient sampling). With longer sampling times, the ambient data were also compared on 1-hr and 24-hr time increments due to the smaller magnitude concentrations and continuous 2-month record.
A source test used a propane torch as a source of pure NOx with little hydrocarbon due to its efficient combustion. The propane torch was used to promote the formation of thermal NOx in the fume hood where the air inlet was located, which was then sampled by the instruments. The agreement between the instruments was analyzed for the NOx test concentrations generated.
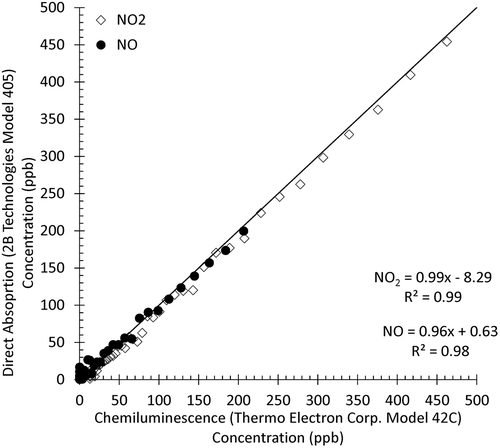
In , the comparison of the two instruments is shown with emissions from a propane torch, a high-temperature flame and thus a significant NOx source. The instruments showed strong agreement (R2 > 0.99) in the range of 0 ppb up to 500 ppb for NO2. The agreement for NO over the range of 0 ppb to 250 ppb was also strong (R2 = 0.98). The offset in NO2 shown in indicates some instrumental differences that may contribute to lower agreement between methods at low NOx concentrations. Field intercomparisons of three NOx instruments during the Albuquerque campaign showed similar agreement (not shown) with slopes and R2 > 0.98, and intercepts < 5 ppb for NO and for NO2 generated via gas-phase titration. Due to its inlet and converters at 10 m the NOy instrument was excluded from this comparison and instead relied upon its standard EPA protocol calibration routine.
Results and discussion
Laboratory biomass burning combustion
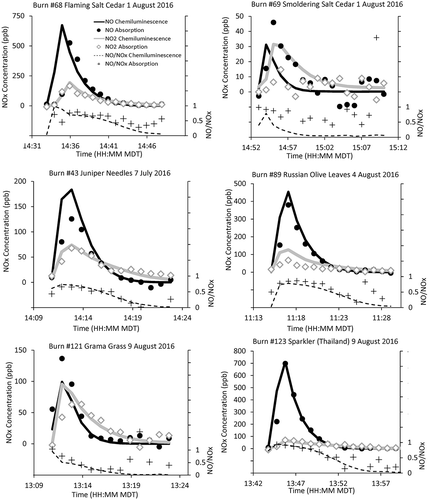
A representative summary of biomass burning combustion experiments is shown in a, a representative subset of experiments with the range of NOx concentrations measured during the laboratory study. For biomass source testing, time series of NOx emissions show NO, NO2, and NO/NOx ratio for both the chemiluminescence and absorption instruments (). Due to the mixing tank shown in , which served to dampen drastic concentration fluctuations, a peak in NOx concentration followed by exponential decay was typically observed (Gomez et al. Citation2018). The combustion tests were further categorized by the ignition method. These included (1) gradual heating with a heat gun at T ~ 450 K, which produced a lower intensity (promoting smoldering) burn, (2) use of a propane torch at T > 750 K, which produced a higher intensity burn with more visible flames and rapid combustion, and (3) ignition with a glowing hot electrical resistance heater or (4) use of butane lighter, as described more fully in Gomez et al. (Citation2018). Burn numbers correspond to the experiments described in Gomez et al. (Citation2018), where more information can be found on methods and fuels. summarizes the correlation of the two methods for NO2, NO, and NOx for each of these experiments.
Table 2. Select detailed combustion test instrument comparisons between direct absorption (model 405) and chemiluminescence (model 42C) instruments for the case studies shown in . For NOx, NO2, and NO, table shows regression statistics for 1-min data and single values for burn integrated data.
Two successive burns with 2 g of salt cedar leaves, an invasive fuel in the southwestern United States, provide an interesting contrast (a and 5b), with the only controlled difference of the high- versus low-temperature ignition methods. Burn 68 was ignited from above the fuel bed with a preheated and glowing hot electrical resistance heater, resulting in a burn dominated by a faster, higher intensity burn with visible flames. Burn 69 was ignited with heat gun, in a more gradual manner and at lower temperature, lacking the full ignition of a flaming phase. NOx concentrations for burn 68 vs. burn 69 reached 866 ppb versus 62 ppb, showing the importance of combustion temperature for NOx generation (). It was observed in the companion combustion experiments (not shown here) that higher temperature and more visibly flaming burns consistently produced much higher concentrations of NOx, consistent with other biomass combustion studies (Benedict et al. Citation2017). At low concentrations characteristic of burn 69, instrument intercomparison showed greater discrepancies. To put this in context, lab testing of the instruments using zero air showed a (2σ) Lower Detection Limit (LDL) of 7 ppb at 1 min for the direct absorption method. Thus, some of the noise in the 1-min NOx < 50 ppb is expected. Differences in response time () were also a likely factor, as the instruments proceed through a sequence of valve switching with each measurement step. For example, the rapid change in concentration at the beginning of the experiment produced outliers due to differences in instrument response time, and thus the data point before the peak shown in burn 68 was discarded from the regression comparisons shown in , as previously described. Instrument internal adaptive filtering of the data stream may affect the real-time comparisons as well, and thus we looked at the burn-integrated measurements to try to minimize this artifact, as discussed further in the following.
The six combustion experiments shown in represent the range of NOx comparisons noted throughout the biomass burn tests. Higher concentration experiments (above 50 ppb) generally showed better intermethod agreement for NO2, NO, and NOx, indicating that method discrepancies were partially related to the signal magnitude at the short averaging time of 1 min. Depending on burn experiment, either NOx method (chemiluminescence or absorption) exceeded the other for either NO or NO2 (, ). Overall, better agreement was observed comparing total NOx versus speciated NOx, indicating some of the difference mentioned previously was a divergence in which bin, NO or NO2, that the instrument categorized NOx species. For brevity, we show agreement between methods for NO2 (the regulated species) and NO for a subset of burns in and give the corresponding regression statistics for NO2, NO, and NOx in . Select, though not all, burns showed lower NO2 via the direct absorption method, possibly indicative of interfering species affecting chemiluminescence. For example, burns 69 and 89 show a factor of ~2 difference in NO2 chemiluminescence versus absorption (, ). However, these were intermittent and of the same magnitude as the discrepancies in NO in other burns, as shown in . Examining the entire burn data set shows this is likely instrumental variability in these dynamic experiments based on the spread of burn integrated NOx, NO, and NO2 values both above and below the 1:1 line (). Although the contribution of interfering species cannot be ruled out, the differences are likely a result of low signal at short averaging times combined with some variability between instruments in the distinction between NO2 and NO. Overall, a repeating and systematic divergence between the two methods was not observed during the combustion experiments. The results suggest caution in NOx measurements with highly variable sources at averaging times of 1 min or less.
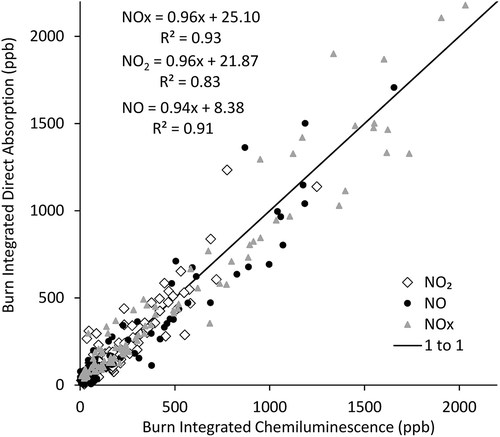
A summary of the burn-integrated NOx measurements via the direct absorption and chemiluminescence methods is shown in for 95 laboratory burns. We have limited the comparison to NO concentrations less than 2 ppm (as measured by chemiluminescence), the upper limit specified by the manufacturer (). The burn-integrated NO2 comparisons here too give little evidence of systematic and consistent interferences or biases in the methods as well. In the intercomparison of burn-integrated signals for NOx, NO, and NO2, slopes > 0.94 and R2 values > 0.83. The somewhat lower R2 for NO2 may relate to signal magnitude (fresh emissions are NO rich, thus giving a larger magnitude for both NO and NOx, as shown in ). It should be noted that the burn-integrated data do show considerable scatter about the 1:1 line throughout the range of 0 to 2 ppm. Similarly, the offsets between instruments (8 to 25 ppb) give indication of significant discrepancies significant at low concentrations (). We attribute some of this again to the response times of the instruments, in the range of 30 to 50 sec as given in the methods section (). This is relatively long compared to the typical 10-min experiment length, which was limited by the quantity of fuel combusted and smoke storage chamber. Though individual burns may vary more, similar overall agreement between NOx, NO and NO2 channels does give an indication that NO2 interferences for either method are not causing a significant NO2 artifact. Comparisons over longer time-frame comparisons for biomass smoke (e.g., ambient smoke events) are recommended to further explore this relationship. Additionally, the difference in response time may be further reduced by having the instruments measure only one NOx species rather than switching between different modes.
Ambient measurements in the urban core of Albuquerque, NM
and show the ambient data collected in February–March 2017 in Albuquerque, NM, at the U.S. EPA NCORE station operated by the city of Albuquerque, NM, and Bernalillo County, NM. A time-series comparison of all four NOx methods over the entire 2-month sampling period averaged to 1-hr intervals is given in , while regression plots for NO2 are given in . All four methods showed quite consistent results for both NO and NO2 with slopes >0.95, intercepts <2 ppb, and R2 values >0.97 comparing 1-hr data (). The 24-hr comparison is similar, and the comparison degrades only modestly for 1 min (). These are agreements similar to those that were observed comparing the instruments to known standards as discussed in the quality control section.
Table 3. Ambient data regression statistics comparing NOx techniques (model number from in parentheses) for February–March 2017 in Albuquerque, NM.
Figure 7. Comparison of ambient (a) NO and (b) NO2 concentrations at the EPA NCORE site in Albuquerque, NM, during February–March 2017 (1-hr average).
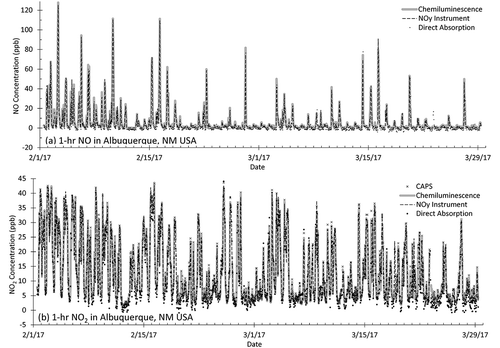
Figure 8. Comparison of ambient NO2 at the EPA NCORE site in Albuquerque, NM, for chemiluminescence (T200), NOy instrument (T200U-NOy), direct absorption (405), and CAPS (T500U) instruments (1-hr average for February–March 2017).
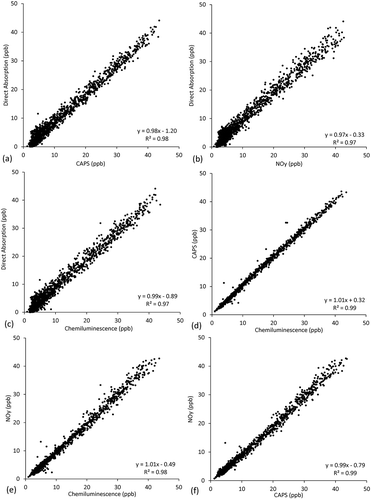
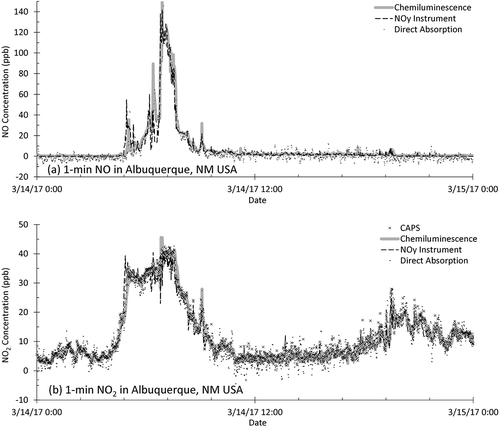
One example of a daily ambient NOx profile is shown in , illustrating agreement among the four techniques with 1-min average data at the Albuquerque NCORE site. In this case, the absorption instrument data has a 3-min running average applied to make it more comparable to the other methods that use an internal adaptive filtering implemented in the chemiluminescence instruments (Thermo Electron Corporation Citation2004). For 1-min data during the episode shown in , interinstrument correlation coefficients range from 0.89 < R2 < 0.95 for NO. These are similar to though slightly lower than the NO2 range of 0.9 < R2 < 0.97, as well as the entire study averages given in . The occasional discrepancies between instruments for NO as seen in b give a recurrent hint as to the high time-varying (NO due to its primary emission) effects on instrument intercomparison observed during the combustion tests. Typical peaks in NO occurred in the early mornings, as shown in and detailed in , consistent with a traffic source and low mixing height at that time. NO2 peaks occurred at this site starting in late afternoon and often persisted overnight and into the next morning, indicative of its more secondary nature. At the finer time scale, all four methods compared well, as summarized in the 1-min regression statistics in . Absorption versus chemiluminescence methods diverged slightly more at the lowest concentrations, as seen from the spread of values of ±2 ppb near the origin in , , and . The absorption method showed the lowest NO2 values, though not beyond measurement uncertainties. It is difficult to discern whether the small offsets of ~1 ppb shown in are suggestive of interfering species at low concentrations (e.g., direct absorption vs. chemiluminescence for 1-hr NO2 gives an offset of −0.89 ppb). Observing similar magnitude offsets between the more specific NO2 methods of direct absorption and the CAPS instrument (as well as similar magnitude NO offsets for all) suggests calibration differences or instrumental precision as opposed to interfering species.
Figure 9. Time series of ambient (a) NO and (b) NO2 at the EPA NCORE site in Albuquerque, NM, for chemiluminescence (T200), NOy instrument (T200U-NOy), direct absorption (405), and CAPS (T500U) instruments on 14 March 2017 (1-min data).
In the Albuquerque urban measurements, the NOy instrument’s NOy channel (not shown graphically here) tracked closely (R2 > 0.99) to the NOx measurement from the same instrument —the two separated by an average of 0.1 ± 0.3 ppb. This indicated that NOy (18.4 ± 21.2 ppb for 1-hr data averaged over 2 months) in this urban area was almost entirely NOx (18.3 ± 21.1 ppb). This high NOx/NOy ratio shows a dominance of local sources (Kleinman et al. Citation2007), and suggests a limited influence of possible NOz (NOy – NOx) interfering species in this environment.
This contrasts with past measurements in a photochemically active urban area and an aged air mass rural area. Measurements by Dunlea et al. (Citation2007) in Mexico City showed a significant positive bias of NO2 via chemiluminescence that was attributed to HNO3 and gas-phase alkyl nitrate interferences generated during periods of high photochemistry. Production of these species was only important at high ozone concentrations (O3 up to 160 ppb during their study), and we attribute the general lack of NOz species here to limited photochemical production (modest O3 and low OH radical concentrations in this dry environment) during this season in Albuquerque.
Dunlea et al. (Citation2007) defined an interference as the chemiluminescence NO2 minus the NO2 from direct methods. Comparing to NO2 chemiluminescence, here the interferent signal was 1.0 ± 5.2 ppb via direct absorption NO2 versus −0.4 ± 2.0 ppb via CAPS NO2 (1-min data). The former difference indicates that while a small interference in the NO2 measurement is possible, it is of similar magnitude to instrumental uncertainties suggested by the latter (and negative) difference. Dunlea et al. (Citation2007) found that the interference signal correlated strongly to high ozone; here, with late winter measurements, the ozone concentrations in Albuquerque were low and exceeded 60 ppb for only 6 hr total over the 2-month period. Additionally, suspected low OH radical concentrations resulting from limited water vapor likely reduced NOx formation in Albuquerque, NM. Differences of up to 20% in summer NO2 concentrations were seen comparing chemiluminescence to the CAPS method in the more photochemically active summertime Beijing environment (Ge et al. Citation2013). In the Beijing study, relative humidity (RH) was higher than the desert southwestern United States and the ozone concentrations were consistently exceeding 60 ppb and at times as high as 120 ppb.
Measurements in rural locations have shown considerable differences in NOy versus NOx indicative of NOz species as well. Including a likely contribution from aged biomass smoke, NOy exceeded NOx in a rural, mountainous location in the western United States, with 1.37 versus 0.66 ppb for NOy versus NOx (Prenni et al. Citation2014). Despite the small magnitudes, Prenni et al. (Citation2014) showed NOy to NOx ratio ~2. The ratio for Albuquerque measurements in our study was close to 1.0; the NOy speciation was dominated by NO and NO2 during this period and showed little influence suggestive of interfering NOz species. We attribute this to (a) the measurements very close to the sources and thus fresh emissions and (b) the late winter season outside of the photochemical season.
Summary and conclusion
Ambient nitrogen oxides (NOx) are important to atmospheric photochemistry, human health, and atmospheric light attenuation, and now face more stringent regulation including a 1-hr standard. Here we intercompared four NOx techniques in ambient measurements in the urban core of Albuquerque, NM. These included a traditional chemiluminescence monitor, a direct absorption NO2 monitor, a NOy chemiluminescence instrument, and a cavity attenuated phase shift method. All methods had good agreement with known calibration concentrations and during propane torch emissions tests.
A subset of these instruments including the direct absorption and chemiluminescent technique were also compared during source testing from biomass combustion. For the rapidly changing laboratory measurements, the agreement of the two instruments degraded particularly for concentrations below approximately 100 ppb, typical of slow smoldering combustion. For example, similar burn conditions (fuel, mass), the higher temperature flaming burn produced NOx concentrations an order of magnitude higher (>800 ppb) than the lower temperature smoldering burn (<65 ppb). The discrepancies between chemiluminescence and direct absorption methods as seen with the smoldering burn in were subject to considerable instrumental noise, likely combined with some variability in the instruments’ ability to distinguish between NO2 and NO due to instrumental response times. These averaging times (1 min) were, however, short compared to those used for typical ambient measurements (1 to 24 hr). The two methods showed better agreement (slopes of 0.94 to 0.96) when, for concentrations below 2 ppm, signals were integrated over the course of an entire burn, though scatter was still considerable (R2 of 0.83 to 0.93). Overall, discrepancies were observed for both NO and NO2 and were not biased towards either instrument, suggesting that instrumental differences likely contributed to instrumental uncertainties and time response as much as any interference.
For ambient measurements in the urban core of Albuquerque, NM, the agreement between the four techniques was strong and within expected experimental uncertainties (R2 and slopes >0.95 for 1-hr NO2 comparisons). Even for an averaging time of 1 min, the regression statistics saw only modest degradation for the more slowly varying ambient measurements. Though small perturbations affected all techniques sporadically, none of the NOx instruments was a clear outlier for either NO or NO2. For the ambient measurements, we likewise and more definitively observed little evidence for significant interfering species. This was found for the direct and indirect NO2 methods and was confirmed with the “NOz” measurement from the NOy instrument, suggesting very little non-NOx species in this environment. These measurements provide insight into several commercially available NOx techniques, including two newer methods of direct light absorption and cavity attenuated phase shift instruments.
Acknowledgment
The authors acknowledge the constructive input of three anonymous reviewers to making this paper stronger.
Additional information
Funding
Notes on contributors
Caroline Allen
Caroline Allen earned a B.S. in environmental engineering at the New Mexico Institute of Mining and Technology and is currently a J.D. student at University of Missouri–Kansas City.
Christian M. Carrico
Christian M. Carrico is an associate professor in the Department of Civil and Environmental Engineering the New Mexico Institute of Mining and Technology.Samantha L. Gomez is an M.S. student in the Department of Civil and Environmental Engineering of the New Mexico Institute of Mining and Technology and a scientist at Los Alamos National Laboratory.
Samantha L. Gomez
Christian M. Carrico is an associate professor in the Department of Civil and Environmental Engineering the New Mexico Institute of Mining and Technology.Samantha L. Gomez is an M.S. student in the Department of Civil and Environmental Engineering of the New Mexico Institute of Mining and Technology and a scientist at Los Alamos National Laboratory.
Peter C. Andersen
Peter C. Andersen, Andrew A. Turnipseed, Craig J. Williford, and John W. Birks are scientists at 2B Technologies, Inc.
Andrew A. Turnipseed
Peter C. Andersen, Andrew A. Turnipseed, Craig J. Williford, and John W. Birks are scientists at 2B Technologies, Inc.
Craig J. Williford
Peter C. Andersen, Andrew A. Turnipseed, Craig J. Williford, and John W. Birks are scientists at 2B Technologies, Inc.
John W. Birks
Peter C. Andersen, Andrew A. Turnipseed, Craig J. Williford, and John W. Birks are scientists at 2B Technologies, Inc.
Dwayne Salisbury
Dwayne Salisbury, Richard Carrion, Dan Gates, and Fabian Macias are air quality specialists for the City of Albuquerque, NM.
Richard Carrion
Dwayne Salisbury, Richard Carrion, Dan Gates, and Fabian Macias are air quality specialists for the City of Albuquerque, NM.
Dan Gates
Dwayne Salisbury, Richard Carrion, Dan Gates, and Fabian Macias are air quality specialists for the City of Albuquerque, NM.
Fabian Macias
Dwayne Salisbury, Richard Carrion, Dan Gates, and Fabian Macias are air quality specialists for the City of Albuquerque, NM.
Thom Rahn
Thom Rahn, Allison C. Aiken, and Manvendra K. Dubey are scientists at the Los Alamos National Laboratory.
Allison C. Aiken
Thom Rahn, Allison C. Aiken, and Manvendra K. Dubey are scientists at the Los Alamos National Laboratory.
Manvendra K. Dubey
Thom Rahn, Allison C. Aiken, and Manvendra K. Dubey are scientists at the Los Alamos National Laboratory.
References
- Beckerman, B., M. Jerrett, J.R. Brook, D.K. Verma, M.A. Arain, and M.M. Finkelstein. 2008. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos. Environ. 42:275–290. doi:10.1016/j.atmosenv.2007.09.042.
- Benedict, K.B., A.J. Prenni, C.M. Carrico, A.P. Sullivan, B.A. Schichtel, and J.L. Collett. 2017. Enhanced concentrations of reactive nitrogen species in wildfire smoke. Atmos. Environ. 148:8–15. doi:10.1016/j.atmosenv.2016.10.030.
- Birks, J.W., P.C. Andersen, C.W. Williford, A.A. Turnipseed, C.E. Ennis, and E. Mattson. 2018. Folded tubular photometer for atmospheric measurements of NO2 and NO. Atmos. Meas. Tech. 11:2821–2835. doi:10.5194/amt-11-2821-2018.
- Carrico, C.M., S.L. Gomez, M.K. Dubey, and A.C. Aiken. 2018. Low Hygroscopicity of Ambient Fresh Carbonaceous Aerosols from Pyrotechnics Smoke. Atmos. Environ. 178:101–108. doi:10.1016/j.atmosenv.2018.01.024.
- Dunlea, E.J., S.C. Herndon, D.D. Nelson, R.M. Volkamer, F. San Martini, P.M. Sheehy, M.S. Zahniser, J.H. Shorter, J.C. Wormhoudt, B.K. Lamb, E.J. Allwine, J.S. Gaffney, N.A. Marley, M. Grutter, C. Marquez, S. Blanco, B. Cardenas, A. Retama, C.R.R. Villegas, C.E. Kolb, L.T. Molina, and M.J. Molina. 2007. Evaluation of nitrogen dioxide chemiluminescence monitors in a polluted urban environment. Atmospheric Chem. Phys. 7:2691–2704. doi:10.5194/acp-7-2691-2007.
- Fowler, D., M. Coyle, U. Skiba, M.A. Sutton, N. Cape, S. Reis, L.J. Sheppard, A. Jenkins, B. Grizzetti, J.N. Galloway, P. Vitousek, A. Leach, A.F. Bouwman, K. Butterbach-Bahl, F. Dentener, D. Stevenson, M. Amann, and M. Voss. 2013. The global nitrogen cycle in the twenty-first century. Philosophical Trans. Royal Soc. 368:20130164. doi:10.1098/rstb.2013.0164.
- Ge, B., Y. Sun, Y. Liu, H. Dong, D. Ji, Q. Jiang, J. Li, and Z. Wang. 2013. Nitrogen dioxide measurement by cavity attenuated phase shift spectroscopy (CAPS) and implications in ozone production efficiency and nitrate formation in Beijing, China. J. Geophys. Res. Atmos. 118:9499–9509. doi:10.1002/jgrd.50757.
- Gomez, S.L., C.M. Carrico, C. Allen, J. Lam, S. Dabli, A.P. Sullivan, A.C. Aiken, T. Rahn, D. Romonosky, P. Chylek, S. Sevanto, and M.K. Dubey. 2018. Southwestern U.S. biomass burning smoke hygroscopicity: The role of phenology, chemistry and combustion properties. J Geophys. Res. Atmos. 123. doi:10.1029/2017JD028162.
- Jacob, D. 1999. Introduction to Atmospheric Chemistry, 170–176. Princeton, USA: Princeton Press.
- Jimenez, A.S., M.R. Heal, and I.J. Beverland. 2011. Intercomparison study of NOx passive diffusion tubes with chemiluminescence analysers and evaluation of bias factors. Atmos. Environ. 45:3062–3068. doi:10.1016/j.atmosenv.2011.03.011.
- Kampa, M., and E. Castanas. 2008. Human health effects of air pollution. Environ. Pollut. 151:362–367. 10.1016/j.envpol.2007.06.012.
- Kebabian, P.L., E.C. Wood, S.C. Herndon, and A. Freedman. 2008. A practical alternative to chemiluminescence-based detection of Nitrogen Dioxide: Cavity attenuated phase shift spectroscopy. Environ. Sci. Technol. 42(16):6040–6045. doi:10.1021/es703204j.
- Kebabian, P.L., S.C. Herndon, and A. Freedman. 2005. Detection of nitrogen dioxide by cavity attenuated phase shift spectroscopy. Anal. Chem. 77(2):724–728. doi:10.1021/ac048715y.
- Kleinman, L.I., P.H. Daum, Y.-N. Lee, G.I. Senum, S.R. Springston, J. Wang, C. Berkowitz, J. Hubbe, R.A. Zaveri, F.J. Brechtel, J. Jayne, T.B. Onasch, and D. Worsnop. 2007. Aircraft observations of aerosol composition and ageing in New England and Mid-Atlantic States during the summer 2002 New England Air Quality Study field campaign. J. Geophys. Res. 112:D09310. doi:10.1029/2006JD007786.
- Minarro, M.D., E.G. Ferradas, C. Romero-Trigueros, and J.B. Rico. 2011. Do pressure changes have an influence on ambient air chemiluminescence NOx measurements? Atmos. Environ. 45:5366–5375. doi:10.1016/j.atmosenv.2011.06.048.
- NIFC, National Interagency Fire Center. 2017. Statistics www.nifc.gov. (accessed July 11, 2018).
- Prenni, A.J., E.J.T. Levin, K.B. Benedict, A.P. Sullivan, M.I. Schurman, K.A. Gebhart, D.E. Day, C.M. Carrico, W.C. Malm, B.A. Schichtel, J.L. Collett, and S.M. Kreidenweis. 2014. Gas-phase reactive nitrogen near Grand Teton National Park: Impacts of transport, anthropogenic emissions, and biomass burning. Atmos. Environ. 89:749–756. doi:10.1016/j.atmosenv.2014.03.017.
- Rocca, M.E., P.M. Brown, L.H. MacDonald, and C.M. Carrico. 2014. Climate change impacts on fire regimes and key ecosystem services in rocky mountain forests. Forest. Eco. Manage. 327:290-305. doi: 10.1016/j.foreco.2014.04.005.
- Thermo, E. C. 2004. Model 42C NO-NO2-NOx Analyzer. 1-20. www.thermo.com/eid (accessed July 11, 2018).
- Tidona, R.J., A.A. Nizami, and N.P. Cernansky. 1988. Reducing Interference Effects in the Chemiluminescent Measurement of Nitric Oxides from Combustion Systems. JAPCA 38(6):806–811. doi:10.1080/08940630.1988.10466421.
- U.S. EPA, Office of Air Quality Planning and Standards. 2017. Air Quality Assessment Division Quality Assurance Handbook for Air Pollution Measurement Systems Volume II, Ambient Air Quality Monitoring Program, EPA-454/B-17-001, RTP, NC.